The prevalence of obesity in westernized societies is now considered by epidemiologists to be at an epidemic level. Slightly over a third of adult Americans are considered obese because they have a body mass index of more than 30 kg/m2 (1). Additionally, the percentage of the U.S. population that has a body mass index of more than 25 (considered “overweight”) is almost 70%, which gives even more “food” for thought (1). The consistent failure, for reasons of compliance or adverse actions, of multiple weight-management strategies is a worrying trend. In this issue of Endocrinology, Lockie et al. (2) potentially offer rational modifications to one of these “failed” treatments, rimonabant, that may allow a more positive reception of what was considered an effective, but flawed, weight-management drug.
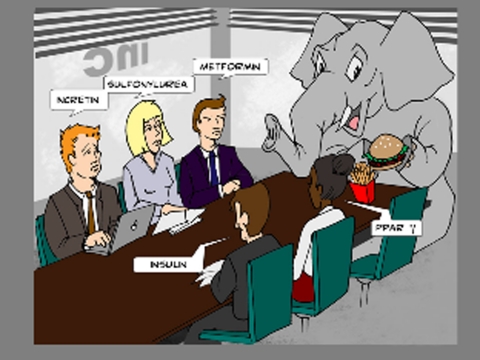
The pathophysiological impact of obesity is enormous: long-term excess body weight is strongly associated with an increased incidence of coronary heart disease, generalized atherosclerosis, hepatic steatosis, chronic inflammation, obstructive sleep apnea, hypertension, various cancer forms, osteoarthritis, inability to exercise, and predisposition to type 2 diabetes mellitus (T2DM) (3). The now epidemic status of T2DM, in which almost 26 million Americans suffer from diabetes, will likely induce a secondary wave of increased pathologies, due firstly to the strong connectivity between obesity and T2DM and secondly to the often poor weight-management efficacy of T2DM therapeutics. In fact, some glucose-lowering therapeutics are positively proobesogenic, e.g. exogenous insulin, sulfonylureas, and PPAR-γ agonists (Fig. 1). The burden of primary and comorbidities that obesity places on healthcare and the economy is almost impossible to fully appreciate (4).
Fig. 1.
Cartoon of glucose-lowering drug discovery. Although several new classes of glucose-lowering agents have become available in the past several years, the prevalence of the root cause of type 2 diabetes, namely obesity (the elephant in the room when any new hypoglycemic agents are being developed), has continued to increase unabated, and use of some diabetes medications actually results in increased body weight (exogenous insulin, sulfonylureas, PPAR-γ agonists).
Of course obesity prevention, targeted to children, as exemplified by Michelle Obama's “Let's Move!” campaign, is the best strategy for future weight management. Taxing “junk” (proobesogenic) food so that it becomes pricier than “healthy” food might be another strategy worth considering by our policy makers (5). However, to prevent the subsequent adverse effects that obesity has on the health of people who are presently obese, effective and well-tolerated fat-resolving treatments would seem to be needed right now. As we have stated, the most conservative, least expensive, and least invasive way to lose weight is best, i.e. eat less calories than one needs to maintain ones prevailing body weight. However, with any therapy, compliance is the prime facilitator of regime efficacy. It is clear with the steadily increasing prevalence of obesity, and with already obese patients becoming even more obese, that simply instructing an obese patient to eat less than they normally do is unfortunately unlikely to enact a radical change to this epidemic. This intractability has led to the increasing popularity of bariatric surgery as a weight-management treatment, because it offers a fast-acting and physically enforced form of calorie restriction. It is not, however, without its own set of problems associated with surgical procedures and considerable postoperative lifestyle alterations.
Therefore, a viable, pragmatic pharmacotherapeutic solution seems the best option to stem the tide of obesity. As increasing weight gain, leading to obesity, results from a systemic imbalance between energy intake and energy expenditure, weight-management agents have targeted several components of this energy homeostasis axis to achieve weight loss, e.g. appetite suppression, inhibition of dietary fat absorption, and stimulation of energy management/thermogenesis (6). Anorectic agents employed include phentermine, sibutramine, diethylpropion, fluoxetine, and rimonabant. Agents that mediate functional metabolic amelioration of obesity include therapeutics targeting the T2DM component of obesity, e.g. metformin, incretin-mimetics (exenatide and liraglutide), and pramlintide. With respect to reduction of fat absorption, Orlistat (Xenical), a reversible gastrointestinal inhibitor of pancreatic lipases, has demonstrated some functional efficacy. Although over 100 different forms of weight-management drugs have been investigated for their efficacy, only Orlistat is now approved by the U.S. Food and Drug Administration (FDA) for the treatment of obesity. Drug safety, however, has become an Achilles heel for most of the recently investigated weight-management medications. Phentermine, diethylpropion, fluoxetine, and sibutramine have all been linked to generation of intractable headaches, insomnia, elevated irritability, palpitations, nervousness, and agitation and even major adverse cardiovascular events (7–10). In addition to these deleterious “off-target” effects, anorectic effects are often accompanied by endogenous compensatory mechanisms elicited through central and peripheral feedback networks to reduce actual energy expenditure, which successively attenuates any progressive weight loss (11). Such resistive behavior can be exemplified in the use of the cannabinoid type 1 receptor (CB1R) antagonist/inverse agonist, rimonabant. The endocannabinoid receptor has long been identified as a key player in the control of food intake, energy balance, lipid and glucose metabolism, and pancreatic structure function (12–14). This status suggests that the cannabinoid receptor system could act as a functional keystone in the complex network of systems controlling metabolism.
Endogenous endocannabinoids, anandamide, and 2-arachidonoylglycerol are lipid mediators that activate two cannabinoid G protein-coupled receptor (GPCR) types (CB1 and CB2). The CB1R is expressed in most tissues, including the central nervous system (CNS), and multiple peripheral cell types, including adipocytes, myocytes, hepatocytes, and insulin-secreting β-cells, whereas the CB2 is almost exclusively expressed in immune and hematopoietic cells and specific neuronal areas (14). Concerted investigation of endocannabinoid function demonstrated that an excessive activation of the CB1R system is closely associated with the presentation of obesity (15). The generation of the selective CB1R antagonist/inverse agonist, rimonabant (16), then opened the possibility of interdicting this excessive CB1R drive in obesity. Preclinically, rimonabant transiently decreased initial food intake in animals while maintaining a sustained weight loss, suggesting an increase in systemic energy expenditure (17). In rats with diet-induced obesity, administration of rimonabant also seemed to elevate the metabolic rate in these animals and resulted in their losing weight (18). There have been four substantial human clinical trials of rimonabant in the treatment of obesity (19–22). All four of these randomized, double-blind, placebo-controlled trials demonstrated similar effects of rimonabant upon weight loss (>4 kg after 1 yr) (14) and attenuation of cardiovascular risk factors. After these initial trials, rimonabant was approved as a weight-management treatment by the European Medicines Agency (EMA). Unfortunately, with increasing patient use, reports emerged outlining deleterious effects of this promising agent: rimonabant was associated with CNS side effects, including anxiety, depression, and potentiation of suicidal behavior (23). Eventually, the suspension of the license for rimonabant was recommended by the EMA in 2008 (24). Approval of rimonabant for patient use in the United States was denied by the FDA (14), because it was considered that the CNS side effects of the first generation of CB1R modifiers outweighed their therapeutic benefits. Other CB1R antagonist drug trials in the pipeline of development were also subsequently halted. Parenthetically, bariatric surgery was also reported to be associated with about a 5-fold incidence increase of successful suicidal events than in a control age- and sex-matched population, of which almost 70% occurred within 3 yr of surgery (25): bariatric surgery is not regulated by EMA or FDA. Because the major impediment to the continued employment of CB1R-directed agents for obesity treatment seems to be their prodepressive effects, it is interesting to note that a strong functional synergy exists between the CB1R system and the endogenous opioid GPCR system (μ, δ, and κ receptors), which can also control both psychiatric, depressive and appetite-related issues. For example, naltrexone, a long-lasting opioid receptor antagonist, demonstrates antidepressive activity (26, 27) and an ability to reduce short-term food intake and formed part of dual weight-management pharmacotherapy with bupropion that was rejected by the FDA as a treatment for obesity pending outcome of long-term studies of the duo's effects on cardiovascular function. Excessive food intake, leading to obesity, has often been likened to an addictive phenotype, and it is interesting to note that naltrexone is also currently demonstrating efficacy for treatment of addiction to various stimulants or repetitive behaviors (28–31). These two receptor systems also demonstrate a tight functional association in many other diverse ways, e.g. opioid-cannabinoid control of hallucinogen or antinociceptive effects (32, 33), heterodimerization of opioid and CB1R (34), as well as colocalization of expression and control of neurotransmitter release in the nucleus accumbens core (35).
In this issue of Endocrinology, Lockie et al. (2) provide a novel functional insight into the hidden complexity of cannabinoid-mediated responses in the sphere of multifactorial physiological processes, such as energy metabolism and emotional behavior. They investigated the functional interaction between two crucially important neurotransmitter GPCR signaling systems, the endocannabinoid and the endogenous opioid. The authors demonstrated that multiple receptor-based components of the endogenous opioid system, i.e. μ and κ receptors, can subtly and diversely modulate the metabolic and behavioral effects of the CB1R antagonist/inverse agonist rimonabant. In generalized terms, it appears from Lockie et al. that rimonabant's actions upon metabolism (i.e. weight reduction) were functionally antagonized by μ opioid receptor (MOR) signaling, whereas the negative psychological effects of CB1R inverse agonism were modulated by κ opioid receptor (KOR) activity. Hence, inhibition of central MOR activity, either via intracerebroventricular or ip introduction of an MOR antagonist (naloxone) or via genetic deletion of MOR, potentiated the ability of coadministered peripheral rimonabant to induce weight loss and reduce food intake. Modulation of the KOR system in the presence of rimonabant had no effects upon the appetite/metabolic actions of this drug but effectively attenuated behavioral effects associated with rimonabant, i.e. prodepressive activity. Simultaneous ip administration of CB1R (rimonabant), MOR (naloxone), and KOR (nor-Binaltorphimine) antagonist agents were able to induce a significant reduction of mouse food intake while simultaneously removing negative prodepressive effects. Therefore, simultaneous comodulation of multiple neurotransmitter receptor systems may provide a starting point for a more complex and nuanced practical approach to the design of pharmacotherapeutic treatments of multifactorial disorders such as metabolic syndrome and obesity.
Although the control of CB1R function appears to be indeed a potent mechanism to effect multiple aspects of metabolic disorders, it is clear from this and previous findings that there are still multiple hurdles to clear before a specific and tractable series of CB1R modulators can be routinely used. In addition to the indications here that multisimultaneous receptor modulation may facilitate usable CB1R exploitation, considerable advances are also being made concerning additional forms of CB1R signal “conditioning.” For example, other forms of coligand regulation, e.g. CB1R modulation of leptin sensitivity are being investigated (36). In addition, the creation of more subtle CB1R ligands, such as allosterically acting ligands (37), synthesis of non-CNS penetrant CB1R antagonists (38), and an enhanced appreciation of the pharmacogenomic basis of CB1R-mediated psychiatric effects (39), may all help to facilitate the manipulation and suitable therapeutic conditioning of the CB1R system for the treatment of metabolic disorders (Fig. 2). Using the whole CB1R system as an example of a functional network, which possesses multiple levels of synergistic physiological control, both in the periphery and CNS, we can demonstrate the potential strength of manipulating such multidimensional receptor systems in combating multifaceted disorders such as obesity. We can consider the combined physiological and pharmacological perturbations created by an obese syndrome as a form of “pathophysiological network,” also with its own feedback systems and homeostatic tendencies. Accepting this posit, it is clear that perhaps our ability to pharmacologically readjust this pathophysiological network with a monolithic form of drug therapy is unrealistic, as we have seen with rimonabant. This situation then leads us to the necessity to increase our understanding of how multiple receptor and ligand systems work together at the molecular level in the cell as well as all the way up to the macro level, across multiple tissues in the same organism. The quality of our appreciation of the nature of GPCR connectivity, contextual signaling, and ligand interaction behavior, in addition to ligand biases in signal transduction and multisite network actions of simultaneously applied agents, may strongly correlate with our ability to develop efficacious and well-tolerated pharmacotherapeutics for complex, multidimensional disorders such as obesity and T2DM (40–42).
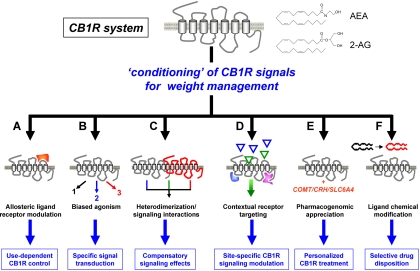
Fig. 2.
Mechanistic conditioning of CB1R activity may facilitate its use as an a weight-management agent. To exploit the multidimensional effects of the CB1R system (comprising CB1R and endogenous agonists, anandamide, and 2-arachidonoylglycerol) upon regulation of obesity and energy management while diminishing the negative effects of simple CB1R antagonism, several signal conditioning steps can be taken. A, Creation of allosteric modulators (red modulator) of the CB1R, rather than simple antagonists, may allow for more subtle regulation of CB1R signaling. B, Ligands that can selectively control the specific forms of downstream G protein coupling (e.g. 1–3) through the CB1R may also be used to generate more specific potency and fewer unwanted side effects. C, Simultaneous ligand application or targeting specific CB1R heterodimers (with red GPCR) may allow efficient mitigation of deleterious CNS side effects. D, With an enhanced understanding of the tissue-type-specific presentation of the CB1R (blue and pink associated proteins) tissue-specific CB1R activation may be attainable (e.g. allowing green but not blue triangle ligand activation). E, Appreciation of how other functional systems [COMT, Catechol-O-methyltransferase; SLC6A4, solute carrier family 6 (neurotransmitter transporter, serotonin), member 4] assist in the development of the multiple facets of CB1R-mediated effects will help specific tailoring of more patient-suitable CB1R-controlling agents. F, Derivation of CB1R antagonists with specific physicochemical properties (red vs. black) that specify a desired tissue distribution (e.g. peripheral only) may also mitigate the side effects of global CB1R antagonism.
Oh, that this too too solid flesh would melt
Thaw and resolve itself into a dew. (Shakespeare)
Acknowledgments
We thank Jimmy Burril of National Institutes of Health Visual Media Department for his contribution to Fig. 1.
This work was supported by the Intramural Research Program of the National Institute on Aging/National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
For article see page 3661
- CB1R
- Cannabinoid type 1 receptor
- CNS
- central nervous system
- EMA
- European Medicines Agency
- FDA
- Food and Drug Administration
- GPCR
- G protein-coupled receptor
- KOR
- κ opioid receptor
- MOR
- μ opioid receptor
- T2DM
- type 2 diabetes mellitus.
References
- 1. Flegal KM, Carroll MD, Ogden CL, Curtin LR. 2010. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303:235–241 [DOI] [PubMed] [Google Scholar]
- 2. Lockie SH, Czyzyk TA, Chaudhary N, Perez-Tilve D, Woods SC, Oldfield BJ, Statnick MA, Tschoep M. 2011. CNS opioid signaling separates cannabinoid receptor 1 mediated effects on body weight and mood-related behavior in mice. Endocrinology 152:3661–3667 [DOI] [PubMed] [Google Scholar]
- 3. Haslam DW, James WP. 2005. Obesity. Lancet 366:1197–1209 [DOI] [PubMed] [Google Scholar]
- 4. Scarborough P, Bhatnagar P, Wickramasinghe KK, Allender S, Foster C, Rayner M. 11 May 2011. The economic burden of ill health due to diet, physical inactivity, smoking, alcohol and obesity in the UK: an update to 2006-07 NHS costs. J Public Health (Oxf) 10.1093/pubmed/fdr033 [DOI] [PubMed] [Google Scholar]
- 5. Bittman M. 2011. Bad food? Tax it, and subsidize vegetables. New York Times, July 23, 2011; 1–7 [Google Scholar]
- 6. Hussain SS, Bloom SR. 2011. The pharmacological treatment and management of obesity. Postgrad Med 123:34–44 [DOI] [PubMed] [Google Scholar]
- 7. Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. 1997. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 337:581–588 [DOI] [PubMed] [Google Scholar]
- 8. Nisoli E, Carruba MO. 2003. A benefit-risk assessment of sibutramine in the management of obesity. Drug Saf 26:1027–1048 [DOI] [PubMed] [Google Scholar]
- 9. Cercato C, Roizenblatt VA, Leança CC, Segal A, Lopes Filho AP, Mancini MC, Halpern A. 2009. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int J Obes 33:857–865 [DOI] [PubMed] [Google Scholar]
- 10. Wise SD. 1992. Clinical studies with fluoxetine in obesity. Am J Clin Nutr 55:181S–184S [DOI] [PubMed] [Google Scholar]
- 11. Spiegelman BM, Flier JS. 2001. Obesity and the regulation of energy balance. Cell 104:531–543 [DOI] [PubMed] [Google Scholar]
- 12. Di Marzo V, Bifulco M, De Petrocellis L. 2004. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 3:771–784 [DOI] [PubMed] [Google Scholar]
- 13. Kim W, Doyle ME, Liu Z, Lao Q, Shin YK, Carlson OD, Kim HS, Thomas S, Napora JK, Lee EK, Moaddel R, Wang Y, Maudsley S, Martin B, Kulkarni RN, Egan JM. 2011. Cannabinoids inhibit insulin receptor signaling in pancreatic β-cells. Diabetes 60:1198–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Butler H, Korbonits M. 2009. Cannabinoids for clinicians: the rise and fall of the cannabinoid antagonists. Eur J Endocrinol 161:655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Marzo V, Matias I. 2005. Endocannabinoid control of food intake and energy balance. Nat Neurosci 8:585–589 [DOI] [PubMed] [Google Scholar]
- 16. Rinaldi-Carmona M, Barth F, Héaulme M, Alonso R, Shire D, Congy C, Soubrié P, Brelière JC, Le Fur G. 1995. Biochemical and pharmacological characterization of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci 56:1941–1947 [DOI] [PubMed] [Google Scholar]
- 17. Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. 2003. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology 167:103–111 [DOI] [PubMed] [Google Scholar]
- 18. Herling AW, Kilp S, Elvert R, Haschke G, Kramer W. 2008. Increased energy expenditure contributes more to the body weight-reducing effect of rimonabant than reduced food intake in candy-fed Wistar rats. Endocrinology 149:2557–2566 [DOI] [PubMed] [Google Scholar]
- 19. Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. RIO-Diabetes Study Group 2006. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomized controlled study. Lancet 368:1660–1672 [DOI] [PubMed] [Google Scholar]
- 20. Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S. RIO-Europe Study Group 2005. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365:1389–1397 [DOI] [PubMed] [Google Scholar]
- 21. Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J; RIO-North America Study Group 2006. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 295:761–775 [DOI] [PubMed] [Google Scholar]
- 22. Després JP, Golay A, Sjöström L; Rimonabant in Obesity-Lipids Study Group 2005. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353:2121–2134 [DOI] [PubMed] [Google Scholar]
- 23. Di Marzo V, Després JP. 2009. CB1 antagonists for obesity—what lessons have we learned from rimonabant? Nat Rev Endocrinol 5:633–638 [DOI] [PubMed] [Google Scholar]
- 24. Li M, Cheung BM. 2009. Pharmacotherapy for obesity. Br J Clin Pharmacol 68:804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tindle HA, Omalu B, Courcoulas A, Marcus M, Hammers J, Kuller LH. 2010. Risk of suicide after long-term follow-up from bariatric surgery. Am J Med 123:1036–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rahman S. 2010. Targeting opioid receptors: a new treatment for brain disorders. CNS Neurol Disord Drug Targets 9:128. [DOI] [PubMed] [Google Scholar]
- 27. Herman BH, Holtzman SG. 1984. Repeated administration of naltrexone and diprenorphine decreases food intake and body weight in squirrel monkeys. Life Sci 34:1–12 [DOI] [PubMed] [Google Scholar]
- 28. Anton RF, Oroszi G, O'Malley S, Couper D, Swift R, Pettinati H, Goldman D. 2008. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the combined pharmacotherapies and behavioral interventions for alcohol dependence (COMBINE) study. Arch Gen Psychiatry 65:135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fontenelle LF, Oostermeijer S, Harrison BJ, Pantelis C, Yücel M. 2011. Obsessive-compulsive disorder, impulse control disorders and drug addiction: common features and potential treatments. Drugs 71:827–840 [DOI] [PubMed] [Google Scholar]
- 30. Smith SG, Gupta KK, Smith SH. 1995. Effects of naltrexone on self-injury, stereotypy, and social behavior of adults with developmental disabilities. J Dev Phys Disabil 7:137–146 [Google Scholar]
- 31. Grant JE, Kim SW, Odlaug BL. 2009. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatr 65:600–606 [DOI] [PubMed] [Google Scholar]
- 32. Capasso R, Borrelli F, Cascio MG, Aviello G, Huben K, Zjawiony JK, Marini P, Romano B, Di Marzo V, Capasso F, Izzo AA. 2008. Inhibitory effect of salvinorin A, from Salvia divinorum, on ileitis-induced hypermotility: cross-talk between κ-opioid and cannabinoid CB(1) receptors. Br J Pharmacol 155:681–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pacheco Dda F, Klein A, Perez AC, Pacheco CM, de Francischi JN, Reis GM, Duarte ID. 2009. Central antinociception induced by mu-opioid receptor agonist morphine, but not δ- or κ-, is mediated by cannabinoid CB1 receptor. Br J Pharmacol 158:225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hojo M, Sudo Y, Ando Y, Minami K, Takada M, Matsubara T, Kanaide M, Taniyama K, Sumikawa K, Uezono Y. 2008. mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: electrophysiological and FRET assay analysis. J Pharmacol Sci 108:308–319 [DOI] [PubMed] [Google Scholar]
- 35. Schoffelmeer AN, Hogenboom F, Wardeh G, De Vries TJ. 2006. Interactions between CB1 cannabinoid and mu opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacology 51:773–781 [DOI] [PubMed] [Google Scholar]
- 36. Boustany-Kari CM, Jackson VM, Gibbons CP, Swick AG. 2011. Leptin potentiates the anti-obesity effects of rimonabant. Eur J Pharmacol 658:270–276 [DOI] [PubMed] [Google Scholar]
- 37. Navarro HA, Howard JL, Pollard GT, Carroll FI. 2009. Positive allosteric modulation of the human cannabinoid (CB) receptor by RTI-371, a selective inhibitor of the dopamine transporter. Br J Pharmacol 156:1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu YK, Yeh CF, Ly TW, Hung MS. 2011. A new perspective of cannabinoid 1 receptor antagonists: approaches toward peripheral CB1R blockers without crossing the blood-brain barrier. Curr Top Med Chem 11:1421–1429 [DOI] [PubMed] [Google Scholar]
- 39. Lazary J, Juhasz G, Hunyady L, Bagdy G. 2011. Personalized medicine can pave the way for the safe use of CB1 receptor antagonists. Trends Pharmacol Sci 32:270–280 [DOI] [PubMed] [Google Scholar]
- 40. Martin B, Golden E, Keselman A, Stone M, Mattson MP, Egan JM, Maudsley S. 2008. Therapeutic perspectives for the treatment of Huntington's disease: treating the whole body. Histol Histopathol 23:237–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maudsley S, Martin B, Luttrell LM. 2005. The origins of diversity and specificity in g protein-coupled receptor signaling. J Pharmacol Exp Ther 314:485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang L, Martin B, Brenneman R, Luttrell LM, Maudsley S. 2009. Allosteric modulators of g protein-coupled receptors: future therapeutics for complex physiological disorders. J Pharmacol Exp Ther 31:340–334 [DOI] [PMC free article] [PubMed] [Google Scholar]