Abstract
The genetic etiologies of male infertility remain largely unknown. To identify genes potentially involved in spermatogenesis and male infertility, we performed genome-wide mutagenesis in mice with N-ethyl-N-nitrosourea and identified a line with dominant hypogonadism and patchy germ cell loss. Genomic mapping and DNA sequence analysis identified a novel heterozygous missense mutation in the kinase domain of Polo-like kinase 4 (Plk4), altering an isoleucine to asparagine at residue 242 (I242N). Genetic complementation studies using a gene trap line with disruption in the Plk4 locus confirmed that the putative Plk4 missense mutation was causative. Plk4 is known to be involved in centriole formation and cell cycle progression. However, a specific role in mammalian spermatogenesis has not been examined. PLK4 was highly expressed in the testes both pre- and postnatally. In the adult, PLK4 expression was first detected in stage VIII pachytene spermatocytes and was present through step 16 elongated spermatids. Because the homozygous Plk4I242N/I242N mutation was embryonic lethal, all analyses were performed using the heterozygous Plk4+/I242N mice. Testis size was reduced by 17%, and histology revealed discrete regions of germ cell loss, leaving only Sertoli cells in these defective tubules. Testis cord formation (embryonic day 13.5) was normal. Testis histology was also normal at postnatal day (P)1, but germ cell loss was detected at P10 and subsequent ages. We conclude that the I242N heterozygous mutation in PLK4 is causative for patchy germ cell loss beginning at P10, suggesting a role for PLK4 during the initiation of spermatogenesis.
Although infertility affects about one in 10 couples, the cause is often unknown (1). Genetic abnormalities that affect the reproductive axis or spermatogenesis account for about 10–15% of male infertility (2). These include mutations in genes that regulate gonadotropin production, testis development, and spermatogenesis, including Y chromosome microdeletions (3–6). However, in 40–50% of cases, the underlying cause is unknown, and the diagnosis remains idiopathic (7, 8). Approximately one in 25 mammalian genes is expressed in the male germ line, reflecting the complexity of male reproduction and the numerous candidates for mutations (9).
There are currently over 400 mouse models with reproductive defects (10), and about 150 of these result in male infertility (11). The majority of these models were created by targeted mutagenesis of candidate genes (10). Alternatively, whole genome mutagenesis, coupled with screening for specific phenotypes, has the potential to identify novel genes previously unknown to play a role in a particular pathway (12). N-ethyl-N-nitrosourea (ENU) is a potent mutagen used for forward mutagenesis in a variety of species, including mice (13–16). It introduces single nucleotide transversions and transitions, favoring A/T→T/A transversions and A/T→G/C transitions, which typically result in missense mutations. In addition to unbiased identification of gene function, ENU mutations can also display a range of phenotypic effects (17).
In a screen for ENU-induced mutations that cause male hypogonadism, we identified Polo-like kinase 4 (Plk4) (also known as Sak, Snk/Plk-akin kinase) as a candidate gene. PLK4 has high homology in its N-terminal region to the Polo subfamily of serine/threonine kinases. However, the C-terminal domain is distinct, containing only one Polo box domain and three PEST domains [proline (P), glutamic acid (E), serine (S), and threonine (T)] (18). The Polo box domain is predicted to be a substrate binding site and is involved in homodimerization and subcellular localization (19). The PLK4 homolog in yeast (Saccharomyces cerevisiae), cell division cycle 5 (CDC5), is involved in sister chromatid separation (20) and exit from mitosis (21–24). Mouse PLK4 is most highly expressed in actively dividing tissues in the embryo and adult (18) and is expressed in a cell cycle-dependent manner with highest expression beginning at late G1 phase and declining upon completion of mitosis (25). Down-regulation of PLK4 in Drosophila and human cells leads to loss of centrioles. In Drosophila Sak mutants, centrioles are lost during the mitotic divisions preceding male meiosis, but the same number of primary spermatocytes is produced. However, the majority of spermatids lacks centrioles and is therefore unable to make axonemes (26). Overexpression of PLK4 in human cells results in centrosome amplification (27). Amplification is inhibited by degradation of PLK4 (28). In the presence of endogenous wild-type PLK4, stable expression of kinase-dead PLK4 results in centriole overduplication (29). Mimicking autophosphorylation of S305 enhances the ability of overexpressed PLK4 to induce centriole amplification and sequesters PLK4 in the centrosome (30). Overexpression of murine PLK4 in Chinese hamster ovary cells results in loss of viability over several cell divisions and shows a significant increase in multinucleation (25). Targeted knockout of Plk4 in mice (Plk4−/−) is embryonic lethal with developmental arrest after gastrulation [embryonic day (E)7.5] and evidence of increased mitotic and apoptotic cells (31). Homozygous Plk4−/− mouse embryonic fibroblasts and embryonic stem cells are nonviable. Heterozygous Plk4+/− embryonic fibroblasts have aneuploidy due to increased numbers of centrosomes and multipolar spindle formation. Plk4+/− mice show a 15-fold increase in liver and lung cancer (32). In humans, PLK4 has been implicated in hepatomas (32), hepatocellular carcinoma (33), and colorectal cancer (34).
In this report, we demonstrate that Plk4 plays a functional role in murine spermatogenesis and describe an unusual type of germ cell loss in mice harboring an ENU-induced missense mutation alerting an isoleucine to asparagine at residue 242 (I242N).
Materials and Methods
Animal husbandry and ENU mutagenesis
Plk4+/GT mice were generated with the assistance of the Northwestern University Transgenic and Targeted Mutagenesis Laboratory using a gene trap Plk4 ES cell line (XK603) from the International Gene Trap Consortium (35). Plk4+/I242N mice were generated via ENU mutagenesis as previously described (36). Mutagenesis was performed on a C57BL/6J background, and G1 males and females were crossed to wild-type C57BL/6J mice. For dominant crosses, 6- to 8-wk-old F1 progeny from 1014 G1 mice underwent a phenotypic screen for urogenital defects, which included body weight measurement, analysis of internal and external urogenital morphology, testis weight measurement, and testicular and epididymal histology. For studies of embryonic testes, breeders were placed in the same cage at 1600 h, and vaginal plugs were inspected at 0800 h the next day. The first morning was designated 0.5 days post coitus. All animals were bred in-house and fed a standard Harlan Teklad irradiated diet (Harlan Laboratories, Inc., Indianapolis, IN). All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Animals, and all studies were approved by the Northwestern University Animal Care and Use Committee.
Testicular weight, histology, and staging
Male Plk4+/+ and Plk4+/I242N mice were killed at E13.5, postnatal day (P)1, P10, P15, P21, P31, and 6 and 8 wk of age. Testis and body weights were recorded for all postnatal animals. Photographs of the testes of affected and unaffected littermates were taken at 6 wk. E13.5 animals were formalin fixed, paraffin embedded, and 5-μm sections were taken through the whole embryo and stained with hematoxylin and eosin (H&E). Postnatal testes were formalin fixed, paraffin embedded, and 5-μm cross-sections were stained with H&E or periodic acid Schiff and hematoxylin (PAS-H). Images were taken with a Zeiss Axioskop and an Optronics camera (Zeiss, Oberkochen, Germany). Staging of tubules was completed for seven affected and seven unaffected 6-wk-old animals. One testis cross-section per animal was stained with PAS-H, and all tubules were assigned a stage (I–XII) as described previously (37). Tubules that contained some germ cells but could not be staged were designated “unstaged.” Tubules containing only Sertoli cells but lacking germ cells were designated “Sertoli cell only” (SCO). Tubules completely devoid of all cells (germ and Sertoli) were designated “empty.” Additionally, each tubule was scored based on the presence or absence of specific germ cell populations. The scoring system was divided into five categories: all germ cells present, missing more than 50% of elongated spermatids, missing more than 50% of round spermatids, missing more than 50% of spermatocytes, and missing more than 50% of spermatogonia. The percentage of tubules within each stage and the analysis of the germ cell populations were then compared between unaffected and affected mice.
Sperm counts and motility
Eight-week-old Plk4+/+ (n = 6) and Plk4+/I242N (n = 5) males were euthanized by cervical dislocation after anesthesia, and one whole epididymis was dissected and placed into 1× PBS prewarmed to 37 C. The epididymis was cut, and the sperm were eluted for 30 min at 37 C. A 15-μl aliquot of a 1:10 dilution of the suspension was counted on a hemocytometer. Sperm that showed tail motion were designated as motile.
Serum hormone analysis
Ten animals of each genotype were used for serum hormone analyses. All hormones were assayed at the University of Virginia School of Medicine Ligand Assay and Analysis Core (Charlottesville, VA). LH levels were measured by a two-site sandwich immunoassay as previously described (38, 39). FSH levels were measured by RIA as previously described (40). Testosterone levels were measured by RIA using a Siemens Coat-A-Count Total Testosterone kit (TKTT2) (Siemens, Los Angeles, CA).
Genomic mapping
The G1 male was crossed to a wild-type DBA female to produce F1 progeny. Genomic DNA from 13 hypogonadal F1 mice was sent to the laboratory of David Beier at Harvard Medical School (Boston, MA) and analyzed using a 768 single nucleotide polymorphism (SNP) panel spanning the genome (41). Additional SNP mapping was completed by PCR amplification and sequencing. Genomic databases were used to identify all genes in the region, and exons of several genes were PCR amplified from genomic DNA of affected males and sequenced. A mutation was identified in exon 5 of Plk4. C57BL/6J and DBA background strains were sequenced to ensure the mutation was not a SNP. Nucleotide and amino acid numbers were designated according to GenBank reference sequence NM_011495.
Embryonic lethality and complementation analysis
To establish embryonic lethality, Plk4+/I242N male mice were bred to Plk4+/I242N female mice, and 25 progeny were genotyped. For the gene trap line, Plk4+/GT male mice were bred to Plk4+/GT female mice, and 36 progeny were genotyped. To determine complementation of the Plk4I242N and Plk4GT alleles, Plk4+/I242N male mice were bred to Plk4+/GT females, and 38 progeny were genotyped.
LacZ enzyme histochemistry
Embryos were dissected at E18.5, and organs were removed, fixed, and stained as previously described (42). Individual seminiferous tubule staining was completed by incubation of testes in PBS with 0.1% collagenase at 37 C for 1 h with rocking. Tubules were dissected further and placed in cold 2% paraformaldehyde/piperazine-N,N′-bis-2-ethanesulfonic acid (PFA/PIPES) buffer at 4 C for 1 h with rocking. Tubules were washed in concentrated rinse buffer for 10 min at 4 C with rocking. Testes were incubated in X-gal for 18 h at 37 C. Testes were rinsed in 1× PBS, and photographs were taken. Testes sections were stained by incubating dissected testes in 2% PFA/PIPES buffer for 3 h at 4 C with rocking. Testes were then incubated in 27% sucrose + 2 mm MgCl2 in 1× diethylpyrocarbonate PBS overnight at 4 C with rocking. Testes were embedded in OCT and cut into 14-μm sections on a cryostat. Sections were postfixed in 2% PFA/PIPES buffer on ice for 10 min, placed in concentrated rinse buffer on ice for 10 min, and stained with X-gal overnight. Sections were rinsed in tap water, stained with PAS-H, and staged as described above.
Immunohistochemistry and immunofluorescence
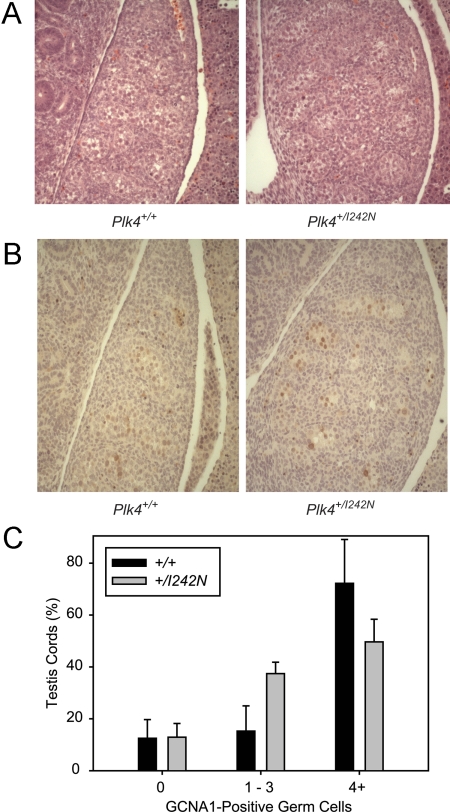
Germ cell immunohistochemistry was completed on 5-μm E13.5 or P15 testes sections from Plk4+/+ and Plk4+/I242N mice. Sections were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol. Antigen unmasking was completed in 10 mm sodium citrate in a microwave for 10 min. Endogenous peroxidases were blocked in 3% peroxide for 10 min. Slides were blocked in 10% normal goat serum for 15 min at room temperature. Sections were stained with undiluted antigerm cell nuclear antigen (GCNA)1 antibody (43) for 90 min at 33 C, rinsed, and then incubated in biotinylated goat antirat antibody (BA-9400; Vector Laboratories, Burlingame, CA) diluted 1:200 for 30 min at room temperature. Slides were incubated in RTU Horseradish Peroxidase Streptavidin (SA-5704; Vector Laboratories) for 15 min at room temperature. Slides were then visualized using diaminobenzidine and counterstained with hematoxylin. Testis cords from each E13.5 embryo section were classified as having 0, 1–3, or 4 or more germ cells. Counts were then converted into percentages based on the number of testis cords counted and compared between genotypes.
Sertoli cell immunofluorescence was completed on 5-μm P15 testes sections from Plk4+/+ and Plk4+/I242N mice. Sections were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol. Antigen unmasking was completed in 10 mm sodium citrate in a microwave for 15 min. Slides were blocked in 10% normal donkey serum for 1 h at room temperature. Sections were stained with anti-GATA4 (sc-1237; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:50 for 18 h at 4 C, rinsed, and then incubated in Alexa Fluor 488 (A11034; Invitrogen, Carlsbad, CA) diluted 1:100 for 1 h at room temperature. Slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI).
PLK4 immunohistochemistry was completed on 5-μm testes sections from 6-wk-old Plk4+/+ and Plk4+/I242N mice. Sections were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol. Antigen unmasking was completed in 10 mm sodium citrate in a microwave for 10 min. Endogenous peroxidases were blocked in 3% peroxide for 10 min. Slides were blocked in 5% normal goat serum for 1 h at room temperature. Sections were stained with anti-PLK4 antibody (12952-1-AP; Proteintech Group, Inc., Chicago, IL) for 20 h at 4 C, rinsed, and then incubated in biotinylated goat antirabbit antibody (BA-1000; Vector Laboratories) diluted 1:200 for 1 h at room temperature. Slides were incubated in RTU Horseradish Peroxidase Streptavidin (SA-5704; Vector Laboratories) for 15 min at room temperature. Slides were then visualized using diaminobenzidine.
Laminin immunofluorescence to assess testis cord organization was completed on 5-μm E13.5 sections from Plk4+/+ and Plk4+/I242N mice. Sections were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol. Antigen unmasking was completed in 10 mm sodium citrate in a microwave for 10 min. Slides were blocked in a mixture of 5% normal goat serum and 5% normal donkey serum for 1 h at room temperature. Sections were stained with antilaminin antibody (L9393; Sigma-Aldrich, St. Louis, MO) diluted 1:200 for 1.5 h at 33 C, rinsed, and then incubated in Alexa Fluor 594 antibody (A11037; Invitrogen) diluted 1:100 for 2 h at room temperature. Slides were counterstained with DAPI.
Centriole number was analyzed using immunofluorescence with an anti-γ-tubulin antibody. Five-micrometer sections from 6-wk-old Plk4+/+ and Plk4+/I242N mice were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol. Antigen unmasking was completed in 10 mm sodium citrate in a microwave for 15 min. Slides were blocked in 5% normal horse serum for 1 h at room temperature. Sections were stained with anti-γ-tubulin antibody (ab11316; Abcam, Cambridge, MA) diluted 1:100 overnight at 4 C, rinsed, and then incubated in fluorescein horse antimouse (FI-2000; Vector Laboratories) diluted 1:200 for 3 h at room temperature. Slides were counterstained with DAPI. Stage XII tubules were identified based on the presence of metaphase spreads visualized using DAPI. The number of centrioles per meiotic cell was counted within all stage XII tubules in one testis section. The percentage of meiotic cells with 0, 1, 2, or more than or equal to 3 centrioles was then compared between Plk4+/+ and Plk4+/I242N mice.
Statistical analysis
In all cases, comparisons were made between two groups and were assessed for normality. Data that showed a normal distribution were compared using a t test. Data that did not show a normal distribution were analyzed using a nonparametric Mann-Whitney U test.
Results
Testicular weight and histology
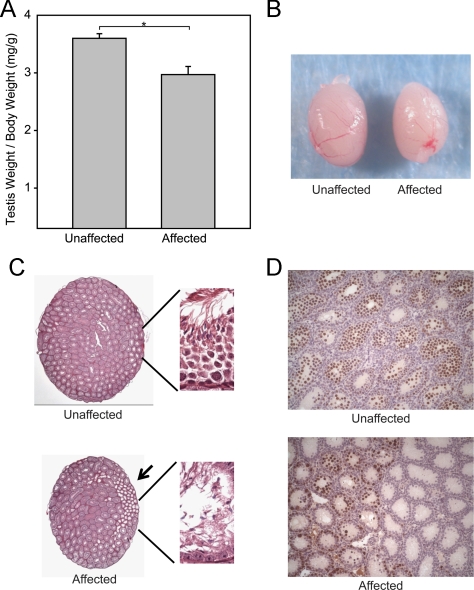
Testis to body weight ratios of affected animals were reduced 17.5% compared with unaffected littermates at 8 wk of age (Fig. 1, A and B). Histologically, affected animals showed discrete regions of abnormal seminiferous tubules (Fig. 1C, arrow), which were not found in unaffected littermates. P15 testes sections from affected mice stained with an anti-GCNA1 antibody showed that the defective tubules were devoid of germ cells, whereas adjacent tubules contained germ cells. Equivalent sections from unaffected mice showed a full complement of germ cells within all the tubules (Fig. 1D). P15 testes sections from affected mice revealed that the defective tubules contained GATA4 and DAPI positive cells, identifying them as Sertoli cells and verifying a SCO feature of the defective tubules. These tubules did not contain cells that stained only with DAPI (germ cells). Normal tubules within the affected mice and all the tubules within the unaffected mice contained both Sertoli cells and germ cells (data not shown).
Fig. 1.
Phenotypic features of the ENU mutant. A, Quantification of testis weights at 6 wk of age. B, Gross examination of whole testes at 6 wk of age. C, Histological features of testis cross-sections at 6 wk of age (magnification, ×2.5). Higher magnification (×63) views are shown in the insets, which include a region of defective tubules (black arrow). D, P15 sections from affected animals stained with an anti-GCNA1 antibody.
To determine whether there was a stage-specific defect, one testis cross-section from seven affected and seven unaffected 6-wk-old animals was stained with PAS-H, and each tubule was assigned a stage (I–XII). Tubules that could not be staged due to germ cell depletion, empty tubules, and SCO tubules were labeled as such. Additionally, each tubule was scored based on the presence or absence of specific germ cell populations (elongated spermatids, round spermatids, spermatocytes, and spermatogonia). The percentage of tubules within each stage and the analysis of the germ cell populations were then compared between affected and unaffected mice. The only statistically significant differences in stage were a small decrease in the percentage of tubules in stage I and stage VII in the affected mice (12.9 ± 1.6 and 10.1 ± 0.8%, respectively) compared with the unaffected mice (18.1 ± 0.5 and 14.6 ± 1.0%, respectively). Within stage I tubules, there was a slight but statistically significant difference between the percentage of stage I tubules containing all types of germ cells (unaffected 100% and affected 97.1 ± 1.1%) and those missing more than 50% of elongated spermatids (unaffected 0% and affected 2.9 ± 1.1%).
Serum hormones and sperm counts
Comparison of serum hormones between Plk4+/+ and Plk4+/I242N littermates did not show a significant difference in either LH or testosterone. In the Plk4+/I242N mice, FSH was increased 2-fold compared with Plk4+/+ littermates. Sperm counts and motility were not significantly different between Plk4+/+ and Plk4+/I242N littermates (Table 1).
Table 1.
Serum Hormone and Sperm Analysis. Serum hormone, sperm count and motility measurements from Plk4+/+ and Plk4+/I242N littermates at 6 weeks of age
| Testosterone (ng/dl) | LH (ng/ml) | FSH (ng/ml) | Total sperm (104/ml) | Motile sperm (%) | |
|---|---|---|---|---|---|
| Plk4+/+ | 259.4 ± 122.4 | 0.23 ± 0.07 | 19.6 ± 1.3 | 465 ± 93.6 | 27.0 ± 16.9 |
| Plk4+/I242N | 412.9 ± 127.8 | 0.45 ± 0.14 | 38.5 ± 1.7 | 395 ± 78.6 | 26.6 ± 8.8 |
| NS | NS | P < 0.001 | NS | NS |
NS, Not significant.
Genomic mapping
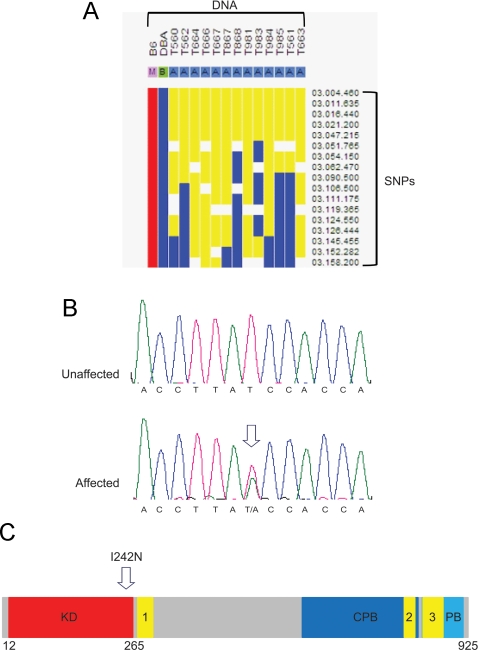
For genomic mapping, the founder C57BL/6J G1 male was crossed to a wild-type DBA female and DNA from the F1 progeny was analyzed using a genome-wide 768 SNP panel. The SNP panel yielded a 24-Mb region of heterozygosity on chromosome 3 (Fig. 2A). Further SNP and microsatellite mapping was completed using PCR amplification and sequencing, reducing the region to 8 Mb. Using an in silico approach, 12 candidate genes in the region were identified and included: Spry1, Intu, Sclt1, Phf17, Larp2, 3110057O12Rik, Slc25a31, Hspa4l, Plk4, D4Ertd751e, Mfsd8, and Pcdh10. Exons and exon-intron boundaries were sequenced, and no mutations were identified except in Plk4, in which there was a potential heterozygous missense mutation in exon 5, altering thymine 1028 to adenine, and changing amino acid 242 from isoleucine to asparagine (I242N) (Fig. 2B). The I242N amino acid substitution was located within the kinase domain (Fig. 2C). The remainder of the exons and exon-intron boundaries of Plk4 contained no alterations. Sequencing of parental strains used in the ENU mutagenesis and breeding (C57BL/6J and DBA) confirmed that the base change was not a strain-specific SNP (data not shown).
Fig. 2.
Identification of the ENU-induced Plk4 I242N heterozygous mutation. A, Genome-wide SNP mapping of DNA from affected F1 mice. Yellow indicates heterozygosity. B, Sequence variant in exon 5 of Plk4. C, Functional domains of PLK4 schematic. KD, Kinase domain; CPB, cryptic polo box; PB, polo box.
Genetic analysis
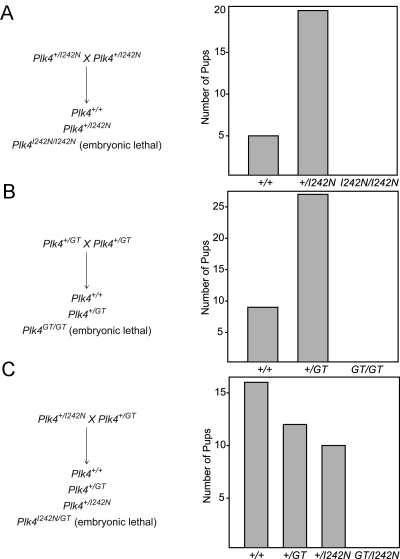
In progeny derived from crosses between two Plk4+/I242N mice, the ratio of Plk4+/+ progeny to Plk4+/I242N progeny was 1:4, and no viable Plk4I242N/I242N were identified (Fig. 3A), suggesting normal viability in the heterozygous state but embryonic lethality in the homozygous state. All males heterozygous for the mutation had hypogonadism and patchy germ cell loss. Conversely, all affected males were heterozygous for this mutation. Fertility was preserved in the Plk4+/I242N males.
Fig. 3.
Genetic complementation analysis of Plk4 alleles. A, Quantification of progeny from crosses between Plk4+/I242N mice. B, Quantification of progeny from crosses between Plk4+/GT mice. C, Quantification of progeny from crosses between Plk4+/I242N and Plk4+/GT mice.
Complementation analysis with the Plk4GT allele
Complementation analysis was completed using mice with a gene trap Plk4 allele (Plk4+/GT). The Plk4GT allele resulted in premature termination of the Plk4 gene after exon 8 and addition of a β-geo gene (fusion of β-galactosidase and neomycin transferase) at that location. Similar to the Plk4I242N/I242N mice, the Plk4GT/GT mice were embryonic lethal (Fig. 3B). To verify causality of the I242N mutation, Plk4+/I242N mice were bred with Plk4+/GT mice. Plk4+/+, Plk4+/GT, and Plk4+/I242N progeny was viable. However, the Plk4GT/I242N progeny were embryonic lethal (Fig. 3C). The complementation analysis therefore verified that the Plk4I242N allele was causative.
Plk4 expression
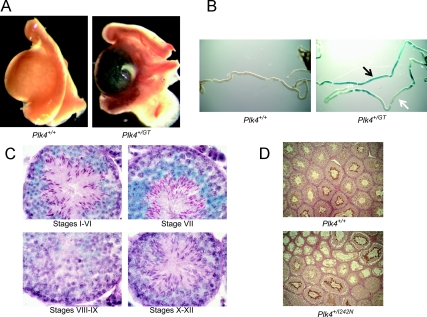
Whole mount LacZ staining of organs from E18.5 Plk4+/GT mice demonstrated LacZ expression within the testis (Fig. 4A), kidney, thymus, heart, and regions of the brain (data not shown). Expression in these organs was not present in Plk4+/+ littermates. High background in the stomach, small intestine, colon, and lung precluded assessment of these organs. Whole mount LacZ staining of individual tubules from 6-wk-old Plk4+/GT mice showed a varying pattern of expression along the length of the tubule (Fig. 4B). Some areas along the tubule showed intense blue staining (Fig. 4B, black arrow), whereas others lacked staining (Fig. 4B, white arrow). Staining was not present in Plk4+/+ littermates. Frozen sections from 6-wk-old Plk4+/GT mice stained with LacZ and PAS-H showed gene expression from the Plk4 locus starting in the stage VIII pachytene spermatocytes and continuing through stage VIII elongated spermatids (Fig. 4C). Staining was also present in the Leydig cells (data not shown). Immunohistochemical staining of testes sections from 6-wk-old Plk4+/+ and Plk4+/I242N mice with an anti-PLK4 antibody showed PLK4 protein in the tails of elongated spermatids in unaffected tubules in both Plk4+/I242N and Plk4+/+ mice (Fig. 4D). Expression was lowest in step 8 round spermatids in stage VIII and increased progressively until reaching maximum intensity in step 16 elongated spermatids in stage VII. The defective tubules of the Plk4+/I242N mice did not demonstrate any PLK4 staining.
Fig. 4.
Plk4 expression in the testis. A, Whole mount testis LacZ staining from E18.5 Plk4+/GT mice. B, Whole mount LacZ staining of seminiferous tubules dissected from testes of 6-wk-old Plk4+/GT mice. Black arrow indicates areas of high expression. White arrow indicates areas without expression. C, Sections from 6-wk-old Plk4+/GT mice stained with LacZ and PAS-H. D, Immunohistochemical staining of testes sections from 6-wk-old Plk4+/+ and Plk4+/I242N mice with an anti-PLK4 antibody.
Centriole number
Because Plk4 has been shown to regulate centriole replication (26, 27, 29, 30), centriole number was counted in testes sections from 6-wk-old Plk4+/+ and Plk4+/I242N mice stained with an anti-γ-tubulin antibody. An equivalent number of metaphase cells was counted for both genotypes (115 for Plk4+/+ and 113 for Plk4+/I242N). The average percent of meiotic cells with no centrioles (out of the plane of section) was 29 ± 5% in Plk4+/+ mice and 28 ± 5% in Plk4+/I242N mice. The average percent of meiotic cells with one centriole (one in the plane of section and one out of the plane of section) was 48 ± 4% in Plk4+/+ mice and 54 ± 7% in Plk4+/I242N mice. The average percent of meiotic cells with two centrioles was 23 ± 6% in Plk4+/+ mice and 19 ± 5% in Plk4+/I242N mice. There were no meiotic cells with supernumerary centrioles in either genotype. Overall, there were no significant differences in centriole number between the two genotypes.
Testis cord organization
H&E-stained sections of E13.5 Plk4+/I242N testes did not differ in testis cord structure, specifically in the organization of the testis cords or germ cell complement, compared with Plk4+/+ littermates (Fig. 5A). Assessment of basement membrane structure using immunofluorescence with an antilaminin antibody did not demonstrate disorganization of the basement membrane of the testis cords in the Plk4+/I242N embryos compared with Plk4+/+ littermates (data not shown). Germ cell number within each testis cord also did not differ between the two genotypes based on immunohistochemistry with an anti-GCNA1 antibody (Fig. 5, B and C).
Fig. 5.
Testis cord development in Plk4+/+ and Plk4+/I242N embryos. A, H&E-stained sections of E13.5 Plk4+/+ and Plk4+/I242N testes. B, GCNA1-stained sections of E13.5 Plk4+/+ and Plk4+/I242N testes. C, Quantification from GCNA-stained sections.
Onset of hypogonadism and germ cell loss
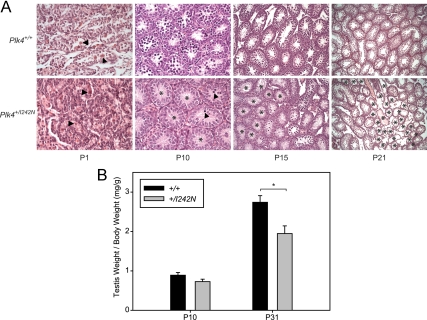
To establish the onset of the hypogonadism and germ cell loss, testis to body weight ratios and histology were analyzed at P1, P10, P15, P21, and P31. The patchy germ cell loss was not present at P1 but was seen at P10 and was seen at all subsequent ages (P15 and P21) (Fig. 6A). Hypogonadism, based on testis weight, was not detected at P10 but was present at P31 (Fig. 6B).
Fig. 6.
Time course for the onset of hypogonadism and germ cell loss. A, H&E testis sections from Plk4+/+ and Plk4+/I242N mice. Arrowheads denote germ cells, and asterisks denote tubules devoid of germ cells. P1 and P10 sections were visualized with a ×40 objective, P15 sections were visualized with a ×20 objective, and P21 sections were visualized with a ×10 objective. B, Quantification of testis weights.
Discussion
The goal of this study was to further our knowledge of male reproduction by generating mice with ENU-induced mutations in genes required for spermatogenesis. Previous mutagenesis studies in reproduction have demonstrated the utility of such screens (14–16). However, many genes involved in spermatogenesis remain unknown. Because ENU mutagenesis is random, a G1 male may harbor 30–50 mutations throughout the genome. It is therefore plausible that the phenotype observed may be due to a different linked mutation. To minimize this possibility, mice were bred for several generations, reducing the number of mutations by half every generation. Once an approximately 5-Mb region containing the mutation has been mapped, the likelihood of a second mutation is low (44). Although this approach is reassuring, genetic complementation studies, using an independent gene trap Plk4 mutation (Plk4+/GT) were used to validate the effect of the ENU-induced missense mutation. Plk4I242N/I242N and Plk4GT/GT mice were each shown to be embryonic lethal. Compound heterozygous (Plk4GT/I242N) pups were also not identified, confirming the I242N mutation in PLK4 as causative for the observed phenotype.
Due to the embryonic lethality of the homozygous Plk4I242N/I242N mice, the I242N mutation could only be studied in the heterozygous state. It is likely that the testis phenotype is less severe than would have been observed in the homozygous animals. Specifically, the patchy germ cell loss and reduction in testis size present in the heterozygotes may be a partial phenotype. The effect of a homozygous Plk4 mutation in the testis could be investigated using a conditional knockout model. However, one benefit of using a heterozygous mouse model is that human mutations are also likely to involve heterozygous base changes or small deletions that cause spermatogenic failure.
Hormonal alterations (testosterone, LH, and FSH) could also cause spermatogenic defects (10). To ensure the patchy germ cell loss phenotype was not secondary to hormonal changes, we measured testosterone, LH, and FSH in the heterozygous Plk4+/I242N mice and in wild-type Plk4+/+ littermates. Although there was not a statistically significant difference in testosterone or LH, there was a slight increase in FSH in the Plk4+/I242N mice. It is possible that damage to the testes could result in decreased inhibin production, leading to increased FSH. In rodents, FSH is thought to stimulate spermatogenesis through an increase in spermatogonial number and entry into meiosis (45). During the first wave of spermatogenesis, FSH is protective against germ cell apoptosis (46). It would therefore be possible that low FSH could result in germ cell loss. However, the Plk4+/I242N mice had increased FSH. It therefore seems more likely that the increase in FSH is the result of germ cell loss as opposed to causative for the defect.
The mutation in PLK4 results in a change from isoleucine to asparagine at amino acid 242. Because isoleucine is an aliphatic, hydrophobic amino acid whereas asparagine is polar and hydrophilic, it is likely that this substitution results in a conformational change in the kinase domain. PLK4 is able to homodimerize and trans-autophosphorylate, promoting self-destruction (30, 47). Homodimerization between kinase dead and wild-type PLK4 disrupts trans-autophosphorylation of wild-type PLK4 by kinase dead PLK4, thereby shielding wild-type PLK4 from degradation (29). It is therefore possible that homodimerization between wild-type and mutant I242N PLK4 proteins could result in decreased trans-autophosphorylation of the wild-type PLK4 and subsequent prolongation of its half-life and activity. These issues remain to be explored further using in vitro models.
Several members of the Plk family are expressed in the gonads and have roles in meiosis. CDC5 is involved in sister-kinetochore coorientation and chromosome segregation during meiosis I (48, 49), and it is required for exit from pachytene (crossover formation and synaptonemal complex breakdown) (50). In Drosophila melanogaster, mutations in POLO result in meiotic germ cells with multipolar spindles and abnormal DNA content (51). D. melanogaster PLK4 mutants lose centrioles during the mitotic divisions preceding male meiosis but still produce the same number of primary spermatocyte cysts as wild type. Spermatids lack centrioles and are unable to produce axonemes (26). The PLK4 homolog in Caenorhabditis elegans, zygote defective: embryonic lethal family member, controls both mitotic and meiotic centriole duplication, but when its C terminus is truncated, there is decreased mitotic centriole duplication and meiotic overamplification (52). In the mouse, Plk1 is expressed in diplotene spermatocytes, secondary spermatocytes, and round spermatids. In the ovary, Plk1 is expressed in growing oocytes and ovulated eggs (53) with subcellular localization that supports a role in meiosis (54). Thus, the polo-like kinases appear to function at several different steps of mitosis and meiosis and may exert other cellular functions (26, 48–54). Human PLK4 is highly expressed in the testis (55).
CDC5 directly phosphorylates REC8, a meiotic cohesin protein required for alignment of the chromosome arms during meiosis I, which allows the cells to move from metaphase I to anaphase I (49). REC8 is activated by hyperphosphorylation, and PLK1 has been shown to phosphorylate REC8 in vitro and promote REC8 cleavage (56). Failure of REC8 to function results in sterility in male mice (56). Based on the consensus phosphorylation motif of murine PLK4 (57), REC8 is a potential target of PLK4 (data not shown). It is therefore possible that mice heterozygous for the I242N mutation in Plk4 alter the hyperphosphorylated state of REC8 in the male germ cells resulting in aberrant meiosis and germ cell loss.
After its initial identification, Plk4 was described as having a role in cell proliferation (18, 25). During development, Plk4 was found in multiple organs during proliferative (mitotic) stages, and in the adult, it was found in areas of high cell division, including the bases of intestinal crypts. Northern blot analysis showed highest expression in the testis (18). Subsequent findings have supported a role for mammalian PLK4 in mitosis (32) and its homologs POLO (Drosophila) (26, 51) and CDC5 (S. cerevisiae) (48–50) in meiosis. However, the role of PLK4 in the mammalian testis remains unclear. Based on the LacZ expression and staining with the PLK4 antibody, PLK4 was not seen in germ cells before the pachytene stage (Fig. 4, C and D). Therefore, it is unlikely that it is expressed in the mitotically active spermatogonial stem cells.
PLK4 has a known role in centriole duplication (58). Plk4+/− embryonic fibroblasts have increased centrosomal amplification (32), and stable overexpression of kinase-dead human PLK4 leads to centriole overduplication (29). Activated (S305 phosphorylated) PLK4 localizes to both mother and daughter centrioles in a cell cycle-specific manner, whereas nonphosphorylated PLK4 does not. Autophosphorylation of PLK4 is thought to have a role in centriole duplication, because mutations that mimic S305 phosphorylation induce centriole amplification (30). Therefore, we considered the possibility that the Plk4+/I242N mice might have defects in centriole replication, thereby altering normal progression through meiosis. However, within the limits of the histological analyses, the number of centrioles in stage XII meiotic spermatocytes was indistinguishable in Plk4+/I242N and Plk4+/+ testes. It remains plausible, however, that PLK4 has a more subtle, or developmentally specific, effect on centriole function during mitosis or meiosis.
Previous reports of PLK4 expression in the mouse testis showed low expression at P8 (before the first meiotic division) and increased expression with age (18). Testes sections showed highest levels of expression in the meiotically dividing spermatocytes and lower levels in the spermatogonia, postmeiotic spermatids, and mature spermatozoa (18). To analyze PLK4 expression further, Plk4+/GT testis sections were stained with LacZ. Staining was present initially in stage VIII pachytene spermatocytes and persisted until the end of spermiogenesis. Whole mount tubule LacZ staining from Plk4+/GT mice showed variation along the tubule, raising the possibility of cyclical or segmental expression. Expression beginning at the pachytene stage would support the possibility that PLK4 has a role in meiosis, because this stage precedes the onset of meiosis. The LacZ staining correlates with the onset of Plk4 promoter activity driving expression of the β-galactosidase gene. As such, it reflects Plk4 expression and does not necessarily correspond to PLK4 protein expression or subcellular localization. Further analysis was therefore conducted using a PLK4 antibody to analyze Plk4+/+ and Plk4+/I242N testis sections. The immunohistochemistry showed staining beginning in step 8 spermatids and continuing through step 16. The discrepancy in expression between the LacZ staining in the Plk4+/GT mice and the PLK4 staining in the Plk4+/+ and Plk4+/I242N mice may reflect a delay in translation, a form of regulation known to occur during mammalian spermatogenesis (59). PLK4 was present in the tails of the elongated spermatids. Although there may be a role for PLK4 in flagellar development and motility, because the motor apparatus of the flagellum is structurally related to the centriole, this subcellular localization does not appear to correlate with the defect seen in the Plk4+/I242N testes. Typically, defects in meiosis in the postpubertal adult testis result in tubules with a postmeiotic block. However, the defective tubules in the Plk4+/I242N mice do not display a postmeiotic block but instead have a SCO phenotype originating at P10. It is plausible that PLK4 has distinct functional roles at different time points throughout spermatogenesis.
The timing of the onset of the testis defect was examined to assess a possible developmental defect caused by the Plk4+/I242N mutation. E13.5 embryos stained with antilaminin and anti-GCNA1 antibodies showed well-organized testis cords containing the normal complement of germ cells in both Plk4+/+ and Plk4+/I242N mice. These experiments verified that the germ cells were initially present but subsequently lost, suggesting that the defect occurs postnatally. Histology was also normal at P1, but germ cells were lost within some tubules at P10, resulting in a patchy SCO phenotype. P10 corresponds to the time when the first wave of spermatogenesis occurs, and it is unique compared with subsequent waves of spermatogenesis. First, instead of germ cells differentiating from Neurogenin-3 positive undifferentiated spermatogonia, as they do in subsequent cycles, the first round of spermatogenesis is initiated directly from gonocytes (60). Second, there is increased apoptosis of spermatogonia during this time (61). Third, the first wave of spermatogenesis occurs in a synchronized manner in all tubules. Although the basis for the patchy germ cell loss is unclear, there is a temporal correlation between the germ cell loss and the onset of the first wave of spermatogenesis. One possibility is that a threshold of PLK4 activity may be required during the first wave of meiosis to maintain spermatogenesis. Of note, the loss of all germ cells within specific tubules during the first wave of spermatogenesis is consistent with the defect not being stage specific, because all tubules are synchronized at that time.
In summary, ENU-induced mutations can result in heritable defects in spermatogenesis. Investigation of mutated genes, such as Plk4, has the potential to identify novel genes and pathways involved in spermatogenesis and male reproduction. The I242N heterozygous mutation in PLK4 is causative for patchy germ cell loss beginning at P10, suggesting a role for PLK4 in the first wave of spermatogenesis.
Acknowledgments
We thank Donna Emge for assistance with histology; Lisa Fisher, Lisa Hurley, and Tim Barrett for assistance with mouse husbandry; and Courtney Finlayson for assistance with genomic mapping. The laboratory of Dr. David Beier performed initial SNP analyses. DNA sequencing was performed using the Northwestern University Genomics Core.
This work was supported by the National Institutes of Health (NIH) Grant U01 HD043425-01. Hormone assays were performed using the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, which is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH (Specialized Cooperative Centers Program in Reproduction and Infertility Research) Grant U54-HD28934.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CDC5
- Cell division cycle 5
- DAPI
- 4′,6-diamidino-2-phenylindole
- E
- embryonic day
- ENU
- N-ethyl-N-nitrosourea
- GCNA
- germ cell nuclear antigen
- H&E
- hematoxylin and eosin
- I242N
- altering an isoleucine to asparagine at residue 242
- PAS-H
- periodic acid Schiff and hematoxylin
- P
- postnatal day
- PFA
- paraformaldehyde
- PIPES
- piperazine-N,N′-bis-2-ethanesulfonic acid
- Plk4
- Polo-like kinase 4
- SCO
- Sertoli cell only
- SNP
- single nucleotide polymorphism.
References
- 1. World Health Organization 1987. Towards more objectivity in diagnosis and management of male infertility. Int J Androl 7(Suppl):1–53 [Google Scholar]
- 2. Ferlin A, Arredi B, Foresta C. 2006. Genetic causes of male infertility. Reprod Toxicol 22:133–141 [DOI] [PubMed] [Google Scholar]
- 3. Kaplan E, Shwachman H, Perlmutter AD, Rule A, Khaw KT, Holsclaw DS. 1968. Reproductive failure in males with cystic fibrosis. N Engl J Med 279:65–69 [DOI] [PubMed] [Google Scholar]
- 4. Hardelin JP. 1997. Kallmann's syndrome. Hum Reprod 12:104–106 [PubMed] [Google Scholar]
- 5. Reijo R, Lee TY, Salo P, Alagappan R, Brown LG, Rosenberg M, Rozen S, Jaffe T, Straus D, Hovatta O. 1995. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet 10:383–393 [DOI] [PubMed] [Google Scholar]
- 6. Oates RD. 2003. Clinical and diagnostic features of patients with suspected Klinefelter syndrome. J Androl 24:49–50 [PubMed] [Google Scholar]
- 7. Lipshultz LI, Howards SS. 1997. Infertility in the male. St. Louis: Mosby-Year Book, Inc [Google Scholar]
- 8. De Kretser DM, Baker HW. 1999. Infertility in men: recent advances and continuing controversies. J Clin Endocrinol Metab 84:3443–3450 [DOI] [PubMed] [Google Scholar]
- 9. Schultz N, Hamra FK, Garbers DL. 2003. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc Natl Acad Sci USA 100:12201–12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matzuk MM, Lamb DJ. 2008. The biology of infertility: research advances and clinical challenges. Nat Med 14:1197–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Bryan MK, de Kretser D. 2006. Mouse models for genes involved in impaired spermatogenesis. Int J Androl 29:76–89; discussion 105–108 [DOI] [PubMed] [Google Scholar]
- 12. Papathanasiou P, Perkins AC, Cobb BS, Ferrini R, Sridharan R, Hoyne GF, Nelms KA, Smale ST, Goodnow CC. 2003. Widespread failure of hematolymphoid differentiation caused by a recessive niche-filling allele of the Ikaros transcription factor. Immunity 19:131–144 [DOI] [PubMed] [Google Scholar]
- 13. Russell WL, Kelly EM, Hunsicker PR, Bangham JW, Maddux SC, Phipps EL. 1979. Specific-locus test shows ethylnitrosourea to be the most potent mutagen in the mouse. Proc Natl Acad Sci USA 76:5818–5819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lessard C, Pendola JK, Hartford SA, Schimenti JC, Handel MA, Eppig JJ. 2004. New mouse genetic models for human contraceptive development. Cytogenet Genome Res 105:222–227 [DOI] [PubMed] [Google Scholar]
- 15. Handel MA, Lessard C, Reinholdt L, Schimenti J, Eppig JJ. 2006. Mutagenesis as an unbiased approach to identify novel contraceptive targets. Mol Cell Endocrinol 250:201–205 [DOI] [PubMed] [Google Scholar]
- 16. Lessard C, Lothrop H, Schimenti JC, Handel MA. 2007. Mutagenesis-generated mouse models of human infertility with abnormal sperm. Hum Reprod 22:159–166 [DOI] [PubMed] [Google Scholar]
- 17. Justice MJ, Noveroske JK, Weber JS, Zheng B, Bradley A. 1999. Mouse ENU mutagenesis. Hum Mol Genet 8:1955–1963 [DOI] [PubMed] [Google Scholar]
- 18. Fode C, Motro B, Yousefi S, Heffernan M, Dennis JW. 1994. Sak, a murine protein-serine/threonine kinase that is related to the Drosophila polo kinase and involved in cell proliferation. Proc Natl Acad Sci USA 91:6388–6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leung GC, Hudson JW, Kozarova A, Davidson A, Dennis JW, Sicheri F. 2002. The Sak polo-box comprises a structural domain sufficient for mitotic subcellular localization. Nat Struct Biol 9:719–724 [DOI] [PubMed] [Google Scholar]
- 20. Alexandru G, Uhlmann F, Mechtler K, Poupart MA, Nasmyth K. 2001. Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell 105:459–472 [DOI] [PubMed] [Google Scholar]
- 21. Stegmeier F, Visintin R, Amon A. 2002. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell 108:207–220 [DOI] [PubMed] [Google Scholar]
- 22. Visintin R, Stegmeier F, Amon A. 2003. The role of the polo kinase Cdc5 in controlling Cdc14 localization. Mol Biol Cell 14:4486–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rahal R, Amon A. 2008. The Polo-like kinase Cdc5 interacts with FEAR network components and Cdc14. Cell Cycle 7:3262–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang F, Jin F, Liu H, Wang Y. 2009. The molecular function of the yeast polo-like kinase Cdc5 in Cdc14 release during early anaphase. Mol Biol Cell 20:3671–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fode C, Binkert C, Dennis JW. 1996. Constitutive expression of murine Sak-a suppresses cell growth and induces multinucleation. Mol Cell Biol 16:4665–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM. 2005. SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol 15:2199–2207 [DOI] [PubMed] [Google Scholar]
- 27. Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA. 2007. Plk4-induced centriole biogenesis in human cells. Dev Cell 13:190–202 [DOI] [PubMed] [Google Scholar]
- 28. Cunha-Ferreira I, Rodrigues-Martins A, Bento I, Riparbelli M, Zhang W, Laue E, Callaini G, Glover DM, Bettencourt-Dias M. 2009. The SCF/Slimb ubiquitin ligase limits centrosome amplification through degradation of SAK/PLK4. Curr Biol 19:43–49 [DOI] [PubMed] [Google Scholar]
- 29. Guderian G, Westendorf J, Uldschmid A, Nigg EA. 2010. Plk4 trans-autophosphorylation regulates centriole number by controlling βTrCP-mediated degradation. J Cell Sci 123:2163–2169 [DOI] [PubMed] [Google Scholar]
- 30. Sillibourne JE, Tack F, Vloemans N, Boeckx A, Thambirajah S, Bonnet P, Ramaekers FC, Bornens M, Grand-Perret T. 2010. Autophosphorylation of polo-like kinase 4 and its role in centriole duplication. Mol Biol Cell 21:547–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hudson JW, Kozarova A, Cheung P, Macmillan JC, Swallow CJ, Cross JC, Dennis JW. 2001. Late mitotic failure in mice lacking Sak, a polo-like kinase. Curr Biol 11:441–446 [DOI] [PubMed] [Google Scholar]
- 32. Ko MA, Rosario CO, Hudson JW, Kulkarni S, Pollett A, Dennis JW, Swallow CJ. 2005. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nat Genet 37:883–888 [DOI] [PubMed] [Google Scholar]
- 33. Rosario CO, Ko MA, Haffani YZ, Gladdy RA, Paderova J, Pollett A, Squire JA, Dennis JW, Swallow CJ. 2010. Plk4 is required for cytokinesis and maintenance of chromosomal stability. Proc Natl Acad Sci USA 107:6888–6893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Macmillan JC, Hudson JW, Bull S, Dennis JW, Swallow CJ. 2001. Comparative expression of the mitotic regulators SAK and PLK in colorectal cancer. Ann Surg Oncol 8:729–740 [DOI] [PubMed] [Google Scholar]
- 35. Nord AS, Chang PJ, Conklin BR, Cox AV, Harper CA, Hicks GG, Huang CC, Johns SJ, Kawamoto M, Liu S, Meng EC, Morris JH, Rossant J, Ruiz P, Skarnes WC, Soriano P, Stanford WL, Stryke D, von Melchner H, Wurst W, Yamamura K, Young SG, Babbitt PC, Ferrin TE. 2006. The international gene trap consortium website: a portal to all publicly available gene trap cell lines in mouse. Nucleic Acids Res 34:D642–D648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Siepka SM, Takahashi JS. 2005. Forward genetic screens to identify circadian rhythm mutants in mice. Methods Enzymol 393:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Russel LD, Ettlin RA, Sinha Hikim AP, Clegg ED. 1990. Histological and histopathological evaluation of the testis. St. Louis: Cache River Press [Google Scholar]
- 38. Fallest PC, Trader GL, Darrow JM, Shupnik MA. 1995. Regulation of rat luteinizing hormone β gene expression in transgenic mice by steroids and a gonadotropin-releasing hormone antagonist. Biol Reprod 53:103–109 [DOI] [PubMed] [Google Scholar]
- 39. Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I. 1993. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology 132:1687–1691 [DOI] [PubMed] [Google Scholar]
- 40. Gay VL, Midgley AR, Jr, Niswender GD. 1970. Patterns of gonadotropin secretion associated with ovulation. Fed Proc 29:1880–1887 [PubMed] [Google Scholar]
- 41. Moran JL, Bolton AD, Tran PV, Brown A, Dwyer ND, Manning DK, Bjork BC, Li C, Montgomery K, Siepka SM, Vitaterna MH, Takahashi JS, Wiltshire T, Kwiatkowski DJ, Kucherlapati R, Beier DR. 2006. Utilization of a whole genome SNP panel for efficient genetic mapping in the mouse. Genome Res 16:436–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eldredge LC, Gao XM, Quach DH, Li L, Han X, Lomasney J, Tourtellotte WG. 2008. Abnormal sympathetic nervous system development and physiological dysautonomia in Egr3-deficient mice. Development 135:2949–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Enders GC, May JJ., 2nd 1994. Developmentally regulated expression of a mouse germ cell nuclear antigen examined from embryonic day 11 to adult in male and female mice. Dev Biol 163:331–340 [DOI] [PubMed] [Google Scholar]
- 44. Keays DA, Clark TG, Flint J. 2006. Estimating the number of coding mutations in genotypic- and phenotypic-driven N-ethyl-N-nitrosourea (ENU) screens. Mamm Genome 17:230–238 [DOI] [PubMed] [Google Scholar]
- 45. O'Shaughnessy PJ, Monteiro A, Verhoeven G, De Gendt K, Abel MH. 2010. Effect of FSH on testicular morphology and spermatogenesis in gonadotrophin-deficient hypogonadal mice lacking androgen receptors. Reproduction 139:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meachem SJ, Ruwanpura SM, Ziolkowski J, Ague JM, Skinner MK, Loveland KL. 2005. Developmentally distinct in vivo effects of FSH on proliferation and apoptosis during testis maturation. J Endocrinol 186:429–446 [DOI] [PubMed] [Google Scholar]
- 47. Holland AJ, Lan W, Niessen S, Hoover H, Cleveland DW. 2010. Polo-like kinase 4 kinase activity limits centrosome overduplication by autoregulating its own stability. J Cell Biol 188:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee BH, Amon A. 2003. Role of Polo-like kinase CDC5 in programming meiosis I chromosome segregation. Science 300:482–486 [DOI] [PubMed] [Google Scholar]
- 49. Clyne RK, Katis VL, Jessop L, Benjamin KR, Herskowitz I, Lichten M, Nasmyth K. 2003. Polo-like kinase Cdc5 promotes chiasmata formation and cosegregation of sister centromeres at meiosis I. Nat Cell Biol 5:480–485 [DOI] [PubMed] [Google Scholar]
- 50. Sourirajan A, Lichten M. 2008. Polo-like kinase Cdc5 drives exit from pachytene during budding yeast meiosis. Genes Dev 22:2627–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Herrmann S, Amorim I, Sunkel CE. 1998. The POLO kinase is required at multiple stages during spermatogenesis in Drosophila melanogaster. Chromosoma 107:440–451 [DOI] [PubMed] [Google Scholar]
- 52. Peters N, Perez DE, Song MH, Liu Y, Müller-Reichert T, Caron C, Kemphues KJ, O'Connell KF. 2010. Control of mitotic and meiotic centriole duplication by the Plk4-related kinase ZYG-1. J Cell Sci 123:795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Matsubara N, Yanagisawa M, Nishimune Y, Obinata M, Matsui Y. 1995. Murine polo like kinase 1 gene is expressed in meiotic testicular germ cells and oocytes. Mol Reprod Dev 41:407–415 [DOI] [PubMed] [Google Scholar]
- 54. Pahlavan G, Polanski Z, Kalab P, Golsteyn R, Nigg EA, Maro B. 2000. Characterization of polo-like kinase 1 during meiotic maturation of the mouse oocyte. Dev Biol 220:392–400 [DOI] [PubMed] [Google Scholar]
- 55. Karn T, Holtrich U, Wolf G, Hock B, Strebhardt K, Rubsamen-Waigmann H. 1997. Human SAK related to the PLK/polo family of cell cycle kinases shows high mRNA expression in testis. Oncology Rep 4:505–510 [DOI] [PubMed] [Google Scholar]
- 56. Kudo NR, Anger M, Peters AH, Stemmann O, Theussl HC, Helmhart W, Kudo H, Heyting C, Nasmyth K. 2009. Role of cleavage by separase of the Rec8 kleisin subunit of cohesin during mammalian meiosis I. J Cell Sci 122:2686–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leung GC, Ho CS, Blasutig IM, Murphy JM, Sicheri F. 2007. Determination of the Plk4/Sak consensus phosphorylation motif using peptide spots arrays. FEBS Lett 581:77–83 [DOI] [PubMed] [Google Scholar]
- 58. Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. 2005. The Polo kinase Plk4 functions in centriole duplication. Nat Cell Biol 7:1140–1146 [DOI] [PubMed] [Google Scholar]
- 59. Kini HK, Vishnu MR, Liebhaber SA. 2010. Too much PABP, too little translation. J Clin Invest 120:3090–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, Nabeshima Y. 2006. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 133:1495–1505 [DOI] [PubMed] [Google Scholar]
- 61. Mori C, Nakamura N, Dix DJ, Fujioka M, Nakagawa S, Shiota K, Eddy EM. 1997. Morphological analysis of germ cell apoptosis during postnatal testis development in normal and Hsp 70–2 knockout mice. Dev Dyn 208:125–136 [DOI] [PubMed] [Google Scholar]