Abstract
Deiodinases are selenoproteins that activate or inactivate thyroid hormone. During vertebrate development, these pathways control thyroid hormone action in a cell-specific fashion explaining how systemic thyroid hormone can affect local control of tissue embryogenesis. Here we investigated the role of the thyroid hormone-inactivating deiodinase (D3) in pancreatic islet function and glucose homeostasis. D3 expression was determined by real-time PCR, immunofluorescence, and enzyme activity. Embryonic and adult wild-type mice and Mice with targeted disruption of Dio3 gene (D3KO) as well as human fetal pancreas and adult islets were studied. Insulin secretion was evaluated in adult mouse isolated islets. We found Dio3 gene expression and protein highly expressed in embryonic and adult pancreatic islets, predominantly in β-cells in both humans and mice. However, mRNA levels were barely detectable for both the thyroid hormone-activating deiodinases types 1 and 2. D3KO animals were found to be glucose intolerant due to in vitro and in vivo impaired glucose-stimulated insulin secretion, without changes in peripheral sensitivity to insulin. D3KO neonatal (postnatal day 0) and adult pancreas exhibited reduced total islet area due to reduced β-cell mass, insulin content, and impaired expression of key β-cells genes. D3 expression in perinatal pancreatic β-cells prevents untimely exposure to thyroid hormone, the absence of which leads to impaired β-cell function and subsequently insulin secretion and glucose homeostasis. An analogous role is likely in humans, given the similar D3 expression pattern.
The role of thyroid hormone in glucose homeostasis is controversial (1, 2). Systemic hypothyroidism in mice promotes fasting hyperglycemia and reduces plasma insulin levels after glucose stimulation (3). Subsequently this delays the onset of type 2 diabetes in the leptin receptor-deficient rat (4). On the other hand, systemic hyperthyroidism reduces glucose tolerance and the insulin-secretory capacity of β-cells (5, 6), with the prevalence of diabetes mellitus being approximately doubled among hyperthyroid patients. These inconsistent observations have yet to be addressed and therefore might stem from the fact that none of these studies took into consideration the role played by the deiodinases in the pancreas.
Deiodinases constitute a group of three thyoredoxin-containing selenoproteins (7) that function as homodimers and control thyroid hormone signaling during development on a cell-specific basis (8). The type 2 deiodinase (D2) activates T4 to T3, the metabolically active form of thyroid hormone, and amplifies thyroid hormone signaling in developing tissues. On the other hand, the type 3 deiodinase (D3) inactivates T4 and T3 to rT3 and reverse T2, respectively, dampening thyroid hormone signaling in a cell-specific fashion (8). Thus, in vertebrates, deiodinases allow for rapid control of thyroid hormone action according to developmental timing, relatively independent of circulating hormone levels (9). This is illustrated in the developing chicken bone and brown adipose tissue as well as postnatally in cochlea and retina (10–13).
In fact, previous studies have shown that thyroid hormone and its thyroid hormone receptor-β-selective analog play a role in pancreatic development in Xenopus laevis (14), murine pancreatic β-cell mass regulation (15, 16), and pancreatic acinar tissue proliferation (17). However, a unifying model of thyroid hormone signaling in pancreatic islet function is missing. Thus, a fundamental question is whether intracellular changes in thyroid hormone signaling mediated via the deiodinases could affect pancreatic islet cells development, function, and/or glucose homeostasis.
In the present studies, we show how the local control of thyroid hormone action via D3 plays an important role in pancreatic β-cell biology. Furthermore, D3 is expressed in differentiating and adult pancreatic mouse and human islets. Mice with targeted disruption of Dio3 gene (D3KO) exhibit smaller pancreatic islets, reduced absolute β-cell mass, and decreased insulin content, with lower expression of key β-cell genes involved in glucose sensing, insulin synthesis, and exocytosis. As a result, D3KO animals are glucose intolerant due to impaired glucose-stimulated insulin secretion in vivo and in perifusion studies. Thus, reducing thyroid hormone signaling via the D3 pathway is critical for normal maturation and function of pancreatic β-cell. Together this study highlights D3 as a potentially important pathway in insulin secretion and glucose homeostasis.
Materials and Methods
Animals
All animal studies were approved by the Institutional Animal Care and Use Committed of University of Miami, Miller School of Medicine. Mice were fed ad libitum with a standard mouse chow diet under a 12-h light, 12-h dark cycle. Mice with disruption of the Dio3 gene (D3KO) were obtained from Dartmouth Medical School (Lebanon, NH). As described previously, these animals have perinatal hyperthyroidism followed by central hypothyroidism after weaning, reduced fertility, and increased rates of perinatal mortality (18). Genotyping of all offspring was performed by PCR on genomic DNA isolated from tail fragment of 3-wk-old mice. The primers used for the genotyping were (forward and reverse) 5′-GGAGTCCTGCTGCTTTTGTG-3′ and 5′-CGAGCCTCTCTGCAATTCAG-3′. The antithyroid drugs used were methimazole 0.05% (wt/vol) (Sigma-Aldrich, Inc., St. Louis, MO) and potassium perchlorate 1% (wt/vol) (Sigma-Aldrich), added to the drinking water for 21 d. All experiments were performed in male animals with 129/Sv/C57BL/6 mixed genetic backgrounds, obtained from heterozygous mating.
To minimize the effect of the mixed background present in the D3KO mice, we used only males siblings generated from heterozygous mating. Under these conditions, we are able to obtain wild-type, heterozygous, and homozygous D3KO animals. However, a complicating factor is that Dio3 behaves as an imprinted gene in different tissues, i.e. only the paternally inherited allele is active. Thus, crossing heterozygous does not allow for the determination of the Dio3 source. In mice the levels of imprinting of this gene may be different between tissues with the paternal allele contributing 84% of the expression in the embryo and 50–607% in the in the placenta (19, 20). Thus, heterozygous animals in our setting do not represent a model of haploinsuficiency. It represents a mix of animals that might have different levels of D3 activity in different cells. Thus, in our study heterozygous animals were not included.
Glucose and insulin tolerance tests
For glucose tolerance tests, mice were fasted for approximately 18 h, and blood samples were obtained from the tail vein to measure glucose levels using a portable glucometer (One Touch Ultra2; LifeScan Inc., Milpitas, CA) immediately before and 3, 5, 10, 15, 20, 30, 45, 60, 90, and 120 min after ip injection 2 g/kg glucose in 0.9% sodium chloride. For insulin tolerance tests, fed mice were injected with insulin 0.75 U/kg (Novolin; Novo Nordisk, Inc., Copenhagen, Denmark). A drop of blood was collected from the cut tail vein before the injection of insulin and after 15, 30, and 60 min to measure glucose levels as described above. Insulin tolerance tests were performed at 1400 h.
Immunofluorescence
Mouse and human pancreata were fixed by immersion in 4% paraformaldehyde overnight at 4 C, cryoprotected in 30% sucrose overnight at 4 C, and embedded with Tissue-Tek compound (Sakura Finetek USA, Inc., Torrance, CA). Sections (8 μm) were cut in cryostat. The following primary antibodies were used: guinea pig antiinsulin (1:50; DakoCytomation, Carpinteria, CA), rabbit antiglucagon (1:50; Dako Cytomation), mouse antiglucagon (1:1200; Sigma-Aldrich), mouse anticytokeratin19 (1:50; Covance Inc., Vienna, VA), rabbit antihuman D3 (1:500; kindly provided by Domenico Salvatore, University of Naples, Naples, Italy) (21), rabbit antimouse D3 (1:1000; Novus Biologicals, Littleton, CO). D3 control peptide NMP1-05767 (Novus Biologicals) was used as negative control for the rabbit antimouse D3 antibody, No staining was observed when the antibody was preincubated with the peptide (Supplemental Fig. 1E, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). All secondary antibodies used were Alexa conjugated (1:400; Molecular Probes, Inc., Eugene, OR). Images were obtained using a Zeiss LSM 510 scanning confocal microscope (Carl Zeiss, Inc., Thornwood, NY). The optical thicknesses of the channels were equalized before image acquisition.
Islet histomorphometry
To quantify the number of endocrines cells in the islet, longitudinal sections of total pancreas were immunostained as describe above. Pictures were taken using ×10 objective lens in an ApoTome microscope (Carl Zeiss), and the total area of the islet was measured using ImageJ software 1.43 (National Institutes of Health, Bethesda, MD). The total areas of insulin and glucagon cell were quantified using MetaMorph image analysis software (Molecular Devices, Sunnyvale, CA). Four to five pancreata from 2-month-old D3KO and wild-type littermates were processed. At least six sections, separated by 250 μm, were analyzed for each pancreas.
Quantitative real-time PCR
Human islet were obtained from deceased donor pancreata for which consent for research use was obtained and that were processed at the Diabetes Research Institute Human Cell Processing Facility-Islet Cell Resources Consortium (Miami, FL). RNA was extracted from total pancreas and mouse or human islets using RiboPure kit (Ambion, Austin, TX), and cDNA was prepared using a high-capacity cDNA reverse transcriptase kit (Applied Biosystems, Foster City, CA), following the manufacturer's protocols. PCR were run in duplicate using Taqman gene expression assays (Applied Biosystems) in a StepOnePlus real-time PCR system (Applied Biosystems). The relative quatification of the gene expression was measured based on the equation relative quatification = 2^-ΔCt, where ΔCt is the difference between the cycle threshold (Ct) value (number of cycles at which amplification for a gene reaches a threshold) of the target gene and the Ct value of the ubiquitous housekeeping gene 18s. Primers were validated using a standard curve prepared with a mixture of cDNA from various mouse pancreata (r2 > 0.99 and efficiency approximately 100%). The primers used are listed in Table 1.
Table 1.
Primer sequences
| Gene name (abbreviation) | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Genotyping primer | 5′-GGAGTCCTGCTGCTTTTGTG-3′ | 5′-CGAGCCTCTCTGCAATTCAG-3′ |
| Mouse quantitative RT-PCR primers | ||
| D1 (Dio1) | 5′-CCACCTTCTTCAGCATCC-3′ | 5′-AGTCATCTACGAGTCTCTTG-3′ |
| D2 (Dio2) | 5′-GTCCGCAAATGACCCCTTT-3′ | 5′-CCCACCCACTCTCTGACTTTC-3′ |
| D3 (Dio3) | 5′-GTTTTTGGCTTGCTCTCAGG-3′ | 5′-CAACAAGTCCGAGCTGTGAA-3′ |
| Insulin1(Ins1) | Mm01950294_s1a | |
| Insulin2 (Ins2) | Mm00731595_gHa,b | |
| Insulin promoter factor 1 (PDX1/IPF1) | Mm00435565_m1a | |
| Glucose transporter, member 2 (Glut 2/Abcc8) | Mm00803450_m1a | |
| Glucokinase | Mm00439129_m1a | |
| Neurogenic differentiation 1 (NeuroD1) | Mm01280117_m1a | |
| ATP channel subunit SUR1 | Mm00446224_m1a | |
| ATP channel subunit Kir6.2 | Mm00440050_s1a | |
| Eukaryotic 18 rRNA | 5′-GTAACCCGTTGAACCCCATT-3′ | 5′-CCATCCAATCGGTAGTAGCG-3′ |
| Human quantitative RT-PCR primers | ||
| D1 (Dio1) | 5′-ACCACGACAACTGGATACCG-3′ | 5′-ACTCCCAAATGTTGCACCTC-3′ |
| D2 (Dio2) | 5′-ACTTCCTGCTGGTCTACATTGATG-3′ | 5′-CTTCCTGGTTCTGGTGCTTCTTC-3′ |
| D3 (Dio3) | 5′-TGGGACCAGAGGAAGATAGC-3′ | 5′-AGTCCTAGGCAGAGCTGCTG-3′ |
| Cyclophilin A (Cyclo A) | 5′-GGCAAATGCTGGACCCAACAC-3′ | 5′-TGCCATTCCTGGACCCAAAGC |
Applied Biosystems Taqman assay number.
Recommended assay for this gene.
Islet perifusion
Islets from 2-month-old mouse D3KO and wild-type (WT) littermates (n = 5) were isolated at the DRI Preclinical Cell Processing and Translational Models Core by collagenase digestion (Sigma) followed by ficoll density gradients purification (Cellgro, Manassas, VA) as described. Isolated islets were cultured in CMRL-1066 media (Life Technologies, Inc., Carlsbad, CA) supplemented with 10% fetal bovine serum, 1% glutamax, 100 U/ml penicillin, 100 μg/ml streptomycin, and 25 mm HEPES and incubated in 95% air and 5% carbon dioxide (CO2) at 37 C for 24 h.
Insulin secretion from the isolated islet was assayed using a customized high-capacity, automated perifusion system (Biorep Inc., Miami, FL), as described (22). A low-pulsatility peristaltic pump pushed HEPES-buffered solution (in millimoles):125 NaCl, 5.9 KCl, 2.56 CaCl2, 1 MgCl2, 25 HEPES, and 0.1% BSA (pH 7.4); glucose concentration was adjusted to 3 mm for all experiment at a perifusion rate of 100 μl/min through a column containing 100 pancreatic islets immobilized in Bio-Gel P-4 gel (Bio-Rad, Hercules, CA). Stimuli (11 mm glucose or 30 mm KCl) were applied with the perifusion buffer. The perifusate was collected in an automatic fraction collector designed for a 96-well plate format and was kept at less than 4 C. The column containing islets and the perifusion solutions were kept at 37 C. Perifusates were collected every minute. Insulin release in the perifusate was determined using an ultrasensitive mouse insulin ELISA kit (Mercodia AB, Uppsala, Sweden), and data were normalized by DNA quantification using Quanti-iT PicoGreen double-stranded DNA kit (Molecular Probes) (23).
Hormone measurements
Blood was collected as described above using EDTA as anticoagulant and centrifuged for 10 min at 1000 × g within 30 min of blood collection. Plasma was collected and stored at −80 C. A total of 15 μl of plasma was used to measure thyroid hormones levels by Luminex multiplex technologies with three-plex beads, T3, T4, and TSH (Millipore Corp., Billerica, MA) according to the manufacturer's recommendation. For measurements of insulin, glucagon, and leptin, approximately 50 μl of blood was collected in parallel with glucose levels from the tail using heparinized capillary tube (Scientific Glass Inc., Rockwood, TN) into a microtainer tube with EDTA (Becton Dickinson, Franklin Lakes, NJ) before and 2, 5, 20, and 40 min after ip glucose injection. Plasma levels of insulin, glucagon, and leptin were determined using a Millipex mouse endocrine immunoassay kit (Millipore). Total pancreatic insulin and glucagon content was extracted using acid ethanol (75% EtOH, 0.2 m HCl) and measured by Millipex mouse endocrine immunoassay kit (Millipore).
Deiodinase assays
Type 1 deiodinase (D1) and D2 activities were assayed as described (24). D3 activity was assayed as described after minor adaptations for pancreatic tissue (25). Pancreatic islets were isolated from a pool of six wild-type animals and three D3KO animals. For each group, about 1000 islets were sonicated in a buffer of 10 mm dithiothreitol and 0.25 m sucrose, supplemented with a cocktail of protease inhibitors (Roche Applied Science, Indianapolis, IN). Twenty micrograms of protein for each sample in duplicate were incubated at 37 C for 6 h with outer-ring-labeled 125I-T3, 0.1 nm T3 substrate, 1 mm propylthiouracil, and 10 mm dithiothreitol. Deiodination products were resolved and quantified by Ultra Performance Liquid Chromatography (ACQUITY; Waters Corp., Milford, MA). All samples for each group were alternatively incubated with 100 nm T3 to determine background levels.
Statistical analysis
Statistical comparisons were performed using Student's t test for pair-wise comparisons or one-way ANOVA for the analysis of multiple groups followed by Tukey or Dunnett analysis (GraphPad Prism Software, San Diego, CA). Differences with a P < 0.05 were considered statistically significant.
Results
D3 is the predominant deiodinase expressed in mouse and human pancreas
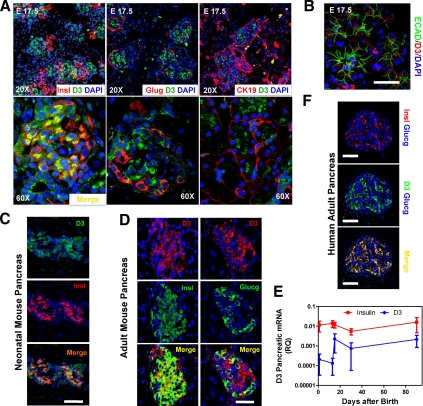
D3 expression as assessed by immunofluorescence was first observed in mouse pancreatic tissue at embryonic day (E) 17.5 (Fig. 1A). At this time point, D3 protein was found in cells expressing the epithelial markers cytokeratin 19 (CK19) (Fig. 1A) and E-cadherin (Fig. 1B). CK19 has been found in ductal cells of normal pancreas (26), which have endocrine pancreatic precursor/stem cell potential (27–29). Colocalization of D3 with glucacon-expressing cells was rare (Fig. 1A). D3-expressing insulin positives cells were found at this stage but not abundantly (Fig. 1A). At early neonatal stage [postnatal day (P) 0], the D3 expression pattern changed and was restricted to the clusters of islet cells, mostly colocalizing with insulin-producing cells (Fig. 1C). Later, in 2-month-old mice, islet D3 expression persisted, predominantly in insulin-expressing cells and less in glucagon-expressing cells (Fig. D). D3 mRNA expression in mice peaked at P15 and remained at similar levels throughout adulthood, mirroring the pattern observed for insulin mRNA (Fig. 1E). There was measurable D3 activity of approximately 0.025 fmol T3 per minute per milligram proteins in isolated adult islets, which is about 2 orders of magnitude less than brain or placenta (30). No measurable mRNA for D1 or D2 was present in developing or adult mouse pancreas (data not shown).
Fig. 1.
D3 expression in mouse and human pancreas. A, Immunofluorescence of E17.5 wild-type embryonic pancreas with α-insulin, α-glucagon, or CK19 antibodies separately, all in red together with α-D3antibody (green) and 4′,6′-diamino-2-phenylindole (DAPI) staining for nuclei (blue). Upper panel, ×20 objective; lower panel, ×60 objective. B, Immunofluorescence of E17.5 wild-type embryonic pancreas with αE-cadherin antibody, a marker of epithelial cells (green), and α-D3 antibodies (red). Bar, 50 μm. C, Confocal immunofluorescence images of neonatal mouse pancreatic tissue stained with α-D3 (green), α-insulin (red) antibodies, and DAPI staining for nuclei (blue). Bars, 50 μm. D, Immunofluorescence of 2-month-old wild-type mouse pancreas with α-D3 (red), α-insulin or α-glucagon (green) antibodies, and DAPI (blue). Bars, 50 μm. E, Quantitative RT-PCR analyses of Dio3 and insulin mRNA; cDNA was prepared from total pancreas of wild-type mice (n = 4 for each age analyzed). Error bars, sem. F, Confocal analysis of human adult pancreas stained with α-D3 (green), α-insulin (red), and α-glucagon (blue) antibodies. Bars, 50 μm.
D3 mRNA was present in the human pancreas, with substantial expression observed at 11 wk of gestational age (Supplemental Fig. 1A), a critical time point characterized by sprouting followed by exponential increase in hormone expression (31). D3 mRNA was also found in human adult islets (Supplemental Fig. 1B), and D3 protein was detected by immunofluorescence in human insulin-positive cells (Fig. 1F). D2 mRNA in human fetal pancreas was highest at 8 wk of gestational age and progressively decreased with time (Supplemental Fig. 1C). Notably, quantitative RT-PCR Ct for D2 was about 40, indicating very low expression of D2 in this tissue.
Thus, the predominance of D3 expression in mouse and human insulin-positive cells indicates that, from the perinatal period to adulthood, thyroid hormone signaling is kept at low levels in β-cells, relatively independent of systemic thyroid hormone levels.
Neonatal and adult D3KO islets are smaller and have less insulin content
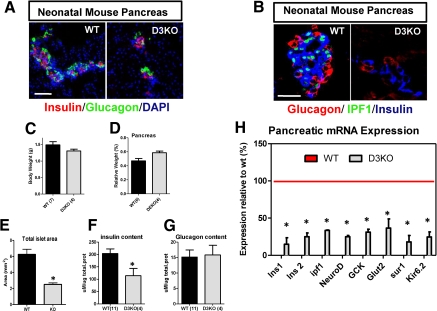
The role played by D3 in the islets was investigated in D3KO mice. At early neonatal stage (P0), the total pancreatic islet area was approximately 50% smaller in the D3KO animals (Fig. 2, A and E). However, at P0, D3KO mice exhibited similar body weight and relative pancreatic weight when compared with wild-type animals (Fig. 2, C and D). Remarkably, insulin content was approximately 35% less in the D3KO mouse (Fig. 2F), whereas glucagon content was not affected (Fig. 2G). Furthermore, D3KO pancreas obtained within the first 24 h after birth had marked reduction in the expression of key genes involved in glucose sensing (GCK, Glut2), insulin expression (Ins1 1, Insl 2, NeuroD, Pdx1), and secretion (sur1, Kir6.2), suggesting a developmental defect in the islet of these animals (Fig. 2H). ipf1/pdx1 expression by immunofluorescence was undetectable in pancreas from two different D3KO mice (Fig. 2B). This is in agreement with the very low levels of ipf1 mRNA levels found in the D3KO and may explain the high mortality rate observed in these animals (18). Homozygous ipf1 gene knockout mice survive fetal development but fail to develop a pancreas and die a few days after birth (32). In humans, mutations in the ipf1 homeobox gene produce maturity onset diabetes of the young type 4 (33). Despite the findings in the D3KO pancreas, blood glucose levels in fed P0 D3KO animals were similar to those of littermate wild-type mice (Supplemental Fig. 1D).
Fig. 2.
D3KO neonatal pancreatic islets are smaller and have less insulin content. A, Confocal immunofluorescence images of wild-type (WT) and homozygous D3KO (D3KO) neonatal mouse pancreatic tissue (P0) stained with α-insulin (red) and α-glucagon (green) antibodies, and 4′,6′-diamino-2-phenylindole (DAPI; blue). Bars, 50 μm. B, Confocal immunofluorescence images of wild-type (WT) and homozygous D3KO (D3KO) neonatal mouse pancreatic tissue (P0) stained with α-glucagon (red), α-ipf1/pdx1 (green), and α-insulin (blue) antibodies. Bars, 50 μm. C, Neonatal (P0) body weight in wild-type (WT; n = 7, back bar) and homozygous D3KO (n = 4, gray bar). Each bar represents mean ± sem. D, Relative pancreatic weight in wild-type (WT, n = 6, back bar) and homozygous D3KO (n = 4, gray bar). Each bar represents mean ± sem of total pancreatic weight normalized by body weight. E, Quantification of total neonatal (P0) pancreatic islet area in pancreatic sections from wild-type (WT; n = 5) and homozygous D3KO (KO; n = 4). Data represent mean ± sem. *, P < 0.05. F and G, Measurement of total insulin and glucagon content in isolated pancreas from (P0) neonatal wild-type (WT; n = 11, and homozygous D3KO, n = 4). Data represent mean ± sem. *, P < 0.05. H, Quantitative RT-PCR gene expression analysis of the indicated genes were performed using cDNA prepared from total pancreata of P0 wild-type (WT) and homozygous D3KO (D3KO). Data represented as percentage of expression relative to wild-type are considered 100% (red line). *, P < 0.05.
We next studied the pancreas of 2-month-old D3KO mice and found that the defects observed in the newborns persisted through adulthood. First, we confirmed that D3 activity in isolated islets of D3KO animals was at background levels (Fig. 3A). Subsequently the D3KO animals were found to be approximately 20% lighter (Fig. 3B) and to have normal relative weight of the pancreas (Fig. 3C). The distribution of the endocrine cells was unchanged in the D3KO islets, with insulin-expressing cells being the most abundant and clustered in the core of the islets, and glucagon-positive cells localized in the periphery (Fig. 3D). The total islet area (Fig. 3E), the absolute area of insulin-positive cells (Fig. 3E) and the total insulin content of the pancreas (Fig. 3F) were reduced in the D3KO mice. No changes in glucagon content were observed (Fig. 3G), indicating that D3 inactivation did not affect glucagon-expressing cells. There was a dramatic reduction in the isolated islet expression of key genes involved in glucose-stimulated insulin secretion (Fig. 3H), including glucose sensing (GCK, Glut2), insulin expression (Ins1, Ins 2, NeuroD, Pdx1), and secretion (sur1, Kir6.2).
Fig. 3.
Reduction in total islet area and insulin content persist until adulthood in D3KO mice. A, Adult pancreatic islet D3 activity. Bars, mean ± sem of sonicated islets isolated from a pool of wild-type (n = 7, black bar) and D3KO−/− (n = 3, gray bar) run in duplicate. *, P < 0.05. B, Body weight in 2-month-old wild-type (back bar) and homozygous D3KO (gray bar) mice. Each bar represents mean ± sem (n = 5). *, P < 0.05. C, Relative pancreatic weight in adult wild-type (n = 5, black bar) and homozygote D3KO (n = 5, gray bar). Each bar represents mean ± sem of total pancreatic weight normalized by body weight. D, Immunofluorescence of 2-month-old mouse pancreas with α-insulin (green) and α-glucagon (red) antibodies in wild-type (WT) and D3KO−/− (D3KO) mice. Bar, 35 μm. E, Quantification of total pancreatic islet area and absolute are of insulin- and glucagon-expressing cells in pancreatic section from wild-type (n = 4, black bar) and D3KO−/− (n = 4, gray bar) in 2-month-old mice. Data represented as percentage of expression relative to wild-type are considered 100%. *, P < 0.05. F and G, Measurement of total insulin and glucagon content in isolated total pancreas from adult wild-type (n = 5, black bar) and D3KO−/− (n = 6, gray bar) mice. Data represent mean ± sem. *, P < 0.05. H, Quantitative RT-PCR gene expression analysis of the indicated genes were performed using islet cDNA prepared from islet of 2-month-old wild-type (n = 5) and D3KO−/− (n = 4) mice. Data represented as percentage of expression in the D3KO relative to wild-type are considered 100%. *, P < 0.05.
To test whether these genes were a direct target of T3 transcriptional effects, isolated adult wild-type pancreatic islets were incubated with the D3 inhibitor iopanoic acid (20 μm) (25) and/or to 100 nm T3 for 24 h, but all failed to respond to the treatment with T3 (Supplemental Fig. 2D).
D3KO mice exhibit intolerance to glucose due to impaired insulin secretion
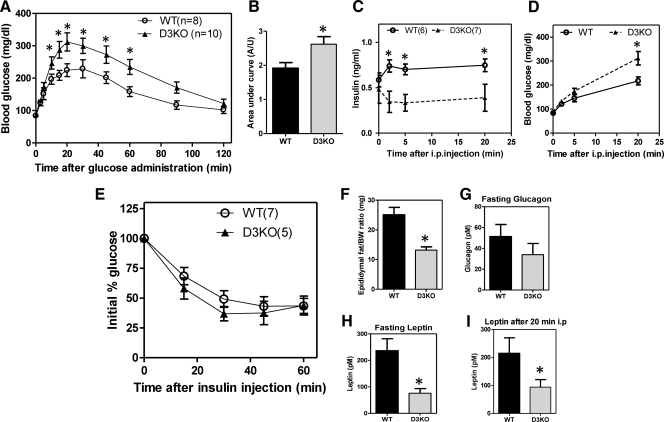
Although the fasting blood glucose levels of the D3KO mouse are normal (Fig. 4A), the ip glucose tolerance test indicated that these animals are glucose intolerant. Starting at 10 min after the glucose injection, D3KO mice had persistent and significantly higher levels of blood glucose (through 60 min; Fig. 4A). Analysis of the integrated area under the curve confirmed the glucose intolerance of the D3KO animals (Fig. 4B). Plasma insulin levels after overnight fasting were normal in the D3KO animal (Fig. 4C). However, at every time point after glucose administration, D3KO plasma insulin levels were significantly reduced when compared with wild-type mice (Fig. 4C). At the same time, in these very same animals, there was a significant increased in blood glucose levels in D3KO mice at the 20-min time point (Fig. 4D). Notably, the insulin tolerance test was unaffected in the D3KO animals (Fig. 4E), indicating that changes in peripheral insulin sensitivity are not present in these animals at the early age of 2 months. It remains to be determined whether insulin sensitivity is modified in older animals. In fact, D3KO mice have less adiposity as evidenced by an approximately 50% reduction in epididymal fat (Fig. 4F) and leptin levels, both before and 20 min after glucose injection (Fig. 4, H and I). Fasting glucagon levels were also normal in the D3KO mice (Fig. 4G). To rule out the possibility that systemic hypothyroidism per se could play a role in the D3KO phenotype, we studied adult wild-type mice made hypothyroid to exhibit serum T4 similar to D3KO animals [but elevated serum TSH (Supplemental Fig. 2A-B) having found a normal ip glucose tolerance test (Supplemental Fig. 2C)]. Taken together, these results indicate that local (pancreatic islet) dampening of thyroid hormone signaling, rather than systemic disruption in thyroid hormone homeostasis, is the key element playing a role in the D3KO pancreatic phenotype.
Fig. 4.
Mice with target disruption of Dio3 gene are glucose intolerants. A, Blood glucose concentrations at the indicated time points after ip glucose injection in 2-month-old wild-type (WT; n = 8) and homozygous D3KO (D3KO; n = 10) animals. Data represent the mean ± sem. *, P < 0.05. B, Quantification of the area under the curve in A. Data represent mean ± sem. *, P < 0.05. C, Plasma insulin levels before and after ip glucose injection in homozygous D3KO (n = 6) and wild-type (WT; n = 7). D, Blood glucose concentration simultaneously at the time point of blood collection in C. Data represent mean ± sem. *, P < 0.05. E, Blood glucose concentration at the indicated time points before and after ip injection of regular human insulin (0.75 U/kg body weight) in adult wild-type (WT; n = 7) and D3KO−/− littermate mice (n = 5). F, Average weight of epididymal fat in adult wild-type, (black bar, n = 4) and D3KO−/− (gray bar, n = 4). Data represent mean ± sem. *, P < 0.05. G, Fasted plasma levels of glucagon in wild-type (WT; n = 12) and D3KO−/− (D3KO; n = 10). Data represent mean ± sem. H and I, Plasma levels of leptin in overnight fasted wild-type (black bar, n = 12) and D3KO−/− (gray bar, n = 10) animals and after 20 min of ip of glucose injection. Data represent the mean ± sem. *, P < 0.05.
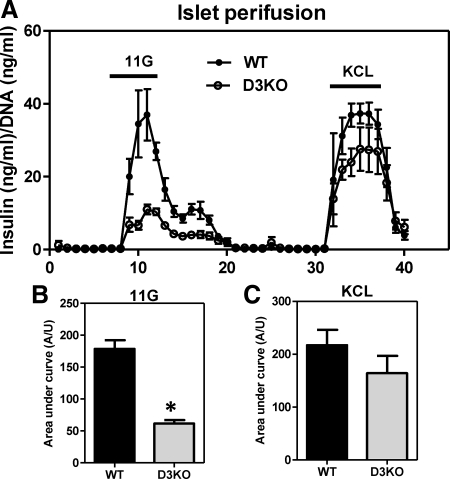
To confirm that the defect observed in the D3KO animals involves impaired insulin secretion, in vitro perifusion studies were performed in D3KO isolated islet. Remarkably, insulin release in response to 11 mm glucose was markedly reduced in D3KO islets, whereas the subsequent response to 30 mm KCl was minimally affected, not reaching significant levels (Fig. 5, A–C).
Fig. 5.
D3KO adult mice display impaired glucose-stimulated insulin secretion. A, Perifusion assay shows that 30 mm of KCL induces insulin secretion in both wild-type and D3KO adult islets. However, a stimulatory dose of 11 mm glucose (11G) for 15 min was unable to stimulate insulin secretion in D3KO−/− adult islets. Graph shows an average (n = 3 mouse islet perifusion experiment and error bar indicated sem). B and C, Area under the curve of the region generate after the stimulus with (11 mm) glucose and (30 nm) KCL, respectively. Data represent the mean ± sem. *, P < 0.05.
Discussion
D3 is highly expressed in the pregnant uterus, placenta, and mammalian fetal tissues, in which it has been implicated in the maternal-fetal transfer of thyroid hormone and development of the brain, skin, liver, bone, ovary, testis, intestine, and brown adipose tissue (10, 34, 35). The present study reveals that in the β-cells thyroid hormone signaling is kept to a minimum via D3 expression from late embryonic development throughout adulthood (Fig. 1). Based on what is known about D3's role in other systems (25, 36), it is likely that the absence of D3 in the β-cells, such as in the D3KO mouse, leads to an untimely exposure to thyroid hormone that disrupts normal β-cell maturation. This is associated with reduced total islet area, decreased absolute β-cells area and insulin content, and lower expression of key genes involved in glucose sensing, insulin expression, and exocytosis (Figs. 2 and 3). These alterations are physiologically significant and explain the phenotype of glucose intolerance resulting from impaired insulin secretion observed in the adult D3KO mouse (Figs. 4 and 5). Notably, despite significant reduction in the D3KO epididymal fat (Fig. 4G) and in leptin levels (Fig. 4H), the peripheral sensitivity to insulin was not affected in the D3KO animal (Fig. 4E).
A conceivable mechanism explaining the pancreatic phenotype in the D3KO mice is that absence of D3 in perinatal β-cells leads to exposure of high levels of thyroid hormone. Two potential factors contribute with this increase, i.e. a systemic factor due to increase in plasma T3 levels and a local factor due to D3 inactivation. However, the D3KO mouse model does not allow for the determination of the relative importance of systemic vs. a local mechanism. The fact that the body weight of the P0 newborns is normal (Fig. 2C) and that the relative pancreatic weight is normal (Fig. 2D) indicates that the systemic thyrotoxicosis during embryogenesis is not a major determinant in this animal model. Investigation of serum T3 levels at E19 reveal that they are only moderately elevated (18). At the same time, the defect is selective to β-cells (Fig. 3E) and their insulin content (Figs. 2F and 3F), which strongly support the idea that localized D3 activity on a cell-specific basis is playing the major role in the determination of the D3KO pancreas phenotype. Importantly, the reduced expression of Glut2 and in particular GKC (37) in the D3KO (Figs. 2H and 3H) suggests that the basic defect in insulin secretion observed in D3KO islet (Fig. 5) is due to a deficiency in glucose metabolism rather that a defect in the exocytosis machinery. This conclusion is supported by the normal KCl-induced insulin secretion in the perifused D3KO islets (Fig. 5).
Thyroid hormone acts by regulating gene transcription, and thus, it is logical to expect that one or more critical genes in β-cell development and glucose metabolism are responsive to T3. Our analysis of multiple key β-cell genes revealed a number of potential candidate genes found to be highly dysregulated in the neonatal and adult D3KO pancreas (Figs. 2H and 3H). However, all of these genes failed to respond to direct in vitro stimulation with 100 nm T3 for 24 h in isolated adult wild-type islets (Supplemental Fig. 2D), indicating that there is a developmental window during which these genes are T3 sensitive or indirectly regulated by T3. In addition, we dismissed adult hypothyroidism in the D3KO mouse as a potential mechanism, given that hypothyroidism (induced by antithyroid drugs) in wild-type animals failed to promote glucose intolerance (Supplemental Fig. 2, A–C).
Notably, the pattern of D3 expression in pancreas (Fig. 1) is the opposite of that observed in most embryonic tissues, in which D3 activity peaks early and decreases toward the end of gestation (30). This is interesting because during the mouse pancreas development, glucagon-positive cells appear first, by approximately E9.5, whereas insulin-positives cells become visible around E14.5 (38, 39). Thus, the emergence of D3 expression in insulin positive cells at E17.5 is in agreement with it playing a role in β- and not α-cell development. It is conceivable that the late D3 induction is connected to a specific embryonic mechanism in β-cells, such as the retinoic acid pathway that is active in late development, a period during which high production of retinoic acid promotes the generation of Ngn3+ endocrine progenitor cells and their further differentiation into β-cells (40). In fact, Dio3 expression has been shown to be activated by the retinoic acid pathway in astrocytes (41).
Although the islet phenotype in the D3KO newborns (Fig. 2) strongly indicates a developmental role for D3 in β-cell, persistence of D3 expression in adulthood (Fig. 1), suggests a role for D3 in this setting as well. In fact, previous observations in adult rats indicate that transient thyrotoxicosis reduces insulin content and insulin secretion in response to glucose stimulation (5). This supports our findings that increased thyroid hormone signaling to the β-cell is detrimental for insulin secretion and thus the possible role played by D3 in adulthood. In the adult β-cell, however, the persistence of D3 expression is likely to be due to other mechanisms, possibly including the Hedgehog signaling and/or the hypoxia-inducible factor-1α (HIF-1α), both pathways known to activate Dio3 expression (25, 42). An active Hedgehog pathway has been reported in mature mouse pancreatic islets in a setting in which the use of the Hedgehog antagonist cyclopamine decreases insulin synthesis and secretion (43). Accordingly, Dio3 is potently up-regulated by Hedgehog signaling through a Gli-2-dependent mechanism in keratinocytes and basal cell carcinomas, in which it plays a role in cell proliferation (42).
At the same time, hypoxia and hypoxia mimetic agents induce Dio3 expression in different cell lines in which it decreases thyroid hormone signaling and the rate of energy expenditure (25). Hypoxia and HIF-1α also underlie the up-regulation of D3 expression in animal models of myocardial infarction (44), right ventricular hypertrophy (25), and brain ischemia (45). Thus, it is notable that HIF-1α was recently found in normoxic mouse and human islet β-cells, in which it plays a role in insulin secretion (46). Animals with β-cell-specific deletion of HIF-1α gene are glucose intolerant with impaired glucose-stimulated insulin secretion (46), a phenotype that is strikingly similar to the one we describe here for the D3KO mouse. These findings raise the interesting possibility that in the β-cell, D3 might be an important component of the HIF-1α pathway.
In conclusion, the present findings provide evidence that reduced thyroid hormone signaling in pancreatic β-cells during development (and potentially adulthood) is important for normal islet function and glucose homeostasis. The leading defect caused by the untimely exposure to thyroid hormone in the D3KO is impaired insulin secretion in response to glucose stimulation. D3 is also expressed in human fetal pancreas and adult pancreatic islet, suggesting that an analogous role is likely in human β-cells. Because D3 expression remains throughout adulthood in human and mouse β-cells, it would be interesting to explore the possibility that dysregulation of Dio3 could play a role in states of impaired insulin secretion. Moreover, suppression of thyroid hormone signaling could potentially be used as a strategy to improve β-cell function. These data therefore suggest that D3 could be a novel target for therapeutic intervention in insulin related pathologies, especially diabetes.
Acknowledgments
We thank the members of our laboratory for technical instruction, suggestion, and helpful discussion; Matthew L. Rosene, Kevin Johnson, and Dr. George McNamara for technical assistance; the Human Islet Cell Processing Facility at the Diabetes Research Institute (University of Miami) for providing the human adult pancreatic tissue and islets; Karen Bookbinder and Dr. Gerald Applegate for assistance with human fetal tissue procurement; Dr. Domenico Salvatore (University of Naples, Naples, Italy) for antihuman D3 antibody; Dr. Alejandro Caicedo and Barry Hudson (University of Miami) for critical reading of the manuscript. M.C.M., A.C.B., H.E., and A.P. conceived and designed the experiments; M.C.M., J.M., and Y.G. performed the experiments; A.F., G.S., and A.H. contributed with reagents/material/analysis tools; M.C.M. and A.C.B. wrote the manuscript; and A.H., H.E., A.P., and A.C.B. reviewed/edited the manuscript.
This work was supported in part by National Institutes of Health Grants DK077148-03 (to A.C.B.), DK077148-03S1 (to M.C.M.), MH083220 (to A.H.) and ATA 2010-021-R1 (to M.C.M). Dr. Domenico Salvatore (D.S.) from the University of Naples, Naples, Italy kindly provided the anti-human D3 antibody; the generation of this antibody was supported by grant DK79946.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- CK19
- Cytokeratin 19
- Ct
- cycle threshold
- D1
- type 1 deiodinase
- D2
- type 2 deiodinase
- D3
- type 3 deiodinase
- D3KO
- targeted disruption of Dio3 gene
- E
- embryonic day
- HIF-1α
- hypoxia-inducible factor-1α
- P
- postnatal day.
References
- 1. Lenzen S, Bailey CJ. 1984. Thyroid hormones, gonadal and adrenocortical steroids and the function of the islets of Langerhans. Endocr Rev 5:411–434 [DOI] [PubMed] [Google Scholar]
- 2. Chidakel A, Mentuccia D, Celi FS. 2005. Peripheral metabolism of thyroid hormone and glucose homeostasis. Thyroid 15:899–903 [DOI] [PubMed] [Google Scholar]
- 3. Taguchi Y, Tasaki Y, Terakado K, Kobayashi K, Machida T, Kobayashi T. 2010. Impaired insulin secretion from the pancreatic islets of hypothyroidal growth-retarded mice. J Endocrinol 206:195–204 [DOI] [PubMed] [Google Scholar]
- 4. Hwang IK, Kim IY, Kim YN, Yi SS, Lee YH, Ju EJ, Lee IS, Park IS, Won MH, Yoon YS, Seong JK. 2009. Effects of methimazole on the onset of type 2 diabetes in leptin receptor-deficient rats. J Vet Med Sci 71:275–280 [DOI] [PubMed] [Google Scholar]
- 5. Lenzen S, Panten U, Hasselblatt A. 1975. Thyroxine treatment and insulin secretion in the rat. Diabetologia 11:49–55 [DOI] [PubMed] [Google Scholar]
- 6. Ximenes HM, Lortz S, Jörns A, Lenzen S. 2007. Triiodothyronine (T3)-mediated toxicity and induction of apoptosis in insulin-producing INS-1 cells. Life Sci 80:2045–2050 [DOI] [PubMed] [Google Scholar]
- 7. Callebaut I, Curcio-Morelli C, Mornon JP, Gereben B, Buettner C, Huang S, Castro B, Fonseca TL, Harney JW, Larsen PR, Bianco AC. 2003. The iodothyronine selenodeiodinases are thioredoxin-fold family proteins containing a glycoside hydrolase clan GH-A-like structure. J Biol Chem 278:36887–36896 [DOI] [PubMed] [Google Scholar]
- 8. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC. 2008. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown DD. 2005. The role of deiodinases in amphibian metamorphosis. Thyroid 15:815–821 [DOI] [PubMed] [Google Scholar]
- 10. Hall JA, Ribich S, Christoffolete MA, Simovic G, Correa-Medina M, Patti ME, Bianco AC. 2010. Absence of thyroid hormone activation during development underlies a permanent defect in adaptive thermogenesis. Endocrinology 151:4573–4582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ng L, Hernandez A, He W, Ren T, Srinivas M, Ma M, Galton VA, St. Germain DL, Forrest D. 2009. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology 150:1952–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu A, Ng L, Ma M, Kefas B, Davies TF, Hernandez A, Chan CC, Forrest D. 2009. Retarded developmental expression and patterning of retinal cone opsins in hypothyroid mice. Endocrinology 150:1536–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dentice M, Bandyopadhyay A, Gereben B, Callebaut I, Christoffolete MA, Kim BW, Nissim S, Mornon JP, Zavacki AM, Zeöld A, Capelo LP, Curcio-Morelli C, Ribeiro R, Harney JW, Tabin CJ, Bianco AC. 2005. The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol 7:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mukhi S, Horb ME, Brown DD. 2009. Remodeling of insulin producing β-cells during Xenopus laevis metamorphosis. Dev Biol 328:384–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Columbano A, Pibiri M, Deidda M, Cossu C, Scanlan TS, Chiellini G, Muntoni S, Ledda-Columbano GM. 2006. The thyroid hormone receptor-β agonist GC-1 induces cell proliferation in rat liver and pancreas. Endocrinology 147:3211–3218 [DOI] [PubMed] [Google Scholar]
- 16. Furuya F, Shimura H, Yamashita S, Endo T, Kobayashi T. 2010. Liganded thyroid hormone receptor-α enhances proliferation of pancreatic β-cells. J Biol Chem 285:24477–24486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kowalik MA, Perra A, Pibiri M, Cocco MT, Samarut J, Plateroti M, Ledda-Columbano GM, Columbano A. 2010. TRβ is the critical thyroid hormone receptor isoform in T3-induced proliferation of hepatocytes and pancreatic acinar cells. J Hepatol 53:686–692 [DOI] [PubMed] [Google Scholar]
- 18. Hernandez A, Martinez ME, Fiering S, Galton VA, St. Germain D. 2006. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest 116:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. da Rocha ST, Edwards CA, Ito M, Ogata T, Ferguson-Smith AC. 2008. Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends Genet 24:306–316 [DOI] [PubMed] [Google Scholar]
- 20. Hernandez A, Fiering S, Martinez E, Galton VA, St. Germain D. 2002. The gene locus encoding iodothyronine deiodinase type 3 (Dio3) is imprinted in the fetus and expresses antisense transcripts. Endocrinology 143:4483–4486 [DOI] [PubMed] [Google Scholar]
- 21. Huang SA, Dorfman DM, Genest DR, Salvatore D, Larsen PR. 2003. Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab 88:1384–1388 [DOI] [PubMed] [Google Scholar]
- 22. Cabrera O, Jacques-Silva MC, Speier S, Yang SN, Köhler M, Fachado A, Vieira E, Zierath JR, Kibbey R, Berman DM, Kenyon NS, Ricordi C, Caicedo A, Berggren PO. 2008. Glutamate is a positive autocrine signal for glucagon release. Cell Metab 7:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jacques-Silva MC, Correa-Medina M, Cabrera O, Rodriguez-Diaz R, Makeeva N, Fachado A, Diez J, Berman DM, Kenyon NS, Ricordi C, Pileggi A, Molano RD, Berggren PO, Caicedo A. 2010. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic β cell. Proc Natl Acad Sci USA 107:6465–6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Christoffolete MA, Ribeiro R, Singru P, Fekete C, da Silva WS, Gordon DF, Huang SA, Crescenzi A, Harney JW, Ridgway EC, Larsen PR, Lechan RM, Bianco AC. 2006. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism. Endocrinology 147:1735–1743 [DOI] [PubMed] [Google Scholar]
- 25. Simonides WS, Mulcahey MA, Redout EM, Muller A, Zuidwijk MJ, Visser TJ, Wassen FW, Crescenzi A, da-Silva WS, Harney J, Engel FB, Obregon MJ, Larsen PR, Bianco AC, Huang SA. 2008. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest 118:975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. 1982. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31:11–24 [DOI] [PubMed] [Google Scholar]
- 27. Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. 2000. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med 6:278–282 [DOI] [PubMed] [Google Scholar]
- 28. Gmyr V, Kerr-Conte J, Belaich S, Vandewalle B, Leteurtre E, Vantyghem MC, Lecomte-Houcke M, Proye C, Lefebvre J, Pattou F. 2000. Adult human cytokeratin 19-positive cells reexpress insulin promoter factor 1 in vitro: further evidence for pluripotent pancreatic stem cells in humans. Diabetes 49:1671–1680 [DOI] [PubMed] [Google Scholar]
- 29. Aye T, Toschi E, Sharma A, Sgroi D, Bonner-Weir S. 2010. Identification of markers for newly formed β-cells in the perinatal period: a time of recognized β-cell immaturity. J Histochem Cytochem 58:369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bates JM, St Germain DL, Galton VA. 1999. Expression profiles of the three iodothyronine deiodinases, D1, D2, and D3, in the developing rat. Endocrinology 140:844–851 [DOI] [PubMed] [Google Scholar]
- 31. Jeon J, Correa-Medina M, Ricordi C, Edlund H, Diez JA. 2009. Endocrine cell clustering during human pancreas development. J Histochem Cytochem 57:811–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. 1997. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet 15:106–110 [DOI] [PubMed] [Google Scholar]
- 33. Stoffers DA, Ferrer J, Clarke WL, Habener JF. 1997. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet 17:138–139 [DOI] [PubMed] [Google Scholar]
- 34. Galton VA. 2005. The roles of the iodothyronine deiodinases in mammalian development. Thyroid 15:823–834 [DOI] [PubMed] [Google Scholar]
- 35. Capelo LP, Beber EH, Huang SA, Zorn TM, Bianco AC, Gouveia CH. 2008. Deiodinase-mediated thyroid hormone inactivation minimizes thyroid hormone signaling in the early development of fetal skeleton. Bone 43:921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pol CJ, Muller A, Zuidwijk MJ, van Deel ED, Kaptein E, Saba A, Marchini M, Zucchi R, Visser TJ, Paulus WJ, Duncker DJ, Simonides WS. 2011. Left-ventricular remodeling after myocardial infarction is associated with a cardiomyocyte-specific hypothyroid condition. Endocrinology 152:669–679 [DOI] [PubMed] [Google Scholar]
- 37. Grupe A, Hultgren B, Ryan A, Ma YH, Bauer M, Stewart TA. 1995. Transgenic knockouts reveal a critical requirement for pancreatic β cell glucokinase in maintaining glucose homeostasis. Cell 83:69–78 [DOI] [PubMed] [Google Scholar]
- 38. Pictet RL, Clark WR, Williams RH, Rutter WJ. 1972. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol 29:436–467 [DOI] [PubMed] [Google Scholar]
- 39. Edlund H. 2002. Pancreatic organogenesis—developmental mechanisms and implications for therapy. Nat Rev Genet 3:524–532 [DOI] [PubMed] [Google Scholar]
- 40. Oström M, Loffler KA, Edfalk S, Selander L, Dahl U, Ricordi C, Jeon J, Correa-Medina M, Diez J, Edlund H. 2008. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into β-cells. PLoS One 3:e2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pallud S, Ramaugé M, Gavaret JM, Lennon AM, Munsch N, St. Germain DL, Pierre M, Courtin F. 1999. Regulation of type 3 iodothyronine deiodinase expression in cultured rat astrocytes: role of the Erk cascade. Endocrinology 140:2917–2923 [DOI] [PubMed] [Google Scholar]
- 42. Dentice M, Luongo C, Huang S, Ambrosio R, Elefante A, Mirebeau-Prunier D, Zavacki AM, Fenzi G, Grachtchouk M, Hutchin M, Dlugosz AA, Bianco AC, Missero C, Larsen PR, Salvatore D. 2007. Sonic hedgehog-induced type 3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc Natl Acad Sci USA 104:14466–14471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thomas MK, Rastalsky N, Lee JH, Habener JF. 2000. Hedgehog signaling regulation of insulin production by pancreatic beta-cells. Diabetes 49:2039–2047 [DOI] [PubMed] [Google Scholar]
- 44. Olivares EL, Marassi MP, Fortunato RS, da Silva AC, Costa-e-Sousa RH, Araújo IG, Mattos EC, Masuda MO, Mulcahey MA, Huang SA, Bianco AC, Carvalho DP. 2007. Thyroid function disturbance and type 3 iodothyronine deiodinase induction after myocardial infarction in rats a time course study. Endocrinology 148:4786–4792 [DOI] [PubMed] [Google Scholar]
- 45. Freitas BC, Gereben B, Castillo M, Kalló I, Zeöld A, Egri P, Liposits Z, Zavacki AM, Maciel RM, Jo S, Singru P, Sanchez E, Lechan RM, Bianco AC. 2010. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J Clin Invest 120:2206–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cheng K, Ho K, Stokes R, Scott C, Lau SM, Hawthorne WJ, O'Connell PJ, Loudovaris T, Kay TW, Kulkarni RN, Okada T, Wang XL, Yim SH, Shah Y, Grey ST, Biankin AV, Kench JG, Laybutt DR, Gonzalez FJ, Kahn CR, Gunton JE. 2010. Hypoxia-inducible factor-1α regulates β cell function in mouse and human islets. J Clin Invest 120:2171–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]