Abstract
Melanocortins (MC) trigger a vagus nerve-mediated cholinergic-antiinflammatory pathway projecting to the testis. We tested whether pharmacological activation of brain MC receptors might protect the testis from the damage induced by ischemia-reperfusion. Adult male rats were subjected to 1-h testicular ischemia, followed by 24-h reperfusion [testicular ischemia-reperfusion (TI/R)]. Before TI/R, groups of animals were subjected to bilateral cervical vagotomy, or pretreated with the nicotinic acetylcholine receptor antagonist chlorisondamine or the selective MC4 receptor antagonist HS024. Immediately after reperfusion, rats were ip treated with saline or the MC analog [Nle4,D-Phe7]α-melanocyte-stimulating hormone (NDP-α-MSH) (340 μg/kg). We evaluated testicular IL-6 and TNF-α by Western blot analysis and organ damage by light microscopy. Some experimental groups were prepared for neural efferent activity recording along the vagus nerve starting 30 min after treatment with NDP-α-MSH or saline, and for a 30-min period. Additional groups of TI/R rats were treated for 30 d with saline, NDP-α-MSH, chlorisondamine plus NDP-α-MSH, or HS024 plus NDP-α-MSH to evaluate spermatogenesis, organ damage, and the apoptosis machinery. After a 24-h reperfusion, in TI/R saline-treated rats, there was an increase in IL-6 and TNF-α expression and a marked damage in both testes. NDP-α-MSH inhibited IL-6 and TNF-α expression, decreased histological damage, and increased neural efferent activity. Furthermore, NDP-α-MSH administration for 30 d greatly improved spermatogenesis, reduced organ damage, and inhibited apoptosis. All positive NDP-α-MSH effects were abrogated by vagotomy, chlorisondamine, or HS024. Our data suggest that selective MC4 receptor agonists might be therapeutic candidates for the management of testicular torsion.
Cerebral structures are connected transneuronally with the gonads and control gonadal function through a pituitary-independent, purely neural mechanism (1, 2). In both sexes, the gonads and other organs of the reproductive system are innervated by sympathetic and parasympathetic efferent (motor) fibers. These nerves of the autonomic nervous system possess afferent (visceral sensory) fibers that carry information toward the central nervous system (CNS) (1, 2). In particular, the central connections of the testicular nerves have been investigated by means of the transneuronal viral tracing technique (3–5). The involvement of the vagus nerve in the direct innervation of the reproductive organs is suggested by the fact that, after virus injection into the testis, labeling (virus infection) in preganglionic sympathetic neurons and in the parasympathetic vagal nuclei appears almost simultaneously, despite the longer course. Indeed, already at the initial stage of infection, when no other cerebral structures are labeled (virus infected), a few immunopositive neurons can be detected in the dorsal motor nucleus of the vagus. Moreover, highlighting the close anatomical connection between the vagus and gonads, in animals subjected to vagotomy, no infected cells were detected in the vagal nuclei (2). Lee et al. (1), after the injection of PRV (pseudorabies) virus to one testis, demonstrated the presence of the virus in the medulla, including the dorsal motor nucleus of the vagus.
The testis is innervated by the superior spermatic nerve and the inferior spermatic nerve. The superior spermatic nerve, the major contributor of testicular innervations, originates from the celiac and aortic plexuses and runs alongside the testicular vessels. Cell bodies of the preganglionic sympathetic fibers are located in the thoracic segments 10 and 11 of the spinal cord, whereas the parasympathetic component of the nerve belongs to the vagus nerve. In the past, the vagus nerve was only considered a part of the parasympathetic system with specific functions. However, more recent data have disclosed previously unrecognized functions of the vagus nerve. More specifically, efferent vagus nerve signaling contributes to modulation of inflammation, and several studies have given evidence that the parasympathetic nervous system can influence the systemic inflammatory response, thus leading to the identification of the “cholinergic antiinflammatory pathway,” the motor arm of the inflammatory reflex (6–10).
Testicular torsion is caused by twisting of the spermatic cord and represents a surgical emergence affecting newborns, children, and adolescent boys (11). The two most important determinants of the pathology are the degree of twisting and the early onset of a surgical maneuver to counterrotate both testis and spermatic cord for allowing reperfusion. This surgical intervention is essential to treat the acute ischemia but may cause a reperfusion injury involving complex and multifaceted molecular signaling pathways.
Several mechanisms play a role in the development of testicular damage after the torsion and detorsion process (12). The testis produces several inflammatory cytokines, including TNF-α and IL-1β (13). The enhanced levels of these cytokines are an indirect measurement of tissue inflammation. TNF-α and IL-1β serve as proinflammatory cytokines to induce IL-6 production and activate stress-related intracellular and extracellular pathways (14, 15). These cytokines are produced by testicular cells as well as by activated interstitial macrophages (13). Besides inflammation, testicular twisting and untwisting causes activation of the apoptotic machinery (16), and this composite pathological cascade is responsible for the testicular atrophy and impaired spermatogenesis that is observed at a later stage. In this condition, the balance between the proapoptotic protein B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax), and the antiapoptotic protein Bcl-2 plays an important role (11).
Melanocortins (MC), bioactive peptides derived by the posttranslational cleavage of proopiomelanocortin (17–19), activate the motor arm of the vagal reflex by stimulating brain MC3 and MC4 receptors, thus blunting the inflammatory cascade and the apoptotic reaction and exerting a strong protection in several ischemia experimental models (20–22).
We investigated whether triggering of the cholinergic antiinflammatory pathway by MC might protect the testis from early and late damage induced by ischemia and reperfusion.
Materials and Methods
Animals and treatments
All procedures complied with the standards for care and use of animal subjects as stated in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy of Sciences, Bethesda, MD). Male Sprague Dawley rats weighing 250–300 g were used. The animals were fed a standard diet ad libitum and had free access to tap water. They were maintained on a 12-h light, 12-h dark cycle at 21 C. Rats were anesthetized with an ip injection of 50 mg/kg of pentobarbital sodium. Testicular ischemia-reperfusion (TI/R) injury was induced by torsion of the left testis, with a 720° twisting of the spermatic cord so as to produce a total occlusion of testis for 1 h. The same testis was then detorted. Sham-operated rats underwent the same surgical procedures as TI/R rats except for testicular occlusion (sham). Groups of animals were pretreated, 2 min before induction of TI/R, with the peripheral nicotinic acetylcholine receptor antagonist chlorisondamine (0.25 mg/kg sc) or with the selective MC4 receptor antagonist HS024 (130 μg/kg ip), or subjected, 2 min before induction of TI/R, to bilateral cervical vagotomy by tying two silk sutures around each vagal trunk, one above the other, and transecting each trunk between the sutures. The sutures served to ensure complete cutting and to facilitate postmortem verification of disconnection. Animals were then randomized to receive, immediately after detorsion, vehicle (1 ml/kg ip of a 0.9% NaCl solution) or the MC analog [Nle4,D-Phe7]α-melanocyte-stimulating hormone (NDP-α-MSH) (340 μg/kg ip). All these groups of animals were killed 24 h later to investigate early damage induced by ischemia and reperfusions. To this end, testes were removed and frozen in liquid nitrogen.
Additional groups of animals (sham and TI/R rats) were used to evaluate the effects of the several treatment on the late testis damage (30 d) induced by testicular twisting. In this experiment, TI/R animals were daily treated for 30 d with vehicle (1 ml/kg ip of a 0.9% NaCl solution) or NDP-α-MSH (340 μg/kg ip) alone or together with either HS024 (130 μg/kg ip) or chlorisondamine (0.25 mg/kg, sc). Both HS024 and chlorisondamine were administered 2 min before NDP-α-MSH injection. All these groups of animals were killed 30 d after testicular torsion.
Finally to record vagal efferent activity, groups of animals underwent neural efferent activity along vagus nerve monitoring by means of a standard system for extracellular recordings (20). Left rostral vagal trunk was placed on a silver wire electrode (recording electrode) and an indifferent electrode was positioned nearby in the wound. Vagal action potentials fed into an AC amplifier (model 1800; A-M Systems, Sequim, WA) and displayed on an oscillope screen. Signals were passed through a band-pass filter (100–1000 Hz), and filtered nerve activity was recorded and analyzed using a Pentium II computer equipped with Digidata 1322A data acquisition system and Axoscope 8.1 software (Axon Instruments, Foster City, CA). The efferent impulses were integrated at constant times with Clampfit software so to evaluate total efferent vagal activity. Efferent vagus nerve activity was recorded starting 30 min after treatment with vehicle (1 ml/kg ip of a 0.9% NaCl solution) or NDP-α-MSH (340 μg/kg ip), and for a 30-min period. For data comparison, we used integrated values obtained at the end of a 100-msec period for each electrophysiological recording and normalized to sham + vehicle (100%) (20).
Isolation of cytoplasmatic proteins
Briefly, pulverized testis samples were homogenized in 1 ml of lysis buffer [25 mm Tris/HCl (pH 7.4), 1.0 mm EGTA, 1.0 mm EDTA, 0.5 mm phenyl methylsulfonyl fluoride, aprotinin, leupeptin, pepstatin A (10 μg/ml each), and Na3VO4 100 mm], with a Dounce homogenizer (15). The homogenate was subjected to centrifugation at 15,000 × g for 15 min. The supernatant was collected and used for protein determination using the Bio-Rad protein assay kit (Bio-Rad, Richmond, CA).
Determination of IL-6, TNF-α, Bax, and Bcl-2 by Western blot analysis
Protein samples (50 μg) were denatured in reducing buffer [62 mm Tris (pH 6.8), 10% glycerol, 2% sodium dodecyl sulfate, 5% β-mercaptoethanol, and 0.003% bromophenol blue] and separated by electrophoresis on an sodium dodecyl sulfate (12%) polyacrylamide gel. The separated proteins were transferred onto a nitrocellulose membrane using the transfer buffer [39 mm glycine, 48 mm Tris (pH 8.3), and 20% methanol] at 200 mA for 1 h. The membranes stained with Ponceau's (0.005% in 1% acetic acid) to confirm equal amounts of protein and were blocked with 5% nonfat dry milk in Tris-buffered saline (TBS)-0.1% Tween for 1 h at room temperature, washed three times for 10 min each in TBS-0.1% Tween, and incubated with a primary antibody for IL-6, TNF-α, Bax, and Bcl-2 (Chemicon, Temecula, CA) in TBS-0.1% Tween overnight at 4 C, diluted 1:1000. After being washed three times for 10 min each in TBS-0.1% Tween, the membranes were incubated with a secondary antibody peroxidase-conjugated goat antirabbit IgG (Pierce, Rockford, IL) for 1 h at room temperature diluted 1:20,000. After washing, the membranes were analyzed by the enhanced chemiluminescence system according to the manufacture's protocol (Amersham, Little Chalfont, UK). Equal loading of proteins was assessed on stripped blots by immunodetection of β-actin with a rabbit monoclonal antibody (Cell Signaling, Beverly, MA) diluted 1:500 and peroxidase-conjugated goat antirabbit IgG (Pierce) diluted 1:15000. All antibodies were purified by protein A and peptide affinity chromatography. The IL-6, TNF-α, Bax, and Bcl-2 protein signal was quantified by scanning densitometry using a bio-image analysis system (Bio-Profil Celbio, Milan, Italy) and was expressed as integrated intensity in comparison with that of control normal animals measured with the same batch (10, 21–23).
Histology
Excised rat testes were longitudinally sectioned, formalin fixed, and paraffin embedded. Five serial sections (4 μm) for each sample were stained with hematoxylin and eosin. Testicular microscopy was performed in 20 high-magnification fields, by a pathologist without knowledge of the sample's identification. Histological lesions were evaluated in both the tubular and extratubular compartments, using a semiquantitative method, as previously performed (14). Venular ectasia was quantified using an ocular micrometer and a Zeiss Axioplan microscope (Zeiss, Oberkochen, Germany). Spermatogenesis was also quantified in 50 tubular cross-sections for each testis, using the Johnsen's score system (24). Briefly, a score of 1–10 was given to each tubule according to the maturity of the germ cells: 10, complete spermatogenesis, with many spermatozoa, and germinal epithelium organized with a regular thickness, leaving an open lumen; 9, many spermatozoa present but germinal epithelium disorganized with marked sloughing or obliteration of the lumen; 8, only few spermatozoa (fewer than 5–10) present; 7, no spermatozoa but many spermatids present; 6, no spermatozoa and only few spermatids (fewer than 5–10) present; 5, no spermatozoa and no spermatids but several or many spermatocytes present; 4, only few spermatocytes (fewer than five) and no spermatids or spermatozoa present; 3, spermatogonia the only germ cells present; 2, no germ cells but Sertoli cells present; and 1, no cells present in tubular sections.
Statistical analysis
All data are expressed as the mean ± sd. For the histological results, statistical analysis was performed using the Kruskal-Wallis test followed by the Mann-Whitney U test. All other data were analyzed by ANOVA followed by the Dunn multiple comparison post hoc test. In all cases, a probability error of less than 0.05 was selected as a criterion for statistical significance.
Drugs
NDP-α-MSH, agonist at MC1, MC3, MC4, and MC5 receptors, with long-lasting biological activity (19, 20, 22), was kindly provided by Paolo Grieco (University of Naples Federico II, Naples, Italy). Cys-Nle-Arg-His-D-Nal-Arg-Trp-Gly-Cys (HS024; cyclic MSH analog with S bridge between two Cys) and chlorisondamine diiodide were supplied by Tocris (Bristol, UK). HS024 is a potent and selective MC4 receptor antagonist (22, 25). Chlorisondamine is an irreversible antagonist at all nicotinic receptors subtypes able, at the dose used here, to block only peripheral receptors (9, 10, 23). All substances were dissolved in a 0.9% NaCl solution, prepared fresh daily, and administered in a volume of 1 ml/kg. Drug doses were chosen on the basis of a pilot study and our previous investigations in experimental models of circulatory shock, myocardial ischemia, and ischemic stroke (9, 10, 22, 23, 25).
Results
NDP-α-MSH inhibits early IL-6 and TNF-α expression
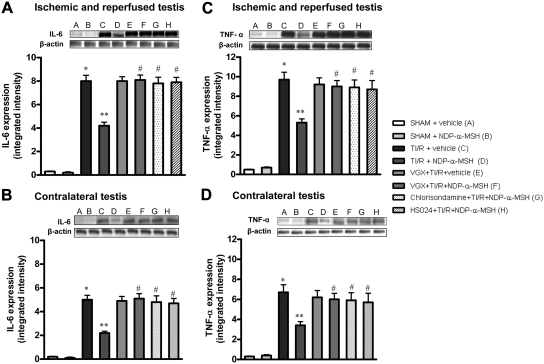
TI/R injury caused a significant increase in IL-6 and TNF-α protein expression in both ipsilateral and contralateral testes after 24 h of reperfusion (Fig. 1). Administration of NDP-α-MSH, agonist at MC1, MC3, MC4, and MC5 receptors, markedly decreased cytokine expression in the ipsilateral testis and contralateral one (Fig. 1). In contrast, cervical vagotomy and pretreatment with either the nicotinic acetylcholine receptor antagonist chlorisondamine (at a dose able to block peripheral receptors only), or the selective MC4 receptor antagonist HS024, abrogated the protective effects of NDP-α-MSH (Fig. 1). Chlorisondamine or HS024 alone did not modify cytokine expression in TI/R rats (results not shown).
Fig. 1.
Western blot analysis of IL-6 (A and B) and TNF-α (C and D) in samples of both ipsilateral (A and C) and contralateral (B and D) testes of rats undergone ischemia and reperfusion and treated with vehicle (1 ml/kg of a 0.9% NaCl solution) or NDP-α-MSH (340 μg/kg) ip administered immediately after reperfusion. Groups of animals were subjected to bilateral cervical vagotomy (VGX) 2 min before induction of ischemia. Another group of animals was pretreated with the nicotinic receptor antagonist chlorisondamine (0.25 mg/kg, sc) and another with the selective MC4 receptor antagonist HS024 (130 μg/kg ip) 2 min before induction of ischemia. Bars represent mean values ± sd of seven animals. *, P < 0.005 vs. sham + vehicle; **, P < 0.005 vs. TI/R + vehicle; #, P < 0.005 vs. TI/R + NDP-α-MSH.
No significant change in IL-6 and TNF-α expression was detected in both testes of sham treated with vehicle or NDP-α-MSH (Fig. 1).
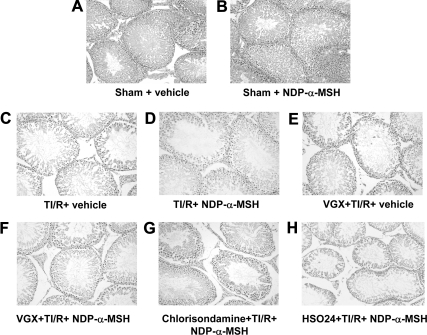
NDP-α-MSH attenuates the early histological damage
The testes from the sham rats (Fig. 2, A and B) showed an unmodified testicular morphology. TI/R injury produced a moderate edema, venular ectasia, and lobular coagulative necrosis after 24 h of reperfusion (Fig. 2C and Table 1). In contralateral testes, ischemia and reperfusion produced a minor degree of histological damage (Table 1). NDP-α-MSH attenuated the histological damage in both ipsilateral and contralateral testes (Fig. 2D and Table 1). Cervical vagotomy and pretreatment with either the nicotinic acetylcholine receptor antagonist chlorisondamine, or the selective MC4 receptor antagonist HS024, abrogated the effects of NDP-α-MSH on the histological alterations induced by TI/R injury (Fig. 2, E–H, and Table 1). Chlorisondamine or HS024 alone did not modify the histological damage caused by testicular torsion (results not shown).
Fig. 2.
Light microscopy at 24 h. A and B, Ipsilateral testis from sham rats treated with vehicle (1 ml/kg of a 0.9% NaCl solution) or NDP-α-MSH (340 μg/kg) ip administered immediately after reperfusion: normal appearance in both the tubular and extratubular compartments. C–H, Ipsilateral testis collected from rats subjected to TI/R injury and treated with vehicle or NDP-α-MSH as above. Bilateral cervical vagotomy (VGX) and pretreatment with the nicotinic receptor antagonist chlorisondamine (0.25 mg/kg, sc) or with the selective MC4 receptor antagonist HS024 (130 μg/kg ip) were performed 2 min before induction of ischemia. Notice the mild interstitial edema, venular ectasia, and lobular coagulative necrosis in D. On the contrary, moderate interstitial edema, venular ectasia, and lobular coagulative necrosis are present in C and E–H. Hematoxylin and Eosin stain, original magnification, ×200.
Table 1.
Histological score of both ischemic-reperfused (TI/R) and contralateral testes, at 24 h
| Experimental groups | Extratubular edema | Venular ectasia | Lobular coagulative necrosis |
|---|---|---|---|
| Ischemic and reperfused testis | |||
| Sham + vehicle | 0 | 0 | 0 |
| Sham + NDP-α-MSH | 0 | 0 | 0 |
| TI/R + vehicle | 1.8 ± 0.5a | 2.3 ± 0.8a | 0.4 ± 0.5a |
| TI/R + NDP-α-MSH | 0.8 ± 0.4b | 1.1 ± 0.5b | 0.3 ± 0.5 |
| VGX + TI/R + vehicle | 1.7 ± 0.8 | 1.9 ± 0.9 | 0.4 ± 0.8 |
| VGX + TI/R + NDP-α-MSH | 1.6 ± 0.5c | 2.0 ± 0.6c | 0.7 ± 0.5 |
| Chlorisondamine + TI/R + NDP-α-MSH | 1.9 ± 0.6c | 2.0 ± 0.8c | 0.4 ± 0.8 |
| HS024 + TI/R + NDP-α-MSH | 2.0 ± 0.6c | 2.3 ± 0.5c | 0.4 ± 0.5 |
| Contralateral testis | |||
| Sham + vehicle | 0 | 0 | 0 |
| Sham + NDP-α-MSH | 0 | 0 | 0 |
| TI/R + vehicle | 1.1 ± 0.5a | 1.6 ± 0.7a | 0.2 ± 0.1a |
| TI/R + NDP-α-MSH | 0.5 ± 0.4b | 0.8 ± 0.4b | 0.09 ± 0.04 |
| VGX + TI/R + vehicle | 1.2 ± 0.5 | 1.5 ± 0.6 | 0.1 ± 0.03 |
| VGX + TI/R + NDP-α-MSH | 1.3 ± 0.3c | 1.7 ± 0.8c | 0.4 ± 0.1c |
| Chlorisondamine + TI/R + NDP-α-MSH | 1.3 ± 0.6c | 1.6 ± 0.8c | 0.2 ± 0.09c |
| HS024 + TI/R + NDP-α-MSH | 1.6 ± 0.6c | 1.8 ± 0.5c | 0.2 ± 0.07c |
Each group included seven animals. Histologic grading was based on the following scale: 0 (absent), 1 (mild), 2 (moderate), and 3 (severe). VGX, Vagotomy. Data are presented as means ± sd.
P < 0.005 vs. sham + vehicle.
P < 0.005 vs. TI/R + vehicle.
P < 0.005 vs. TI/R + NDP-α-MSH.
No morphological alterations were found in both testes of sham rats treated with vehicle or NDP-α-MSH (Fig. 2, A and B, and Table 1).
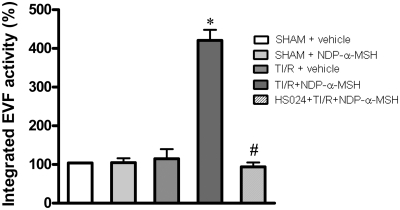
NDP-α-MSH activates efferent vagal fibers
Action potential recordings in TI/R rats showed low levels of efferent vagal activity, which were not different from those of both sham + vehicle and sham + NDP-α-MSH rats (Fig. 3). Interestingly, NDP-α-MSH administration in TI/R rats produced a strong enhancement in efferent vagal fiber activity, as recorded starting 30 min after injection (Fig. 3). These effects remained quite stable during the 30-min observation period. Pretreatment with the MC4 receptor antagonist HS024 prevented the MC-induced increase in neural vagal activity (Fig. 3). HS024 plus vehicle injection did not significantly modify the efferent vagal activity in TI/R rats (results not shown).
Fig. 3.
Efferent vagal fiber (EVF) activity in rats subjected to TI/R injury and treated with vehicle (1 ml/kg of a 0.9% NaCl solution) or NDP-α-MSH (340 μg/kg) ip administered immediately after reperfusion. A group of animals was pretreated with the selective MC4 receptor antagonist HS024 (130 μg/kg) ip administered 2 min before induction of ischemia. Action potentials were recorded starting 30 min after treatment with NDP-α-MSH and for a 30-min period. Bars represent mean values ± sd of the integrated EVF activity of seven animals, normalized to sham + vehicle (100%). For data comparison, we used integrated values obtained at the end of a 100-msec period for each electrophysiologic recording. *, P < 0.005 vs. TI/R + vehicle; #, P < 0.005 vs. TI/R + NDP-α-MSH.
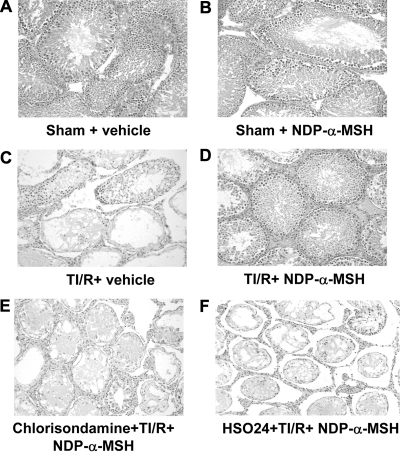
Effects NDP-α-MSH on late histological damage, spermatogenesis, and apoptosis
Thirty days after TI/R injury, groups of animals were evaluated for late histological damage, spermatogenesis, and apoptosis. Testes from sham rats treated with vehicle or NDP-α-MSH showed unmodified testicular morphology (Fig. 4, A and B, and Table 2). Twisted testes after 30 d had a severe grade of extratubular edema, venular ectasia, and lobular coagulative necrosis (Fig. 4C and Table 2). Contralateral testes showed also a moderate damage (Table 2). NDP-α-MSH significantly ameliorated the consequences of torsion/reperfusion on testis morphology, causing a quite absent lobular coagulative necrosis and an appreciable reduction of either extratubular edema or venular ectasia (Fig. 4D and Table 2). Chlorisondamine or HS024 alone did not modify the histological damage caused by testicular torsion (results not shown). However, the combined chlorisondamine and HS024 treatment abated the positive effects of NDP-α-MSH (Fig. 4, E and F, and Table 2). NDP-α-MSH protected also the contralateral testis, and this effect was reverted by either chlorisondamine and HS024 (Table 2).
Fig. 4.
Light microscopy at 30 d. A and B, Ipsilateral testis from sham rats treated with vehicle (1 ml/kg of a 0.9% NaCl solution) or NDP-α-MSH (340 μg/kg) ip administered starting immediately after reperfusion, once daily for 30 d: normal appearance in both the tubular and extratubular compartments. C–F, Ipsilateral testis collected from rats subjected to TI/R injury and treated with vehicle or NDP-α-MSH as above. Pretreatment with the nicotinic receptor antagonist chlorisondamine (0.25 mg/kg, sc) or with the selective MC4 receptor antagonist HS024 (130 μg/kg ip) were performed 2 min before induction of ischemia. Notice the mild interstitial edema, venular ectasia, and lobular coagulative necrosis in D. On the contrary, severe interstitial edema, venular ectasia, and lobular coagulative necrosis are present in C, E, and F. Hematoxylin and Eosin stain, original magnification, ×200.
Table 2.
Histological score of both ischemic-reperfused (TI/R) and contralateral testes, at d 30
| Experimental groups | Extratubular edema | Venular ectasia | Lobular coagulative necrosis |
|---|---|---|---|
| Ischemic and reperfused testis | |||
| Sham + vehicle | 0 | 0 | 0 |
| Sham + NDP-α-MSH | 0 | 0 | 0 |
| TI/R + vehicle | 2.9 ± 0.4a | 2.8 ± 0.5a | 2.9 ± 0.7a |
| TI/R + NDP-α-MSH | 1.0 ± 0.8b | 1.4 ± 1.0b | 0.3 ± 0.5b |
| Chlorisondamine + TI/R + NDP-α-MSH | 2.6 ± 0.8c | 2.7 ± 0.3c | 2.8 ± 0.4c |
| HS024 + TI/R + NDP-α-MSH | 2.7 ± 0.5c | 2.8 ± 0.2c | 2.7 ± 0.5c |
| Contralateral testis | |||
| Sham + vehicle | 0 | 0 | 0 |
| Sham + NDP-α-MSH | 0 | 0 | 0 |
| TI/R + vehicle | 1.9 ± 0.6a | 1.4 ± 0.4a | 1.6 ± 0.5a |
| TI/R + NDP-α-MSH | 0.6 ± 0.3b | 0.9 ± 0.7b | 0.09 ± 0.01b |
| Chlorisondamine + TI/R + NDP-α-MSH | 1.4 ± 0.5c | 1.6 ± 0.6c | 1.5 ± 0.6c |
| HS024 + TI/R + NDP-α-MSH | 1.7 ± 0.6c | 1.3 ± 0.5c | 1.7 ± 0.7c |
Each group included seven animals. All treatments were ip carried out starting immediately after reperfusion, once daily for 30 d. Histologic grading was based on the following scale: 0 (absent), 1 (mild), 2 (moderate), and 3 (severe). Data are presented as means ± sd.
P < 0.005 vs. sham + vehicle.
P < 0.005 vs. TI/R + vehicle.
P < 0.005 vs. TI/R + NDP-α-MSH.
Spermatogenesis in sham rats, evaluated by the Johnsen's score system, was normal and unaffected (Table 3). TI/R injury after 30 d produced a severe depletion of germ cells in basal and luminal compartments (Table 3). Treatment with NDP-α-MSH markedly improved spermatogenesis, and the germ cell morphology resulted well preserved (Table 3). Chlorisondamine or HS024 alone did not change the impairment in spermatogenesis caused by testicular torsion (results not shown), but both the antagonists reverted the beneficial effect of NDP-α-MSH.
Table 3.
Evaluation of spermatogenesis (Johnsen's score) in TI/R rats after 30 d of different treatments
| Experimental groups | Johnsen's score |
|---|---|
| Sham + vehicle | 9.6 ± 0.5 |
| Sham + NDP-α-MSH | 9.7 ± 0.5 |
| TI/R + vehicle | 3.0 ± 1.0a |
| TI/R + NDP-α-MSH | 6.9 ± 3.4b |
| Chlorisondamine + TI/R + NDP-α-MSH | 2.7 ± 0.8c |
| HS024 + TI/R + NDP-α-MSH | 2.4 ± 1.0c |
All treatments were ip carried out starting immediately after reperfusion, once daily for 30 d. Data are presented as means ± sd.
P < 0.005 vs. sham + vehicle.
P < 0.005 vs. TI/R + vehicle.
P < 0.005 vs. TI/R + NDP-α-MSH.
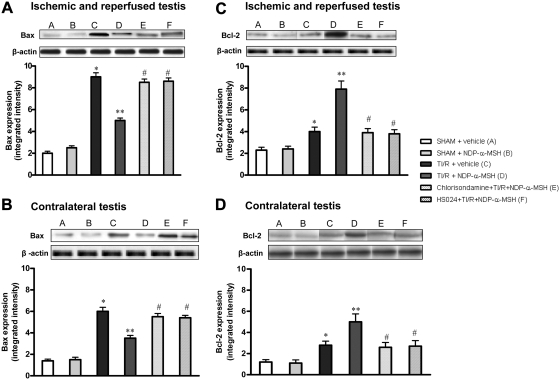
No significant difference in the basal expression of Bax and Bcl-2 was detected between testes of sham rats treated with vehicle or NDP-α-MSH (Fig. 5, A–D). TI/R injury after 30 d caused, in both testes, a marked increase in the expression of the proapoptotic protein Bax and a lesser (although significant) rise in the antiapoptotic protein Bcl-2 (Fig. 5). By contrast, these changes in the proapoptotic protein Bax were significantly blunted in the ipsilateral and contralateral testes of TI/R rats treated with NDP-α-MSH, whereas Bcl-2 expression greatly increased (Fig. 5). Chlorisondamine or HS024 alone did not modify the changes in the apoptosis machinery caused by testicular torsion (results not shown), but both antagonists abated the effects of NDP-α-MSH on apoptosis (Fig. 5).
Fig. 5.
Western blot analysis of Bax (A and B) and Bcl-2 (C and D) in samples of both ipsilateral (A and C) and contralateral (B and D) testes of rats undergone ischemia and reperfusion and ip treated with vehicle (1 ml/kg of a 0.9% NaCl solution) or NDP-α-MSH (340 μg/kg) immediately after reperfusion. A group of animals was pretreated with the nicotinic receptor antagonist chlorisondamine (0.25 mg/kg sc), and another with the selective MC4 receptor antagonist HS024 (130 μg/kg ip), 2 min before induction of ischemia. Bars represent mean values ± sd of seven animals. *, P < 0.005 vs. sham + vehicle; **, P < 0.005 vs. TI/R + vehicle; #, P < 0.005 vs. TI/R + NDP-α-MSH.
Discussion
The main complication of testicular torsion is the functional loss of the testis, which may lead to impaired fertility. Early surgical management increases the chance for testicular salvage. However, testicular salvage with surgical maneuver does not cause a fully resolution in all patients and, as a consequence, they may suffer infertility later in life.
The mechanisms causing infertility are an immediate damage prompted by ischemia during torsion and a late effect due to reperfusion during the untwisting of the spermatic cord (1, 2). Given the complexity of the underlying pathophysiology, several experimental pharmacological approaches, including antioxidants (25–29), dehydroepiandrosterone (30), erythropoietin (31, 32), and peroxisome proliferator-activated receptor agonists (33), have shown, at least experimentally, beneficial effects. These therapeutic approaches attempted to interrupt the two most important pathological mechanisms triggered by the restoration of blood flow to the testis: more specifically, they were addressed against either the inflammatory cascade and the activation of the apoptosis machinery (14, 16, 33). Both of these events take place during TI/R injury and may be responsible, at later stages, for the impairment in spermatogenesis. There is, therefore, a strong interest in identifying new pharmacological strategies able to counteract the exaggerated inflammatory reaction and the augmented apoptotic machinery, in an effort to reduce the impact of this pathology on male fertility.
It has been recognized that the vagus nerve signals the gonads trough neuronal impulses that originate from very different sites of the CNS (1, 2). Bilateral vagotomy induces an inhibitory effect on gonadotropin release triggered by orchidectomy (34). Moreover, unilateral cutting of the vagus nerve causes a marked increase in LH (35), and a combination of right-sided interruption of the vagus nerve with a right-sided hemiorchidectomy reduces serum testosterone and gonadotropin production (36). This experimental evidence strongly indicates that the efferent motor arm of the vagus nerve influences, in a pituitary-independent manner, testis function.
The presence of a cholinergic antiinflammatory pathway as a self-defense mechanism is now well recognized (6–10, 20, 37). This pathway consists of a vagal reflex, and electrical stimulation of the efferent vagal arm contributes to the reduction of the systemic and local inflammation. Furthermore, vagus nerve stimulation is able to down-regulate the apoptotic machinery triggered by the inflammatory and ischemic stimuli (23, 37). These effects are mediated by acetylcholine, the main vagus nerve neurotransmitter, which binds to a specific nicotinic receptor expressed on several types of inflammatory cells and bearing the α7-subunit (6–9). Interestingly, several nicotinic receptors are present in the sperm (38), and in particular, knock-out mice for the α7-subunit of the nicotinic receptor show an altered and disturbed sperm function (39).
These experimental findings led us to hypothesize that the stimulation of the vagus nerve motor arm might be a rational approach to reduce inflammation, to blunt the exaggerated apoptotic reaction, and to improve the impaired spermatogenesis after testicular torsion. As far as we know, no experimental study has specifically addressed this scientific hypothesis.
MC were used to activate the cholinergic antiinflammatory pathway to the testes (20, 21, 23, 37). These endogenous peptides, such as ACTH [ACTH-(1-24)], α-MSH, shorter fragments, and synthetic analogs as NDP-α-MSH, modulate the inflammatory response in several conditions, including low-flow states, such as circulatory shock, myocardial ischemia, and ischemic stroke (20, 21, 23, 37). MC bind different G protein-linked, seven transmembrane receptors (MC1 to MC5), and all receptors stimulate adenylate cyclase as a second messenger. MC cross the blood-brain barrier; MC3 and MC4 receptors are largely expressed in various brain areas with CNS concentration of MC4 broader than that of MC3 receptors (18). MC3 and MC4 receptor stimulation activates the cholinergic antiinflammatory pathway, the latter being responsible for the MC beneficial effects in circulatory shock and ischemic stroke (20, 21, 37).
In the present study, we used 1) NDP-α-MSH, agonist at MC1, MC3, MC4 and MC5 receptors, to stimulate MC receptors, including cerebral ones; 2) the selective MC4 receptor antagonist HS024 to identify the MC receptor subtype involved in the pharmacological effects; 3) vagotomy; and 4) the nicotinic acetylcholine receptor antagonist chlorisondamine (at a dose able to block peripheral receptors only) to trace the final step of the cholinergic antiinflammatory pathway. Here, we show that NDP-α-MSH attenuated the early histological damage caused by ischemia and reperfusion of the testis. Treatment with the MC agonist also inhibited activation of the early inflammatory cascade that leads to organ damage, by reducing the expression of the inflammatory cytokines TNF-α and IL-6. These effects were abrogated by bilateral cervical vagotomy, HS024, and chlorisondamine, thus confirming the important role played by the activation of the brain MC4 receptors at the first level of the vagus nerve-mediated antiinflammatory pathway and the involvement of a cholinergic nicotinic receptor at the final step of the triggered efferent vagal activity. To further ascertain and corroborate the hypothesis that also in TI/R injury brain MC receptor stimulation may prompt the vagus nerve-mediated cholinergic antiinflammatory pathway, we also recorded the neural activity along vagus nerve. We found that NDP-α-MSH markedly augmented efferent vagal activity, and this activity was abrogated by HS024, thus clearly confirming that the protective effects of MC observed in rats subjected to TI/R injury are the consequence of a MC4 receptor-triggered activation of the cholinergic antiinflammatory pathway. This points out that brain MC4 and peripheral nicotinic receptors are key components of the cholinergic antiinflammatory pathway. However, also the splenic (sympathetic) nerve seems to play an important role in the last step of the cholinergic antiinflammatory pathway. Indeed, the splenic nerve is required for mediating the antiinflammatory effects of the vagus nerve stimulation (37, 40, 41).
Overall, these findings would indicate that the activation of the vagus nerve motor arm may represent a rational therapeutic strategy to limit testicular damage during the early phases of the testicular torsion and may be combined to the surgical maneuver to improve the rate of testicular salvage. However, the main goal in the treatment of acute testicular torsion is to limit the long-lasting impact on fertility. Again, as far as we know, no study has been carried out to investigate whether activation of the cholinergic antiinflammatory pathway may influence spermatogenesis, at least experimentally. To investigate this point, we evaluated spermatogenesis 30 d after testicular torsion. Spermatogenesis was markedly impaired, with significant alteration in the germ cell line and absence of mature spermatozoa, an histological picture that resembles that observed in human male infertility. Vagus nerve motor arm activation by the MC NDP-α-MSH significantly improved spermatogenesis, whereas this beneficial effect was blunted by either HS024 and chlorisondamine pretreatment, thus confirming that, in our experimental conditions, brain MC4 and peripheral nicotinic receptors play a critical role in the vagus nerve-mediated cholinergic protective pathway. The mechanism by which activation of the vagus nerve is able to display protection against the late effects on spermatogenesis induced by testicular twisting seems to be a modulation of the apoptosis reaction. In fact, NDP-α-MSH reduced the expression of the proapoptotic proteins Bax and enhanced the expression of the antiapoptotic protein Bcl-2, thus confirming the idea that activation of the apoptosis machinery is one of the most important mechanisms underlying the disturbed spermatogenesis induced by testicular torsion.
In conclusion, these findings could have major clinical implications, because they indicate endogenous agents able to activate a physiological self-defense mechanism, namely selective MC4 receptor agonists, as an attractive therapeutic alternative for the management of testicular torsion. It is of note, however, that MC4 receptor agonists at doses much higher than those used in our present study, as compared within the same animal species, initiate penile erection (42), which may worsen the pain and complications of patients with testicular torsion. Where necessary, therefore, a concomitant treatment with an analgesic drug, not belonging to the opioid family, should be performed. Indeed, the MC and opioid systems regulate nociception in an opposite manner (43).
Acknowledgments
This study was supported by grants from Department and Ministero dell'Università e della Ricerca, Italy.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Bax
- Bcl-2-associated X protein
- Bcl-2
- B-cell lymphoma 2
- CNS
- central nervous system
- MC
- melanocortin
- NDP-α-MSH
- [Nle4,D-Phe7]α-melanocyte-stimulating hormone
- TBS
- Tris-buffered saline
- TI/R
- testicular ischemia-reperfusion.
References
- 1. Lee S, Miselis R, Rivier C. 2002. Anatomical and functional evidence for a neural hypothalamic-testicular pathway that is independent of the pituitary. Endocrinology 143:4447–4454 [DOI] [PubMed] [Google Scholar]
- 2. Gerendai I, Banczerowski P, Halász B. 2005. Functional significance of the innervation of the gonads. Endocrine 28:309–318 [DOI] [PubMed] [Google Scholar]
- 3. Gerendai I, Tóth IE, Boldogkoi Z, Medveczky I, Halász B. 2000. Central nervous system structures labelled from the testis using the transsynaptic viral tracing technique. J Neuroendocrinol 12:1087–1095 [DOI] [PubMed] [Google Scholar]
- 4. Gerendai I, Tóth IE, Boldogkoi Z, Medveczky I, Halász B. 1998. Neuronal labeling in the rat brain and spinal cord from the ovary using viral transneuronal tracingtechnique. Neuroendocrinology 68:244–256 [DOI] [PubMed] [Google Scholar]
- 5. Gerendai I, Kocsis K, Halász B. 2002. Supraspinal connections of the ovary:structural and functional aspects. Microsc Res Tech 59:474–483 [DOI] [PubMed] [Google Scholar]
- 6. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458–462 [DOI] [PubMed] [Google Scholar]
- 7. Tracey KJ. 2002. The inflammatory reflex. Nature 420:853–859 [DOI] [PubMed] [Google Scholar]
- 8. Tracey KJ. 2007. Physiology and immunology of the cholinergic anti-inflammatory pathway. J Clin Invest 117:289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, Marini H, Squadrito G, Minutoli L, Bertolini A, Marini R, Adamo EB, Venuti FS, Squadrito F. 2003. Efferent vagal fibre stimulation blunts nuclear factor-kB activation and protects against hypovolemic hemorrhagic shock. Circulation 107:1189–1194 [DOI] [PubMed] [Google Scholar]
- 10. Altavilla D, Guarini S, Bitto A, Mioni C, Giuliani D, Bigiani A, Squadrito G, Minutoli L, Venuti FS, Messineo F, De Meo V, Bazzani C, Squadrito F. 2006. Activation of the cholinergic anti-inflammatory pathway reduces NF-κB activation, blunts TNF-α production, and protects againts splanchic artery occlusion shock. Shock 25:500–506 [DOI] [PubMed] [Google Scholar]
- 11. Ringdahl E, Teague L. 2006. Testicular torsion. Am Fam Physician 74:1739–1743 [PubMed] [Google Scholar]
- 12. Filho DW, Torres MA, Bordin AL, Crezcynski-Pasa TB, Boveris A. 2004. Spermatic cord torsion, reactive oxygen and nitrogen species and ischemia-reperfusion injury. Mol Aspects Med 25:199–210 [DOI] [PubMed] [Google Scholar]
- 13. Lysiak JJ. 2004. The role of tumor necrosis factor-α and interleukin-1 in the mammalian testis and their involvement in testicular torsion and autoimmune orchitis. Reprod Biol Endocrinol 10:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Minutoli L, Antonuccio P, Romeo C, Nicòtina PA, Bitto A, Arena S, Polito F, Altavilla D, Turiaco N, Cutrupi A, Zuccarello B, Squadrito F. 2005. Evidence for a role of mitogen-activated protein kinase 3/mitogen-activated protein kinase in the development of testicular ischemia-reperfusion injury. Biol Reprod 73:730–736 [DOI] [PubMed] [Google Scholar]
- 15. Antonuccio P, Minutoli L, Romeo C, Nicòtina PA, Bitto A, Arena S, Altavilla D, Zuccarello B, Polito F, Squadrito F. 2006. Lipid peroxidation activates mitogen-activated protein kinases in testicular ischemia-reperfusion injury. J Urol 176:1666–1672 [DOI] [PubMed] [Google Scholar]
- 16. Minutoli L, Antonuccio P, Polito F, Bitto A, Squadrito F, Di Stefano V, Nicotina PA, Fazzari C, Maisano D, Romeo C, Altavilla D. 2009. Mitogen-activated protein kinase3/mitogen.activated protein kinase 1 activates apoptosis during testicular ischemia-reperfusion injury in a nuclear factor-κB-independent manner. Eur J Pharmacol 604:27–35 [DOI] [PubMed] [Google Scholar]
- 17. Tatro JB. 1996. Receptor biology of the melanocortins, a family of neuroimmunomodulatory peptides. Neuroimmunomodulation 3:259–284 [DOI] [PubMed] [Google Scholar]
- 18. Catania A, Gatti S, Colombo G, Lipton JM. 2004. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev 56:1–29 [DOI] [PubMed] [Google Scholar]
- 19. Corander MP, Fenech M, Coll AP. 2009. Science of self-preservation: how melanocortin action in the brain modulates body weight, blood pressure and ischemic damage. Circulation 120:2260–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guarini S, Cainazzo MM, Giuliani D, Mioni C, Altavilla D, Marini H, Bigiani A, Ghiaroni V, Passaniti M, Leone S, Bazzani C, Caputi AP, Squadrito F, Bertolini A. 2004. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc Res 63:357–365 [DOI] [PubMed] [Google Scholar]
- 21. Mioni C, Bazzani C, Giuliani D, Altavilla D, Leone S, Ferrari A, Minutoli L, Bitto A, Marini H, Zaffe D, Botticelli AR, Iannone A, Tomasi A, Bigiani A, Bertolini A, Squadrito F, Guarini S. 2005. Activation of an efferent cholinergic pathway produces strong protection against myocardial ischemia/reperfusion injury in rats. Crit Care Med 33:2621–2628 [DOI] [PubMed] [Google Scholar]
- 22. Giuliani D, Mioni C, Altavilla D, Leone S, Bazzani C, Minutoli L, Bitto A, Cainazzo MM, Marini H, Zaffe D, Botticelli AR, Pizzala R, Savio M, Necchi D, Schiöth HB, Bertolini A, Squadrito F, Guarini S. 2006. Both early and delayed treatment with melanocortin 4 receptor-stimulating melanocortins produces neuroprotection in cerebral ischemia. Endocrinology 147:1126–1135 [DOI] [PubMed] [Google Scholar]
- 23. Ottani A, Giuliani D, Mioni C, Galantucci M, Minutoli L, Bitto A, Altavilla D, Zaffe D, Botticelli AR, Squadrito F, Guarini S. 2009. Vagus nerve mediates the protective effects of melanocortins against cerebral and systemic damage after ischemic stroke. J Cereb Blood Flow Metab 29:512–523 [DOI] [PubMed] [Google Scholar]
- 24. Johnsen SG. 1970. Testicular biopsy score count—a method for registration of spermatogenesis in human testis: normal values and results in 335 hypogonadal males. Hormones 1:2–25 [DOI] [PubMed] [Google Scholar]
- 25. Bitto A, Polito F, Altavilla D, Irrera N, Giuliani D, Ottani A, Minutoli L, Spaccapelo L, Galantucci M, Lodi R, Guzzo G, Guarini S, Squadrito F. 2011. Melanocortins protect against multiple organ dysfunction syndrome in mice. Br J Pharmacol 162:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Romeo C, Antonuccio P, Esposito M, Marini H, Impellizzeri P, Turiaco N, Altavilla D, Bitto A, Zuccarello B, Squadrito F. 2004. Raxofelast, a hydrophilic vitamin E-like antioxidant, reduces testicular ischemia-reperfusion injury. Urol Res 32:367–371 [DOI] [PubMed] [Google Scholar]
- 27. Wei SM, Yan ZZ, Zhou J. 2008. Taurine reduces testicular ischemia/reperfusion-induced neutrophil recruitment to testis probably by downregulation of pro-inflammatory cytokines and E-selectin. Urology 72:464–465 [DOI] [PubMed] [Google Scholar]
- 28. Wei SM, Yan ZZ, Zhou J. 2009. Curcumin attenuates ischemia-reperfusion injury in rat testis. Fertil Steril 91:271–277 [DOI] [PubMed] [Google Scholar]
- 29. Payabvash S, Salmasi AH, Kiumehr S, Tavangar SM, Nourbakhsh B, Faghihi SH, Dehpour AR. 2007. Salutary effects of N-acetylcysteine on apoptotic damage in a rat model of testicular torsion. Urol Int 79:248–254 [DOI] [PubMed] [Google Scholar]
- 30. Aksoy H, Yapanoglu T, Aksoy Y, Ozbey I, Turhan H, Gursan N. 2007. Dehydroepiandrosterone treatment attenuates reperfusion injury after testicular torsion and detorsion in rats. J Pediatr Surg 42:1740–1744 [DOI] [PubMed] [Google Scholar]
- 31. Akcora B, Altug ME, Kontas T, Atik E. 2007. The protective effect of darbepoetin alfa on experimental testicular torsion and detorsion injury. Int J Urol 14:846–850 [DOI] [PubMed] [Google Scholar]
- 32. Yazihan N, Ataoglu H, Koku N, Erdemli E, Sargin AK. 2007. Protective role of erythropoietin during testicular torsion of the rats. World J Urol 25:531–536 [DOI] [PubMed] [Google Scholar]
- 33. Minutoli L, Antonuccio P, Polito F, Bitto A, Squadrito F, Irrera N, Nicotina PA, Fazzari C, Montalto AS, Di Stefano V, Romeo C, Altavilla D. 2009. Peroxisome proliferator activated receptor β/δ activation prevents extracellular regulated kinase 1/2 phosphorylation and protects the testis from ischemia and reperfusion injury. J Urol 181:1913–1921 [DOI] [PubMed] [Google Scholar]
- 34. Allen LG, Hodson CA, Burden HW, Lawrence IE., Jr 1983. Effect of vagotomy on postcastration gonadotropin secretion in male rats. Proc Soc Exp Biol Med 173:613–619 [DOI] [PubMed] [Google Scholar]
- 35. Gerendai I, Motta M. 1990. Effect of unilateral vagotomy on serum gonadotropin concentration in rats with two testes and in hemicastrates. Endocrinol Exp 24:325–332 [PubMed] [Google Scholar]
- 36. Gerendai I, Csaba Z, Vokó Z, Csernus V. 1995. Involvement of a direct neural mechanism in the control of gonadal functions. J Steroid Biochem Mol Biol 53:299–305 [DOI] [PubMed] [Google Scholar]
- 37. Giuliani D, Ottani A, Altavilla D, Bazzani C, Squadrito F, Guarini S. 2010. Melanocortins and the cholinergic anti-inflammatory pathway. Adv Exp Med Biol 681:71–87 [DOI] [PubMed] [Google Scholar]
- 38. Kumar P, Meizel S. 2005. Nicotinic acetylcholine receptor subunits and associated proteins in human sperm. J Biol Chem 280:25928–25935 [DOI] [PubMed] [Google Scholar]
- 39. Bray C, Son JH, Kumar P, Meizel S. 2005. Mice deficient in CHRNA7, a subunit of the nicotinic acetylcholine receptor, produce sperm with impaired motility. Biol Reprod 73:807–814 [DOI] [PubMed] [Google Scholar]
- 40. Rosas-Ballina M, Tracey KJ. 2009. Cholinergic control of inflammation. J Intern Med 265:663–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tracey KJ. 2010. Understanding immunity requires more than immunology. Nat Immunol 11:561–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wikberg JE, Mutulis F. 2008. Targeting melanocortin receptors: an approach to treat weight disorders and sexual dysfunction. Nat Rev Drug Discov 7:307–323 [DOI] [PubMed] [Google Scholar]
- 43. Vrinten DH, Kalkman CJ, Adan RA, Gispen WH. 2001. Neuropathic pain: a possible role for the melanocortin system? Eur J Pharmacol 429:61–69 [DOI] [PubMed] [Google Scholar]