Abstract
While the stimulatory effect of alcohol on the rat hypothalamic-pituitary-adrenal (HPA) axis is well known, the mechanisms underlying this influence remain poorly understood. Here, we tested the hypothesis that brain catecholamines play an important role in this response. As expected, the acute intragastric administration of alcohol to adult male rats elevated plasma ACTH levels and activated hypothalamic corticotropin-releasing factor neurons. Novel findings pertain to the effect of alcohol on, and the role played by, brain adrenergic circuits. We first observed that alcohol upregulated c-fos signals in the locus coeruleus (LC), the main noradrenergic brain cell group; and that it activated (nor)adrenergic medullary cells called A1–A2/C1–C3. Evidence for the role played by these catecholaminergic circuits then came from the observation that blockade of α1-, but not β-, adrenergic receptors interfered with alcohol-induced ACTH secretion; and that depletion of catecholaminergic input to the PVN by the toxin 6-hydroxydopamine, significantly decreased the ACTH response to alcohol. Finally, destruction of the A1–A2/C1–C3 region with the immunotoxin anti-DBH-saporin interfered with the catecholaminergic input to the PVN. Collectively, our work extends our knowledge of the ability of this drug to upregulate catecholamine circuitry in the rat brain. It also shows that medullary catecholamine innervation of the hypothalamus plays an important role in modulating the stimulatory effect of alcohol on the HPA axis, an effect exerted through activation of α1-adrenergic receptors.
Keywords: alcohol, norepinephrine, corticotropin-releasing factor, hypothalamus, brain stem
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis plays a critical role in the organism’s response to stressors. It comprises neurons in the paraventricular nucleus (PVN) of the hypothalamus that manufacture corticotropin-releasing factor (CRF), which upon release from the median eminence, induces ACTH release (1–4). The HPA axis is activated by many stimuli, including alcohol (5), and the PVN plays a critical role in this response (6, 7). However, the mechanisms through which this drug exerts its influence on the HPA axis remain surprisingly elusive.
While in isolated systems, alcohol acts directly on the CRF promoter through cAMP-PKA-dependent pathways (8), its effect in intact animals likely involves intermediates. There is evidence that alcohol releases dopamine (9) and stimulates fos signals in catecholamine-rich brain regions [see for example (10–12)]. However, with the exception of nitric oxide (13), the physiological importance of specific modulators is unknown. Here we focused on norepinephrine, a bioamine that stimulates the PVN (14, 15) through activation of α1-adenergic receptors, and whose release within this nucleus is critical for many HPA axis responses to stressors (16). Aminergic pathways to the PVN originate from the lower brain stem in the ventrolateral medulla, the nucleus of the solitary tract (NTS) and the locus coeruleus (LC). As this latter only provides sparse direct innervation to the PVN (17), its influence on the HPA axis response to stressors is thought to involve innervation of the prefrontal cortex (18, 19), while the NTS and the related A1–A2/C1–C3 cell groups represent the most important direct catecholaminergic input (2, 20, 21). However, at present, the importance of adrenergic circuits in mediating the HPA axis response to alcohol remains largely untested. Importantly, because categorically distinct stressors stimulate the HPA axis through different circuits and mechanisms (22), results obtained with other stimuli cannot automatically be considered relevant during alcohol exposure. We therefore first evaluated the effect of this drug on medulla noradrenergic cells and on afferent circuits to the PVN, using freely-moving rats bearing intragastric (ig) cannulae (23). This protocol has the advantage over many previous studies, that alcohol is administered without the stress of handling the animals and is delivered to the stomach rather than the abdominal cavity, which can release pro-inflammatory cytokines whose effect on the HPA axis (24) interferes with the interpretation of the results. We then investigated the role played by adrenergic-dependent pathways with type-specific adrenergic receptor antagonists, with specific emphasis on the α1 subtype (14, 16). Finally, we used the toxins 6-hydroxydopamine (6-OHDA) to destroy the catecholaminergic input provided to the PVN by the ascending bundle, and anti-DBH-saporin to eliminate >65% DBH-PNMT staining in the A1–A2/C1–C3 regions [see (18, 25–28)]. Collectively, our results demonstrate a hitherto unreported response of the A1–A2/C1–C3 regions to alcohol and show that catecholamines in general, and the brain stem in particular, regulate the HPA axis response to this drug through activation of α1-adrenergic receptors.
Materials and Methods
Animals
Male Sprague-Dawley rats, aged 60–65 days at the time of the experiments, were kept under standard lighting regimens (12 h lights on:12 h lights off, lights on at 0630) and provided rat chow and water ad libitum. The animals were housed individually after surgery. All protocols were approved by the Salk Institute IACUC.
Procedures
Surgeries and assays
Permanent indwelling ig, intravenous (iv) and/or intracerebroventricular (icv) cannulae were implanted under ketamine/acepromazine/xylazine (icv) or isoflurane (iv or ig) anesthesia as previously described (29, 30). Icv and ig cannulae were put in place 7–8 days, and iv cannulae 2 days prior to the experiments. Correct placement of the icv or microinfusion cannulae was checked in coronal sections of the brains after injections of dye, and only rats with correct placement were included in the statistical analysis. On the day of the experiment, the rats were individually transferred from their home cages to individual opaque, plastic buckets. Their icv, iv and/or ig cannulae were connected to tubing that extended through a hole in the top of the bucket and attached to syringes for injection of test materials and/or blood withdrawal. These procedures avoid subjecting the animals to handling stress during the experiment. A 2.5–3 h rest was then allowed before the experiments started between 0930 and 1000. Blood volumes withdrawn were 0.35 ml, and were replaced with apyrogenic saline.
Alcohol injection
Alcohol was diluted with water to ≤18% v/v and slowly injected via the ig cannulae over a 90 sec period into freely-moving, non-handled animals. The dose, 4.5 g/kg, was chosen because in our hands, it induces the consistent endocrine responses required in our protocols. While the volumes used depended on the animals' weights, they averaged 5.0 ml. As controls were administered a comparable volume, gastric distension is not believed to contribute to the ability of alcohol itself to induce brain stem activation. Changes in blood alcohol levels observed under these conditions were 257.9 ± 20.0 mg% 2 hrs after alcohol ig injection, which corresponds to those we previously reported (23, 31). While the animals appeared somewhat intoxicated, they remained conscious and ambulatory. Controls were injected with the vehicle.
Reagents
Anti-dopamine-B-hydroxylase (DBH)-saporin was purchased from Advanced Targeting Systems (San Diego, CA). Six-hydroxydopamine, phenoxybenzamine, propranolol and corynanthine were purchased from Sigma-Aldrich (St. Louis, MO). Phenoxybenzamine was first dissolved in DMSO, then in 0.04 M PBS, pH 7.4, while all other reagents were dissolved directly in PBS (for systemic injection) or apyrogenic water (for icv injections). Absolute, reagent grade alcohol was purchased from Aaper EtOH and Chemical Co. (Shelbyville, KY). R/hCRF, synthesized as previously described (32), was generously provided by Dr. Jean Rivier (The Salk Institute, La Jolla, CA). It was dissolved in 0.04 M PBS, pH 7.4, that contained 0.1% crystalline BSA and 0.01% ascorbic acid.
Hormone assays
ACTH
Plasma ACTH levels were determined by a commercially available two-site immunoradiometric assay from DiaSorin Inc. (Stillwater, MN). The lower detection limit of this assay is 15 pg/ml and samples in which ACTH levels were less than 15 pg/ml were assigned that value for statistical analysis. The intra- and inter-assay coefficients of variation are 7 and <15%, respectively.
Corticosterone
Plasma corticosterone levels were determined using a commercially available radioimmunoassay kit (MP Biomedicals, Orangeburg, NY). The lowest limit of detectability is 5 ng/ml. Intra- and interassay coefficients of variation are 7.3 and 13.2%, respectively.
Catecholamine blockade
Antagonists
An array of compounds blocking α-adrenergic receptors is available, including prazosin, phentolamine and phenoxybenzamine. In our hands, the α-adrenergic antagonist prazosin, albeit widely used, produces a strong elevation of basal plasma ACTH levels, which probably at least in part results from significant changes in blood pressure and the neuronal activity of the related catecholaminergic afferents to the PVN (33). We therefore used phenoxybenzamine instead, a non-reversible general α-adrenergic antagonist (34) (10 mg/kg, iv - 3 h) that penetrates the brain following systemic injection (35) and only induces a slight increase in basal ACTH release (C. Rivier, unpublished and current work). Specific blockade of α1- and β-adrenergic receptors was achieved with corynanthine and propranolol, respectively, at doses and regimens chosen from the published literature (14, 29, 36). These were 150 µg icv/rat for corynanthine and 2.5 mg/kg iv for propranolol, all injected 45 min prior to alcohol or its vehicle.
Chemical lesions
Destruction of PVN catecholamine terminals was done by injecting 6-OHDA dissolved in sterile apyrogenic water with 0.2% ascorbic acid. As 6-OHDA does not alter serotonin levels, it is considered a specific neurotoxin for catecholamine (noradrenergic and dopamine) cells. Also, it displays a higher regional specificity for LC axons than for lateral tegmental noradrenergic neurons (28). We used a 100 µg dose icv, which does not alter behavior and, in our hands as well as those of others, depletes synaptic norepinephrine as well as dopamine levels by ≥90% (29, 37). The 100 µg dose (or vehicle for control rats) was administered into the right ventricle daily for two consecutive days at the rate of 1 µl/20 µg/10 sec (total volume: 5 µl), and the animals were used 48 h later. Only animals with lesions determined to have destroyed ≥90% of catecholaminergic terminals [measured by decreases in levels of NE and tyrosine hydroxylase (TH) immunoreactivity in the PVN as described in (37)], were used in the statistical analysis of the data.
Immunological lesions
As 6-OHDA can also destroy dopaminergic neurons (38–41), we also used the axonally transported immunotoxin anti-dopamine-β-hydroxylase-saporin (anti-DBH-saporin), which is an antibody to DBH (the enzyme responsible for NE synthesis) coupled to saporin. This toxin, which arrests protein synthesis in the neuronal cell body, specifically eliminates noradrenergic as well as adrenergic terminals and their neurons while preserving dopaminergic and cholinergic cell bodies (26). The toxin was microinfused directly in the A1/C1 and A2-C2/C3 region of the brain stem (see details below) in order to provide more discrete lesions that those caused by icv treatment, which also destroys noradrenergic neurons of the LC (42). And indeed, this approach indicated that the LC was spared, as illustrated by positive DBH staining of this region (data not shown). Preliminary experiments were conducted on the basis of published data [see for example (18, 25–28, 42–44)] to establish the dose of toxin required to eliminate ≥65% DBH/PNMT staining in the A1–A2/C1–C3 regions (see below). Also, while we conducted preliminary experiments to specifically target one area only (i.e., A1/C1, A2/C2 or C3), results were not satisfactory, probably because the shape of these nuclei precludes lesions that are both specific to one area, and complete for this area only. Consequently, the micropipette was first lowered into the A2/C2/C3 area, then into the A1/C1 area. We microinfused 60 nl (vehicle or 33 ng toxin) bilaterally via a micropipette (inner diameter, 10–20 mm) using a pressure ejection system (picospritzer II, World Precision Instruments) that delivered treatments at the following stereotaxic coordinates: A1/C1; anteriorposterior −10.5 mm, mediolateral ± 1.4 mm, dorsoventral −9.4 mm, A2/C2–C3; anteriorposterior −10.5 mm, mediolateral ± 0.4 mm, dorsoventral − 7.1 mm. Treatments were injected over 30 seconds, and the pipette was left in situ for 5 minutes following completion of the injection. Thereafter, the pipette was slowly removed, the incision was sutured and the animal was allowed to recover from anesthesia under a heat lamp. Effectiveness of lesions of the A/C cell groups was evaluated by measuring a significant (≥65%) reduction in the density of the DBH-immunoreactive (ir) innervation (i.e., terminal fields) of the PVN (18, 28, 45) as well as in the number of the ventrolateral A1/C1 and dorsomedial A2/C2/C3 neurons positively stained for DBH or PNMT (26).
Anti-DBH-saporin lesions both noradrenergic and adrenergic cells in the medulla. The approach exploits the fact that DBH on the inner surface of synaptic vesicles is exposed to the extracellular space during transmitter release, providing a target for antiserum to bind the enzyme, and toxin to be internalized, in the normal course of vesicle recycling. DBH converts dihydoxyphenylalanine to NE; immunolabeling for this enzyme represents both NE and E fibers and terminals, whereas staining for PNMT is specific to E. Immunohistochemical techniques were performed to show that these immunotoxin lesions did cause dramatic DBH cell loss in medullary cell groups. Also, DBH was used to identify E and NE fibers in the PVN [see for example (46)]. Since we are interested in the medullary C cell groups that project to the PVN and affect the HPA axis, we measured PNMT-ir cells on the C1, C2, and C3 areas after alcohol injection. Immunotoxin treatment produced a marked depletion of the aminergic innervation of the PVN and nor/adrenergic cell loss in medullary cell groups. The observation that some neurons survive even at high doses of the toxin, agrees with previously published reports (26–28). The animals were used 2 weeks later to allow maximum degeneration of aminergic inputs.
Immunohistochemistry
The animals were randomly divided into vehicle or alcohol injection groups (N = 5–6 each). All experiments were started between 0930 and 1000 following a 2.5 – 3 h period during which the rats were left undisturbed. The rats were sacrificed 2 h after the injection of the vehicle or alcohol (4.5 g/kg, ig), which our preliminary studies indicated as corresponding to peak protein responses. In a few select studies, we also included a 3 h time point to ensure that we did not miss delayed responses, but as this was not the case, these results are not included in the present work. The animals were deeply anesthetized with 35% chloral hydrate and then perfused transcardially with saline followed by 4% paraformaldehyde/0.1 M borate buffer, pH 9.5. The perfused brains were transferred to 10% sucrose in phosphate buffer overnight before sectioning. Brain sections were created by freezing sucrose-treated tissue with dry ice (−80°C) and mounting on a Leica microtome. Sections were cut in the coronal plane at 30 µm thickness and placed in cryoprotectant solution (50% 0.1M phosphate-buffered saline, 30% ethylene glycol, and 20% glycerol) until histochemical analysis. Each analysis consists of every 4th section from the PVN, locus coeruleus, and brain stem. Single DAB-labeled staining was performed on free-floating sections. Sections were rinsed in 0.1 M KPBS and then placed in 0.1% NaBH4 in KPBS. Residual NaBH4 was rinsed with KPBS. Sections were then incubated in mouse anti-DBH (1:1,000, Chemicon, Temecula, CA), mouse anti-TH (1:20,000, Novus Biologicals, Littleton, CO) or sheep anti-PNMT (1:7500, Chemicon, Temecula, CA) for 48 hr at 4°C. Sections were rinsed and placed in appropriate biotinylated secondary antibody (goat anti-rabbit, or rabbit anti-sheep 1:500, Vector Laboratories, Burlingame, CA) for 1 h at room temperature, then rinsed and incubated in avidin–biotin complex (ABC kit, Vectastain, Burlingame, CA) for 1 h at room temperature. After the incubation, sections were rinsed in KPBS and 0.1 M Sodium Acetate (NaOAc) and developed with a Ni-DAB solution and coverslipped. Double immunohistochemistry consisted of rabbit or goat anti-c-fos antibody (1:10,000, Calbiochem, San Diego, CA or Santa Cruz Biotechnology, Santa Cruz, CA) as the first primary antibody and one of the following antibodies as the second primary antibody [mouse anti-DBH (1:1,000, Chemicon, Temecula, CA), rabbit anti-CRF (1:13,000, RC-70, W. Vale, Salk Institute, La Jolla, CA) or mouse anti-TH (1:20,000, Novus Biologicals, Littleton, CO)]. Sections were rinsed and placed in appropriate biotinylated secondary antibody (goat anti-rabbit, goat anti-mouse, or rabbit anti-sheep 1:500, Vector Laboratories, Burlingame, CA) for 1 h at room temperature, rinsed, and incubated with avidin–biotin complex (ABC kit, Vectastain, Burlingame, CA) for 1 h at room temperature. Sections were then rinsed in KPBS and 0.1 M NaOAc. Staining for c-fos was done using Ni-DAB solution in a glucose oxidase reaction for 120–180 seconds. The sections were again rinsed in NaOAc and KPBS and then placed in blocking solution (3–5% normal serum) for 30 min and then incubated with appropriate second primary antibody (see above) for 24–48 hrs at 4°C. After rinsing, sections were incubated in appropriate secondary antibody, then in ABC complex at room temperature, 1 h each and rinsed with KPBS and 0.05 M Tris saline. Reaction was carried out in DAB–H2O2 solution for 120–180 sec. The sections were then rinsed in Tris-buffered saline and KPBS. Stained sections were then mounted on gelatin-coated sub slides, dehydrated, and cover slipped using DPX mounting media (Fluka Biochemika, Ronkonkoma, NY). Negative controls without primary or secondary antibody were included. The pictures were taken on a bright-field microscope at 100X magnification.
Quantification of immunohistochemistry
The PVN and the medullary sections were captured using a Coolsnap Photometrics digital video camera on a bright-field Nikon Eclipse microscope at 200X. The images were rendered using the Real Micro Color Capture program (CRI, Boston, MA). The resulting stains gave the first antibody (c-fos) black stain and the second antibody (DBH, CRF, TH or PNMT) brown stain. The black (c-fos) stain indicates activated nuclei whereas the brown DBH, CRF, TH, or PNMT-ir stain shows cytoplasmic signals. Immuno-labeled cells were counted using Image J 1.34s (NIH, Bethesda, MD) and edited using Canvas 8.0 (Deneba Systems, Miami, FL). The contrast and brightness were adjusted for optimum visualization, and the signals of each image were manually counted and those counts were adjusted for the background counts. Total number of cells in the PVN and the medulla were counted per section using a 20X dry objective, and the mean values were determined in 3 sections for each rat and for each brain region. The data was expressed as the number of Fos-positive cells colocalized with CRF, DBH or TH-positive cell bodies. For quantitative assessments of lesion effectiveness, measurements were done on the intact and lesioned slides. Bright-field photomicrographs of DBH-ir in the PVN were collected at 100X with the same camera and a microscope as above and saved as 1300 × 1030-pixel, 16-bit grayscale Tiff files. The Tiffs were analyzed by using the Image J, to estimate the areal fraction of the PVN occupied by DBH-ir fibers. The border of the PVN was traced to estimate its area in each section. Images were thresholded to the default auto-function and all particles in the PVN area were included and the data was averaged across 3 sections per animal.
Statistical Analysis
Data were analyzed by one- or two-way analysis of variance (ANOVA) followed by Newman-Keuls’ test or the least squares means test as a post-hoc test. Each value was expressed as the mean ± SEM, and statistical significance was accepted for P < 0.05.
Results
Influence of acute alcohol injection on hypothalamic and amygdala neurons
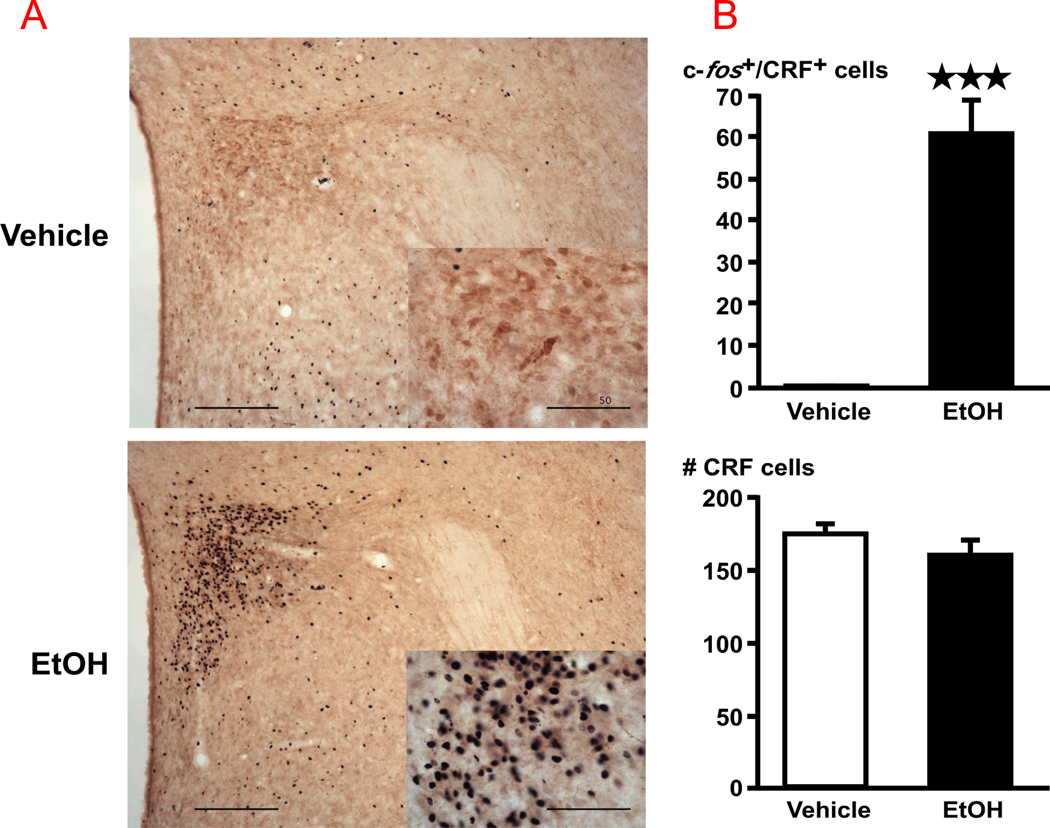
In vehicle-injected rats, CRF-ir neurons were found exclusively in the parvocellular part of the paraventricular hypothalamic nucleus (pPVN) (Fig. 1). Alcohol (EtOH) induced a significant (P<0.01) increase in c-fos signals in pPVN CRF, measured 2 h later. In addition, and in agreement with previous reports using other alcohol protocols (47, 48), we found that ig alcohol increased c-fos signals in the central nucleus of the amygdala (vehicle; 47.6 ± 21.5, alcohol, 136.8 ± 13.3, P < 0.01).
Figure 1.
(A) Acute alcohol (EtOH) increases c-fos levels in CRF-positive cells of the PVN. Bright-field photographs through the PVN from vehicle controls (top), and rats challenged with alcohol (bottom). Images show a representation of the double immunohistochemical techniques that stained c-fos-ir in black nuclei and CRF-ir in brown cytoplasm with a 10X dry objective (scale bar = 200 µm) and insets with a 40X objective (scale bar = 50 µm). (B) Cell counts were obtained for c-fos-ir and CRF-ir positive cells throughout the rostral-caudal extent of the PVN. Mean ± SEM levels of c-fos signals in CRF–immunoreactive cells in the PVN (top right) and total number of CRF cells (bottom right) of 5–6 rats injected with vehicle or EtOH. ***, P<0.001.
Ability of acute alcohol injection to activate catecholaminergic-rich brain stem/medullary regions
Noradrenergic A1–A2 and adrenergic C1–C3 cell groups
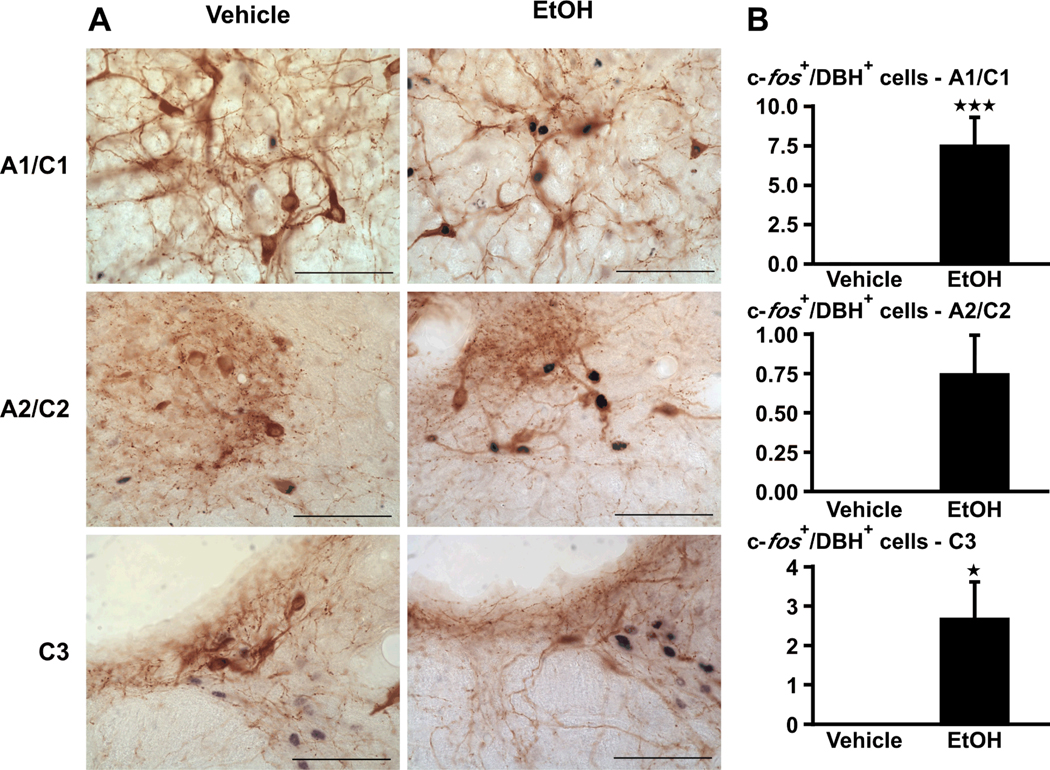
We focused on DBH, the enzyme that catalyzes the conversion of dopamine to noradrenaline and is expressed selectively in noradrenaline (A1–A2) and adrenaline (C1–C3) neurons. In these regions, alcohol (EtOH) significantly (P<0.01) increased c-fos levels in DBH-ir cells, measured 2 hr later (Fig. 2).
Figure 2.
(A) Acute alcohol (EtOH) increases c-fos signals in the A1–A2/C1–C3 cell region, identified by DBH. Bright-field photographs through the A1–A2/C1–C3 area of brain stem from vehicle controls (left), and rats challenged with alcohol (right). Images show a representation of the double immunohistochemistry that stained c-fos-ir in black and DBH-ir in brown (scale bar = 200 µm). (B) Cell counts were obtained for c-fos immunoreactivity in DBH-ir cells. Mean ± SEM levels of c-fos signals in DBH–ir cells in the A1/C1, A2/C2 and C3 area of 5–6 rats injected with vehicle or alcohol. *, P<0.05; ***, P<0.001.
LC
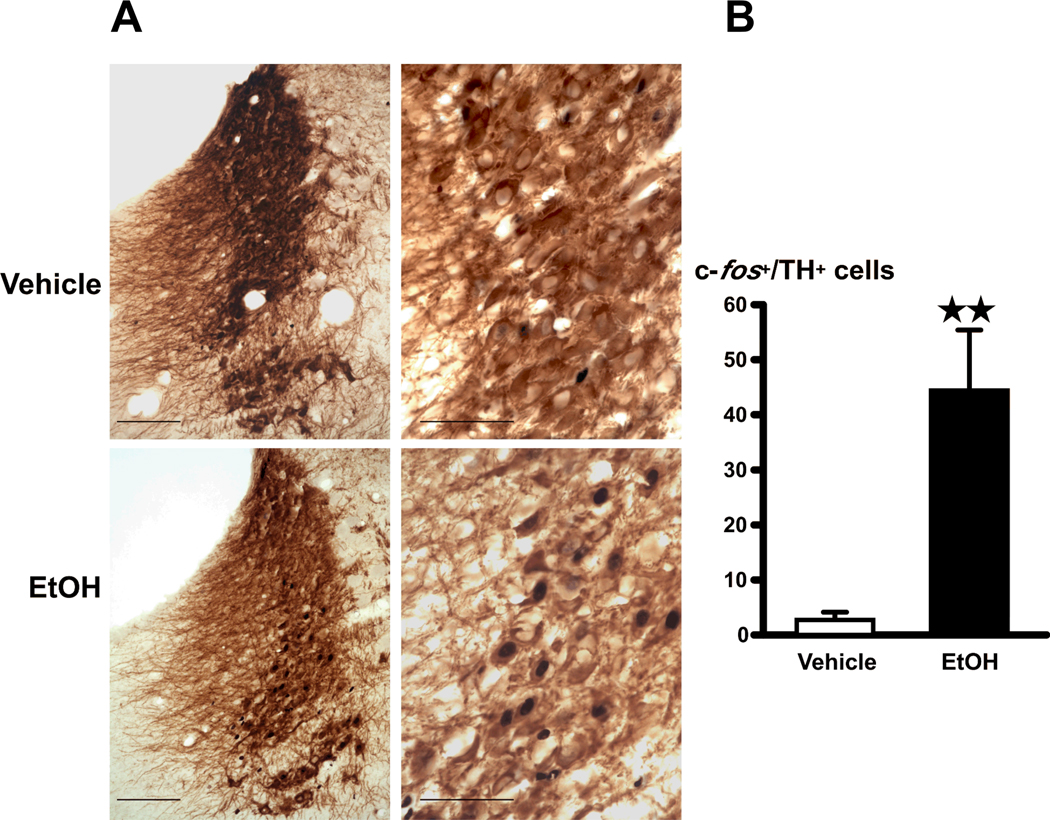
Previously published work reported increased c-fos signals in the LC of rats administered alcohol (10, 49). However, these investigators not only administered the drug intraperitoneally, which can cause cytokine release due to local inflammation, they also handled the animals during injection, which can cause an additional stress. Results obtained with this approach may therefore not be entirely due to the effect of alcohol itself. For these studies, we focused on TH, the rate limiting enzymes in biosynthesis of dopamine and norepinephrine. We show here that the stress-free injection of alcohol (EtOH) into the stomach of freely-moving rats significantly (P<0.01) upregulated c-fos signals in TH-immunoreactive cells in the LC (A6), measured 2 h later (Fig. 3).
Figure 3.
(A) Acute alcohol (EtOH) increases c-fos signals in the LC, identified by TH-positive cells. Bright-field photographs through the LC from vehicle controls (top), and rats challenged with alcohol (bottom) with a 20X objective (scale bar = 100 µm) (left) and a 60X objective (scale bar = 50 µm) (right). (B) Cell counts of c-fos positive nuclei in TH-positive cells in the LC. Mean ± SEM levels of c-fos positive signals in TH–immunoreactive cells in the LC of 5–6 rats injected with vehicle or alcohol (right). **, P<0.01.
Consequences of interfering with the catecholaminergic innervation of the PVN
Depletion of hypothalamic NE
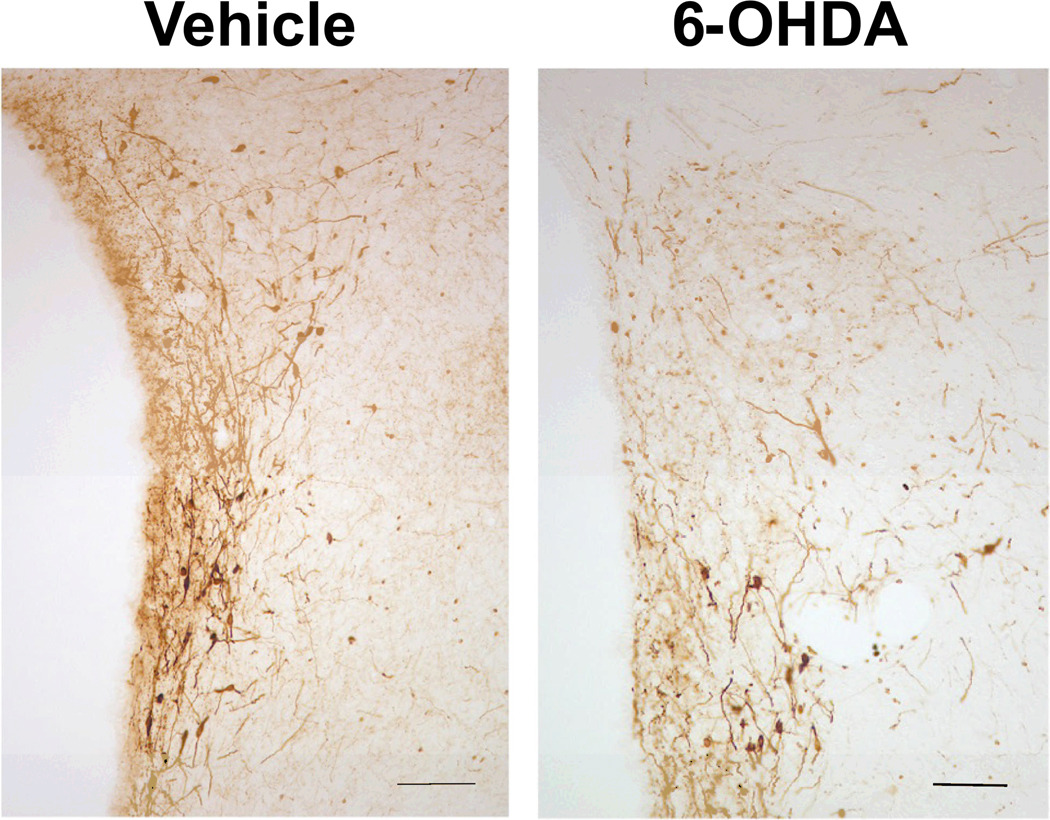
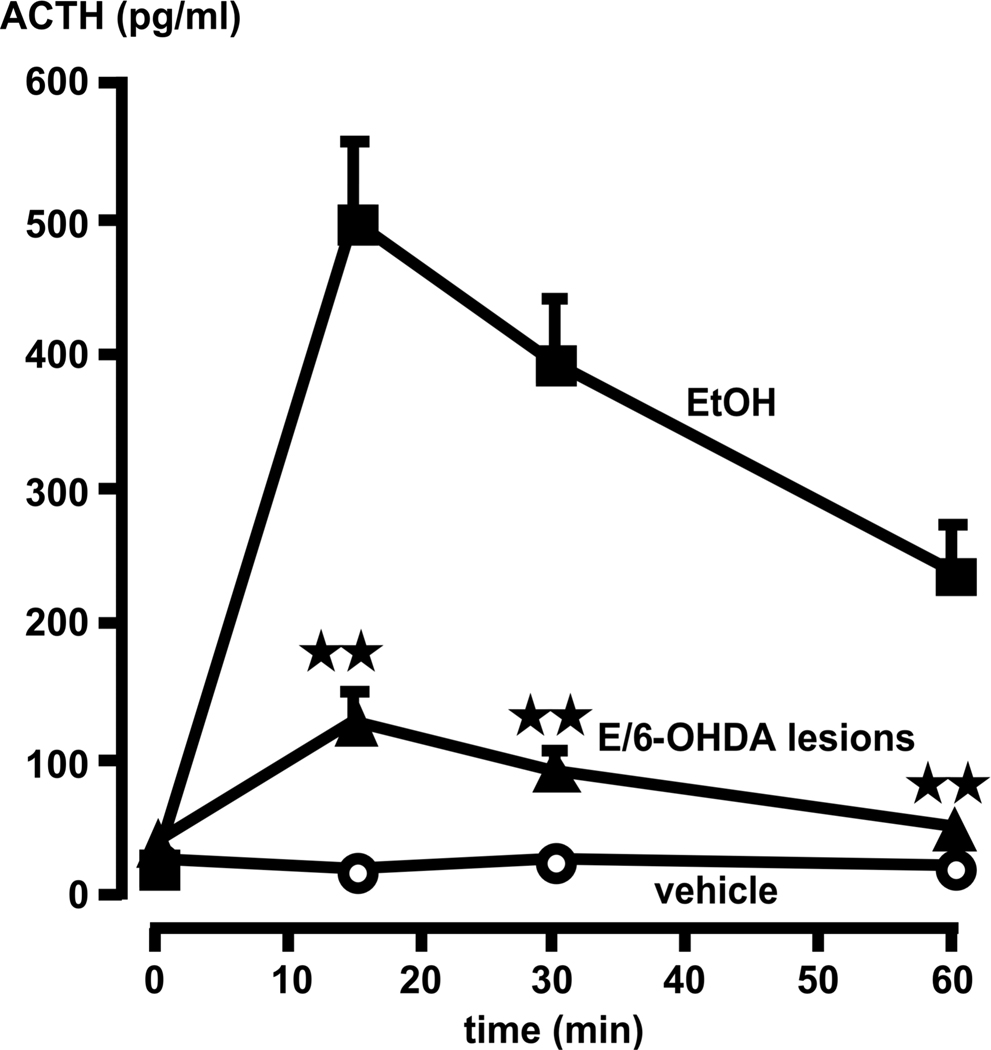
A representative illustration of this type of 6-OHDA lesion is presented in Fig. 4. In sham-lesioned rats, acute alcohol injection induced the expected rise in plasma ACTH levels (Fig. 5). The loss of noradrenergic terminals to the PVN (6-OHDA treatment) significantly (P<0.01), but not completely reduced this response. We also measured corticosterone levels even though the rapid saturation of this curve at relatively low ACTH values [see (50)] makes this steroid a less informative parameter of HPA axis activity because even relatively modest increases in ACTH release prompt large elevations in corticosterone levels. While basal corticosterone levels remained <40 ng/ml in all animals, peak values in alcohol-injected rats, measured 30 min post-drug, were 312 ± 43 ng/ml in the vehicle-pretreated group, and 157 ± 23 ng/ml in animals with 6-OHDA lesions.
Figure 4.
Representative bright-field photographs showing TH-ir fibers in the PVN of 6-OHDA treated rats, compared to those injected with the vehicle (scale bar = 100 µm).
Figure 5.
Compared to rats without lesions, 6-OHDA lesions significantly blunt the ACTH response to alcohol (EtOH). Time = 0 indicates plasma ACTH levels immediately prior to alcohol administration. Each point illustrates the means ± SEM of 5–7 rats. **, P<0.01 vs. ACTH response in corresponding EtOH intact controls.
Elimination of A1–A2/C1–C3 neurons
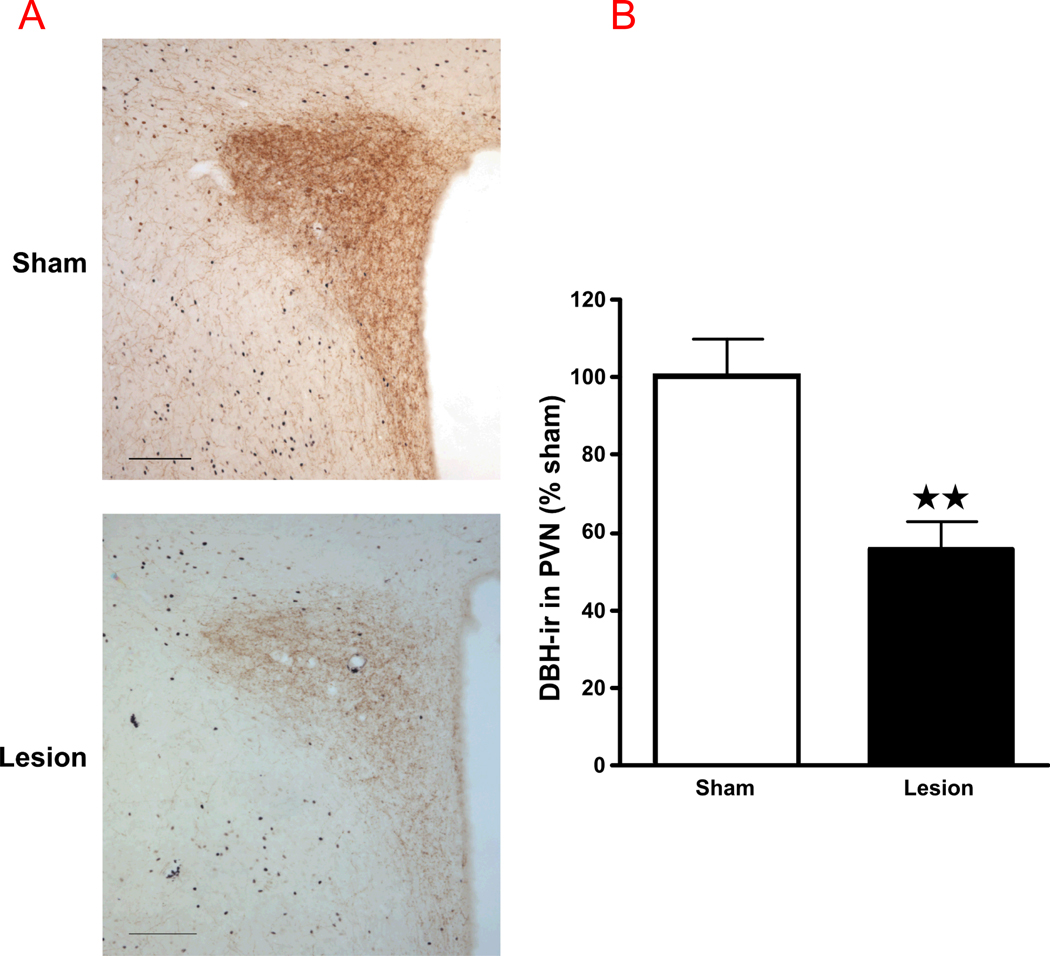
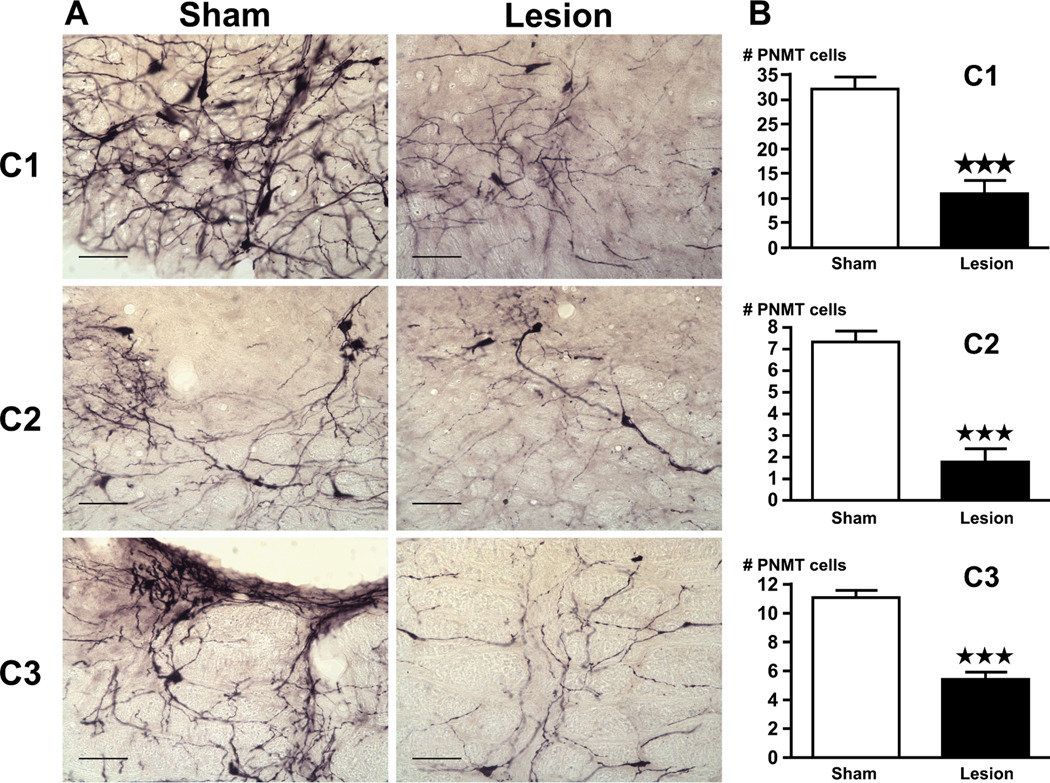
Compared to results obtained in rats that did not receive anti-DBH-saporin, the toxin induced the following changes in brain tissues obtained 2 hr after alcohol (EtOH) injection: they significantly decreased the density of DBH-ir fibers in the PVN (Fig 6), indicating the loss of catecholaminergic input to this hypothalamic region; and they also lowered the number of positive immunoreactive cells for PNMT (Fig. 7) in the C1–C3 region of the brain stem.
Figure 6.
(A) Anti-DBH-saporin lesions significantly decrease the number of DBH-positive fibers in the PVN, compared to sham-operated rats. Bright-field photographs through the PVN from sham controls (top) and lesion rats (bottom) challenged with alcohol. Images show a representation of the immunohistochemical procedures that stained DBH fibers in brown (scale bar = 200 µm). (B) The density of DBH-ir fibers in the PVN. Mean ± SEM density of DBH-ir fibers in the PVN of sham and lesion rats injected with alcohol. ***, P<0.001.
Figure 7.
(A) Lesions caused by the anti-DBH-saporin toxin significantly decreases the number of PNMT-positive cells in the C1–C3 cell groups, compared to rats pretreated with the vehicle. Bright-field photographs through the C1–C3 area of brain stem from sham controls (left) and lesion (right) rats challenged with alcohol. Images show a representation of the immunohistochemical techniques that stained PNMT-ir in purple (scale bar = 200 µm). (B) Cell counts were obtained for PNMT-ir positive cells in the C1, C2 and C3 area of the brain stem. Mean ± SEM levels of PNMT-ir cells in the C1–C3 area of sham and lesion rats injected with alcohol. ***, P<0.001.
Effect of blockade of specific adrenergic receptors on the ACTH response to alcohol (EtOH)
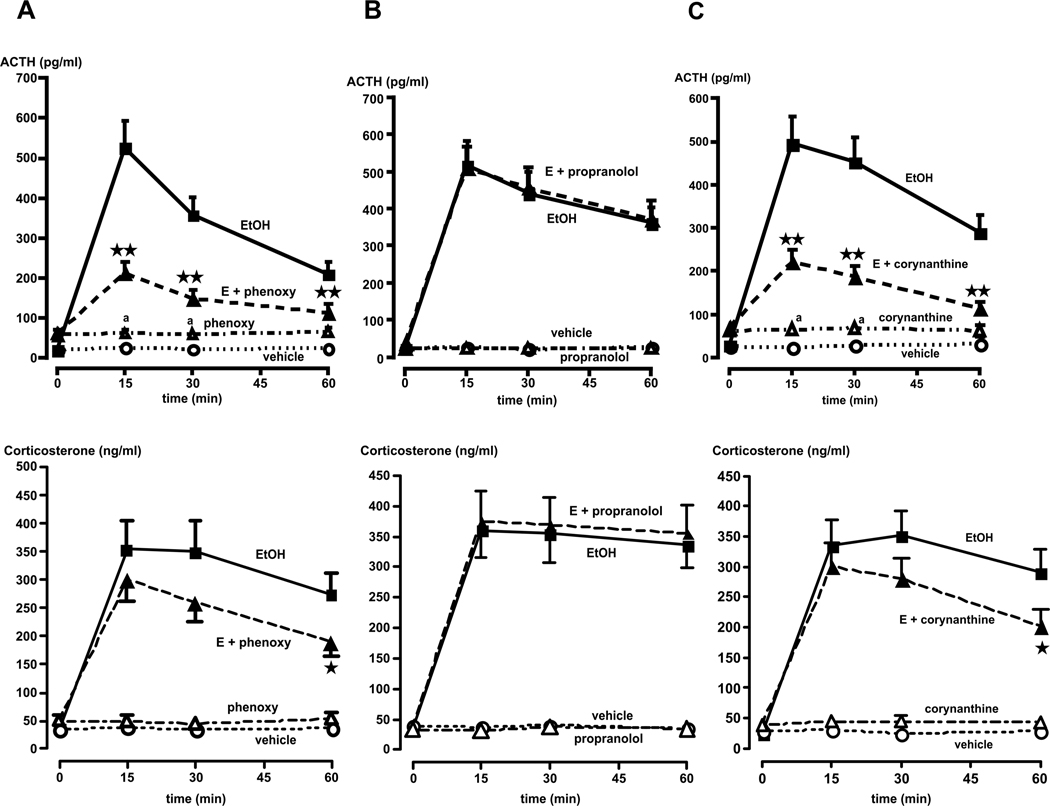
Having shown that adrenergic input was required for the effect of alcohol on the HPA axis, we then identified the type of adrenergic receptors involved in this effect. The roles of α- and β-adrenergic receptors involved in the modulating influence of catecholamines in our model was first investigated with the general α-adrenergic antagonist phenozybenzamine and the general β-adrenergic antagonist propranolol, respectively. While phenoxybenzamine caused a slight increase in basal ACTH levels, it also reduced the ACTH response to alcohol by >65% (P<0.01) (top Fig. 8A). In contrast, propranolol was without effect (top Fig. 8B). These results support a primary role of α, but not β-adrenergic receptors on the pituitary's response to ig injected alcohol. Probably because the corticosterone dose-response curve to ACTH is saturated at relatively low ACTH levels in rats [see (50)], significant decreases in ACTH levels were only accompanied by modest changes in corticosterone release. We then examined the role of the α1 subtype receptor because of its importance in the HPA axis response to a variety of stressors (14, 16). Though blockade of the α1 subtype with corynanthine slightly elevated plasma ACTH and corticosterone levels, it nevertheless significantly (P<0.01) interfered with alcohol-induced ACTH release (top Fig. 8C). In contrast, only the 60 min time point of the corticosterone response for that group was significantly (P<0.05) altered (bottom Fig. 8C).
Figure 8.
Effect of various adrenergic receptor antagonists on the ACTH (top graphs) and corticosterone (bottom graphs) responses to alcohol. (A) Phenoxybenzamine significantly (P<0.01) blunts the ACTH response to alcohol (E + phenoxy) at all times tested, compared to rats injected with alcohol alone (EtOH). In contrast, this antagonist only significantly (P<0.05) decreases the corticosterone response at the 60 min time point. (B) Propranolol does not alter the ACTH or corticosterone responses to alcohol (E + propranolol), compared to rats injected with alcohol alone (EtOH). (C) Corynanthine significantly (P<0.01) blunts the ACTH response to alcohol (E + corynanthine) at all times tested, compared to rats injected with alcohol alone (EtOH). In contrast, this antagonist only significantly (P<0.05) decreases the corticosterone response at the 60 min time point. Time = 0 indicates plasma ACTH/corticosterone levels immediately prior to alcohol administration. Each point illustrates the means ± SEM of 5–7 rats. *, P<0.05 and **, P<0.01 vs. EtOH alone; a, P<0.01 vs. vehicle.
Discussion
PVN CRF perikarya represent the primary site at which alcohol exerts its stimulatory influence on the rodent's HPA axis (6, 51, 52), and the critical role of this peptide on the ensuing ACTH release (7, 53) takes place via activation of pituitary CRF receptors type 1 (54). However, we still do not understand whether the main effect of alcohol is exerted directly on PVN perikarya, or (also?) involves intermediates. The work described here examined the potential role of catecholamines in a model of investigator-controlled alcohol delivery.
Catecholamines primarily stimulate the HPA axis via a local influence on PVN neurons (55), though other sites might also be involved, such as the prefrontal cortex (18, 19). In addition, major afferents to the PVN include adrenergic C1–C3 and noradrenergic A1–A2 cell groups of the brain stem, which have specific projections to, and terminals on, PVN CRF neurons (2, 46, 56, 57). Finally, the LC has wide-spread efferent fibers that supply norepinephrine throughout the central nervous system (58, 59), and while its direct projections to the PVN are considered sparse (60), they nevertheless mediate the HPA axis responses to various stressors (16, 57, 61, 62). However, to our knowledge, there is no information regarding the role played by (nor)adrenergic pathways in mediating alcohol-induced activation of the HPA axis. As a first step, we examined the effect of the acute ig injection of this drug, in a paradigm free of any other stressors, on specific (nor)adrenergic areas. While other investigators have reported a stimulatory effect of alcohol on LC function (10, 11, 49), these models were not stress-free, which makes it difficult to differentiate between the influence of the drug itself, and that of its mode of injection. Here we show that the ig delivery of alcohol to non handled, freely moving rats increased neuronal activity in the LC as well as in the A1–A2/C1–C3 cell groups of the brain stem. This suggests that this drug activates catecholaminergic-rich regions of the brain stem. Furthermore, while gastric distension can activate the brain stem (63), the observation of upregulated medullary neuronal activity in several different models of alcohol exposure (see Introduction), suggests that this represents a response specific to this drug, and not a function of its mode of administration. Collectively, these results provided novel information regarding the stimulatory effect of alcohol on the (nor)adrenergic circuitry, and they also guided us in the subsequent functional studies.
(Nor)adrenergic medullary cell groups convey interoceptive information in a stress-specific manner (17, 19, 22, 64) and their lesion, through knife cuts or catecholamine depletion induced by toxins, interferes with the ACTH response to a variety (22, 29), but not all (19) stressors. While we therefore hypothesized that this area might also mediate the HPA axis' response to alcohol, it was important to demonstrate it. Six brain stem cell groups are currently considered to provide the catecholaminergic input required for normal PVN CRF responses to many stressors, i.e., the noradrenergic A1, A2 and A6 (LC) regions on one hand, and the adrenergic C1, C2 and C3 (including the NTS) regions on the other hand (16). As 6-OHDA lesions are considered to interrupt (nor)adrenergic inputs from the brain stem to the PVN, results of this type of experiments give us general information regarding the functional importance of these terminals on CRF neurons. It should be noted, however, that the ability of these lesions to impact (nor)adrenergic circuitry throughout the brain, does not allow us to assess the potential role of regions other than the brain stem, that impact on the PVN. This being said, we show here that indeed, like many other stressors, the ability of alcohol to release ACTH, and by inference to activate PVN CRF perikarya, at least partially depends on this innervation. However, while functional involvement of the LC in the stimulatory effect of alcohol on the HPA axis was of significant interest, it was not entirely unexpected because many stimuli can activate this pontine region [review in (59)]. Indeed, we believe that the most novel finding reported here pertains to the importance of the A1–A2/C1–C3 region. These medullary (nor)adrenergic cell groups provide sensory signals to the PVN (65) such as those related to changes in blood pressure and sodium/water balance (66). They therefore are important homeostatic centers that contribute to the catecholaminergic innervation of the PVN (46). To our knowledge however, their precise role in regulating the HPA axis response to alcohol has not been studied. We had recently reported that female rats exposed to alcohol prenatally and stressed as adults, not only exhibited the documented enhanced HPA axis response, but also showed significantly enhanced neuronal response of their A1/C1 region (67). This had suggested a possible involvement of this brain area in the neuroendocrine effect of alcohol. Additionally, we focused on C1–C3 cells because as they mostly arise from non-LC nuclei (46), we reasoned that they would provide information that complemented those obtained with 6-OHDA lesions. We recently showed that selective destruction of medullary neurons by bilateral microinfusion of the immunotoxin anti-DBH saporin significantly decreased PVN CRF cell number (68), while here we report that it caused the loss of catecholaminergic input to this hypothalamic region. These preliminary observations indicate that the anti-DBH toxin can be used in future experiments to examine the role played by adrenergic projections provided to the neuroendocrine hypothalamus, and that originate in the medullary region, in the HPA axis response to alcohol; and to better understand the hierarchy of the adrenergic circuitry that mediates HPA axis activation by this drug.
Lastly, we examined the type of receptors involved in mediating the influence of (nor)adrenergic pathways in our alcohol model. In general, the bulk of the stimulatory catecholaminergic drive to the central limb of the HPA axis is provided by adrenergic receptors type α [references in (14, 15)], and subtype α1 receptors have been localized in the endocrine PVN (69). However, their central blockade interferes with the effect of only selective stressors (14, 21, 36, 70). Whether this represents poor penetration into the brain of systemically-injected drugs, inadequate reach by these drugs of the brain areas that are important in the model studied, or a differential reliance of various stressors on catecholaminergic inputs into the PVN remains unclear, though the issue of the blood-brain barrier was circumvented in some studies by the icv administration of the antagonists (14, 71). Alpha1 adrenergic receptors are also reported to mediate CRF release from the median eminence (72). We found that blockade of β receptors did not significantly alter the ability of alcohol to release ACTH, and that on the other hand this drug was not capable of inducing ACTH secretion in the absence of functional α1-adrenergic receptors. We had previously shown that alcohol acts on PVN CRF neurons and not directly on the pituitary (7, 8). As corynanthine does not significantly interfere with ACTH release induced by exogenous CRF (Rivier, unpublished), we propose that the primary site of action of this antagonist is exerted in brain regions protected by the blood-brain barrier. Finally, as alcohol increases circulating levels of epinephrine (73), which may be able to penetrate the brain, one might argue that blood-borne amines might play a role in our model. However, activation of the HPA axis by epinephrine is mediated by β-adrenergic receptors (74). As blockade of these receptors did not alter the effect of alcohol, it seems unlikely that changes in peripheral catecholamines represent an important mechanism in the stimulatory effect of this drug on the HPA axis.
In conclusion, we present here novel information regarding the ability of alcohol to stimulate brain catecholaminergic circuits, and the role that these circuits play in mediating the influence of acute injection of this drug on the HPA axis. While our current experiments focused on the PVN, the ability of the pharmacological manipulations we used to also influence other brain areas, indicates that future studies will need to examine the potential importance of brain sites outside the hypothalamus. Finally it is important to reiterate, as indicated at the beginning of this Discussion, that all our experiments involved non-contingent alcohol administration. Whether drug self-administration influences the HPA axis through mechanisms comparable to the ones we studied in the present work, therefore remains to be determined. Nevertheless, we believe that these findings are important for our overall understanding of the mechanisms that mediate the neuroendocrine influence of alcohol, and also for the development of potential therapies designed to address the adverse consequences of its abuse.
Acknowledgments
The authors are grateful to Irene Choi, Dong Ji, Cristin Roach, Brian Baridon, Yaira Haas, Calvin Lau, Sarah Im and Jonathan Tjong for excellent technical assistance and Dr. Jason Radley for assisting with taking microscope pictures. The project described was supported by Award Numbers AA006420 and AA008924 from the National Institute on Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health.
References
- 1.Watts AG. The impact of physiological stimuli on the expression of corticotropin-releasing hormone (CRH) and other neuropeptide genes. Front Neuroendocrinol. 1996;17:281–326. doi: 10.1006/frne.1996.0008. [DOI] [PubMed] [Google Scholar]
- 2.Sawchenko PE, Brown ER, Chan RKW, Ericsson A, Li H-Y, Roland BL, Kovacs KJ. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. In: Holstege G, Bandler R, Saper CB, editors. Emotional Motor System. Elsevier Science B. V.; 1996. pp. 201–222. [DOI] [PubMed] [Google Scholar]
- 3.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 4.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Rivier C. Alcohol stimulates ACTH secretion in the rat: Mechanisms of action and interactions with other stimuli. Alcoholism: Clin Exp Res. 1996;20:240–254. doi: 10.1111/j.1530-0277.1996.tb01636.x. [DOI] [PubMed] [Google Scholar]
- 6.Rivier C, Lee S. Acute alcohol administration stimulates the activity of hypothalamic neurons that express corticotropin-releasing factor and vasopressin. Brain Res. 1996;726:1–10. [PubMed] [Google Scholar]
- 7.Lee S, Selvage D, Rivier C. Site of action of acute alcohol administration in stimulating the rat hypothalamic-pituitary-adrenal axis: Comparison between the effect of systemic and intracerebroventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology. 2004;145:4470–4479. doi: 10.1210/en.2004-0110. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Kang SS, Lee S, Rivier C. Effect of ethanol on the regulation of corticotropin-releasing factor (CRF) gene expression. Mol Cell Neurosci. 2005;29:345–354. doi: 10.1016/j.mcn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Marinelli PW, Quirion R, Gianoulakis C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology (Berl) 2003;169:60–67. doi: 10.1007/s00213-003-1490-2. [DOI] [PubMed] [Google Scholar]
- 10.Chang SL, Patel NA, Romero AA. Activation and desensitization of fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 1995;679:89–98. doi: 10.1016/0006-8993(95)00210-h. [DOI] [PubMed] [Google Scholar]
- 11.Thiele TE, Cubero I, van Dijk G, Mediavilla C, Bernstein IL. Ethanol-induced c-fos expression in catecholamine- and neuropeptide Y-producing neurons in rat brainstem. Alcoholism: Clin Exp Res. 2000;24:802–809. [PubMed] [Google Scholar]
- 12.Ryabinin A, Criado J, Henriksen S, Bloom F, Wilson M. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiat. 1997;2:32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- 13.Seo DO, Rivier C. Interaction between alcohol and nitric oxide on ACTH release in the rat. Alcoholism: Clin Exp Res. 2003;27:989–996. doi: 10.1097/01.ALC.0000071737.84882.C4. [DOI] [PubMed] [Google Scholar]
- 14.Kiss A, Aguilera G. Role of alpha-1-adrenergic receptors in the regulation of corticotropin-releasing hormone mRNA in the paraventricular nucleus of the hypothalamus during stress. Cell Mol Neurobiol. 2000;20:683–694. doi: 10.1023/A:1007098724683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn AJ, Swiergiel AH. The role of corticotropin-releasing factor and noradrenaline in stress-related responses, and the inter-relationships between the two systems. Eur J Pharmacol. 2008;583:186–193. doi: 10.1016/j.ejphar.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wittmann G. Regulation of hypophysiotrophic corticotrophin-releasing hormone- and thyrotrophin-releasing hormone-synthesising neurones by brainstem catecholaminergic neurones. J Neuroendocrinol. 2008;20:952–960. doi: 10.1111/j.1365-2826.2008.01748.x. [DOI] [PubMed] [Google Scholar]
- 17.Sawchenko P. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. In: Mayer E, Saper C, editors. Progress in Brain Research. Elsevier Science; 2000. pp. 61–78. [DOI] [PubMed] [Google Scholar]
- 18.Radley J, Williams B, Sawchenko P. Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J Neurosci. 2008;28:5806–5816. doi: 10.1523/JNEUROSCI.0552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dayas CV, Buller KM, Day TA. Medullary neurones regulate hypothalamic corticotropin-releasing factor cell responses to an emotional stressor. Neuroscience. 2001;105:707–719. doi: 10.1016/s0306-4522(01)00213-5. [DOI] [PubMed] [Google Scholar]
- 20.Sved AF, Cano G, Passerin AM, Rabin BS. The locus coeruleus, Barrington's nucleus, and neural circuits of stress. Physiol Behav. 2002;77:737–742. doi: 10.1016/s0031-9384(02)00927-7. [DOI] [PubMed] [Google Scholar]
- 21.Douglas AJ. Central noradrenergic mechanisms underlying acute stress responses of the Hypothalamo-pituitary-adrenal axis: adaptations through pregnancy and lactation. Stress. 2005;8:5–18. doi: 10.1080/10253890500044380. [DOI] [PubMed] [Google Scholar]
- 22.Li H-Y, Ericsson A, Sawchenko PE. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc Natl Acad Sci USA. 1996;93:2359–2364. doi: 10.1073/pnas.93.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogilvie K, Lee S, Rivier C. Effect of three different modes of alcohol administration on the activity of the rat hypothalamic-pituitary-adrenal axis. Alcoholism: Clin Exp Res. 1997;21:467–476. doi: 10.1111/j.1530-0277.1997.tb03792.x. [DOI] [PubMed] [Google Scholar]
- 24.Turnbull AVT, Rivier CL. Regulation of the hypothalamo-pituitary-adrenal (HPA) axis by cytokines: Actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Blessing WW, Lappi DA, Wiley RG. Destruction of locus coeruleus neuronal perikarya after injection of anti-dopamine-B-hydroxylase immunotoxin into the olfactory bulb of the rat. Neurosci Lett. 1998;243:85–88. doi: 10.1016/s0304-3940(98)00090-1. [DOI] [PubMed] [Google Scholar]
- 26.Wrenn CC, Picklo MJ, Lappi DA, Robertson D, Wiley RG. Central noradrenergic lesioning using anti-DBH-saporin: anatomical findings. Brain Res. 1996;740:175–184. doi: 10.1016/s0006-8993(96)00855-4. [DOI] [PubMed] [Google Scholar]
- 27.Madden C, Ito S, Rinaman L, Wiley R, Sved A. Lesions of the C1 catecholaminergic neurons of the ventrolateral medulla in rats usinig anti-DbH-saporin. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1063–R1075. doi: 10.1152/ajpregu.1999.277.4.R1063. [DOI] [PubMed] [Google Scholar]
- 28.Schiltz J, Sawchenko P. Specificity and generality of the involvement of catecholaminergic afferents in hypothalamic responses to immune insults. J Comp Neurol. 2007;502:455–467. doi: 10.1002/cne.21329. [DOI] [PubMed] [Google Scholar]
- 29.Seo D, Lee S, Rivier C. Role of specific adrenergic receptors in mediating the ACTH response to increased nitric oxide levels. Journal of Neuroendocrinology. 2003;15:530–537. doi: 10.1046/j.1365-2826.2003.01027.x. [DOI] [PubMed] [Google Scholar]
- 30.Rivier C, Grigoriadis D, Rivier J. Role of corticotropin releasing factor receptors type 1 and 2 in modulating the rat adrenocorticotropin response to stressors. Endocrinology. 2003;144:2396–2403. doi: 10.1210/en.2002-0117. [DOI] [PubMed] [Google Scholar]
- 31.Rivier C, Lee S. Effect of repeated exposure to alcohol on the response of the hypothalamic-pituitary-adrenal axis of the rat: II. Role of the length and regimen of alcohol treatment. Alcoholism: Clin Exp Res. 2001;25:106–111. [PubMed] [Google Scholar]
- 32.Kornreich WD, Galyean R, Hernandez J-F, Craig AG, Donaldson CJ, Yamamoto G, Rivier C, Vale W, Rivier J. Alanine series of ovine corticotropin releasing factor (oCRF): A structure-activity relationship study. J Med Chem. 1992;35:1870–1876. doi: 10.1021/jm00088a024. [DOI] [PubMed] [Google Scholar]
- 33.Chan R, Sawchenko P. Hemodynamic regulation of tyrosine hydroxylase messenger RNA in medullary catecholamine neurons: a c-fos-guided hybridization histochemical study. Neuroscience. 1995;66:377–390. doi: 10.1016/0306-4522(94)00600-a. [DOI] [PubMed] [Google Scholar]
- 34.Li J, French BA, Fu P, Bardag-Gorce F, French SW. Catecholamines are involved in the mechanism of the urinary alcohol level cycle in rats fed ethanol intragastrically at a constant rate. Life Sci. 2004;75:3043–3051. doi: 10.1016/j.lfs.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 35.Kehne JH, Sorenson CA. The effects of pimozide and phenoxybenzamine pretreatments on amphetamine and apomorphine potentiation of the acoustic startle response in rats. Psychopharmacology (Berl) 1978;58:137–144. doi: 10.1007/BF00426896. [DOI] [PubMed] [Google Scholar]
- 36.Pardon MC, Ma S, Morilak DA. Chronic cold stress sensitizes brain noradrenergic reactivity and noradrenergic facilitation of the HPA stress response in Wistar Kyoto rats. Brain Res. 2003;971:55–65. doi: 10.1016/s0006-8993(03)02355-2. [DOI] [PubMed] [Google Scholar]
- 37.Selvage DJ, Parsons L, Rivier C. Role played by brainstem neurons in regulating testosterone secretion via a direct neural pathway between the hypothalamus and the testes. Endocrinology. 2006;147:3070–3075. doi: 10.1210/en.2005-1358. [DOI] [PubMed] [Google Scholar]
- 38.Kostrzewa RM, Jacobowitz DM. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974;26:199–288. [PubMed] [Google Scholar]
- 39.Javitch JA, Uhl GR, Snyder SH. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: characterization and localization of receptor binding sites in rat and human brain. Proc Natl Acad Sci USA. 1984;81:4591–4595. doi: 10.1073/pnas.81.14.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu SC, Chang FW, Sung YJ, Hsu WM, Lee EH. Neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the substantia nigra and the locus coeruleus in BALB/c mice. J Pharmacol Exp Ther. 1991;259:1379–1387. [PubMed] [Google Scholar]
- 41.Heikkila RE, Hess A, Duvoisin RC. Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice. Science. 1984;224:1451–1453. doi: 10.1126/science.6610213. [DOI] [PubMed] [Google Scholar]
- 42.Rohde DS, Basbaum AI. Activation of coeruleospinal noradrenergic inhibitory controls during withdrawal from morphine in the rat. J Neurosci. 1998;18:4393–4402. doi: 10.1523/JNEUROSCI.18-11-04393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Estacio MA, Tsukamura H, Reyes BA, Uenoyama Y, I'Anson H, Maeda K. Involvement of brainstem catecholaminergic inputs to the hypothalamic paraventricular nucleus in estrogen receptor alpha expression in this nucleus during different stress conditions in female rats. Endocrinology. 2004;145:4917–4926. doi: 10.1210/en.2004-0469. [DOI] [PubMed] [Google Scholar]
- 44.Rinaman L, Dzmura V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1495–R1503. doi: 10.1152/ajpregu.00393.2007. [DOI] [PubMed] [Google Scholar]
- 45.Amelita M, Estacio C, Tukamura H, Reyes B, Uenoyama Y, I'Anson H, Maeda K. Involvement of brainstem catecholaminergic inputs to the hypothalamic paraventricular nucleus in estrogen receptor a expression in thie nucleus during different stress conditions in female rats. Endocrinology. 2004;145:4917–4926. doi: 10.1210/en.2004-0469. [DOI] [PubMed] [Google Scholar]
- 46.Mejias-Aponte C, Drouin C, Aston-Jones G. Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral filed: Prominent inputs from medullatry homeostatic centers. J Neurosci. 2009;29:3613–3626. doi: 10.1523/JNEUROSCI.4632-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morales M, Criado JR, Sanna PP, Henriksen SJ, Bloom FE. Acute ethanol induces c-fos immunoreactivity in GABAergic neurons of the central nucleus of the amygdala. Brain Res. 1998;798:333–336. doi: 10.1016/s0006-8993(98)00457-0. [DOI] [PubMed] [Google Scholar]
- 48.Knapp DJ, Braun CJ, Duncan GE, Qian Y, Fernandes A, Crews FT, Breese GR. Regional specificity of ethanol and NMDA action in brain revealed with FOS-like immunohistochemistry and differential routes of drug administration. Alcoholism: Clin Exp Res. 2001;25:1662–1672. [PubMed] [Google Scholar]
- 49.Thiele TE, van Dijk G, Brenstein IL. Ethanol-induced c-Fos expression in rat lines selected for low and high alcohol consumption. Brain Res. 1997;756:278–282. doi: 10.1016/s0006-8993(97)00228-x. [DOI] [PubMed] [Google Scholar]
- 50.Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: variations on a theme of B. Recent Prog Horm Res. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- 51.Lee S, Lee K-F, Bradbury M, Vale W, Rivier C. Alcohol increases steady-state gene expression of corticotropin-releasing factor in the paraventricular nucleus of CRF receptor 1-deficient mice. Alcoholism: Clin Exp Res. 1999;23S:22A. [Google Scholar]
- 52.Madeira MD, Paula-Barbosa MM. Effects of alcohol on the synthesis and expression of hypothalamic peptides. Brain Res Bull. 1999;48:3–22. doi: 10.1016/s0361-9230(98)00131-2. [DOI] [PubMed] [Google Scholar]
- 53.Rivier C. Gender, sex steroids, corticotropin-releasing factor, nitric oxide and the HPA response to stress. Pharmacol Biochem Behav. 1999;64:739–751. doi: 10.1016/s0091-3057(99)00148-3. [DOI] [PubMed] [Google Scholar]
- 54.Lee S, Smith G, Vale W, Lee K-F, Rivier C. Mice that lack corticotropin-releasing factor (CRF) receptors type 1 show a blunted ACTH response to acute alcohol despite up-regulated constitutive hypothalamic CRF gene expression. Alcoholism: Clin Exp Res. 2001;25:427–433. [PubMed] [Google Scholar]
- 55.Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci. 2002;22:959–969. doi: 10.1523/JNEUROSCI.22-03-00959.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 57.Day TA. Defining stress as a prelude to mapping its neurocircuitry: no help from allostasis. Prog Neuropsychopharmacol Biol Psychiat. 2005;29:1195–1200. doi: 10.1016/j.pnpbp.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Reyes BA, Valentino RJ, Xu G, Van Bockstaele EJ. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur J Neurosci. 2005;22:93–106. doi: 10.1111/j.1460-9568.2005.04197.x. [DOI] [PubMed] [Google Scholar]
- 59.Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- 61.Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valentino RJ, Curtis AL, Page ME, Pavcovich LA, Florin-Lechner SM. Activation of the locus ceruleus brain noradrenergic system during stress: circuitry, consequences, and regulation. Adv Pharmacol. 1998;42:781–784. doi: 10.1016/s1054-3589(08)60863-7. [DOI] [PubMed] [Google Scholar]
- 63.Olson BR, Freilino M, Hoffman GE, Stricker EM, Sved AF, Verbalis JG. c-Fos Expression in Rat Brain and Brainstem Nuclei in Response to Treatments That Alter Food Intake and Gastric Motility. Mol Cell Neurosci. 1993;4:93–106. doi: 10.1006/mcne.1993.1011. [DOI] [PubMed] [Google Scholar]
- 64.Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis an dparaventricular nucleus o the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. J Neurosci. 2006;26:11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cunningham E, Bohn M, Sawchenko P. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1990;292:651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- 66.Guyenet P. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 67.Choi I, Lee S, Rivier C. Novel role of adrenergic neurons in the brain stem in mediating the hypothalamic-pituitary axis hyperactivity caused by prenatal alcohol exposure. Neuroscience. 2008;155:888–901. doi: 10.1016/j.neuroscience.2008.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allen CD, Waser B, Körner M, Reubi JC, Lee S, Rivier C. Neuropeptide Y acts within the rat testis to inhibit testosterone secretion. Neuropeptides. 2010;45:55–61. doi: 10.1016/j.npep.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Day H, Campeau S, Watson S, Akil H. Expression of a1b adrenoreceptor mRNA in corticotropin-releasing hormone-containing cells of the rat hypothalamus and its regulation by corticosterone. J Neurosci. 1999;19:10098–10106. doi: 10.1523/JNEUROSCI.19-22-10098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Douglas AJ, Meddle SL, Toschi N, Bosch OJ, Neumann ID. Reduced activity of the noradrenergic system in the paraventricular nucleus at the end of pregnancy: implications for stress hyporesponsiveness. J Neuroendocrinol. 2005;17:40–48. doi: 10.1111/j.1365-2826.2005.01272.x. [DOI] [PubMed] [Google Scholar]
- 71.Szafarczyk A, Malaval F, Laurent A, Gibaud R, Assenmacher I. Further evidence for a central stimulatory action of catecholamines on adrenocorticotropin release in the rat. Endocrinology. 1987;121:883–892. doi: 10.1210/endo-121-3-883. [DOI] [PubMed] [Google Scholar]
- 72.Kiss A, Aguilera A. Participartion of α1-adrenergic receptors in the secretion of hypothalamic corticotropin-releasing hormone during stress. Neuroendocrinology. 1992;56:53–60. doi: 10.1159/000126223. [DOI] [PubMed] [Google Scholar]
- 73.Ireland M, Vandongen R, Davidson L, Beilin L, Rouse I. Acute effects of moderate alcohol consumption on blood pressure and plasma catecholamines. Clin Sci (Lond) 1984;66:643–648. doi: 10.1042/cs0660643. [DOI] [PubMed] [Google Scholar]
- 74.Tilders FHH, Berkenbosch F, Smelik PG. Adrenergic mechanisms involved in the control of pitutary-adrenal activity in the rat: a β-adrenergic stimulatory mechanism. Endocrinology. 1982;110:114–120. doi: 10.1210/endo-110-1-114. [DOI] [PubMed] [Google Scholar]