Abstract
Age‐related deficits have been demonstrated in working memory performance and in the dopamine system thought to support it. We performed positron emission tomography (PET) scans on 12 younger (mean 22.7 years) and 19 older (mean 65.8 years) adults using the radiotracer 6‐[18F]‐fluoro‐L‐m‐tyrosine (FMT), which measures dopamine synthesis capacity. Subjects also underwent functional magnetic resonance imaging (fMRI) while performing a delayed recognition working memory task. We evaluated age‐related fMRI activity differences and examined how they related to FMT signal variations in dorsal caudate within each age group. In posterior cingulate cortex and precuneus (PCC/Pc), older adults showed diminished fMRI deactivations during memory recognition compared with younger adults. Greater task‐induced deactivation (in younger adults only) was associated both with higher FMT signal and with worse memory performance. Our results suggest that dopamine synthesis helps modulate default network activity in younger adults and that alterations to the dopamine system may contribute to age‐related changes in working memory function. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: aging, caudate, fMRI, FMT, PET, AADC
INTRODUCTION
Deficits in frontal lobe‐dependent cognitive function are common during aging [Cohen et al.,1987; De Luca et al.,2003; Gazzaley et al.,2005]. Dopaminergic neurotransmission also may show considerable variation during aging, when loss of dopaminergic function may occur [Kish et al.,1995; Rinne et al.,1990], and during late adolescence and early adulthood, when development of this system may still be underway [Weickert et al.,2007]. Such variations in the dopaminergic system in normal adults may help account for differences in frontal lobe function, including working memory, between subjects and across age groups [Backman et al.,2000; Cools et al.,2008; Volkow et al.,1998]. In particular, presynaptic dopamine metabolism in caudate nucleus and frontal cortex have been linked to cognitive functions thought to rely on frontal lobe [Bruck et al.,2005].
In addition to changes in the dopamine system and in cognition, functional magnetic resonance imaging (fMRI) has uncovered age‐related differences in the brain's response to memory stimuli. In particular, several working memory studies have found that older adults show differences in fMRI activity in prefrontal cortex compared with younger adults [Cabeza,2002; Emery et al.,2008; Mattay et al.,2006; Rajah and D'Esposito,2005]. Likewise, age‐related decreases in fMRI activity at rest have been noted in the default network (a group of medial brain structures that show more fMRI activity at rest compared to during tasks) [Damoiseaux et al.,2008]. This decreased default network activity while subjects are at rest results in greater fMRI deactivations in younger compared with older adults during tasks [Grady et al.,2006; Persson et al.,2007; Sambataro et al.,2008]. We sought to determine whether such age‐related changes in brain function reflected by fMRI activity and cognition were associated with differences in dopamine synthesis capacity that occur with age.
Both dorsolateral prefrontal cortex (DLPFC) and the posterior cingulate cortex/precuneus (PCC/Pc) area are good candidate regions for modulation during working memory by both age and variability in dopamine system function. DLPFC and precuneus are both known to have reciprocal circuit connectivity with dorsal caudate (DCA) [Alexander et al.,1986; Schmahmann and Pandya,1990]. Additionally, several previous studies have found age‐related differences in fMRI activity in both regions during memory tasks. Specifically, during working memory, older adults showed greater fMRI activity in DLPFC compared with younger adults [Cabeza et al.,2004; Rajah and D'Esposito,2005]. In contrast, younger adults tend to deactivate PCC/Pc during memory tasks relative to baseline, while older adults generally do not [Grady et al.,2006; Lustig et al.,2003], demonstrating classic age‐related differences in default network activity. Finally, dopaminergic activity has been shown to be related to fMRI signal variations in prefrontal cortex (PFC) [Apud et al.,2007; Gibbs and D'Esposito,2005; Landau et al.,2009], PCC [Kelly et al.,2009; Nagano‐Saito et al.,2008], and precuneus [Tomasi et al.,2009] in previous studies. This dopaminergic modulation of fMRI signal in PCC may reflect dopamine's control over default network activity and functional connectivity in general in regions connected to striatum [Kelly et al.,2009]. We therefore investigated age‐related differences in the relationship between dopamine synthesis capacity in DCA and fMRI response in these regions distant from, but connected to, DCA.
To perform this investigation, we scanned older and younger adults using positron emission tomography (PET) with the radiotracer 6‐[18F]‐fluoro‐l‐m‐tyrosine (FMT). FMT is a substrate for aromatic amino acid decarboxylase (AADC), which converts levodopa to dopamine. FMT PET signal therefore represents tonic dopamine synthesis capacity given sufficient substrate. FMT is similar to the more widely used radiotracer, fluorodopa (FDOPA), in that both are substrates for AADC. However, decarboxylated FDOPA is taken into vesicles and may be released and metabolized further. Therefore, its signal is affected by both vesicular uptake and post‐release processing [Sossi et al.,2002]. In contrast, metabolized FMT in the form of 6‐[18F]‐fluoro‐m‐tyramine is not a good substrate for vesicular transporters [Endres et al.,1997] and so is not released from vesicles for further processing, resulting in a purer measure of AADC activity [Dejesus et al.,2001].
In a previous study of the subjects examined here, we found that in our older group, subjects showed an increase in FMT signal in DCA with increasing age, while in the younger group, FMT signal decreased with age [Braskie et al.,2008]. Additionally, in the younger adults alone, higher FMT signal was associated with worse performance on tests of frontal lobe function. We had hypothesized that elevated FMT signal represented nonoptimal dopamine levels. We suggested that in the older adults, the higher FMT signal represented (possibly ineffective) AADC compensation for well‐established decreases in dopamine receptor expression and binding that occurs with aging [Kaasinen et al.,2000; Seeman et al.,1987; Severson et al.,1982; Volkow et al.,1996; Weickert et al.,2007], while in the younger adults, higher FMT signal indicated a still‐developing dopamine system not yet honed to provide optimal adult dopamine levels [Braskie et al.,2008].
In the current study, in addition to the FMT‐PET scanning reported on previously, subjects underwent functional magnetic resonance imaging (fMRI) scans while performing a verbal delayed recognition working memory task shown to be sensitive to variations in the dopamine system [Landau et al.,2009]. We then examined how well striatal FMT signal within each age group explained variations in fMRI signal within group as well as differences in fMRI signal between groups.
We previously found that FMT signal showed age‐related differences in its relationship to cognitive performance on tasks thought to rely on frontal lobe (such as working memory and executive function). We therefore hypothesized that during a working memory task, fMRI activity in our candidate regions (DLFPC and PCC/Pc) would likewise show age‐related differences in its relationship with striatal FMT signal. We further hypothesized that in these brain regions, FMT signal in DCA would help explain established differences in fMRI activation between older and younger adults during a working memory task.
MATERIALS AND METHODS
Subjects
Included in the study were 19 older (57–85 years; mean 65.8) and 12 younger (20–30 years; mean 22.7) cognitively intact adults (Table I), recruited through newspaper ads and postings. Each potential subject also underwent neuropsychological testing that included tests of executive function, working memory, language, episodic verbal and spatial memory, motor response, and general cognitive function. All subjects included in this study scored at least 26 on the Mini Mental State Exam (MMSE), and scored low average or better for their age on both the Wechsler Memory Scale (WMS) auditory immediate age‐adjusted summed score (which includes WMS logical memory immediate score and WMS verbal paired associates immediate score) and the Wechsler Adult Intelligence Digit Span age‐adjusted summed score (which includes Digit Span Forward and Digit Span Backward). No subject had a Geriatric Depression Rating (21 item) score greater than 10. In addition, volunteers were excluded based on a number of other criteria: they were not current smokers, hypertensive, taking medications that could affect cognition or FMT signal, or unable to undergo MRI or PET scanning for safety reasons. Volunteers with a history of neurological or psychological disorders, major systemic disease, drug or alcohol abuse, or serious head injury (loss of consciousness >15 min) were also excluded. Functional MRI scans that were unusable due to excessive motion (>2 mm) or severe artifacts were not included in the current study. To ensure that the number of correct trials across age groups was similar and to ensure that the subjects were properly attempting the task, we excluded any subject whose total accuracy of attempted items was less than 75% or whose correct items as a percent of total presented items in useable scans was less than 55% (at least 16 useable items per load level). Subjects in the older group were significantly more educated than those in the younger group (Mann‐Whitney two‐tailed test P = 0.0004); however, all subjects in the younger group who had completed fewer than 16 years of education were still active students. Therefore, these differences were unlikely to be related to underlying intellectual ability or socio‐economic factors.
Table I.
Demographic characteristics of participants
| Older | Younger | |
|---|---|---|
| Number of participants | 19 | 12 |
| Agea , b | 65.8 ± 7.1 | 22.7 ± 2.8 |
| Males/Females | 6/13 | 3/9 |
| Education (years)a , b | 18.5 ± 2.1 | 15.8 ± 1.2 |
| Mini‐mental state exama | 29.3 ± 1.1 | 29.3 ± 0.9 |
| Sternberg task: Accuracy (attempted trials)a | 0.95 ± 0.04 | 0.95 ± 0.05 |
| Sternberg task: High‐load accuracy (six‐item attempted trials)a | 0.93 ± 0.08 | 0.96 ± 0.05 |
| Sternberg task: Low load accuracy (two‐item attempted trials)a | 0.95 ± 0.05 | 0.96 ± 0.05 |
| Sternberg task: Reaction time slope (correct items: ms)a , c | 38 ± 24 | 51 ± 16 |
| Sternberg task: Average reaction time (correct items: ms)a , b | 1251 ± 186 | 1083 ± 129 |
| Sternberg task: 0‐load average reaction time (ms)a , d | 537 ± 138 | 542 ± 104 |
Demographic features are listed as mean ± standard deviation.
Age groups are significantly different (P < 0.05) as determined using a two‐tailed Mann‐Whitney test.
Calculated as the slope of the line created when the average reaction time for each load was plotted against load size (i.e. two, four, and six items).
Not available for two older adults.
Of the 19 older subjects in this study, 18 were reported on in a previous fMRI study [Landau et al.,2009], which included only older adults. All 31 subjects included in the current study were also formerly reported on in a study that evaluated the relationship between FMT signal and aging [Braskie et al.,2008] but did not report fMRI findings. Two subjects from that study (one older and one younger) were excluded from the current study because of fMRI task scores that were below our cut‐off. One older subject from that study was excluded from the current study due to excessive fMRI motion, and two younger subjects were excluded from the current study due to technical problems with their fMRI scans.
The IRBs at participating institutions approved the current study, and all subjects provided written informed consent prior to enrolling in the study.
Imaging Procedures
Within 6 months of their PET scans, we performed MRI scans on all subjects in both age groups using a Varian Unity/Inova whole body 4.0 T scanner (Palo Alto, CA) with a TEM send‐and‐receive head coil (MR Instruments, Minneapolis, MN). Whole brain axial T1‐weighted “Magnetization Prepared Fast Low‐Angle Shot” (MPFLASH) 3D MRI sequences were obtained for use in partial volume correction of PET scans and region of interest (ROI) demarcation (1.55 mm slices/0 mm gap; 256 × 256 [0.875 × 0.875 mm2] in‐plane resolution; field of view [FOV] = 224 × 224; repetition time [TR] = 9 ms; echo time [TE] = 5 ms). We also acquired 18‐slice whole brain functional MRI scans using a two‐shot gradient echo, echo‐planar scan sequence while the subjects performed a Sternberg working memory task (5 mm slices/1 mm gap; 64 × 64 [3.5 × 3.5 mm2] in‐plane resolution; FOV = 224 × 224; TR = 2,000 ms; TE = 28 ms; flip angle [FA] = 20°). We acquired “Gradient Echo Multi‐Slice” (GEMS) scans (256 × 256 [0.875 × 0.875 mm2] in‐plane resolution; FOV = 224 × 224; TR = 200 ms; TE = 5 ms) coplanar to the functional scans to aid in coregistration of fMRI scans to a standard Montreal Neurologic Institute (MNI) brain.
PET Data Acquisition
FMT was synthesized at the Lawrence Berkeley National Laboratory using a procedure previously described [VanBrocklin et al.,2004]. Approximately 1 h prior to PET scanning, subjects ingested 2.5 mg kg−1 of carbidopa to minimize peripheral FMT metabolism. PET scans containing 47 parallel slices (4‐mm thick; 3.6 mm in‐plane voxel size) were acquired on a Siemens ECAT EXACT HR PET scanner in 3D acquisition mode. A 10‐min transmission scan was obtained for attenuation correction, followed by a bolus injection of ∼2.5–3.0 mCi of FMT into an antecubital vein. A dynamic acquisition sequence in 3D mode was then obtained: 4 × 1 min, 3 × 2 min, 3 × 3 min, and 14 × 5 min for a total of 89 min of scan time. We reconstructed data using an ordered subset expectation maximization (OSEM) algorithm with weighted attenuation, an image size of 256 × 256, and six iterations with 16 subsets. We applied a Gaussian filter with 6 mm full width at half maximum and scatter correction. Quality control minimized subject motion and assured adequate count rates.
PET Reference Region
On the coronal plane of each subject's structural MRI image, we manually delineated cerebellar masks that included two thirds of the slices posterior to the last slice in which the superior cerebellar peduncles stretched superiorly. Including only these most posterior slices ensured that FMT signal from the substantia nigra and ventral tegmental area did not contaminate the reference region. The cerebellum then was segmented automatically using the “FSL Automatic Segmentation Tool” (FAST) software [Zhang et al.,2001] from version 3.3 of the “Analysis Group at the Oxford Centre for Functional MRI of the Brain” (FMRIB) software library (FSL) to exclude large white matter tracts. As reported previously, the FMT signal in the cerebellum did not contribute to the striatal FMT differences between our age groups [Braskie et al.,2008].
PET Data Analysis
To adjust for between‐frame subject motion, we realigned each PET frame to the summed image of the first 10 frames using “Statistical Parametric Mapping” (SPM2; Wellcome Department of Cognitive Neurology, University College London, London, UK). Individual MRI scans (along with their accompanying brain matter masks and cerebellar gray matter masks) were then coregistered to their respective summed PET images. Utilizing Patlak graphical analysis for irreversible binding [Patlak and Blasberg,1985], K i images were created from PET frames corresponding to 24 to 89 min. The individual cerebellar gray matter masks defined the PET reference regions.
Partial Volume Correction
Correction for partial volume errors is necessary to render the data comparable among brains of different sizes. We performed partial volume correction using a two‐compartment model as described previously [Meltzer et al.,1999]. Briefly, the PET scanner point spread function (empirically derived from our scanner) was applied to each subject's brain matter binary mask by convolving the MRI brain matter mask with a Gaussian kernel. Each subject's observed PET scan in native space then was divided by the coregistered and convolved brain matter mask to arrive at the corrected PET scan [Meltzer et al.,1999]. In the current study, all FMT signal values reported or used in analysis have been partial volume corrected (PVC).
FMT Regions of Interest (ROIs)
DCA ROIs were defined on the individual MRI scans using guidelines outlined previously [Mawlawi et al.,2001]. After individual MRI scans were coregistered to the corresponding PET images, average FMT counts associated with each ROI were extracted from the PVC K i image. Reliability on these ROIs has been reported previously [Braskie et al.,2008]. Tonic striatal AADC activity in healthy adults has been shown previously to be stable over a period of months [Vingerhoets et al.,1994].
Memory Activation and Control Task
During fMRI scanning, participants were tested on a letter stimuli version of the Sternberg delayed recognition task [Sternberg,1966]. Before entering the scan room, they performed a 15 trial computer‐based practice version of the task. During the cue phase for each trial, subjects encoded 2, 4, or 6 capital letters presented simultaneously on the screen for 4 s. The cue phase was followed by a 12‐s delay during which they fixated on a +. After the delay phase, a lower case probe letter was presented for 2 s, and subjects pressed one of two buttons to indicate whether or not the probe letter was among the letters previously encoded in the current trial. A jittered interstimulus interval from 8‐ to 12‐s long followed each probe. All subjects were tested using the same six versions of the task, although trials within and across scan runs contained discrete sets of letters to encode. Each scan run contained five trials at each letter load for a total of 15 trials per scan. Letter loads were counterbalanced within run. Although by nature of this task design, phases of the task are fairly collinear, the FSL software we used automatically and explicitly accounts for this collinearity when computing the variance of the estimated contrast. Additionally, we have not made any comparisons between events that are partially collinear. Therefore, any reported significant result is valid.
While in the scanner, subjects also performed a control version of the Sternberg task in which the letters that normally were presented during the cue and the probe phases were replaced with Xs. Subjects were instructed to press both response buttons as quickly as possible when the probe appeared. No fMRI data was collected during this task; it was performed solely to collect information on button press reaction time during a task that required attention and motor speed without engaging working memory processes.
Percent correct (accuracy) on the working memory task was calculated for total attempted items, and attempted 6, 4, and 2 item trials separately. We also calculated average reaction time (RT) for correct items in the Sternberg delayed recognition task and for all responses in the 0‐load task (to evaluate attention and motor speed). Finally, because better accuracy of working memory responses may occur at the expense of reaction time, we created a normalized performance score that considered both speed and accuracy. This Sternberg performance score was created from the working memory task data by multiplying the Z score for the Sternberg RT (on correct items) by −1 (to represent better performance) and averaging the product with the Z score for Sternberg accuracy (on attempted items). Although task accuracy was calculated from all responses and fMRI activity was based on correct responses only, it is reasonable that, on a task such as the current one, task accuracy would be related to fMRI activity. Task accuracy does not reflect any one trial, but rather the subjects' performance across all trials. If, on average, subjects are getting more trials wrong, then they are most likely also having a harder time even on the trials where they are responding correctly. This would be reflected in the fMRI signal even for the correct trials. For instance, those subjects having lower accuracy would likely have either a larger proportion of their correct responses be associated with lower certainty, or a diminished ability to monitor their own responses.
Stimuli were presented using E‐Prime presentation software (Psychology Software Tools, Inc.; Pittsburgh, PA,; http://www.pstnet.com/eprime) and were projected onto a screen that was fastened above each subject's abdomen.
Functional MRI Data Analysis
We performed all fMRI data analyses using FSL tools. Skulls were first stripped from the coplanar GEMS scan using FSL “Brain Extraction Tool” [BET; Smith,2002]. We used FSL “FMRI Expert Analysis Tool” (FEAT) to perform individual preprocessing and individual and group statistical analysis. Preprocessing included both brain extraction using BET and motion correction with FSL's “Motion Correction using FMRIB's Linear Image Registration Tool” [MCFLIRT; Jenkinson et al.,2002]. Each functional scan was coregistered to its GEMS and MPFLASH scans using six parameter rigid body transformations. The MPFLASH scans were normalized to the MNI standard brain using 12 parameter affine transformations. Functional scans were high‐pass temporally filtered (55 s), and images were spatially smoothed using a Gaussian smoothing kernel of 7 mm full width half maximum.
Functional MRI Regions of Interest
Because of variations in lateral ventricle sizes in an aging population, obtaining valid normalizations of adjacent subcortical structures for use in a voxel‐wise fMRI analysis is difficult. We therefore also obtained average fMRI percent signal change for right and left caudate in native space for each subject.
Functional MRI signal dropout and distortion artifacts affected orbitofrontal cortex and the most anterior portions of caudate on some subjects. Therefore, the DCA ROIs used in our PET analyses were not appropriate for obtaining reliable fMRI percent signal change values within the caudate. Instead, we drew new right and left caudate ROIs for use in the fMRI experiments. ROIs were drawn on the structural MRI (MPFLASH) scans in native space such that they encompassed primarily caudate body, and some dorsal caudate head. The anterior boundary of the caudate for each ROI was one coronal slice anterior to the first in which the anterior commissure was visible. The posterior boundary was selected in the axial view and was represented by the most posterior point within the caudate that bordered the anterior horns of the lateral ventricles at the level of the thalamus. These boundaries were chosen for maximal reliability in the ROIs without compromising the ROIs by including areas with fMRI signal dropout or distortion in any subject. ROIs were drawn using coronal and axial slices. High test‐retest reliability was established across five subjects (average ICC = 0.996 interrater; R 2 = 1.00 intrarater).
Statistical Analysis
Individual scan statistical analyses were performed within FEAT using “FMRIB's Improved Linear Model” (FILM) [Woolrich et al.,2001]. FILM applies the general linear model and corrects for dependence between time points after nonparametric estimation of time series autocorrelation. For each individual, we contrasted fMRI activity during the interstimulus intervals with activity during each trial phase: cue, delay, and probe. Signal that varied linearly with increasing working memory load was also investigated for each phase. For the remainder of this document, activity during each phase of the task will refer to load‐independent activity unless otherwise specified.
We next compared load‐dependent and ‐independent fMRI activity during the cue, delay, and probe phases between older and younger adults using FSL “FMRIB's Local Analysis of Mixed Effects” [FLAME; Beckmann et al.,2003] to generate Z statistic images (Z > 2.3, adjusted to P < 0.05 using cluster thresholding to correct for multiple comparisons) across subjects. This analysis did not include an interaction term between group and task. For all comparisons, only the trials associated with correct responses were considered.
We then sought to evaluate whether the differences in fMRI activity between age groups could be explained by variations in FMT signal. To do this, we first investigated the relationship between FMT signal and fMRI activity within each age group, using whole brain regression and limiting our search to masked regions where fMRI activity was significantly different between groups. In other words, we examined the conjunction between fMRI activity that differed significantly by age group and fMRI activity that significantly correlated with FMT signal. Performing separate analyses for each group and then comparing the resulting relationships between groups allowed for the detection of within‐group relationships that may occur in opposite directions between groups. Because our previous study showed both that FMT signal was significantly correlated with age (in opposite directions) in the two age groups, and because we previously found correlations between frontal lobe dependent cognitive function and FMT signal only in the younger group, it would be inappropriate to regress FMT signal with fMRI signal across all subjects in both age groups [Braskie et al.,2008]. Such an analysis would yield results only if a linear relationship existed between FMT and fMRI across the entire age range, which we had reason to believe, based on our previous results, may not be the case. We therefore included FMT signal values from bilateral DCA as a covariate in our fMRI analyses separately for older and younger adults. Because FMT signal was significantly correlated with age within each age group, we also controlled for age within each age group. The within‐group age adjustment ensured that any relationship that emerged between FMT signal and fMRI activity within each age group would not be confounded by age‐related factors other than FMT signal.
With the exception of the voxel‐wise analysis, which were performed using the statistical parametric mapping within the FSL software, all statistical comparisons were performed using nonparametric tests (with an alpha criterion of P < 0.05) unless otherwise noted. Normal approximations of the two‐tailed Mann‐Whitney tests were used to compare values between groups, and Spearman rank correlations were used to measure the relationships between continuous variables within each group. Age was controlled for in such relationships using Spearman partial correlation tests [Schemper,1991], which are analogous to parametric multiple regression tests. Nonparametric tests do not require data to be normally distributed, and they minimize the effects of any existing data outliers. Therefore, although these tests are conservative and may diminish the ability to detect a relationship if one exists, they are appropriate for small sample sizes, whose data are rarely normally distributed.
Functional ROI Analysis
The initial analysis comparing fMRI activation between young and old subjects showed significant differences in PCC/Pc during the probe presentation. For this reason, we elected to examine relationships between activation in PCC/Pc and dopaminergic function. We used as a functionally defined ROI a binary mask of all voxels that were both significantly different between young and old subjects during probe presentation and were significantly correlated with FMT in DCA. We then performed “FEAT query” for each subject to extract the average fMRI activity per contrast within the ROI. Finally, we performed a Spearman partial correlation nonparametric test to evaluate the relationships between the ROI's fMRI signal and the DCA FMT signal across subjects, while controlling for age within group.
RESULTS
Behavioral Performance—Main Effect of Memory Load
Across all subjects, a Friedman's nonparametric ANOVA showed that RT on correct items increased with increasing load (Q = 47.03, P < 0.001). This increase remained significant in younger adults (Q = 20.17, P < 0.001), and older adults (Q = 26.95, P < 0.001). Across all subjects (Q = 2.05) or in older (Q = 4.11) or younger adults alone (Q = 0.29), accuracy did not significantly change with memory load (P > 0.05).
Behavioral Performance—Main Effect of Age
Using Mann‐Whitney two‐tailed tests to compare performance on the Sternberg task between the older and younger groups, we found no significant differences for overall accuracy (P = 0.52), accuracy for six items (P = 0.28), for four items (P = 0.37), or for two items (P = 0.94). Mann‐Whitney tests also showed that average RT on correct items was slower in older adults when all working memory loads were considered together (P = 0.008), and for each memory distinct level: (P = 0.03) for six items, (P = 0.02) for four items, and (P = 0.003) for two items. However, there was no significant difference between age groups using the Sternberg 0 load (attention and motor) task (P = 0.96), suggesting that the RT difference on the memory task related to cognitive processes.
A Student's t‐test (appropriate for use on normalized data) showed that younger adults had significantly better overall Sternberg performance scores, which considered both task accuracy and RT, than older adults (P = 0.048).
Functional MRI Age Effects
We compared fMRI activity during the load‐dependent and ‐independent cue, delay, and probe phases between older and younger adults. During load‐dependent cue and delay, older adults showed greater fMRI activity than younger adults broadly across the brain, including in frontal regions (including dorsal lateral prefrontal cortex during delay), hippocampus and other temporal regions, cingulate gyrus, parietal cortex, occipital cortex, and thalamus (Table II, Fig. 1).
Table II.
Age group‐related differences in fMRI activity during load‐dependent cue and delay phases of the Sternberg task
| R/L | BA | Sample coordinates | Peak Z score | |
|---|---|---|---|---|
| Cue: load‐dependent (older > younger) | ||||
| Superior frontal g. | R/L | 6, 8 | 34, 16, 52 | 4.38 |
| Medial frontal g. | R/L | 6 | 8, −10, 54 | 4.12 |
| Precentral g. | R/L | 4 | −48, −12, 36 | 4.86 |
| Precentral g. | R | 6 | 40, −8, 56 | 3.41 |
| Postcentral g. | R | 5 | 24, −38, 68 | 2.87 |
| Superior temporal g | R | 22 | 44, −48, 18 | 3.45 |
| Middle temporal g. | R | 39 | 48, −56, 14 | 4.01 |
| Inferior temporal g. | R | 19 | 48, −58, −2 | 4.01 |
| Parahippocampal g. | R/L | 19, 37 | −22, −54, −6 | 4.62 |
| Precuneus | R/L | 7, 19 | 22, −76, 36 | 5.31 |
| Supramarginal g. | R/L | 40 | −58, −42, 55 | 3.16 |
| Cuneus | R/L | 19 | 0, −88, 26 | 4.12 |
| Cingulate g. | R/L | 24 | 10, −2, 40 | 3.00 |
| Cingulate g. | L | 31 | −6, −28, 38 | 3.76 |
| Posterior cingulate | R | 30 | 16, −50, 16 | 4.00 |
| Posterior cingulate | L | 31 | −20, −64, 18 | 3.75 |
| Insula | R/L | 13 | 46, −6, 16 | 3.23 |
| Hippocampus | R | 28, −26, −12 | 2.87 | |
| Thalamus | R | 16, −24, 4 | 3.50 | |
| Putamen | R | 34, −10, 2 | 3.78 | |
| Midbrain | R | 10, −34, −10 | 2.93 | |
| Delay: load‐dependent (older > younger) | ||||
| Dorsal lateral prefrontal cortex | R | 9 | 34, 32, 36 | 4.10 |
| Medial frontal g. | R/L | 6 | 22, 6, 52 | 4.64 |
| Precentral g. | R | 6 | 46, 2, 36 | 4.09 |
| Superior temporal g. | R | 22 | 48, −46, 6 | 3.04 |
| Precuneus | R/L | 7, 19 | 0, −70, 44 | 4.04 |
| Supramarginal g. | R/L | 40 | 44, −42, 34 | 4.90 |
| Anterior cingulate g. | R/L | 24 | 2, 26, 20 | 4.70 |
| Cingulate g. | R/L | 23, 31, 32 | 2, 28, 30 | 3.92 |
| Cuneus | R/L | 17 | 4, −82, 6 | 3.83 |
| Middle occipital g. | R/L | 18 | 24, −84, 0 | 4.30 |
| Lingual g. | R | 18 | 20, −54, 4 | 3.28 |
| Thalamus | R | 20, −24, 12 | 3.07 | |
| Hippocampus | R/L | 24, −28, −10 | 2.79 | |
Note: Significance was set at Z= 2.3, corrected for multiple comparisons using cluster thresholding to adjust image‐wise significance to P < 0.05. No voxels showed greater activity in younger compared with older adults during load‐dependent cue or delay. There were no age differences in fMRI activity during the load‐dependent probe phase.
Figure 1.
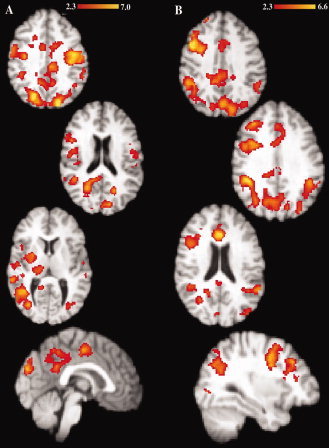
Age‐related load‐dependent fMRI activity differences. Differences in fMRI activity were found between younger and older adults during the load‐dependent (A) cue phase and (B) delay phase when the activity was compared to fMRI signal during the baseline fixation. Functional MRI activity was not significantly different between age groups during the load‐dependent probe phase. Red to yellow scale thresholded Z score masks represent regions in which older adults showed significantly greater fMRI activity than younger adults. Significance was set at Z = 2.3, corrected for multiple comparisons using cluster thresholding to adjust the image‐wise significance to P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
During the load‐independent cue phase, older adults showed greater fMRI activity in precuneus, while younger adults preferentially recruited sensory and motor cortex, parietal cortex, caudate, and thalamus. During the load‐independent delay phase, younger adults showed greater activation in frontal cortex, anterior and posterior cingulate, precuneus, and cerebellum, while older adults did not show greater activation than the younger adults in any region. During the load‐independent probe phase, older adults showed greater activation than younger adults in precuneus, posterior cingulate, supplementary motor area, and motor cortex, while younger adults showed greater activation in basal ganglia, thalamus, and insular cortex (Table III, Fig. 2).
Table III.
Age group‐related differences in fMRI activity during load‐independent cue and delay, and probe phases of the Sternberg task
| R/L | BA | Sample coordinates | Peak Z score | |
|---|---|---|---|---|
| Cue: load‐independent (older > younger) | ||||
| Precuneus | R | 7, 19, 31 | 24, −74, 40 | 3.34 |
| Cue: load‐independent (younger > older) | ||||
| Precentral g. | L | 4 | −32, −26, 62 | 3.25 |
| Postcentral g. | L | 2 | −34, −34, 62 | 4.21 |
| Superior parietal lobule | L | 7 | −26, −54, 54 | 3.10 |
| Inferior parietal lobule | L | 40 | −48, −30, 42 | 2.95 |
| Caudate | R/L | −6, 4, 8 | 5.02 | |
| Thalamus | L | −12, −26, 16 | 4.40 | |
| Delay: load‐independent (younger > older) | ||||
| Superior frontal g. | R | 8 | 16, 42, 46 | 3.38 |
| Medial frontal g. | R | 6 | 6, 44, 36 | 3.98 |
| Medial frontal g. | R/L | 9 | 4, 50,18 | 3.10 |
| Precuneus | R/L | 7/31 | 4, −54, 32 | 2.85 |
| Anterior cingulate | R | 24 | 4, 34, 18 | 2.73 |
| Posterior cingulate | R/L | 23, 30 | 2, −52, 14 | 4.16 |
| Cerebellum | R/L | 4, −56, −2 | 4.02 | |
| Probe: load‐independent (older > younger) | ||||
| Medial frontal g. | R/L | 6 | −2, −22, 64 | 3.61 |
| Precentral g. | R/L | 4 | −14, −28, 68 | 3.84 |
| Precuneus | L | 7 | −6, −42, 44 | 3.18 |
| Precuneus | R/L | 31 | −4, −48, 36 | 3.66 |
| Posterior cingulate | R/L | 23, 31 | 12, −50, 24 | 3.23 |
| Probe: load‐independent (younger > older) | ||||
| Posterior cingulate | R/L | 23 | −2, −20, 26 | 3.41 |
| Insula | R/L | 13 | 42, 20, −8 | 4.01 |
| Thalamus | R/L | 4, −2, 6 | 4.28 | |
| Caudate | R/L | −8, 2, 12 | 4.24 | |
| Putamen | R/L | −24, 0, −8 | 3.00 | |
Note: Significance was set at Z= 2.3, corrected for multiple comparisons using cluster thresholding to adjust image‐wise significance to P < 0.05. There were no voxels that showed significantly greater activity in older than in younger adults during load‐independent delay.
Figure 2.
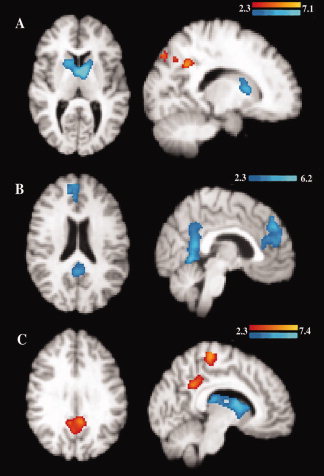
Age‐related load‐independent fMRI activity differences. Differences in fMRI activity were found between younger and older adults during the load‐independent (A) cue phase, (B) delay phase, and (C) probe phase when the activity was compared to fMRI signal during the baseline fixation. Red to yellow scale thresholded Z score masks represent regions in which older adults showed significantly greater fMRI activity than younger adults. Blue scale masks represent regions in which younger adults showed significantly greater fMRI activity. Significance was set at Z = 2.3, corrected for multiple comparisons using cluster thresholding to adjust the image‐wise significance to P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We also compared native space average fMRI percent signal change for bilateral caudate between older and younger adults. Mann‐Whitney tests showed that older adults showed significantly less fMRI activation in bilateral caudate during the cue and probe phases (P = 0.008 and 0.009, respectively) compared with younger adults. There was no significant effect of age on caudate fMRI activity during load‐independent delay or during load‐dependent cue, delay, or probe.
FMT Effects
As previously reported [Braskie et al.,2008] in a sample that included the current subjects, FMT signal in bilateral DCA was higher in the older subjects compared with the younger ones (two‐tailed Mann‐Whitney test P = 0.004). Additionally, FMT signal in bilateral DCA was significantly correlated with age in the older (Spearman ρ = 0.74; P = 0.0003) and younger (ρ = −0.76; P = 0.004) adults. We therefore evaluated the relationship between age‐adjusted FMT signal in bilateral DCA and fMRI signal within each group. Our search was limited to regions that showed a significant difference in fMRI activity between groups during any load‐dependent or load‐independent task phase. We found that during the load‐independent probe phase alone, the relationship between fMRI activity in bilateral PCC/Pc and FMT signal in bilateral DCA was different between older and younger adults. Specifically, after adjusting for age, younger adults having greater FMT signal in bilateral DCA also showed greater fMRI task‐induced deactivations in PCC/Pc (BA 31: peak coordinates 2, −58, 28, peak Z score 3.69; BA 23: peak coordinates −2, −60, 20, peak Z score 3.66; BA 7: peak coordinates 6, −58, 34, peak Z score 3.60). No such significant relationship existed in the older adults (Figs. 3 and 4A,B). The results were verified in young adults using a nonparametric Spearman partial correlation that likewise corrected for age (R 2 [full model] = 0.55; P [full model] = 0.027; R 2 [partial contribution of FMT] = 0.55; P [partial contribution of FMT] = 0.009). Age significantly contributed to this multiple regression (R 2 [partial contribution of age] = 0.34; P [partial contribution of age] = 0.027), although in an individual comparison, age and fMRI signal were not significantly correlated P = 0.92. The PCC/Pc ROI was made into a binary mask, which was then used to obtain percent signal change values in individuals. FMT signal was not significantly correlated with fMRI activity in any other region having age group‐related fMRI differences during the probe or any other task phase (load‐dependent or load‐independent). We did note that in the older adults only, during load‐dependent delay, increased fMRI activity in right DLPFC was associated with increased FMT signal in right DCA, however that effect was no longer significant after controlling for age, suggesting that the results related more to age than to dopamine synthesis.
Figure 3.
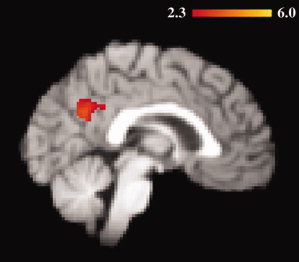
FMT modulates age‐related fMRI activity during the probe phase. The highlighted regions represent the intersection of two analyses: voxels in which the fMRI activity was significantly greater in older than in younger adults during the probe phase (independent of load), and also the relationship between FMT in bilateral DCA and fMRI signal was significantly different between age groups (P = 0.05 after cluster thresholding adjustment for multiple comparisons). The overlap of these contrasts suggests that the dopamine system may play a role in modulating fMRI activity in this region. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 4.
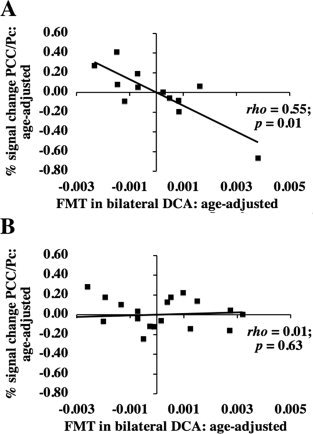
Age‐adjusted relationship between fMRI activity and FMT signal during the probe phase in (A) younger and (B) older adults. Residuals of fMRI in PCC/Pc (after age adjustment) were plotted against residuals of FMT signal in bilateral DCA (after age adjustment). The fMRI ROI was defined functionally as described in the Materials and Methods section. The ρ and P values shown on the graphs represent the individual contributions of FMT in bilateral DCA to describing fMRI activity after adjusting for age using a Spearman partial correlation as described in the Results section. When the outlying data point was removed in (A), the relationship was still significant (P = 0.01). Because these numbers have been age‐adjusted, the distribution of fMRI signal change in the negative versus positive range is different from the distribution of fMRI raw signal values reported in the Results section. Specifically, using raw fMRI signal values, 75% of younger adults, but only 26% of older adults showed any task‐induced deactivation in PCC/Pc during the probe phase.
A two‐tailed Mann‐Whitney test showed that average fMRI activations in PCC/Pc were significantly lower in younger adults compared with older adults during the probe phase (P = 0.005), with 75% of the younger adults (but only 26% of the older adults) showing any level of task‐induced deactivation compared with activity during fixation. FMT signal in bilateral DCA was not significantly correlated with load‐dependent or load‐independent fMRI activity in caudate in either group during any phase of the task.
Correlations Between Sternberg Task Performance and Imaging Measures
Finally, we evaluated whether the Sternberg task performance differences noted between age groups were related either to FMT signal in bilateral DCA or to fMRI activity in the PPC/Pc ROI. In younger adults, fMRI activity in PPC/Pc was more positive (mostly reflecting less deactivation) during the probe phase in those who performed better on the Sternberg task, even after controlling for age using a Spearman partial correlation that included fMRI % signal change (negative for deactivations and positive for activations) and age as independent variables (R 2 [full model] = 0.56; P [full model] = 0.024; R 2 [partial contribution of fMRI % signal change] = 0.53; P [partial contribution of fMRI % signal change] = 0.009) (Fig. 5). It is important to note that fMRI % signal change used here is not an absolute value; therefore, a large task induced deactivation would be reflected as a more negative (lower) number than a mild deactivation, which would be more negative than a task induced activation. In older adults, total Sternberg performance was not significantly correlated with PPC/Pc fMRI activity during the probe phase (R 2 [full model] = 0.09; P [full model] = 0.46; R 2 [partial contribution of fMRI % signal change] = 0.001; P [partial contribution of fMRI % signal change] = 0.88). Because total Sternberg performance scores for each subject reflected both accuracy and speed of response, we next compared speed and accuracy of response separately to fMRI % signal change in order to better determine what aspect of performance may have been driving the results (in younger adults). We found that (after adjusting for age), a more positive fMRI response (mostly reflecting less deactivation) in PCC/Pc during the probe phase showed trends toward significant correlations with both quicker reaction time on correct items (P [full model] = 0.08; P [partial contribution of fMRI % signal change] = 0.03) and higher accuracy on attempted items (P [full model] = 0.13; P [partial contribution of fMRI % signal change] = 0.11) when each was considered alone.
Figure 5.
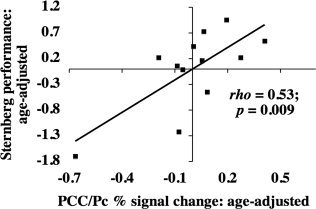
Age‐adjusted relationship between fMRI activity and Sternberg task performance during the load‐independent probe phase in younger adults. Residuals of Sternberg task performance (after age adjustment) were plotted against residuals of fMRI percent signal change in PCC/Pc (after age adjustment). The fMRI ROI was defined functionally as described in the Materials and Methods section. The ρ and P values shown on the graphs represent the individual contributions of fMRI percent signal change in PCC/Pc during probe to describing Sternberg task performance after adjusting for age using a Spearman partial correlation as described in the Results section. When the outlying data point was removed from this analysis, the relationship still strongly trended toward significance (P [full model] = 0.069, P [partial contribution of fMRI % signal change] = 0.046).
Total Sternberg performance was not significantly correlated with FMT signal in bilateral DCA in either age group.
DISCUSSION
Functional MRI activity varied between age groups in many brain regions during the Sternberg task. However, only in PCC/Pc during the probe phase was there also an age‐related dissociation in the relationship between fMRI activity and DCA FMT signal. Specifically, younger adults with higher FMT signal showed greater task‐induced deactivations, a relationship that was not apparent in the older adults. Consistent with prior memory studies [Grady et al.,2006; Lustig et al.,2003], younger adults generally deactivated PCC/Pc during the probe phase, while most older adults showed increased fMRI activity in that region during the task. Task‐induced deactivations occur across a variety of tasks [Hampson et al.,2006; McKiernan et al.,2003], and are believed to be part of a “default network.” The functional significance of the default network is hotly debated, but many believe the deactivations relate to a redistribution of attentional resources during tasks [McKiernan et al.,2003]. This hypothesis is supported by recent research showing that during a visual attention task, greater task load was associated with greater fMRI deactivations in the precuneus [Tomasi et al.,2009]. Taken together, our findings suggest that dopamine plays a role in modulating attention during working memory and that alterations in this system underlie changes in age‐related working memory function.
Our results suggest that in younger adults, DCA dopamine synthesis and PCC/Pc fMRI deactivations are related. These results are consistent with past studies showing both that dopamine modulates some task‐induced deactivations [Argyelan et al.,2008] and that default network activity in PCC/Pc may be abnormally high or low in diseases in which the dopamine system has been implicated [Harrison et al.,2007; Tinaz et al.,2008]. The relationship between striatal dopamine synthesis and resting state fMRI activity in PCC/Pc may be facilitated by the closed loop DCA—pallidal—thalamic—cortical connectivity that includes both DLPFC and precuneus (BA7) [Alexander et al.,1986; Schmahmann and Pandya,1990].
In younger adults, greater probe phase PCC/Pc fMRI deactivations were associated both with greater DCA FMT signal and with worse Sternberg task performance. Our results are consistent with recent research that found that during a visual attention task, adults having more dopamine transporter binding (likely associated with less available dopamine) showed less fMRI deactivation in the precuneus [Tomasi et al.,2009]. Our findings are also consistent with our previous study in which DCA FMT signal and frontal lobe‐dependent cognition scores were inversely correlated only in younger adults [Braskie et al.,2008], lending support to our hypothesis that higher DCA FMT values represent nonoptimal dopaminergic functioning. Only correct trials were considered in our fMRI analysis, and both accuracy (of attempted trials) and RT (on correct trials) contributed to our performance measure, suggesting that fMRI deactivation was related to increased perceived task difficulty rather than to processes specific to task accuracy. Such results are not without precedent. Some previous studies [McKiernan et al.,2003; Persson et al.,2007; Tomasi et al.,2009] have demonstrated that PCC deactivated more during difficult tasks compared with easier ones, possibly due to a greater reallocation of attentional resources needed during more challenging tasks [McKiernan et al.,2003]. Our finding that higher FMT signal in the caudate was associated with worse performance on the memory task is in contrast with a previous study whose authors found that subjects with a low working memory capacity showed less caudate FMT signal [Cools et al.,2008]. However, methodological differences (such as partial volume correction and ROI delineation) between the current study and the previous one are likely to account for our differing results as discussed previously [Braskie et al.,2008].
The association that we found between greater task‐induced deactivations in PCC/Pc and worse memory performance may appear to conflict with recent studies having opposing results [Duverne et al.,2009; Miller et al.,2008], although fundamental methodological differences suggest that the results are complementary, not contradictory. Previous studies contrasted successfully versus unsuccessfully remembered trials during memory encoding [Duverne et al.,2009; Miller et al.,2008], while here, only successful trials were considered and the relationship between fMRI activity and performance during memory retrieval was evaluated by covarying activity with a performance score representing perceived task difficulty. If task‐induced deactivations are indeed related to attentional allocation, it is plausible that in previous studies, greater deactivation occurred during encoding of successful trials because unsuccessful encoding resulted from insufficient attention. In contrast, our fMRI activity was considered only in trials that already had been successfully encoded and retrieved. Subjects who showed greater deactivation required greater attention for a task that was associated with worse performance scores and, therefore, perceived as more difficult. Our results are consistent with those of a recent study that demonstrated that fMRI activity in the posterior midline region (including precuneus and posterior cingulate) was greater when memory retrieval performance was better [Daselaar et al.,2009]. In their same subjects, that study's authors found that successful memory encoding resulted in decreased activity relative to baseline in that region [Daselaar et al.,2009] consistent with the other studies [Duverne et al.,2009; Miller et al.,2008].
Although age was associated with increases in striatal FMT signal in the current study and previously in nonhuman primates, due to differences in the PET tracers, age may still be associated with decreases in signal for the more common tracer, FDOPA. Such divergent results between the two tracers were demonstrated previously using both tracers in the same aging nonhuman primates [Dejesus et al.,2001]. While the effect of age on FDOPA uptake has been tested extensively in humans previously, with inconsistent results [Bhatt et al.,1991; Cordes et al.,1994; Eidelberg et al.,1993; Ishikawa et al.,1996; Kumakura et al.,2008; Martin et al.,1989; Sawle et al.,1990], the study of the effect of age on FMT uptake in humans has been more limited [Braskie et al.,2008]. However, like a previous study in nonhuman primates [Dejesus et al.,2001], we found an increase in striatal FMT signal with age. We suggest that this increased signal represents nonoptimal available dopamine, as discussed previously [Braskie et al.,2008].
The relationships between fMRI deactivation and both FMT signal and memory performance were specific to younger adults. There are several possible (and potentially overlapping) explanations for our results. First, age‐related loss of white matter integrity [Sullivan et al.,2008] in pathways recruited for signaling to DCA or precuneus may interfere with the relationships between dopamine signaling and fMRI activity or cognition in older adults. It also may result in the dedifferentiation of fMRI activity that has been proposed previously in some studies [Li et al.,2001; Rajah and D'Esposito,2005] to explain age‐related regional increases in fMRI activity. Loss of white matter integrity could explain why our older adults (who overall showed little or no PCC/Pc task‐induced deactivations) still performed worse than their younger counterparts even though in younger adults, diminished task‐induced PCC/Pc deactivation was associated with better performance. Second, we previously hypothesized that higher FMT levels represented nonoptimal dopamine processing [Braskie et al.,2008]. Compared with younger adults, these high FMT signal levels in older adults may be less representative of dopaminergic signaling, potentially weakening the relationship between FMT signal and fMRI activity. For instance, AADC activity upregulation in response to deficits in dopaminergic signaling [Hadjiconstantinou et al.,1993] in older adults may not be successful, adding variability to the FMT‐dopamine relationship. Finally, in older adults, factors other than dopamine signaling may create enough variability in memory ability and/or fMRI activity that they are no longer closely correlated with FMT signal. Such factors could include changes in other neurotransmitter systems, varying degrees of brain atrophy, and subclinical levels of disease‐related pathology in some apparently normal older adults.
During load‐dependent cue and delay, older adults showed greater fMRI activity than younger adults in a number of regions (including DLPFC during load‐dependent delay), consistent with some previous studies [Cabeza et al.,2004; Emery et al.,2008; Rajah and D'Esposito,2005]. Although we did not detect effects of FMT signal in the regions of PFC in which fMRI signal was different between age groups, it is still possible that FMT signal and PFC fMRI activity were correlated in regions not demonstrating age‐related fMRI activity differences. Despite the probe phase age differences that we found in fMRI activity overall, load‐dependent probe phase fMRI activity was not significantly different between age groups. This suggests that the age‐related differences we found in probe phase fMRI activity were associated with an aspect of the task that was similar in the high and low load conditions. Of such possible task components, those that could conceivably relate to task performance and also have been shown previously to recruit PCC or precuneus include attentional control [Wagner et al.,2005], memory‐related imagery [Fletcher et al.,1995], and motor planning [Astafiev et al.,2003].
It is difficult to compare our fMRI results in PCC/Pc with those of other age‐related or dopamine‐related working memory studies because of the different methods employed. For instance, several studies evaluated relationships between dopaminergic activity and fMRI signal only in regions in which the task of interest elicited positive activation [Apud et al.,2007; Gibbs and D'Esposito,2005; Landau et al.,2009]. Likewise, of the few aging studies that separately examined cue, delay, and probe in a working memory study, two considered fMRI activity only in a priori ROIs [such as PFC; Park et al.,2003; Rypma et al.,2005]. A third focused mainly on a canonical variates analysis, and their supplementary statistical parametric mapping analysis utilized a very conservative voxel‐wise correction for multiple comparisons, yielding few age‐related differences [Zarahn et al.,2007].
FMT signal measures tonic dopamine synthesis capacity, but our working memory task requires phasic dopamine release (and associated fMRI activity) related to brain activity changes on a milliseconds scale. This raises the question of how FMT and fMRI signal were related given the different temporal dynamics of our measures. However, it is believed that tonic levels of dopamine can affect neural tuning at least in PFC. For instance, D1 receptor‐mediated tonic dopamine release is thought to set a firing threshold for PFC neurons during tasks requiring phasic release [Vijayraghavan et al.,2007]. Thus nonoptimal tonic dopamine release could interfere with modulation of the phasic dopamine release, and therefore with the best allocation of attentional resources to relevant stimuli. Further research focusing on PCC/Pc would be necessary to elucidate whether similar modulation occurs in that region as well.
Structural differences among older subjects and between older and younger subjects may affect the results of this study. For instance, variation in levels of atrophy may cause coregistration to a common template to be morphed more in some subjects. Although coregistrations were all visually checked for reasonableness, our results are limited by the extent to which the imaging software effectively coregistered the scans to a common template.
In our younger adults, FMT signal was significantly correlated with fMRI activity in PCC/Pc only during the probe phase of the Sternberg task. If our results did relate to attentional control, it is unclear why this correlation would be significant only during the probe phase, and not also during the cue phase, when encoding presumably also requires attention. It is possible that the results are truly specific to the probe phase and relate to a cognitive task other than attention. It is also possible that a correlation between FMT signal and fMRI activity exists in the cue phase, but is sub threshold and requires more power before detection is possible. Our a priori hypothesis was not specific to the probe phase; future studies having a greater number of young subjects would be helpful in verifying the specificity of our results.
Because our sample size of twelve useable younger adults was relatively small, it is possible that DCA FMT signal was related to fMRI signal in regions additional to PCC/Pc, but that we lacked the power to detect such relationships. However, our statistically significant results using nonparametric statistical tests appropriate for smaller sample sizes suggest that the results that we did find are unlikely to be due to chance. Future similar studies using larger sample sizes of younger adults would clarify whether our findings were specific to PCC/Pc.
CONCLUSION
In summary, of the regions showing age‐variant fMRI activity, only PCC/Pc activity was significantly correlated with DCA FMT signal, and only in younger adults. Additionally, greater task‐induced probe phase PCC/Pc fMRI deactivations were associated with poorer memory performance in younger adults, supporting our previous hypothesis that higher DCA FMT signal was associated with nonoptimal dopamine synthesis [Braskie et al.,2008]. Task‐induced deactivations in PCC/Pc are consistent with previous studies of the default network, and our results further suggest that dopamine synthesis plays a role in modulating that network in younger adults. We hypothesize that age‐related changes alter the relationship between striatal FMT signal and PCC/Pc fMRI activity during memory retrieval, pointing to the involvement of this system in the pathogenesis of age‐related working memory dysfunction.
Acknowledgements
The authors thank Ms. Jennifer Kluth and Ms. Amynta Hayenga for performing the neuropsychological testing related to this study.
REFERENCES
- Alexander GE,DeLong MR,Strick PL ( 1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381. [DOI] [PubMed] [Google Scholar]
- Apud JA,Mattay V,Chen J,Kolachana BS,Callicott JH,Rasetti R,Alce G,Iudicello JE,Akbar N,Egan MF,Goldberg TE,Weinberger DR ( 2007): Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology 32: 1011–1020. [DOI] [PubMed] [Google Scholar]
- Argyelan M,Carbon M,Ghilardi MF,Feigin A,Mattis P,Tang C,Dhawan V,Eidelberg D ( 2008): Dopaminergic suppression of brain deactivation responses during sequence learning. J Neurosci 28: 10687–10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev SV,Shulman GL,Stanley CM,Snyder AZ,Van Essen DC,Corbetta M ( 2003): Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci 23: 4689–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman L,Ginovart N,Dixon RA,Wahlin TB,Wahlin A,Halldin C,Farde L ( 2000): Age‐related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry 157: 635–637. [DOI] [PubMed] [Google Scholar]
- Beckmann CF,Jenkinson M,Smith SM ( 2003): General multilevel linear modeling for group analysis in FMRI. Neuroimage 20: 1052–1063. [DOI] [PubMed] [Google Scholar]
- Bhatt MH,Snow BJ,Martin WR,Pate BD,Ruth TJ,Calne DB ( 1991): Positron emission tomography suggests that the rate of progression of idiopathic parkinsonism is slow. Ann Neurol 29: 673–677. [DOI] [PubMed] [Google Scholar]
- Braskie MN,Wilcox CE,Landau SM,O'Neil JP,Baker SL,Madison CM,Kluth JT,Jagust WJ ( 2008): Relationship of striatal dopamine synthesis capacity to age and cognition. J Neurosci 28: 14320–14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck A,Aalto S,Nurmi E,Bergman J,Rinne JO ( 2005): Cortical 6‐[18F]fluoro‐L‐dopa uptake and frontal cognitive functions in early Parkinson's disease. Neurobiol Aging 26: 891–898. [DOI] [PubMed] [Google Scholar]
- Cabeza R ( 2002): Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging 17: 85–100. [DOI] [PubMed] [Google Scholar]
- Cabeza R,Daselaar SM,Dolcos F,Prince SE,Budde M,Nyberg L ( 2004): Task‐independent and task‐specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex 14: 364–375. [DOI] [PubMed] [Google Scholar]
- Cohen RL,Sandler SP,Schroeder K ( 1987): Aging and memory for words and action events: Effects of item repetition and list length. Psychol Aging 2: 280–285. [DOI] [PubMed] [Google Scholar]
- Cools R,Gibbs SE,Miyakawa A,Jagust W,D'Esposito M ( 2008): Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci 28: 1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes M,Snow BJ,Cooper S,Schulzer M,Pate BD,Ruth TJ,Calne DB ( 1994): Age‐dependent decline of nigrostriatal dopaminergic function: A positron emission tomographic study of grandparents and their grandchildren. Ann Neurol 36: 667–670. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS,Beckmann CF,Arigita EJ,Barkhof F,Scheltens P,Stam CJ,Smith SM,Rombouts SA ( 2008): Reduced resting‐state brain activity in the “default network” in normal aging. Cereb Cortex 18: 1856–1864. [DOI] [PubMed] [Google Scholar]
- Daselaar SM,Prince SE,Dennis NA,Hayes SM,Kim H,Cabeza R ( 2009): Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front Hum Neurosci 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CR,Wood SJ,Anderson V,Buchanan JA,Proffitt TM,Mahony K,Pantelis C ( 2003): Normative data from the CANTAB. I: Development of executive function over the lifespan. J Clin Exp Neuropsychol 25: 242–254. [DOI] [PubMed] [Google Scholar]
- Dejesus OT,Endres CJ,Shelton SE,Nickles RJ,Holden JE ( 2001): Noninvasive assessment of aromatic l‐amino acid decarboxylase activity in aging rhesus monkey brain in vivo. Synapse 39: 58–63. [DOI] [PubMed] [Google Scholar]
- Duverne S,Motamedinia S,Rugg MD ( 2009): The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb Cortex 19: 733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelberg D,Takikawa S,Dhawan V,Chaly T,Robeson W,Dahl R,Margouleff D,Moeller JR,Patlak CS,Fahn S ( 1993): Striatal 18F‐dopa uptake: Absence of an aging effect. J Cereb Blood Flow Metab 13: 881–888. [DOI] [PubMed] [Google Scholar]
- Emery L,Heaven TJ,Paxton JL,Braver TS ( 2008): Age‐related changes in neural activity during performance matched working memory manipulation. Neuroimage 42: 1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres CJ,Swaminathan S,DeJesus OT,Sievert M,Ruoho AE,Murali D,Rommelfanger SG,Holden JE ( 1997): Affinities of dopamine analogs for monoamine granular and plasma membrane transporters: Implications for PET dopamine studies. Life Sci 60: 2399–2406. [DOI] [PubMed] [Google Scholar]
- Fletcher PC,Frith CD,Baker SC,Shallice T,Frackowiak RS,Dolan RJ ( 1995): The mind's eye—Precuneus activation in memory‐related imagery. Neuroimage 2: 195–200. [DOI] [PubMed] [Google Scholar]
- Gazzaley A,Cooney JW,Rissman J,D'Esposito M ( 2005): Top‐down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci 8: 1298–1300. [DOI] [PubMed] [Google Scholar]
- Gibbs SE,D'Esposito M ( 2005): Individual capacity differences predict working memory performance and prefrontal activity following dopamine receptor stimulation. Cogn Affect Behav Neurosci 5: 212–221. [DOI] [PubMed] [Google Scholar]
- Grady CL,Springer MV,Hongwanishkul D,McIntosh AR,Winocur G ( 2006): Age‐related changes in brain activity across the adult lifespan. J Cogn Neurosci 18: 227–241. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M,Wemlinger TA,Sylvia CP,Hubble JP,Neff NH ( 1993): Aromatic l‐amino acid decarboxylase activity of mouse striatum is modulated via dopamine receptors. J Neurochem 60: 2175–2180. [DOI] [PubMed] [Google Scholar]
- Hampson M,Driesen NR,Skudlarski P,Gore JC,Constable RT ( 2006): Brain connectivity related to working memory performance. J Neurosci 26: 13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ,Yucel M,Pujol J,Pantelis C ( 2007): Task‐induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr Res 91: 82–86. [DOI] [PubMed] [Google Scholar]
- Ishikawa T,Dhawan V,Kazumata K,Chaly T,Mandel F,Neumeyer J,Margouleff C,Babchyck B,Zanzi I,Eidelberg D ( 1996): Comparative nigrostriatal dopaminergic imaging with iodine‐123‐beta CIT‐FP/SPECT and fluorine‐18‐FDOPA/PET. J Nucl Med 37: 1760–1765. [PubMed] [Google Scholar]
- Jenkinson M,Bannister P,Brady M,Smith S ( 2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841. [DOI] [PubMed] [Google Scholar]
- Kaasinen V,Vilkman H,Hietala J,Nagren K,Helenius H,Olsson H,Farde L,Rinne J ( 2000): Age‐related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging 21: 683–688. [DOI] [PubMed] [Google Scholar]
- Kelly C,de Zubicaray G,Di Martino A,Copland DA,Reiss PT,Klein DF,Castellanos FX,Milham MP,McMahon K ( 2009): L‐dopa modulates functional connectivity in striatal cognitive and motor networks: A double‐blind placebo‐controlled study. J Neurosci 29: 7364–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ,Zhong XH,Hornykiewicz O,Haycock JW ( 1995): Striatal 3,4‐dihydroxyphenylalanine decarboxylase in aging: Disparity between postmortem and positron emission tomography studies? Ann Neurol 38: 260–264. [DOI] [PubMed] [Google Scholar]
- Kumakura Y,Vernaleken I,Buchholz HG,Borghammer P,Danielsen E,Grunder G,Heinz A,Bartenstein P,Cumming P ( 2010): Age‐dependent decline of steady state dopamine storage capacity of human brain: An FDOPA PET study. Neurobiol Aging 31: 447–463. [DOI] [PubMed] [Google Scholar]
- Landau SM,Lal R,O'Neil JP,Baker S,Jagust WJ ( 2009): Striatal dopamine and working memory. Cereb Cortex 19: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC,Lindenberger U,Sikstrom S ( 2001): Aging cognition: From neuromodulation to representation. Trends Cogn Sci 5: 479–486. [DOI] [PubMed] [Google Scholar]
- Lustig C,Snyder AZ,Bhakta M,O'Brien KC,McAvoy M,Raichle ME,Morris JC,Buckner RL ( 2003): Functional deactivations: Change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA 100: 14504–14509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR,Palmer MR,Patlak CS,Calne DB ( 1989): Nigrostriatal function in humans studied with positron emission tomography. Ann Neurol 26: 535–542. [DOI] [PubMed] [Google Scholar]
- Mattay VS,Fera F,Tessitore A,Hariri AR,Berman KF,Das S,Meyer‐Lindenberg A,Goldberg TE,Callicott JH,Weinberger DR ( 2006): Neurophysiological correlates of age‐related changes in working memory capacity. Neurosci Lett 392: 32–37. [DOI] [PubMed] [Google Scholar]
- Mawlawi O,Martinez D,Slifstein M,Broft A,Chatterjee R,Hwang DR,Huang Y,Simpson N,Ngo K,Van Heertum R, et al. ( 2001): Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21: 1034–1057. [DOI] [PubMed] [Google Scholar]
- McKiernan KA,Kaufman JN,Kucera‐Thompson J,Binder JR ( 2003): A parametric manipulation of factors affecting task‐induced deactivation in functional neuroimaging. J Cogn Neurosci 15: 394–408. [DOI] [PubMed] [Google Scholar]
- Meltzer CC,Kinahan PE,Greer PJ,Nichols TE,Comtat C,Cantwell MN,Lin MP,Price JC ( 1999): Comparative evaluation of MR‐based partial‐volume correction schemes for PET. J Nucl Med 40: 2053–2065. [PubMed] [Google Scholar]
- Miller SL,Celone K,DePeau K,Diamond E,Dickerson BC,Rentz D,Pihlajamaki M,Sperling RA ( 2008): Age‐related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA 105: 2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano‐Saito A,Leyton M,Monchi O,Goldberg YK,He Y,Dagher A ( 2008): Dopamine depletion impairs frontostriatal functional connectivity during a set‐shifting task. J Neurosci 28: 3697–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC,Welsh RC,Marshuetz C,Gutchess AH,Mikels J,Polk TA,Noll DC,Taylor SF ( 2003): Working memory for complex scenes: Age differences in frontal and hippocampal activations. J Cogn Neurosci 15: 1122–1134. [DOI] [PubMed] [Google Scholar]
- Patlak CS,Blasberg RG ( 1985): Graphical evaluation of blood‐to‐brain transfer constants from multiple‐time uptake data. Generalizations. J Cereb Blood Flow Metab 5: 584–590. [DOI] [PubMed] [Google Scholar]
- Persson J,Lustig C,Nelson JK,Reuter‐Lorenz PA ( 2007): Age differences in deactivation: A link to cognitive control? J Cogn Neurosci 19: 1021–1032. [DOI] [PubMed] [Google Scholar]
- Rajah MN,D'Esposito M ( 2005): Region‐specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain 128: 1964–1983. [DOI] [PubMed] [Google Scholar]
- Rinne JO,Lonnberg P,Marjamaki P ( 1990): Age‐dependent decline in human brain dopamine D1 and D2 receptors. Brain Res 508: 349–352. [DOI] [PubMed] [Google Scholar]
- Rypma B,Berger JS,Genova HM,Rebbechi D,D'Esposito M ( 2005): Dissociating age‐related changes in cognitive strategy and neural efficiency using event‐related fMRI. Cortex 41: 582–594. [DOI] [PubMed] [Google Scholar]
- Sambataro F,Murty VP,Callicott JH,Tan HY,Das S,Weinberger DR,Mattay VS ( 2010): Age‐related alterations in default mode network: Impact on working memory performance. Neurobiol Aging. 31: 839–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawle GV,Colebatch JG,Shah A,Brooks DJ,Marsden CD,Frackowiak RS ( 1990): Striatal function in normal aging: Implications for Parkinson's disease. Ann Neurol 28: 799–804. [DOI] [PubMed] [Google Scholar]
- Schemper M ( 1991): Non‐parametric partial association revisited. Statistician 40: 73–76. [Google Scholar]
- Schmahmann JD,Pandya DN ( 1990): Anatomical investigation of projections from thalamus to posterior parietal cortex in the rhesus monkey: A WGA‐HRP and fluorescent tracer study. J Comp Neurol 295: 299–326. [DOI] [PubMed] [Google Scholar]
- Seeman P,Bzowej NH,Guan HC,Bergeron C,Becker LE,Reynolds GP,Bird ED,Riederer P,Jellinger K,Watanabe S, et al. ( 1987): Human brain dopamine receptors in children and aging adults. Synapse 1: 399–404. [DOI] [PubMed] [Google Scholar]
- Severson JA,Marcusson J,Winblad B,Finch CE ( 1982): Age‐correlated loss of dopaminergic binding sites in human basal ganglia. J Neurochem 39: 1623–1631. [DOI] [PubMed] [Google Scholar]
- Smith SM ( 2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossi V,de La Fuente‐Fernandez R,Holden JE,Doudet DJ,McKenzie J,Stoessl AJ,Ruth TJ ( 2002): Increase in dopamine turnover occurs early in Parkinson's disease: Evidence from a new modeling approach to PET 18 F‐fluorodopa data. J Cereb Blood Flow Metab 22: 232–239. [DOI] [PubMed] [Google Scholar]
- Sternberg S ( 1966): High‐speed scanning in human memory. Science 153: 652–654. [DOI] [PubMed] [Google Scholar]
- Sullivan EV,Rohlfing T,Pfefferbaum A ( 2010): Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: Relations to timed performance. Neurobiol Aging 31: 464–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinaz S,Schendan HE,Stern CE ( 2008): Fronto‐striatal deficit in Parkinson's disease during semantic event sequencing. Neurobiol Aging 29: 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D,Volkow ND,Wang R,Telang F,Wang GJ,Chang L,Ernst T,Fowler JS ( 2009): Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One 4: e6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBrocklin HF,Blagoev M,Hoepping A,O'Neil JP,Klose M,Schubiger PA,Ametamey S ( 2004): A new precursor for the preparation of 6‐[18F]Fluoro‐L‐m‐tyrosine ([18F]FMT): Efficient synthesis and comparison of radiolabeling. Appl Radiat Isot 61: 1289–1294. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S,Wang M,Birnbaum SG,Williams GV,Arnsten AF ( 2007): Inverted‐U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci 10: 376–384. [DOI] [PubMed] [Google Scholar]
- Vingerhoets FJ,Snow BJ,Schulzer M,Morrison S,Ruth TJ,Holden JE,Cooper S,Calne DB ( 1994): Reproducibility of fluorine‐18‐6‐fluorodopa positron emission tomography in normal human subjects. J Nucl Med 35: 18–24. [PubMed] [Google Scholar]
- Volkow ND,Wang GJ,Fowler JS,Logan J,Gatley SJ,MacGregor RR,Schlyer DJ,Hitzemann R,Wolf AP ( 1996): Measuring age‐related changes in dopamine D2 receptors with 11C‐raclopride and 18F‐N‐methylspiroperidol. Psychiatry Res 67: 11–16. [DOI] [PubMed] [Google Scholar]
- Volkow ND,Gur RC,Wang GJ,Fowler JS,Moberg PJ,Ding YS,Hitzemann R,Smith G,Logan J ( 1998): Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 155: 344–349. [DOI] [PubMed] [Google Scholar]
- Wagner AD,Shannon BJ,Kahn I,Buckner RL ( 2005): Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9: 445–453. [DOI] [PubMed] [Google Scholar]
- Weickert CS,Webster MJ,Gondipalli P,Rothmond D,Fatula RJ,Herman MM,Kleinman JE,Akil M ( 2007): Postnatal alterations in dopaminergic markers in the human prefrontal cortex. Neuroscience 144: 1109–1119. [DOI] [PubMed] [Google Scholar]
- Woolrich MW,Ripley BD,Brady M,Smith SM ( 2001): Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14: 1370–1386. [DOI] [PubMed] [Google Scholar]
- Zarahn E,Rakitin B,Abela D,Flynn J,Stern Y ( 2007): Age‐related changes in brain activation during a delayed item recognition task. Neurobiol Aging 28: 784–798. [DOI] [PubMed] [Google Scholar]
- Zhang Y,Brady M,Smith S ( 2001): Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Trans Med Imaging 20: 45–57. [DOI] [PubMed] [Google Scholar]


