Abstract
Insulin resistance (IR) is the major feature of metabolic syndrome, including type 2 diabetes. IR studies are mainly focused on peripheral tissues, such as muscle and liver. There is, however, little knowledge about IR in neurons. In this study, we examined whether neurons develop IR in response to hyperinsulinemia. We first examined insulin signaling using adult dorsal root ganglion neurons as a model system. Acute insulin treatment resulted in time- and concentration-dependent activation of the signaling cascade, including phosphorylation of the insulin receptor, Akt, p70S6K, and glycogen synthase kinase-3β. To mimic hyperinsulinemia, cells were pretreated with 20 nm insulin for 24 h and then stimulated with 20 nm insulin for 15 min. Chronic insulin treatment resulted in increased basal Akt phosphorylation. More importantly, acute insulin stimulation after chronic insulin treatment resulted in blunted phosphorylation of Akt, p70S6K, and glycogen synthase kinase-3β. Interestingly, when the cells were treated with phosphatidylinositol 3-kinase pathway inhibitor, but not MAPK pathway inhibitor, chronic insulin treatment did not block acute insulin treatment-induced Akt phosphorylation. Insulin-induced Akt phosphorylation was lower in dorsal root ganglion neurons from BKS-db/db compared with control BKS-db+ mice. This effect was age dependent. Our results suggest that hyperinsulinemia cause IR by disrupting the Akt-mediated pathway. We also demonstrate that hyperinsulinemia increases the mitochondrial fission protein dynamin-related protein 1. Our results suggest a new theory for the etiology of diabetic neuropathy, i.e. that, similar to insulin dependent tissues, neurons develop IR and, in turn, cannot respond to the neurotrophic properties of insulin, resulting in neuronal injury and the development of neuropathy.
Insulin is a major anabolic hormone playing an essential role in glucose homeostasis by regulating the balance between hepatic glucose production and glucose uptake by muscle and adipose tissue. Insulin resistance (IR) is defined as a state of decreased responsiveness of target tissues to normal circulating levels of insulin. IR is the main contributor to a group of related physiologic dysfunctions known as the metabolic syndrome (glucose intolerance, obesity, dyslipidemia, and hypertension) (1, 2). IR and metabolic syndrome are major risk factors for the development of type 2 diabetes (3).
The world population with diabetes is estimated to increase from 171 million in 2000 to 366 million in 2030 (4). According to the report from the Centers for Disease Control and Prevention, diabetes affects 23.6 million people in the United States (plus another 57 million with prediabetes), costing $174 billion for the U.S. economy in 2007. Diabetes is a complex metabolic disorder characterized by hyperglycemia and associated with microvascular and macrovascular complications (5). Diabetic neuropathy is one of the most common complications for both type 1 and type 2 diabetes (5). The prevalence of diabetic neuropathy depends on the duration of the disease; more than half of patients eventually develop neuropathy (6).
There is a variety of opinions concerning the pathogenesis of diabetic neuropathy (5). One theory suggests that perturbation of neurotrophic factors, such as nerve growth factor or IGF, results in neurodegeneration and damage to supporting Schwann cells, contributing to the pathogenesis of diabetic neuropathy. Insulin is a well-documented growth factor for neurons (7, 8), and its receptors are widely expressed in both the peripheral and central nervous systems (9). Although neurons are not insulin dependent, they are insulin responsive (7–9). Therefore, insulin deficiency in type 1 diabetes and hyperinsulinemia in type 2 diabetes may contribute to the development of diabetic neuropathy.
Akt, a serine/threonine kinase-activated downstream of phosphatidylinositol 3-kinase (PI3-K), is a critical signaling molecule mediating cell survival, growth, and energy homeostasis (10, 11). Full activation of Akt requires phosphorylation of Thr308 in the catalytic domain by phosphoinositide dependent protein kinase (PDK)1 and Ser473 in the C-terminal hydrophobic domain by mammalian target of rapamycin complex (mTORC)2 (10). Considering the important roles of Akt in insulin signaling, it is not surprising that alterations in Akt activity are found in various cells in diabetes and insulin-resistant states. In obese rats, insulin-stimulated Akt1 activity is decreased in muscle and adipose tissue but increased in liver and vice versa for Akt2 (12). Akt phosphorylation is reduced in adipocytes and skeletal muscle of type 2 diabetic patients (13, 14).
IR studies are mainly focused on peripheral tissues, such as muscle and liver. Chronic insulin treatment induces IR with decreased Akt signaling and glucose transporter expression in muscle and adipocytes (15–17). There is, however, little knowledge about IR in neurons. In the late 1990s, our laboratory introduced the idea that glucose-mediated oxidative stress injures sensory neurons, leading to eventual development of diabetic neuropathy (18). Recently, we started to investigate whether hyperinsulinemia-induced IR causes or contributes to similar neuronal injuries. We speculate that like metabolic tissues, such as fat and muscle, neurons develop a form of IR, making them susceptible to glucose-mediated damage. In the current study, we examined possible molecular mechanisms for neuronal IR. We present evidence of chronic insulin stimulation-mediated blunting of Akt and its downstream effectors in adult dorsal root ganglion (DRG) neuron culture. We provide the evidence that hyperinsulinemia results of disruption in mitochondrial biogenesis. We also demonstrate that insulin-mediated Akt phosphorylation is reduced in DRG neurons prepared from a mouse model of type 2 diabetes. We conclude that chronic stimulation of Akt may contribute to reduced insulin signaling in DRG neurons and underlie, in part, the pathogenesis of diabetic neuropathy.
Materials and Methods
Antibodies and chemicals
Antibodies against insulin receptor (InsR), InsR substrate (IRS)-1, and actin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antiglyceraldehyde-3-phosphate dehydrogenase antibody was from Chemicon (Temecula, CA) and antidynamin-related protein (Drp)1 was from Abnova (Walnut, CA). All other antibodies were purchased from Cell Signaling (Beverly, MA). Inhibitors (LY294002 and U0126) were purchased from Calbiochem (La Jolla, CA). Palmitic acid (PA) was purchased from Nu-Chek Prep, Inc. (Elysian, MN). All other chemicals were purchased from either Sigma (St. Louis, MO) or Fisher Scientific (Fair Lawn, NJ).
Adult DRG neuron preparation
DRG neurons were prepared as previously described from adult (>6 wk old) C57Bl6/J mice or Sprague Dawley rats (19). BKS-db/db and db+ (BKS.Cg-m+/+ Leprdb/J, JAX Mice stock no. 000642) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were housed in a pathogen-free environment and cared for following the University of Michigan Committee on the Care and Use of Animals guidelines. All culture reagents were from GIBCO (Grand Island, NY) unless stated otherwise. The neurons were dissociated in 0.2% collagenase for 30 min followed by 1% trypsin for 15 min. Cells were plated on collagen-coated 12-well tissue culture plates in feed media (1:1 mix of DMEM:F-10 media containing 1× B27 additives without antioxidant, 7 μm aphidicolin, and 1000 U/ml penicillin/streptomycin/neomycin) (d 0). The media were changed to fresh feed media on d 2. On d 3 (24 h before treatment), to exclude the effect of insulin contained in B27, culture media were changed to treatment media (feed media without B27). Chronic treatment with insulin, glucose, or palmitate was achieved by adding these agents on d 3 in treatment media for 24 h. Inhibitors were also added to the media of some cultures at the same time, for 24 h.
Western immunoblotting
DRG neuron cultures were lysed in radioimmunoprecipitation assay (RIPA) buffer (Pierce, Rockford, IL) containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Lysates were collected, briefly sonicated, and centrifuged at 13,000 rpm for 15 min at 4 C. Western immunoblotting was performed as described previously (20). Tris-buffered saline with 0.01% Tween 20, and 5% fat-free milk was used for blocking and antibody dilution. Incubations with primary and secondary antibodies were carried out either at room temperature for 2 h or at 4 C overnight. The signal was visualized using enhanced chemiluminescence reagents (Amersham Bioscience, Piscataway, NJ) or SuperSignal West Femto Maximum Sensitivity Substrate (Pierce). Images were captured using the Chemidoc XRS system and analyzed by Quantity One software (Bio-Rad Laboratory, Hercules, CA) (20). All experiments were repeated at least three times, and representative results are presented in the figures. In some experiments, the nitrocellulose membranes were incubated at 60 C for 15 min in stripping solution [2% sodium dodecyl sulfate, 100 mm dithiothreitol, and 100 mm Tris (pH 6.8)], whereupon they were used for immunoblotting with another antibody.
Immunohistochemistry (IHC)
Adult DRG neurons cultured on laminin-coated coverslips were permeabilized with PBS containing 3% BSA, 0.1% Triton X-100, and 1% normal donkey serum (IHC buffer). The cells were incubated with the primary antibodies diluted in IHC buffer at 4 C overnight. After rinsing with PBS three times for 5 min each, the cells were incubated with the antirabbit IgG secondary antibody conjugated with Alexa Fluor 594 (Molecular Probes, Eugene, OR) for 2 h at room temperature. After rinsing with PBS, the coverslips were mounted with ProLong antifade mounting media containing 4′,6-diamidino-2-phenylindole (Molecular Probes). The digital images were captured using a Spot-RT camera (Diagnostic Instrument, Inc., Sterling Heights, MI) attached to Nikon Microphot-FXA microscope (Nikon, Melville, NY).
Statistical analysis
All experiments were repeated at least three times and presented as the mean ± sem. Statistical analysis was performed by either one-way ANOVA with Tukey's post hoc analysis or Student's t test depending on the number of comparison groups, using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA). Statistical significance was defined as P < 0.05.
Results
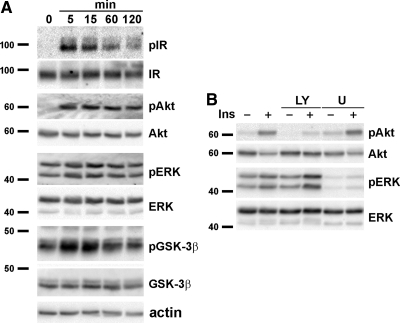
Insulin signaling influences the development of the nervous system by promoting neurogenesis, survival, and differentiation (21–24). The MAPK and PI3-K/Akt-pathways are the major intracellular signaling cascades that mediate insulin's effects (9, 25). As a first step, insulin signaling in adult DRG neurons was examined by Western immunoblotting after treatment with 20 nm insulin for 0–2 h. Insulin stimulation induced a time-dependent increase in the phosphorylation (i.e. activation) of intracellular signaling molecules, including InsR, Akt, and glycogen synthase kinase-3β (GSK-3β) (Fig. 1A). ERK exhibited high background phosphorylation, and insulin stimulation had a minimal effect on its phosphorylation. Each blot was stripped and reprobed with antibodies against total protein as well as actin to confirm equal protein loading. As expected, the PI3-K pathway inhibitor LY294002 reduced insulin-induced Akt phosphorylation, and the MAPK inhibitor U0126 decreased basal ERK phosphorylation (Fig. 1B). We find similar results using rat adult DRG neurons (data not shown).
Fig. 1.
Insulin signaling in cultured adult DRG neurons. A, DRG neurons were prepared from adult C57Bl6/J mice. The cells were cultured for 4 d and stimulated with 20 nm insulin for 0–2 h. B, DRG neurons were treated without or with 20 μm LY294002 (LY) or U0126 (U) for 1 h and then stimulated for 15 min with 20 nm insulin. Cell lysates were prepared in RIPA buffer and immunoblotted with the antibodies against the indicated phosphorylated proteins. The blots were stripped and reprobed for the total proteins. Experiments were repeated at least three times, and a representative result is shown. Ins, Insulin; p, phosphorylated form.
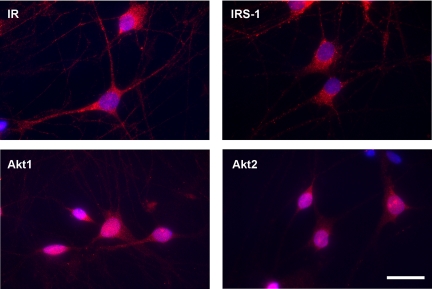
We next examined the localization of the signaling molecules in DRG neurons using IHC. IR and IRS-1 display a punctate pattern of localization within the cytoplasm and along the neurites. Nuclear localization was not detected (Fig. 2). In contrast, Akt1 and Akt2 display more diffuse distribution within the cytoplasm and nucleus. We did not detect any quantifiable changes in the localization of the signaling molecules by insulin treatment (data not shown).
Fig. 2.
Cellular localization of insulin signaling molecules. Adult rat DRG neurons were cultured on laminin-coated coverslides and immunolabeled with the indicated antibodies (red). Nuclei were visualized by 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bar, 10 μm.
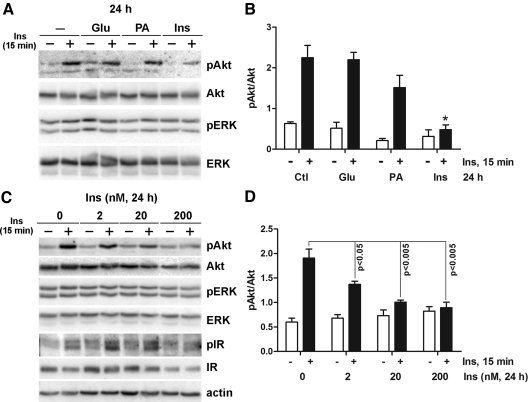
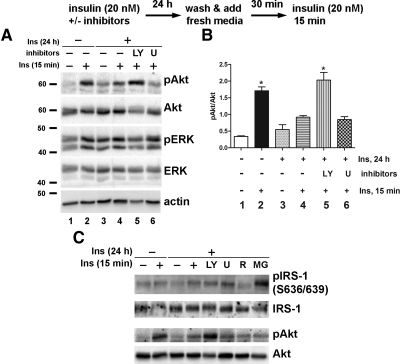
IR may be induced by hyperglycemia, dyslipidemia, or hyperinsulinemia, which are the characteristics of type 2 diabetes and the metabolic syndrome (1, 26, 27). Therefore, we next explored whether these factors contribute the development of IR in DRG neurons. To create an in vitro model of the metabolic syndrome and assess IR, adult DRG neurons were treated with 25 mm added glucose, 0.2 mm PA, or 20 nm insulin for 24 h. The media were then replaced with fresh treatment media for 30 min, and the cells were stimulated with 20 nm insulin for another 15 min. Akt was phosphorylated after 15 min of acute insulin treatment in DRG neurons not given a 24-h pretreatment. Interestingly, when cells were chronically pretreated with 20 nm insulin for 24 h, Akt phosphorylation by subsequent acute insulin treatment was significantly reduced (Fig. 3A). In contrast, 24 h of glucose or PA treatment had no effect on the activation of Akt in response to short-term insulin treatment. Densitometric analysis confirmed that only insulin pretreatment significantly decreased in Akt phosphorylation (Fig. 3B). The effect of chronic insulin pretreatment on Akt phosphorylation was concentration-dependent from 2 to 200 nm (Fig. 3C). Maximal Akt phosphorylation by acute insulin treatment was significantly lower in insulin-pretreated cells compared with nontreated cells. There was also a concentration-dependent increase in basal Akt phosphorylation, but this trend did not reach statistical significance. When the cells were pretreated with 20 or 200 nm insulin, acute insulin treatment failed to elicit any significant increase in Akt phosphorylation over basal phosphorylation (Fig. 3D). Insulin treatment did not induce any noticeable changes in ERK phosphorylation regardless of pretreatment insulin concentration. InsR phosphorylation was not significantly affected by up to 20 nm insulin pretreatment (Fig. 3C). InsR phosphorylation and expression were decreased only at the highest pretreatment insulin concentration (200 nm). These results suggest that development of IR is mainly induced by hyperinsulinemia, rather than hyperglycemia or hyperlipidemia, in DRG neurons.
Fig. 3.
Chronic insulin stimulation mimicking hyperinsulinemia down-regulates the ability of DRG neurons to respond to acute insulin stimulation. A, DRG neurons were incubated with 25 mm glucose (Glu), 0.2 mm PA, or 20 nm insulin (Ins) for 24 h, washed in fresh treatment media for 30 min, and then stimulated with 20 nm insulin for 15 min. B, Densitometric analysis of Akt phosphorylation of A. *, P < 0.05 by one-way ANOVA compared with the other acute insulin-treated conditions. Akt phosphorylation in Glu and PA pretreated cells was not statistically significant compared with control cells. C, DRG neurons were incubated with 0–200 nm insulin for 24 h and then stimulated with 20 nm insulin for 15 min. D, Densitometric analysis of Akt phosphorylation of C. Statistical significance was analyzed by Student's t test. Cell lysates were prepared in RIPA buffer and immunoblotted with the indicated antibodies. Antiactin immunoblot demonstrates the equal loading of the proteins. Ctl, Control; p, phosphorylated form.
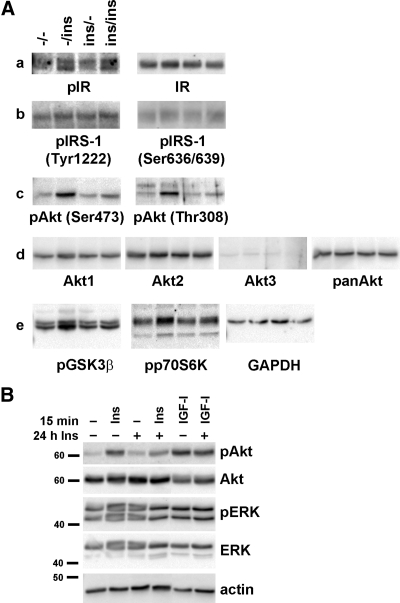
We next examined the detailed changes in insulin signaling by insulin pretreatment. As shown in the previous figure, IR phosphorylation and protein expression levels were not affected by insulin pretreatment (Fig. 4Aa). IRS-1 phosphorylation was measured by phosphor-specific antibodies at Tyr1222 and Ser636/639 sites. Insulin stimulation induced slight phosphorylation at both locations, and like IR, insulin pretreatment did not affect the phosphorylation (Fig. 4Ab). Full activation of Akt requires sequential phosphorylation of Thr308 and Ser473 by PDK1 and mTORC2, respectively (10). Twenty-four-hour insulin treatment decreased the phosphorylation at both of these sites (Fig. 4Ac). Insulin pretreatment or acute insulin stimulation did not affect the protein expression levels of Akt1 or Akt2 isoforms or total Akt (Fig. 4Ad). The Akt3 isoform was not expressed in DRG neurons. p70S6K and GSK-3β are two important signaling molecules mediating Akt's physiological effects on survival, neuronal differentiation, and metabolism (10, 11). In parallel with the effect on Akt phosphorylation, 24-h chronic insulin pretreatment decreased acute insulin-stimulated p70S6K and GSK-3β phosphorylation (i.e. activation of p70S6K and inactivation of GSK-3β) (Fig. 4Ae). Insulin and IGF-I share common signaling pathways for their physiological actions (9, 25). Even though insulin pretreatment significantly reduced Akt phosphorylation by acute insulin treatment, it did not affect IGF-I-stimulated Akt phosphorylation (Fig. 3B), suggesting that the effect of insulin pretreatment is specific to insulin signaling.
Fig. 4.
Chronic insulin treatment reduces insulin-stimulated Akt phosphorylation and downstream signaling molecules. A, The cells were treated without or with 20 nm insulin for 24 h and stimulated with 20 nm insulin for 15 min. B, The cells were pretreated with insulin for 24 h and then stimulated with 20 nm insulin or IGF-I for 15 min. Cell lysates were prepared in RIPA buffer and immunoblotted with the indicated antibodies. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. panAkt, Total Akt; Ins, insulin; p, phosphorylated form.
We next examined the possible mechanism behind the reduction in Akt activation after chronic insulin treatment using the inhibitors for PI3-K/Akt (LY294002) or ERK (U0126) signaling pathways. During the 24-h chronic insulin pretreatment, DRG neurons were incubated without or with these inhibitors. The cells were then washed with Hank's balanced salt solution and incubated in fresh treatment media (without insulin or inhibitors) for 30 min before stimulation with 20 nm insulin for 15 min. As in the previous experiments, acute insulin treatment-induced Akt phosphorylation was significantly reduced in DRG neurons chronically pretreated with insulin (Fig. 5A). Combined treatment with insulin and LY294002 restored Akt phosphorylation by acute insulin treatment to control levels (Fig. 5A, compare lanes 2, 4, and 5). U0126 had no effect on Akt phosphorylation. Densitometric analysis (Fig. 5B) confirms that LY294002 treatment (lane 5) restored Akt phosphorylation, which was significantly higher than in cells treated without inhibitors (lane 4) or with U0126 (lane 6) treatment, and displayed no statistically significant difference compared with control insulin-treated neurons (lane 2). Insulin-induced IRS-1 serine phosphorylation was not affected by chronic insulin or by LY294002 or U0126. mTOR/p70S6K inhibitor, rapamycin, reduced and proteasome inhibitor, MG132, increased IRS-1 phosphorylation. However, Akt phosphorylation was not changed by rapamycin or MG132 treatment (Fig. 5C). As in the previous experiments, ERK phosphorylation was not significantly affected by a 24-h insulin treatment or by the inhibitors. These results suggest that chronic hyperactivation of Akt by insulin prevents further activation by acute insulin treatment.
Fig. 5.
LY294002 pretreatment prevents hyperinsulinemia-induced down-regulation of Akt stimulation. A, DRG neurons were incubated with insulin along with 20 μm LY294002 (LY) or U0126 (U) for 24 h. The cells were washed and incubated in fresh treatment media for 30 min before acute insulin stimulation for 15 min. Immunoblotting was performed using the antibodies against phosphorylated Akt or ERK and then stripped and reprobed for total protein as well as for actin. B, Densitometric analysis of Akt phosphorylation. *, P < 0.05 by one-way ANOVA compared with other treatment conditions. C, Cells were treated with insulin and/or inhibitors for 24 h and then stimulated with insulin for 15 min in fresh media. R, Rapamycin, 100 nm; MG, MG132, 20 μm. Ins, Insulin; p, phosphorylated form.
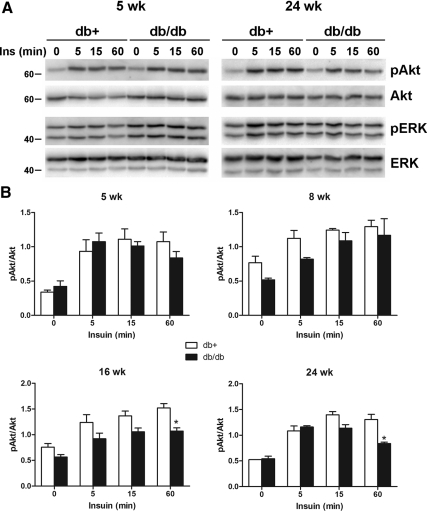
These observations were confirmed using adult DRG from an animal model of type 2 diabetes, the BKS-db/db mouse. The BKS.Cg-m+/+ Leprdb/J, commonly known as BKS-db/db, expresses a homozygous mutation of the leptin receptor and demonstrates typical characteristics of type 2 diabetes, including obesity, hyperinsulinemia, and hyperglycemia (http://jaxmice.jax.org/strain/000642.html). BKS-db+ mice are heterozygous for this mutation and serve as a control. Adult mouse DRG neurons prepared from different ages were cultured in vitro for 4 d and stimulated with 20 nm insulin for 0–60 min. DRG neurons prepared from 5 wk (1 wk diabetes) db+ and db/db mice demonstrated the increased Akt phosphorylation by insulin treatment, which was maintained up to 60 min (Fig. 6A). However, DRG neurons from 24-wk db/db, but not db+, mice displayed decreased Akt phosphorylation at 60 min. This effect was age dependent, with decreased Akt phosphorylation beginning at approximately 16 wk of age (12-wk diabetes) (Fig. 6B).
Fig. 6.
Insulin-stimulated Akt phosphorylation is reduced in DRG neurons from BKS-db/db mice. DRG neurons were prepared from BKS-db+ and BKS-db/db mice at different ages and cultured in vitro for 4 d before stimulation with 20 nm insulin for 0–60 min. A, The representative immunoblots of DRG neurons from 5- and 24-wk-old mice. B, Densitometry analysis of Akt phosphorylation from DRG neurons of 5-, 8-, 16-, and 24-wk-old mice. *, P < 0.05 by Student's t test compared with BKS-db+ at 60 min. Ins, Insulin; p, phosphorylated form.
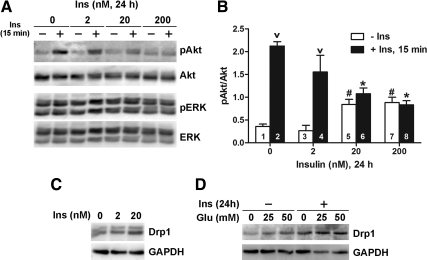
Mitochondria play important roles in cellular energy metabolism and ATP generation (28). High metabolic activity of mitochondria results in the generation of reactive oxygen species (29). We demonstrated the critical role of mitochondria in the progression of hyperglycemia-mediated neuronal damage and diabetic neuropathy (30–32). Previous work from our laboratory implicated Drp1, a GTPase mediating mitochondrial fission, in hyperglycemia-mediated mitochondrial damage in sensory neurons (33, 34). For this experiment, we used rat adult DRG. Consistent with mouse DRG, rat adult DRG neurons display a similar decrease in insulin-induced Akt phosphorylation after chronic insulin treatment (Fig. 7, A and B). When adult DRG neurons were exposed to chronic insulin treatment for 24 h, there was a concentration-dependent increase in Drp1 expression (Fig. 7C). To examine the effect of hyperinsulinemia on glucose-mediated increase in Drp1 expression, the cells were first treated with 20 nm insulin for 24 h. The cells were then washed with HBSS and incubated in fresh treatment media for 30 min before treatment with 25 or 50 mm added glucose for 6 h. In agreement with our previous results (33, 34), glucose treatment induced Drp1 expression, suggesting increased mitochondrial fission (Fig. 7D). When the cells were pretreated with chronic insulin, there was additive effect on glucose-mediated Drp1 expression. Our data suggest that hyperinsulinemia disrupts mitochondrial activity, making the neurons more susceptible to subsequent hyperglycemic insults.
Fig. 7.
Hyperinsulinemia and hyperglycemia increase Drp1 expression in rat adult DRG neuron culture. A, DRG neurons were incubated with 0–200 nm insulin for 24 h and then stimulated with 20 nm insulin for 15 min. B, Densitometric analysis of Akt phosphorylation of A. Statistical significance was analyzed by one-way ANOVA. v, P < 0.05 compared with corresponding no 15-min insulin treatment (bars 1 vs. 2 and 3 vs. 4); #, P < 0.05 compared with 0 and 2 nm 24 h insulin and no 15-min insulin treatment (bars 1 and 3); *, P < 0.05 compared with no 24-h insulin and 15-min insulin treatment (bar 2). C, DRG neurons were incubated with 0, 2, or 20 nm insulin for 24 h. D, DRG neurons were incubated without or with 20 nm insulin for 24 h, washed, and then treated with 25 or 50 mm glucose for the additional 6 h in treatment media. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. Ins, Insulin; p, phosphorylated form.
Discussion
IR due to hyperinsulinemia is one of the main characteristics of type 2 diabetes and other metabolic syndromes (1). IR studies are mostly focused on metabolic tissues, such as adipocytes and muscle. We hypothesized that neurons, similar to metabolic tissues, may develop IR that contributes to the development of neuropathy in diabetic patients. In the current study, we demonstrate that insulin-induced Akt phosphorylation was severely blunted in adult DRG neurons that had been previously exposed to insulin for a long period (24 h). Interestingly, acute Akt signaling was restored when the PI3-K inhibitor, LY294002, was added with 24-h insulin. We also observed that adult DRG prepared from the animal models of type 2 diabetes display reduced insulin-stimulated Akt phosphorylation. Our results suggest that chronic stimulation of PI3-K/Akt pathway is partly responsible for the development of IR.
Stimulation of adult DRG with physiologic levels of insulin resulted in the activation of signaling pathways, including the phosphorylation of InsR, Akt, and its downstream signaling molecules, GSK-3β and p70S6K. We have previously reported the activation of Akt by IGF-I and its critical role in DRG survival (35). In contrast, ERK phosphorylation was only slightly affected by insulin treatment, which was in agreement with the previous reports by ours (35) and other researchers (36, 37). Even though InsR phosphorylation was quickly decreased after approximately 60 min of insulin stimulation, Akt phosphorylation was maintained up to 2 h. Akt is activated downstream of PI3-K. However, it has been suggested that full activation of PI3-K is not necessary for maximal Akt activation (38). Akt activity in skeletal muscle from obese Zucker rats is only slightly decreased, even though PI3-K activity is significantly reduced compared with lean control rats (12). As expected, PI3-K and MAPK signaling pathway inhibitors LY294002 and U0126 decreased insulin-stimulated as well as basal Akt and ERK phosphorylation, respectively. Our results suggest that the Akt-mediated signaling pathway plays a major role in conducting insulin's action in DRG neurons.
Type 2 diabetes and the metabolic syndrome are characterized by hyperglycemia, dyslipidemia, and hyperinsulinemia, all of which contribute the development of IR (1, 26, 27). In the late 1990s, our laboratory introduced the idea that glucose-mediated oxidative stress injures sensory neurons, leading to eventual death and loss of neurons and supporting Schwann cells and the development of diabetic neuropathy (5). We demonstrated that 20–25 mm added glucose induces programmed cell death in DRG neurons (32, 33, 39, 40). Our recent results suggest that dyslipidemia also contributes to diabetic neuropathy by increasing plasma oxidized low density lipoprotein, which directly leads to oxidative stress and injury in DRG neurons via lectin-like oxidized low density lipoprotein receptor (LOX)-1 (41, 42). Even though no data are available for neurons, it has been shown that long-term exposure to insulin suppresses mitochondrial biogenesis and function and leads to increased production of mitochondrial reactive oxygen species in primary hepatocytes (43).
After establishing the normal insulin signaling pathway in DRG neurons, we next examined the possible contributors to IR by treating the neurons with high glucose, fatty acid, or insulin. Of these, only chronic insulin treatment resulted in significant inhibition of Akt activation and was insulin pretreatment concentration dependent. Phosphorylation of both Ser473 and Thr308 sites was severely inhibited by chronic insulin treatment. The total protein level of Akt was not affected by insulin pretreatment. Insulin-induced phosphorylation of downstream signaling molecules GSK-3β and p70S6K was also reduced by insulin pretreatment. This effect was specific to insulin signaling, because IGF-I-induced Akt phosphorylation was not affected by chronic insulin treatment. Our results are in agreement with a recent report using primary hepatocytes (44). When primary hepatocytes were chronically pretreated with insulin, acute insulin-induced Akt phosphorylation was greatly reduced. Glucose pretreatment, however, did not affect insulin-stimulated Akt phosphorylation. In the same report, the authors also demonstrated that continuous treatment of nonobese diabetic mice (NOD/ShiLtj) with a long-acting human insulin analog, insulin detemir, resulted in the development of IR with reduced mitochondrial production and increased lipid accumulation and oxidative stress. Therefore, our results strongly suggest that hyperinsulinemia is the main contributor for the development of IR in DRG neurons.
Insulin-stimulated Akt phosphorylation was examined in DRG neurons prepared from an animal model of type 2 diabetes, the BKS-db/db. These mice are obese and become hyperinsulinemic beginning at approximately 4 wk of age (http://jaxmice.jax.org/strain/000642.html). These animals exhibit increased serum lipids and hyperglycemia similar to that seen in human patients and are the most commonly used model of type 2 diabetes (45). Insulin stimulation resulted in reduced Akt phosphorylation in DRG neurons prepared from BKS-db/db compared with the neurons from BKS-db+ mice. However, the phosphorylation time course was different from the control DRG neurons chronically pretreated with insulin. In DRG neurons from BKS-db/db mice, Akt phosphorylation was initially increased, but it did not reach the maximum, and the phosphorylation was not maintained as in BKS-db+ DRG. These results suggest that there might be different regulation(s) of Akt phosphorylation/dephosphorylation in long-term diabetes (>16 wk) with hyperglycemia and hyperinsulinemia compared with rather short-term (24 h) hyperinsulinemia without hyperglycemia. Our results also suggest that diabetes may cause permanent genetic changes to DRG neurons affecting Akt signaling, considering that the neurons from BKS-db/db mouse were cultured in vitro for 4 d in normal (without high glucose or insulin) media before insulin treatment.
The specific role of PI3-K/Akt signaling for the induction of IR in neurons was confirmed by blocking this pathway with PI3-K inhibitor, LY294002. When DRG neurons were exposed to insulin and LY294002 together for 24 h, acute insulin-induced Akt phosphorylation was restored. Our results are in agreement with a previous report in the L6 muscle cell line (15). Recently, Liu et al. (46) reported similar results using the mice fed a high-fat diet. When fed a high-fat diet, the mice develop IR along with increased oxidative stress. The mice display hyperinsulinemia with normal fasting glucose levels, again suggesting that IR is mainly induced by hyperinsulinemia rather than hyperglycemia (44). The adverse effect of a high-fat diet on oxidative stress and IR were reversed when the mice were treated with LY294002 during the day (when mice usually do not eat). These results and our own suggest that constant insulin signaling increases basal Akt phosphorylation, rendering the target tissues desensitized to insulin and develop IR.
Serine phosphorylation of IRS-1 triggers its degradation; this step is generally considered a negative regulator of insulin signaling that contributes to the development of IR (25). Chronic insulin treatment that resembles IR induces IRS-1 degradation in 3T3-L1 adipocytes (47, 48). PI3-K pathway-mediated reduction in IRS-1/2 expression is responsible for the decreased Akt phosphorylation after chronic insulin stimulation in L6 muscle cells (15). Although IRS-1 serine phosphorylation generally inhibits insulin signaling, recent reports suggest that it may have positive roles. Ser302 phosphorylation is implicated in nutrient-mediated cell growth and mitogenesis (49). Phosphorylation at Ser636, which has negative effect on insulin signaling, is decreased by Ser629 phosphorylation, resulting in enhanced insulin signaling (50). However, our results demonstrate little correlation between IRS-1 phosphorylation/expression and Akt phosphorylation, suggesting that, unlike peripheral tissues, signaling molecule(s) downstream of IRS are responsible for the decreased Akt phosphorylation after chronic insulin treatment in DRG neurons. Full activation of Akt requires phosphorylation of Thr308 in the catalytic domain by PDK1 and Ser473 in the C-terminal hydrophobic domain by mTORC2 (10). The mTORC1 complex regulates cellular homeostasis and is under the control of energy and nutrient input (51). mTOR is the key component of both mTORC1 and mTORC2 complexes and is essential for cell metabolism, growth, and survival (51). AMP-activated protein kinase (AMPK) is cellular energy sensor that is regulated by the AMP/ATP ratio (52). AMPK regulates lipid and glucose metabolism in liver and muscle and, thus, considered a potential pharmacological target for diabetes and metabolic syndrome (53). AMPK influences mTOR activity by regulating tuberous sclerosis protein-1/2 complexes. Tuberous sclerosis protein-1/2 is inhibited by Akt, resulting in feedback regulation of Akt activity (54). Therefore, the AMPK/mTOR-mediated pathway is a possible candidate responsible for the chronic insulin treatment-induced decrease in Akt activation in our experimental setting. We are currently actively pursuing this possibility.
Mitochondrial dysfunction and increased oxidative stress are key pathological features of IR (55). Patients with diabetes and IR display smaller mitochondrial size (56) and decreased mitochondrial DNA content. It has been suggested that increased oxidative stress in skeletal muscle is the major factor behind the mitochondrial dysfunction and development of IR observed in mice fed high fat and sugar (57). Oxidative stress along with mitochondrial dysfunction are also the prominent features of diabetic neuropathy. Our laboratory demonstrated that glucose-mediated oxidative stress causes an imbalance in mitochondrial biogenesis and fission in both in vivo and in vitro models of diabetes, which may contribute to the pathogenesis of diabetic neuropathy (18, 19). Our current and previous results (33, 34) clearly demonstrate increased mitochondrial fission after hyperinsulinemia and hyperglycemia. Disruption of mitochondrial fission-fusion machinery has been demonstrated in several neuropathological conditions; it is a prominent feature of autosomal dominant optic atrophy (58), and altered Drp1 level has been reported from the cortical neurons from Alzheimer's disease patients (59). Furthermore, our results demonstrate the additive effect on Drp1 expression when hyperinsulinemia precedes hyperglycemic treatment. Our data suggest that hyperinsulinemia results in imbalance of mitochondrial biogenesis and making the neurons more susceptible to subsequent injury.
In summary, our experiments demonstrate that chronic insulin stimulation in vivo and in vitro result in the disruption of Akt signaling and mitochondrial biogenesis, a form of IR in DRG neurons. Our results introduce a new concept for the pathogenesis of diabetic neuropathy. Similar to metabolic tissues, neurons develop IR. This results in reduced sensitivity for insulin-induced Akt activation, making neurons more vulnerable to oxidative stress-mediated injury and the development of neuropathy.
Acknowledgments
We thank Dr. Kelli A. Sullivan for her helpful discussion.
This work was supported by National Institutes of Health Grants UO1 DK076160 and R24 DK082840-01 and by the Program for Neurology Research and Discovery.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMPK
- AMP-activated protein kinase
- DRG
- dorsal root ganglion
- Drp
- dynamin-related protein
- GSK-3β
- glycogen synthase kinase-3β
- IHC
- immunohistochemistry
- InsR
- insulin receptor
- IR
- insulin resistance
- IRS
- InsR substrate
- mTORC
- mammalian target of rapamycin complex
- PA
- palmitic acid
- PDK
- phosphoinositide dependent protein kinase
- PI3-K
- phosphatidylinositol 3-kinase
- RIPA
- radioimmunoprecipitation assay.
References
- 1. Bruce KD, Hanson MA. 2010. The developmental origins, mechanisms, and implications of metabolic syndrome. J Nutr 140:648–652 [DOI] [PubMed] [Google Scholar]
- 2. Petersen KF, Shulman GI. 2006. Etiology of insulin resistance. Am J Med 119:S10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taubes G. 2009. Insulin resistance. Prosperity's plague. Science 325:256–260 [DOI] [PubMed] [Google Scholar]
- 4. Wild S, Roglic G, Green A, Sicree R, King H. 2004. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 5. Edwards JL, Vincent AM, Cheng HT, Feldman EL. 2008. Diabetic neuropathy: mechanisms to management. Pharmacol Ther 120:1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, Wilson DM, O'Brien PC, Melton LJ, 3rd, Service FJ. 1993. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester diabetic neuropathy study. Neurology 43:817–824 [DOI] [PubMed] [Google Scholar]
- 7. Xu QG, Li XQ, Kotecha SA, Cheng C, Sun HS, Zochodne DW. 2004. Insulin as an in vivo growth factor. Exp Neurol 188:43–51 [DOI] [PubMed] [Google Scholar]
- 8. Toth C, Brussee V, Martinez JA, McDonald D, Cunningham FA, Zochodne DW. 2006. Rescue and regeneration of injured peripheral nerve axons by intrathecal insulin. Neuroscience 139:429–449 [DOI] [PubMed] [Google Scholar]
- 9. Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. 2009. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 30:586–623 [DOI] [PubMed] [Google Scholar]
- 10. Franke TF. 2008. PI3K/Akt: getting it right matters. Oncogene 27:6473–6488 [DOI] [PubMed] [Google Scholar]
- 11. Matheny RW, Jr, Adamo ML. 2009. Current perspectives on Akt Akt-ivation and Akt-ions. Exp Biol Med 234:1264–1270 [DOI] [PubMed] [Google Scholar]
- 12. Kim YB, Peroni OD, Franke TF, Kahn BB. 2000. Divergent regulation of Akt1 and Akt2 isoforms in insulin target tissues of obese Zucker rats. Diabetes 49:847–856 [DOI] [PubMed] [Google Scholar]
- 13. Brozinick JT, Jr, Roberts BR, Dohm GL. 2003. Defective signaling through Akt-2 and -3 but not Akt-1 in insulin-resistant human skeletal muscle: potential role in insulin resistance. Diabetes 52:935–941 [DOI] [PubMed] [Google Scholar]
- 14. Krook A, Roth RA, Jiang XJ, Zierath JR, Wallberg-Henriksson H. 1998. Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects. Diabetes 47:1281–1286 [DOI] [PubMed] [Google Scholar]
- 15. Pirola L, Bonnafous S, Johnston AM, Chaussade C, Portis F, Van Obberghen E. 2003. Phosphoinositide 3-kinase-mediated reduction of insulin receptor substrate-1/2 protein expression via different mechanisms contributes to the insulin-induced desensitization of its signaling pathways in L6 muscle cells. J Biol Chem 278:15641–15651 [DOI] [PubMed] [Google Scholar]
- 16. Thomson MJ, Williams MG, Frost SC. 1997. Development of insulin resistance in 3T3-L1 adipocytes. J Biol Chem 272:7759–7764 [DOI] [PubMed] [Google Scholar]
- 17. Bertacca A, Ciccarone A, Cecchetti P, Vianello B, Laurenza I, Maffei M, Chiellini C, Del Prato S, Benzi L. 2005. Continually high insulin levels impair Akt phosphorylation and glucose transport in human myoblasts. Metabolism 54:1687–1693 [DOI] [PubMed] [Google Scholar]
- 18. Feldman EL. 2003. Oxidative stress and diabetic neuropathy: a new understanding of an old problem. J Clin Invest 111:431–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vincent AM, Edwards JL, McLean LL, Hong Y, Cerri F, Lopez I, Quattrini A, Feldman EL. 2010. Mitochondrial biogenesis and fission in axons in cell culture and animal models of diabetic neuropathy. Acta Neuropathol 120:477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim B, Backus C, Oh S, Hayes JM, Feldman EL. 2009. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology 150:5294–5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aberg D. 2010. Role of the growth hormone/insulin-like growth factor 1 axis in neurogenesis. Endocr Dev 17:63–76 [DOI] [PubMed] [Google Scholar]
- 22. Duarte AI, Santos P, Oliveira CR, Santos MS, Rego AC. 2008. Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3β signaling pathways and changes in protein expression. Biochim Biophys Acta 1783:994–1002 [DOI] [PubMed] [Google Scholar]
- 23. Sanderson TH, Kumar R, Murariu-Dobrin AC, Page AB, Krause GS, Sullivan JM. 2009. Insulin activates the PI3K-Akt survival pathway in vulnerable neurons following global brain ischemia. Neurol Res 31:947–958 [DOI] [PubMed] [Google Scholar]
- 24. Willaime-Morawek S, Arbez N, Mariani J, Brugg B. 2005. IGF-I protects cortical neurons against ceramide-induced apoptosis via activation of the PI-3K/Akt and ERK pathways; is this protection independent of CREB and Bcl-2? Brain Res Mol Brain Res 142:97–106 [DOI] [PubMed] [Google Scholar]
- 25. Boura-Halfon S, Zick Y. 2009. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab 296:E581–E591 [DOI] [PubMed] [Google Scholar]
- 26. Dekker JM, Girman C, Rhodes T, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ. 2005. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation 112:666–673 [DOI] [PubMed] [Google Scholar]
- 27. Group DR. 1995. Effect of intensive diabetes management on macrovascular events and risk factors in the diabetes control and complications trial. Am J Cardiol 75:894–903 [DOI] [PubMed] [Google Scholar]
- 28. Mironov SL. 2009. Complexity of mitochondrial dynamics in neurons and its control by ADP produced during synaptic activity. Int J Biochem Cell Biol 41:2005–2014 [DOI] [PubMed] [Google Scholar]
- 29. Navarro A, Boveris A. 2007. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292:C670–C686 [DOI] [PubMed] [Google Scholar]
- 30. Vincent AM, Feldman EL. 2004. New insights into the mechanisms of diabetic neuropathy. Rev Endocr Metab Disord 5:227–236 [DOI] [PubMed] [Google Scholar]
- 31. Vincent AM, Russell JW, Low P, Feldman EL. 2004. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 25:612–628 [DOI] [PubMed] [Google Scholar]
- 32. Vincent AM, McLean LL, Backus C, Feldman EL. 2005. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J 19:638–640 [DOI] [PubMed] [Google Scholar]
- 33. Leinninger GM, Backus C, Sastry AM, Yi YB, Wang CW, Feldman EL. 2006. Mitochondria in DRG neurons undergo hyperglycemic mediated injury through Bim, Bax and the fission protein Drp1. Neurobiol Dis 23:11–22 [DOI] [PubMed] [Google Scholar]
- 34. Edwards JL, Quattrini A, Lentz SI, Figueroa-Romero C, Cerri F, Backus C, Hong Y, Feldman EL. 2010. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia 53:160–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leinninger GM, Backus C, Uhler MD, Lentz SI, Feldman EL. 2004. Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. FASEB J 18:1544–1546 [DOI] [PubMed] [Google Scholar]
- 36. Jones DM, Tucker BA, Rahimtula M, Mearow KM. 2003. The synergistic effects of NGF and IGF-1 on neurite growth in adult sensory neurons: convergence on the PI 3-kinase signaling pathway. J Neurochem 86:1116–1128 [DOI] [PubMed] [Google Scholar]
- 37. Edström A, Ekström PA. 2003. Role of phosphatidylinositol 3-kinase in neuronal survival and axonal outgrowth of adult mouse dorsal root ganglia explants. J Neurosci Res 74:726–735 [DOI] [PubMed] [Google Scholar]
- 38. Choi K, Kim YB. 2010. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J Intern Med 25:119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vincent AM, Perrone L, Sullivan KA, Backus C, Sastry AM, Lastoskie C, Feldman EL. 2007. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology 148:548–558 [DOI] [PubMed] [Google Scholar]
- 40. Russell JW, Berent-Spillson A, Vincent AM, Freimann CL, Sullivan KA, Feldman EL. 2008. Oxidative injury and neuropathy in diabetes and impaired glucose tolerance. Neurobiol Dis 30:420–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. 2009. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes 58:2376–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. 2009. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst 14:257–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu HY, Yehuda-Shnaidman E, Hong T, Han J, Pi J, Liu Z, Cao W. 2009. Prolonged exposure to insulin suppresses mitochondrial production in primary hepatocytes. J Biol Chem 284:14087–14095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu HY, Cao SY, Hong T, Han J, Liu Z, Cao W. 2009. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM). J Biol Chem 284:27090–27100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sullivan KA, Lentz SI, Roberts JL, Jr, Feldman EL. 2008. Criteria for creating and assessing mouse models of diabetic neuropathy. Curr Drug Targets 9:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu HY, Hong T, Wen GB, Han J, Zuo D, Liu Z, Cao W. 2009. Increased basal level of Akt-dependent insulin signaling may be responsible for the development of insulin resistance. Am J Physiol Endocrinol Metab 297:E898–E906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Egawa K, Nakashima N, Sharma PM, Maegawa H, Nagai Y, Kashiwagi A, Kikkawa R, Olefsky JM. 2000. Persistent activation of phosphatidylinositol 3-kinase causes insulin resistance due to accelerated insulin-induced insulin receptor substrate-1 degradation in 3T3-L1 adipocytes. Endocrinology 141:1930–1935 [DOI] [PubMed] [Google Scholar]
- 48. Hiratani K, Haruta T, Tani A, Kawahara J, Usui I, Kobayashi M. 2005. Roles of mTOR and JNK in serine phosphorylation, translocation, and degradation of IRS-1. Biochem Biophys Res Commun 335:836–842 [DOI] [PubMed] [Google Scholar]
- 49. Giraud J, Leshan R, Lee YH, White MF. 2004. Nutrient-dependent and insulin-stimulated phosphorylation of insulin receptor substrate-1 on serine 302 correlates with increased insulin signaling. J Biol Chem 279:3447–3454 [DOI] [PubMed] [Google Scholar]
- 50. Luo M, Reyna S, Wang L, Yi Z, Carroll C, Dong LQ, Langlais P, Weintraub ST, Mandarino LJ. 2005. Identification of insulin receptor substrate 1 serine/threonine phosphorylation sites using mass spectrometry analysis: regulatory role of serine 1223. Endocrinology 146:4410–4416 [DOI] [PubMed] [Google Scholar]
- 51. Wullschleger S, Loewith R, Hall MN. 2006. TOR signaling in growth and metabolism. Cell 124:471–484 [DOI] [PubMed] [Google Scholar]
- 52. Zhang BB, Zhou G, Li C. 2009. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 9:407–416 [DOI] [PubMed] [Google Scholar]
- 53. Kraegen EW, Bruce C, Hegarty BD, Ye JM, Turner N, Cooney G. 2009. AMP-activated protein kinase and muscle insulin resistance. Front Biosci 14:4658–4672 [DOI] [PubMed] [Google Scholar]
- 54. Kimball SR. 2006. Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc 38:1958–1964 [DOI] [PubMed] [Google Scholar]
- 55. Turner N, Heilbronn LK. 2008. Is mitochondrial dysfunction a cause of insulin resistance? Trends Endocrinol Metab 19:324–330 [DOI] [PubMed] [Google Scholar]
- 56. Kelley DE, He J, Menshikova EV, Ritov VB. 2002. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950 [DOI] [PubMed] [Google Scholar]
- 57. Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. 2008. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B. 2000. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 26:211–215 [DOI] [PubMed] [Google Scholar]
- 59. Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. 2009. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci 29:9090–9103 [DOI] [PMC free article] [PubMed] [Google Scholar]