Abstract
Cross-sectional epidemiologic scoliosis screening was carried out to determine the current prevalence of scoliosis in the Korean population and to compare with the results of previous studies. Between 2000 and 2008, 1,134,890 schoolchildren underwent scoliosis screening. The children were divided into two age groups, 10–12-year-olds (elementary school) and 13–14-year-olds (middle school), to calculate age- and sex-specific prevalence rates. Children with a scoliometer reading ≥5° were referred for radiograms. Two surgeons independently measured curve types, magnitudes, and Risser scores (inter-observer r = 0.964, intra-observer r = 0.978). Yearly and overall prevalence rates of scoliosis were calculated. There were 584,554 boys and 550,336 girls in the sample, with a male to female ratio of 1.1:1. There were 77,910 (6.2%) children (26,824 boys and 51,086 girls) with scoliometer readings >5°, and 37,339 of them had positive results with Cobb angles ≥10° (positive predictive value, 46.4%). The overall scoliosis prevalence rate was 3.26%; girls had a higher prevalence (4.65%) than boys (1.97%). Prevalence rates increased progressively from 1.66 to 6.17% between 2000 and 2008, with the exception of 2002. According to age and gender, 10–12-year-old girls had the highest scoliosis prevalence rates (5.57%), followed by 13–14-year-old girls (3.90%), 10–12-year-old boys (2.37%), and 13–14-year-old boys (1.42%). In girls and boys, prevalence rates dropped by 64.53 and 60.65% among 10–12-year-olds and 13–14-year-olds, respectively (P = 0.00). The proportion of 10°–19° curves was 95.25 and 84.45% in boys and girls, respectively; and the proportion of 20°–29° curves was 3.91 and 11.28%, which was a significant difference (P = 0.00). Thoracic curves were the most common (47.59%) followed by thoracolumbar/lumbar (40.10%), double (9.09%), and double thoracic (3.22%) curves. A comparison of the curve patterns revealed significant differences between genders (P = 0.00). We present this report as a guide for studying the prevalence of idiopathic scoliosis in a large population, and the increasing trend in the prevalence of idiopathic scoliosis emphasizes the need for awareness.
Keywords: Scoliosis screening, Prevalence rate, Changing trend, Awareness
Introduction
Screening, as defined by the American Commission on Chronic Illness, is the “presumptive identification of unrecognized disease or defect by application of tests, examination, or other procedures which can be applied rapidly” [40]. As resources are limited in many screening programs, it is important that screening for idiopathic scoliosis be targeted at the optimal age group, in whom conservative management, such as bracing, can be instituted to control curve progression and reduce the need for surgery. Early detection by comprehensive screening programs enables early institution of conservative treatment, with the aim of reducing the number of patients with curves reaching a magnitude that requires surgical treatment. Scoliosis screening in schoolchildren has been popularized over the past two decades and is currently carried out in 15 states in the United States and in the Middle East, Sweden, South Africa, and some parts of Japan [9, 24, 28, 36, 42]. School-based screening can be used to identify children who may have scoliosis as well as those who may be at high risk for the disease; however, the screening procedure should not be considered a diagnostic test [41]. Several techniques have been described for the early detection of scoliosis through school-based screening, and the most widely used method is the forward bending test developed by Adams [29, 31, 33].
Prevalence rates of idiopathic scoliosis vary from 0.35 to 13%, depending on the defined Cobb angles, screening age, and sex [3, 7, 35]. In addition, there are regional and population-based differences in prevalence rates [6, 9, 12, 15, 36]. The influence of geography on human biology is determined by socioeconomic and environmental factors, such as temperature, humidity, and lighting, that are transferred and expressed in human cells by specific mediators [12]. The reported prevalence of adolescent idiopathic scoliosis (AIS) in the literature increases in the northern geographic latitudes and decreases as the latitude approaches the equator [15].
Although a wide range in the prevalence of AIS in different countries is demonstrated by various reports, there is no study that reports the prevalence of scoliosis in the Korean population. The significance of specifically observing prevalence in the Korean population may not be obvious, but its evaluation is important because it could be related to a factor that contributes to AIS pathogenesis. The objectives of our study were to determine the current prevalence of scoliosis in the Korean population and to compare these results with those of studies in other countries. Data reflecting curve distribution, magnitude, pattern, sex ratio, symptoms, etiology, and family history were examined.
Materials and methods
A 9-year cross-sectional epidemiologic study was performed to determine the prevalence and distribution of various scoliosis parameters in schoolchildren in Korea. From 2000 to 2008, 1,134,890 children were screened for scoliosis in schools in Seoul and Gyeonggi province. There were 584,554 boys and 550,336 girls in the study, with ages ranging from 10 to 14 years. We selected primary (10–12-year-olds) and middle school (13–14-year-old) children for this study, and the schools were randomly selected with no special consideration for geographic or economic representation. However, all screened children were of Korean origin, with the child and both parents having been born in Korea. There were no duplicate screenings of children or schools.
An experienced team of nurses from our institute performed the initial school-based screening. Children who had been previously diagnosed or who were already being managed for scoliosis were also included in the data analysis. Their records were obtained from hospitals and clinics, and their information was included for analysis. Boys and girls were examined separately. The forward bending test was performed with the child bent forward while allowing the upper extremities to hang freely with the palms opposed in a relaxed manner, and the exposed back was viewed from the front as well as from the side. Children who had axial trunk rotation (ATR) of 5° or more on scoliometer were referred to our institute for further evaluation with whole spine anteroposterior (AP) and lateral radiograms [16, 17]. Final diagnoses of scoliosis were based on Cobb angles measured by two observers (HNM, SWS), and further treatment was advised. The inter-observer and intra-observer correlation coefficients of the Cobb angles of the standing AP radiographs were 0.964 and 0.978, respectively. Curve types, magnitudes, and Risser scores were recorded. The parameters were recorded in a spreadsheet (Microsoft Excel).
Using these data, yearly and overall prevalence rates for scoliosis were calculated for all patients. The percentage of children with positive scoliometer (ATR) readings (>5°) and the presence of scoliosis on radiogram, defined as a Cobb angle >10°, was also calculated. All children with scoliosis were divided to define the prevalence rates according to the severity of the curvature. Risser scores were also noted to differentiate the prevalence rates according to Risser staging. We also divided our study population into two age groups, 10–12-year-olds (elementary school children) and 13–14-year-olds (middle school children), to calculate age- and sex-specific prevalence rates. The statistical analysis was performed using a statistical package (SPSS, version 12, Chicago, IL, USA). P values <0.05 were considered statistically significant.
Results
Between 2000 and 2008, 1,134,890 children were screened for scoliosis in Seoul and Gyeonggi province of South Korea. There were 584,554 boys and 550,336 girls in the study, with a male to female ratio of 1.1:1.0. Table 1 provides a detailed description of all enrolled children for each study year. Among all of the children screened for scoliosis, 77,910 children (6.2%), including 26,824 boys and 51,086 girls, had positive scoliometer readings (>5°) on the forward bending test and were referred for radiograms (Table 2).
Table 1.
Numbers of children enrolled for the study
| Year | Male (n) | Female (n) | Total (n) |
|---|---|---|---|
| 2000 | 4,578 | 4,202 | 8,780 |
| 2001 | 21,512 | 20,350 | 41,862 |
| 2002 | 34,705 | 36,178 | 70,883 |
| 2003 | 117,742 | 112,852 | 230,594 |
| 2004 | 140,835 | 132,235 | 273,070 |
| 2005 | 140,447 | 128,913 | 269,360 |
| 2006 | 44,395 | 40,585 | 84,980 |
| 2007 | 41,858 | 38,802 | 80,660 |
| 2008 | 38,482 | 36,219 | 74,701 |
| Total (n) | 584,554 | 550,336 | 1,134,890 |
Table 2.
Number of ATR (rotations ≥ 5°) and X-ray (Cobb ≥ 10°) positive children with scoliosis along with percentage of referral patients for the radiogram after screening
| Year | No. screened | ATR +ve (M/F) | Referral (%) | X-ray +ve (M/F) |
|---|---|---|---|---|
| 2000 | 8,780 | 405 (191/204) | 4.6 | 147 (49/98) |
| 2001 | 41,862 | 1,727 (735/992) | 4.1 | 1,034 (392/642) |
| 2002 | 70,883 | 2,178 (819/1,359) | 2.9 | 1,056 (368/688) |
| 2003 | 230,594 | 9,663 (3,566/6,097) | 4.2 | 5,526 (1,645/3,881)) |
| 2004 | 273,070 | 22,021 (7,886/14,135) | 8.1 | 9,500 (2,721/6,779) |
| 2005 | 269,360 | 19,167 (6,328/12,839) | 7.1 | 8,321 (2,168/6,153) |
| 2006 | 84,980 | 6,289 (1,807/4,482) | 7.4 | 3,153 (952/2,201) |
| 2007 | 80,660 | 7,421 (2,539/4,882) | 9.2 | 3,993 (1,294/2,699) |
| 2008 | 74,701 | 9,039 (2,953/6,086) | 12.1 | 4,609 (1,499/3,110) |
| Total | 1,134,890 | 77,910 (26,824/51,086) | 6.2 | 37,339 (11,088/26,251) |
+ve, positive; −ve, negative; M/F, male/female
Predictive value of the forward bending test
Of the 77,910 children with a positive forward bending test, 37,339 (46.4%) had positive results on radiograms, with Cobb angles of 10° or more. Thus out of all of the referred children, 53.6% were false positives. The positive predictive value was lower in boys (41%) than girls (51%) in all age groups (Table 2); however, this difference was not statistically significant (P = 0.15, Chi-square test).
Prevalence rate
The prevalence rate by gender is shown in Table 3. The overall prevalence rate of scoliosis in 10–14-year-old schoolchildren (elementary and middle school) with Cobb angles of 10° or more was 3.26%, and girls had a higher prevalence (4.65%) than boys (1.97%). Additionally, one of the interesting findings was that the prevalence rate increased progressively from 1.66 to 6.17% between the years 2000 and 2008, with the exception of 2002, which had a prevalence rate lower than in 2001. Girls had 2.4 times higher prevalence rate than boys in our study. Table 4 explains that there was a similar distribution of Risser stages in both boys and girls in the group with scoliosis of 10° or more (P = 0.99, Chi-square test). Additionally, analysis of the percentage of male and female children with Cobb angles greater than 10° according to the two age groups revealed that 18.49 and 11.20% of boys and 42.78 and 27.52% of girls were in 10–12 and 13–14-year-old groups, respectively (Table 5). Cobb angles were found to be 12.31 ± 3.37° and 13.16 ± 4.34° in boys in the 10–12- and 13–14-year-old age groups, respectively, and Cobb angles were 13.72 ± 4.80° and 15.35 ± 6.08° in girls. Analyses of prevalence rates according to age and gender, showed 10–12-year-old girls had the highest scoliosis prevalence rate of 5.57%, which was followed by 13–14-year-old girls (3.90%), 10–12-year-old boys (2.37%), and 13–14-year-old boys (1.42%). Our results revealed that in both girls and boys in the 10–12- and 13–14-year-old groups, prevalence rates dropped by 64.53 and 60.65%, respectively, which was statistically significant (P = 0.00, Chi-square test).
Table 3.
Percentage of children screened to have scoliosis ≥10°
| Year | Male (%) | Female (%) | Total (%) |
|---|---|---|---|
| 2000 | 1.07 | 2.33 | 1.66 |
| 2001 | 1.78 | 3.08 | 2.41 |
| 2002 | 0.95 | 1.75 | 1.35 |
| 2003 | 1.45 | 3.65 | 2.49 |
| 2004 | 1.88 | 5.39 | 3.53 |
| 2005 | 1.54 | 4.75 | 3.08 |
| 2006 | 2.14 | 5.42 | 3.71 |
| 2007 | 3.09 | 6.95 | 4.95 |
| 2008 | 3.9 | 8.59 | 6.17 |
| Total (%) | 1.97 | 4.65 | 3.26 |
Table 4.
Distribution of the Risser stage in both sexes
| Sex/Risser | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Male | 12.14 | 13.24 | 13.95 | 14.5 | 14.65 | 14.86 |
| Female | 11.96 | 12.37 | 12.91 | 13.39 | 13.9 | 14.84 |
Table 5.
Gender wise number, average Cobb angle and prevalence rate of scoliosis in elementary (age, 10–12 years) and middle school (age, 13–14 years) children
| Sex | Male | Female | ||
|---|---|---|---|---|
| Age (years) | 10–12 | 13–14 | 10–12 | 13–14 |
| Total (n) | 291,357 | 293,197 | 286,728 | 263,608 |
| Scoliosis (n) | 6,904 | 4,184 | 15,974 | 10,277 |
| Cobb angle | 12.31 | 13.16 | 13.72 | 15.35 |
| SD | 3.37 | 4.34 | 4.8 | 6.08 |
| Prevalence (gender) | 2.37 | 1.43 | 5.57 | 3.90 |
Curve distribution
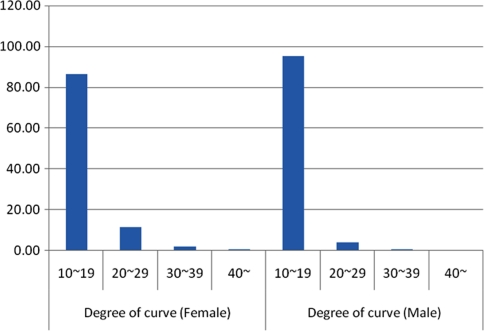
Table 6 presents the distribution of curvatures in each year for both boys and girls. The groups representing the smallest curves, including the 10°–19° and 20°–29° groups, comprised 89.09 and 9.07% of all of the children (combined 98.16%). Only 1.40 and 0.44% (combined 1.88%) of the children had curves 30°–39° and more than 40°, respectively, requiring treatment in with bracing or surgery. This distributions of curvatures remained similar over the 9-year study period (P = 1.0, ANOVA). For boys and girls, the proportions of 10°–19° curves were 95.25 and 84.45%, respectively, while the proportions of 20°–29° curves were 3.91 and 11.28%, which was statistically significant (P = 0.00, Chi-square test). Our results showed that there was a higher prevalence of smaller curves in boys than in girls (Table 7; Fig. 1).
Table 6.
Number of scoliosis children according to severity of Cobb angle
| Year | Degree of curve | Total | |||
|---|---|---|---|---|---|
| 10–19 | 20–29 | 30–39 | 40– | (n) | |
| 2000 | 141 | 5 | 0 | 1 | 147 |
| 2001 | 957 | 72 | 4 | 1 | 1,034 |
| 2002 | 982 | 68 | 4 | 2 | 1,056 |
| 2003 | 4,693 | 683 | 113 | 37 | 5,526 |
| 2004 | 8,587 | 768 | 110 | 35 | 9,500 |
| 2005 | 7,410 | 763 | 113 | 35 | 8,321 |
| 2006 | 2,809 | 281 | 49 | 14 | 3,153 |
| 2007 | 3,559 | 354 | 58 | 22 | 3,993 |
| 2008 | 4,125 | 401 | 67 | 16 | 4,609 |
| Total (n) | 33,267 | 3,385 | 522 | 165 | 37,339 |
Table 7.
The distribution of the curve magnitude in percentage in girls and boys separately
| Year | Degree of curve (female) | Degree of curve (male) | ||||||
|---|---|---|---|---|---|---|---|---|
| 10–19 | 20–29 | 30–39 | 40– | 10–19 | 20–29 | 30–39 | 40– | |
| 2000 | 94.90 | 4.08 | 0.00 | 1.02 | 97.96 | 2.04 | 0.00 | 0.00 |
| 2001 | 90.50 | 8.57 | 0.62 | 0.31 | 94.95 | 4.04 | 1.01 | 0.00 |
| 2002 | 90.57 | 8.85 | 0.29 | 0.29 | 95.10 | 4.36 | 0.54 | 0.00 |
| 2003 | 81.47 | 15.28 | 2.47 | 0.77 | 93.19 | 5.35 | 1.03 | 0.43 |
| 2004 | 88.38 | 9.70 | 1.51 | 0.41 | 95.55 | 3.90 | 0.44 | 0.11 |
| 2005 | 86.84 | 11.18 | 1.53 | 0.46 | 95.39 | 3.41 | 0.88 | 0.32 |
| 2006 | 85.91 | 11.45 | 2.05 | 0.59 | 96.64 | 3.05 | 0.21 | 0.11 |
| 2007 | 86.14 | 11.16 | 1.89 | 0.82 | 95.37 | 4.09 | 0.54 | 0.00 |
| 2008 | 86.37 | 11.23 | 1.92 | 0.48 | 95.79 | 3.41 | 0.73 | 0.07 |
| Total | 86.45 | 11.28 | 1.73 | 0.54 | 95.25 | 3.91 | 0.67 | 0.17 |
Fig. 1.
The distribution of children with scoliosis in girls and boys according to the severity of the curvature
Curve pattern
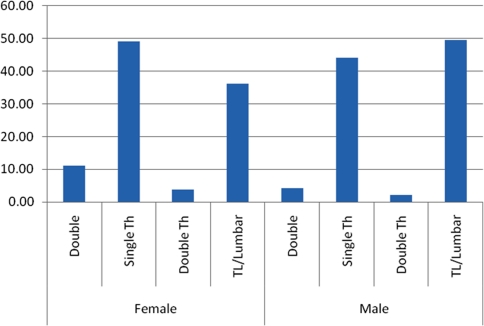
Thoracic curves were the most common (47.59%), followed by thoracolumbar/lumbar curves (40.10%), double curves (9.09%), and double thoracic curves (3.22%). Table 8 shows the distribution of curve patterns in all children by year. Among all study participants, 87.69% of had either thoracic or thoracolumbar/lumbar curves. For boys, the proportions of thoracic, thoracolumbar/lumbar, double, and double thoracic curves were 44.06, 49.55, 4.26 and 2.14%, respectively; for girls, the proportions were 49.10, 36.09, 11.10 and 3.71%. Boys had a higher proportion of thoracolumbar/lumbar curves, while girls had higher proportions of thoracic and double curves (Table 9). The difference between boys and girls in patterns of curve types was statistically significant (P = 0.00, Chi-square test) (Fig. 2).
Table 8.
Number of scoliosis children according to curve pattern
| Year | Type of curve | Total (n) | |||
|---|---|---|---|---|---|
| Double | Thoracic | Double Th | TL/lumbar | ||
| 2000 | 6 | 55 | 0 | 86 | 147 |
| 2001 | 72 | 521 | 4 | 437 | 1,034 |
| 2002 | 76 | 383 | 2 | 595 | 1,056 |
| 2003 | 529 | 2,324 | 36 | 2,637 | 5,526 |
| 2004 | 831 | 4,462 | 202 | 4,005 | 9,500 |
| 2005 | 787 | 4,188 | 307 | 3,039 | 8,321 |
| 2006 | 320 | 1,569 | 100 | 1,164 | 3,153 |
| 2007 | 364 | 1,989 | 236 | 1,404 | 3,993 |
| 2008 | 408 | 2,272 | 317 | 1,612 | 4,609 |
| Total (n) | 3,393 | 17,763 | 1,204 | 14,979 | 37,339 |
Table 9.
The distribution of the curve pattern in percentage in girls and boys separately
| Year | Curve pattern (Female) | Curve pattern (Male) | ||||||
|---|---|---|---|---|---|---|---|---|
| Double | Single Th | Double Th | TL/lumbar | Double | Single Th | Double Th | TL/lumbar | |
| 2000 | 6.12 | 39.80 | 0.00 | 54.08 | 0.00 | 32.65 | 0.00 | 67.35 |
| 2001 | 9.66 | 45.95 | 0.62 | 43.77 | 2.03 | 57.36 | 1.02 | 39.59 |
| 2002 | 8.56 | 35.56 | 0.29 | 55.59 | 4.08 | 37.50 | 0.54 | 57.88 |
| 2003 | 12.04 | 44.74 | 0.64 | 42.58 | 3.53 | 35.89 | 0.67 | 59.91 |
| 2004 | 10.57 | 48.70 | 2.35 | 38.38 | 4.23 | 42.74 | 1.65 | 51.38 |
| 2005 | 11.13 | 52.19 | 4.23 | 32.46 | 4.70 | 45.06 | 2.17 | 48.06 |
| 2006 | 12.32 | 51.73 | 3.82 | 32.14 | 5.15 | 45.32 | 1.68 | 47.84 |
| 2007 | 11.68 | 50.56 | 6.89 | 30.87 | 3.78 | 48.26 | 3.86 | 44.09 |
| 2008 | 10.65 | 50.11 | 8.18 | 31.06 | 5.07 | 48.00 | 4.14 | 42.79 |
| Total | 11.10 | 49.10 | 3.71 | 36.09 | 4.26 | 44.06 | 2.14 | 49.55 |
Fig. 2.
The distribution of children with scoliosis in girls and boys according to the pattern of the curvature
Discussion
Adolescent idiopathic scoliosis (AIS) is present in 2–4% of children between 10 and 16 years of age. It is defined as a lateral curvature of the spine greater than 10° accompanied by vertebral rotation. Scoliosis can be identified by the Adam’s forward bend test during physical examination [38, 43]. In our study, we found a scoliosis prevalence of 3.26% among 1,134,890 schoolchildren, which is similar to previously published reports. However, we believe this is the first study identifying the prevalence rate of scoliosis in such a large population. We carried out this study over a 9-year period using school-based screening, which helped to identify the trends in scoliosis prevalence in the Korean population. Although many studies related have been performed in various populations [4, 8, 12, 13, 17–21, 25, 27, 30, 32, 36, 37, 39, 42, 44–46], no previous study reported the prevalence of scoliosis in the Korean population. Our study is unique because of the large sample size as well as the 9-year study period, which allowed us to determine trends in the prevalence of scoliosis over time.
Wong et al. [30] suggested that screening of 11–12- and 13–14-year-old girls would identify a significant number who could benefit from treatment of scoliosis. The Scoliosis Research Society has recommended annual screening of all children age 10–14 years; the American Academy of Orthopedic Surgeons has recommended screening girls at the ages of 11 and 13 years and boys age 13 or 14; and the American Academy of Pediatrics has recommended routine screening at ages 10, 12, 14, and 16 years [1, 2]. The Bright Futures guidelines recommend noting the presence of scoliosis during the physical examination of adolescents and children who are at least 8-years-old [14]. The screening tool of choice has been the Adam’s forward bending test, which tests for asymmetry via visual inspection [19, 46]. Yawn and Yawn [47] noted that school-based screenings identified some children who went on to receive treatment but referred to many more who did not. The forward bending test is a fast and simple method to detect the presence of minor curvatures in the spine although it may result in false positive rates between 25 and 82%, depending on the screening method and criteria [10, 18, 23, 25, 34, 37, 47]. In our study, a single team of nurses who were experienced at taking ATR readings and performing school-based screenings participated in the initial screening; therefore, possibilities for high false-positive results were minimized. Our final results showed that the positive predictive value for the forward bending test was 46.4%. Additionally, we used a scoliometer reading of 5° as a cutoff in our screening; however, there are several recommendations that suggest a scoliometer reading of 7°–7.5° as a cutoff point to screen for scoliosis [18]. However, our goal was not to miss even a single case of scoliosis. Therefore, the comparatively higher false-positive rate in our study is justified. Another advantage of this cutoff was that it was very easy and convenient to use for the nursing staff, and we could screen for children who needed further investigations.
Ohtsuka et al. [28] published a study of the prevalence of idiopathic scoliosis in 1.24 million Japanese school children who were screened over a period of 8 years. Using a cutoff Cobb angle of 15° or more, they reported prevalence rates of 1.77 and 0.25% in 13–14-year-old girls and boys, respectively. Another aspect of their study was that they used Moiré topography for screening. However, in our study we found prevalences of 1.87 and 0.72% among girls and boys, respectively, which were higher than their study. Additionally, we found these prevalence rates using a Cobb angle cutoff of 10° or more. This demonstrates that using a Cobb angle of 15° as the cutoff point would have resulted in a significantly lower prevalence rate. Another point of difference is that we used a scoliometer reading on the forward bending test, not the Moiré topography, for our screening. They have reported higher prevalence rates in an urban population; however, we could not explore this difference in our study population, which was comprised of only urban children. In that sense, this is the largest screening study in the urban population.
Although there was a sharp decrease in the prevalence rate from the 10–12-year-old to the 13–14-year-old groups, we did not find any significant differences in the ratio of female to male prevalence rates of idiopathic scoliosis between these two age groups (P = 0.97, Chi-square test). In a school-based screening study, Stirling et al. [11] reported prevalence rates of 0.4 and 2.2% in 9–11-year-old and 12–14-year-old English girls, respectively; the boys had prevalence rates of 0.1 and 0.3% in the two age groups. Soucacos et al. [35] reported a prevalence of 1.7% in a screening of 82,900 9 to 14-year-old Greek schoolchildren during a 1-year prospective study. In a point prevalence survey of 72,699 schoolchildren, Wong et al. [43] noted that the prevalence rates were low in 6–7- and 9–10-year-olds but increased rapidly to 1.37 and 2.22% for 11–12- and 13–14-year-old girls, respectively, suggesting increasing prevalence as age increases between the ages of 7 and 14. In their study of age- and sex-specific prevalence among 29,195 children, Morais et al. [25] also noted that prevalence rates increase between ages 8 and 15, more markedly so for girls than for boys. However, our findings are different than all of the above because our study showed prevalence rates in girls and boys fall from 5.57 and 2.37%, respectively, in 10–12-year-olds to 3.90 and 1.42% in 13–14-year-olds (P < 0.001). Many authors have described spontaneous correction of curves in the immature spine [3, 10, 23, 34]. However, we cannot apply this theory to our study as it was a cross-sectional study; therefore, none of the patients were followed for a long period to allow observation of the curve in a particular case. We aimed to observe the prevalence rate in different schoolchildren over a period of 9 years. Another interesting finding, in contrast to Ohtsuka et al. [28], is that we observed a gradual increase in the prevalence of scoliosis from 1.66 to 6.17% between 2000 and 2008, except for the year 2002 which showed a decreased prevalence (Table 3). However, Ohtsuka et al. found the opposite trend between 1979 and 1986, and they also noted that the urban population had an increased prevalence rate. Our study concentrated only on an urban population, and we think that increasing urbanization and development processes in our city between 2000 and 2008 might have impacted the increasing prevalence of scoliosis. However, the ratio of female to male prevalence rates varied between 1.7:1 and 3.1:1 without showing any trend, which is similar to other published reports [11, 42, 43]. Ranshaw [9] noted that the female to male ratio is 2:1 when the Cobb angle is more than 10°; however, it reaches 5:1 or 6:1 when the Cobb angle is more than 20°. We also agree with their findings, as we observed a ratio of 2.1:1 for 10°–19° curves and 6.2:1 for curves greater than 20°. The scoliosis prevalence was found to be 1.99 and 0.93% in girls and boys, respectively, for Cobb angles 10°–19° and 0.31 and 0.05% for Cobb angles greater than 20° (Table 7). Additionally, this ratio further increased to 6.4:1 and 7.4:1 in Cobb angles greater than 30° and 40°, respectively. Notably, the number of children with scoliosis and Cobb angles less than 30° was particularly increased (Tables 6, 7). The number of children who required surgical management (Cobb angle > 40°) was not increased during the study period. Therefore, the rate of scoliosis surgery did not increase proportionately during the same period (National registry information N0447).
Thoracic curves were the most common (47.59%) in this study, followed by thoracolumbar/lumbar curves (40.10%), double curves (9.09%), and double thoracic curves (3.22%). Studies by Wong et al. [43], Moris et al. [25], and Brooks et al. [3] showed that thoracolumbar or lumbar curves were more common than thoracic curves. However, another study by Willner and Uden [42] showed that thoracic scoliosis dominated with a rate of 44%, followed by thoracolumbar curves with 32%, in contrast to the findings of other reports. Our findings agreed with this report, as the observed proportion of thoracic scoliosis was 47.59%. We calculated thoracolumbar and lumbar curves together as a single type (King type 5), and perhaps that caused the rate, 40.10%, to be slightly higher than Willner’s and Uden’s rate. Additionally, when comparing the type of curves between boys and girls, we found that boys had predominantly thoracolumbar/lumbar curves (49.55%), while girls had thoracic curves (49.10%). The difference in patterns of curves between boys and girls was significant (P = 0.00, Chi-square test) (Table 9). This could be reflected by the significant difference in the curve magnitude between boys and girls in our study (P = 0.00, Chi-square test), as 95.25% boys had mild curves (less than 19°) while only 86.45% girls had mild curves.
One important limitation of this study was that it was not a longitudinal study; therefore, we are not able to comment on the spontaneous resolution or progression of curves [3] or the effects of bracing and percentage of subjects receiving operative treatment. Cost effectiveness of scoliosis school-based screening for a large population has also been criticized [5, 26]. However, according to one recent study from Hong Kong performed in a large student population, scoliosis school-based screening proved to be more clinically effective without an obvious increase in expenses when compared with other existing screening protocols [22]. Therefore, we should consider all such points while considering school-based screening. Additionally, it was not possible to study the psychological impact on patients who were diagnosed with clinically minor scoliosis and their parents. We were unable to evaluate how many children had been diagnosed previously or had been previously treated. In a long-term cross-sectional study, especially in a large population, we believe it would be difficult to identify all such issues, and long follow-up in a large study group would be difficult due to several reasons, such as drop-out and loss to follow-up. In our study, we avoided such issues by screening one by one in selected schools, and of course, we were careful to screen each child only once. There is only one published study of the prevalence of scoliosis in a large population [28]; however, that study did not analyze the distribution of curve patterns in the population, which we have successfully analyzed in our study. Therefore, our study presents a further investigation of the unsolved issues regarding the prevalence of idiopathic scoliosis. Additionally we also agree that there was a difference in the numbers of screened children over the years which may act as one of the important confounding factor; however, we observed a trend toward increasing prevalence of idiopathic scoliosis in our study, which underscores the need for awareness among the general population and researchers as well as the policy makers and healthcare providers to initiate further long-term, prospective research to explore possible remedies.
Contributor Information
Seung-Woo Suh, Phone: +82-2-26263087, FAX: +82-2-26261163, Email: spine@korea.ac.kr.
Hitesh N. Modi, Phone: +82-2-26261994, FAX: +82-2-26261163, Email: modispine@yahoo.co.in
References
- 1.American Academy of Pediatrics (1988) Guidelines for health supervision. II. American Academy of Pediatrics, Elk Grove Village
- 2.Scoliosis Research Society (1986) Scoliosis: a handbook for patients. Scoliosis Research Society, Park Ridge
- 3.Brooks HL, Azen SP, Gerberg E, Brooks R, Chan L. Scoliosis: a prospective epidemiological study. J Bone Joint Surg Am. 1975;57:968–972. [PubMed] [Google Scholar]
- 4.Bunnell W. An objective criterion for scoliosis screening. J Bone Joint Surg Am. 1984;66:1381–1387. [PubMed] [Google Scholar]
- 5.Bunnell WP (2005) Selective screening for scoliosis. Clin Orthop Relat Res 40–45 [DOI] [PubMed]
- 6.Burwell R, James N, Johnson F, Webb J, Wilson Y. Standardised trunk asymmetry scores. A study of back contour in healthy school children. J Bone Joint Surg Br. 1983;65:452–463. doi: 10.1302/0301-620X.65B4.6874719. [DOI] [PubMed] [Google Scholar]
- 7.Daruwalla JS, Balasubramaniam P, Chay SO, Rajan U, Lee HP. Idiopathic scoliosis. Prevalence and ethnic distribution in Singapore schoolchildren. J Bone Joint Surg Br. 1985;67:182–184. doi: 10.1302/0301-620X.67B2.3980521. [DOI] [PubMed] [Google Scholar]
- 8.Dickson R. Scoliosis in the community. Br Med J. 1983;19:615–618. doi: 10.1136/bmj.286.6365.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dommisse G. The management of scoliosis. S Afr Med J. 1970;44:1331–1335. [PubMed] [Google Scholar]
- 10.Figueredo U, James J. Juvenile idiopathic scoliosis. J Bone Joint Surg [Br] 1981;63:61–66. doi: 10.1302/0301-620X.63B1.7204475. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg C, Moore D, Fogarty E, Dowling F. Scoliosis: a review. Pediatr Surg Int. 2008;24:129–144. doi: 10.1007/s00383-007-2016-5. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg CJ, Dowling FE, Fogarty EE. Adolescent idiopathic scoliosis—early menarche, normal growth. Spine (Phila Pa 1976) 1993;18:529–535. doi: 10.1097/00007632-199304000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Gore D, Passehl R, Sepic S, Dalton A. Scoliosis screening: results of a community project. Pediatrics. 1981;67:196–200. [PubMed] [Google Scholar]
- 14.Green M. Bright Futures: guidelines for health supervision of infants, children, and adolescents. Arlington, VA: National Center for Education in Maternal and Child Health; 1994. [Google Scholar]
- 15.Grivas T, Vasiliadis E, Moizakis V, Mihas C, Koufopoulos G. Association between adolescent idiopathic scoliosis prevalence and age at menarche in different geographic latitudes. Scoliosis. 2006;1:9. doi: 10.1186/1748-7161-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossman T, Mazur J, Cummings R. An evaluation of the Adams forward bend test and the scoliometer in a scoliosis school screening setting. J Pediatr Orthop. 1995;15:535–538. doi: 10.1097/01241398-199507000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Huang S. Cut-off point of the scoliometer in school scoliosis screening. Spine. 1997;22:1985–1989. doi: 10.1097/00007632-199709010-00007. [DOI] [PubMed] [Google Scholar]
- 18.Katz D. A population-based study of school scoliosis screening. Phyl Ther. 2000;80:315. [Google Scholar]
- 19.Katz D, Durrani A. Factors that influence outcome in bracing large curves in patients with adolescent idiopathic scoliosis. Spine. 2001;26:2354–2361. doi: 10.1097/00007632-200111010-00012. [DOI] [PubMed] [Google Scholar]
- 20.Koukourakis I, Giaourakis G, Kouvidis G, Kivernitakis E, Blazos J, Koukourakis M. Screening school children for scoliosis on the island of Crete. J Spinal Disord. 1997;10:527–531. doi: 10.1097/00002517-199712000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Laulund T, Sojbjerg J, Horlyck E. Moire topography in school screening for structural scoliosis. Acta Orthop Scand. 1982;53:765–768. doi: 10.3109/17453678208992289. [DOI] [PubMed] [Google Scholar]
- 22.Lee CF, Fong DY, Cheung KM, Cheng JC, Ng BK, Lam TP, Mak KH, Yip PS, Luk KD (2010) Costs of school scoliosis screening: a large, population-based study. Spine (Phila Pa 1976). doi:10.1097/BRS.0b013e3181cbcc10 [DOI] [PubMed]
- 23.Lonstein J, Carlson J. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg [Am] 1984;66:1061–1071. [PubMed] [Google Scholar]
- 24.Lonstein JE, Bjorklund S, Wanninger MH, Nelson RP. Voluntary school screening for scoliosis in Minnesota. J Bone Joint Surg Am. 1982;64:481–488. [PubMed] [Google Scholar]
- 25.Morais T, Bernier M, Turcotte F. Age- and sex-specific prevalence of scoliosis and the value of school screening programs. Am J Public Health. 1985;75:1377–1380. doi: 10.2105/AJPH.75.12.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrissy RT. School screening for scoliosis. Spine (Phila Pa 1976) 1999;24:2584–2591. doi: 10.1097/00007632-199912150-00005. [DOI] [PubMed] [Google Scholar]
- 27.Nissinen M, Heliovaara M, Ylikoski M, Poussa M. Trunk asymmetry and screening for scoliosis: a longitudinal cohort study of pubertal schoolchildren. Acta Paediatr. 1993;82:77–82. doi: 10.1111/j.1651-2227.1993.tb12521.x. [DOI] [PubMed] [Google Scholar]
- 28.Ohtsuka Y, Yamagata M, Arai S, Kitahara H, Minami S. School screening for scoliosis by the Chiba University Medical School screening program. Results of 1.24 million students over an 8-year period. Spine (Phila Pa 1976) 1988;13:1251–1257. doi: 10.1097/00007632-198811000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Pin LH, Mo LY, Lin L, Hua LK, Hui HP, Hui DS, Chang BD, Chang YY, Yuan L. Early diagnosis of scoliosis based on school-screening. J Bone Joint Surg Am. 1985;67:1202–1205. [PubMed] [Google Scholar]
- 30.Reamy B, Slakey J. Adolescent idiopathic scoliosis: review and current concepts. Am Fam Physician. 2001;64:111–116. [PubMed] [Google Scholar]
- 31.Renshaw T. Screening school children for scoliosis. Clin Orthop. 1988;229:26–33. [PubMed] [Google Scholar]
- 32.Roach J. Adolescent idiopathic scoliosis. Orthop Clin North Am. 1999;30:353–365. doi: 10.1016/S0030-5898(05)70092-4. [DOI] [PubMed] [Google Scholar]
- 33.Rogala EJ, Drummond DS, Gurr J. Scoliosis: incidence and natural history. A prospective epidemiological study. J Bone Joint Surg Am. 1978;60:173–176. [PubMed] [Google Scholar]
- 34.Soucacos P, Zacharis K, Gelalis J, Soultanis K, Kalos N, Beris A, Xenakis T, Johnson E. Assessment of curve progression in idiopathic scoliosis. Eur Spine J. 1998;7:270–277. doi: 10.1007/s005860050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soucacos PN, Soucacos PK, Zacharis KC, Beris AE, Xenakis TA. School-screening for scoliosis. A prospective epidemiological study in northwestern and central Greece. J Bone Joint Surg Am. 1997;79:1498–1503. doi: 10.2106/00004623-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Span Y, Robin G, Makin M. Incidence of scoliosis in schoolchildren in Jerusalem: proceedings of the Israeli Orthopaedic Society. J Bone Joint Surg Br. 1976;58:379. [Google Scholar]
- 37.Stirling AJ, Howel D, Millner PA, Sadiq S, Sharples D, Dickson RA. Late-onset idiopathic scoliosis in children six to fourteen years old. A cross-sectional prevalence study. J Bone Joint Surg Am. 1996;78:1330–1336. doi: 10.2106/00004623-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Strayer L. The incidence of scoliosis in post-partum female on Cape Cod. J Bone Joint Surg Am. 1973;55A:436. [Google Scholar]
- 39.Weiss H, Weiss G, Schaar H. Incidence of surgery in conservatively treated patients with scoliosis. Pediatr Rehabil. 2003;6:111–118. doi: 10.1080/13638490310001593446. [DOI] [PubMed] [Google Scholar]
- 40.Whitby L. Screening for disease: definitions and criteria. Lancet. 1974;2:819–824. doi: 10.1016/S0140-6736(74)91082-4. [DOI] [PubMed] [Google Scholar]
- 41.Williams J. Criteria for screening: are the effects predictable. Spine. 1988;13:1178–1186. doi: 10.1097/00007632-198810000-00023. [DOI] [PubMed] [Google Scholar]
- 42.Willner S, Uden A. A prospective prevalence study of scoliosis in Southern Sweden. Acta Orthop Scand. 1982;53:233–237. doi: 10.3109/17453678208992208. [DOI] [PubMed] [Google Scholar]
- 43.Wong H, Hui J, Rajan U, Chia H. Idiopathic scoliosis in Singapore schoolchildren: a prevalence study 15 years into the screening program. Spine. 2005;30:1188–1196. doi: 10.1097/01.brs.0000162280.95076.bb. [DOI] [PubMed] [Google Scholar]
- 44.Wong HK, Balasubramaniam P, Rajan U, Chng SY. Direct spinal curvature digitization in scoliosis screening–a comparative study with Moire contourgraphy. J Spinal Disord. 1997;10:185–192. doi: 10.1097/00002517-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Yawn B, Yawn R, Hodge D, Kurland M, Shaughnessy W, Ilstrup D, Jacobsen S. A population-based study of school scoliosis screening. JAMA. 1999;282:1472–1474. doi: 10.1001/jama.282.15.1427. [DOI] [PubMed] [Google Scholar]
- 46.Yawn BP, Yawn RA. The estimated cost of school scoliosis screening. Spine (Phila Pa 1976) 2000;25:2387–2391. doi: 10.1097/00007632-200009150-00019. [DOI] [PubMed] [Google Scholar]
- 47.Zhang G, Li Z, Wei X, et al. Screening for scoliosis among schoolchildren in Beijing. Chin Med J. 1988;101:151–155. [PubMed] [Google Scholar]