Abstract
Priority of neurological decompression was regarded as necessary for scoliosis patients associated with Chiari I malformation in order to decrease the risk of spinal cord injury from scoliosis surgery. We report a retrospective series of scoliosis associated with Chiari I malformation in 13 adolescent patients and explore the effectiveness and safety of posterior scoliosis correction without suboccipital decompression. One-stage posterior approach total vertebral column resection was performed in seven patients with scoliosis or kyphosis curve >90° (average 100.1° scoliotic and 97.1° kyphotic curves) or presented with apparent neurological deficits, whereas the other six patients underwent posterior pedicle screw instrumentation for correction of spinal deformity alone (average 77.3° scoliotic and 44.0° kyphotic curves). The apex of the scoliosis curve was located at T7–T12. Mean operating time and intraoperative hemorrhage was 463 min and 5,190 ml in patients undergoing total vertebral column resection, with average correction rate of scoliosis and kyphosis being 63.3 and 71.1%, respectively. Mean operating time and intraoperative hemorrhage in patients undergoing instrumentation alone was 246 min and 1,450 ml, with the average correction rate of scoliosis and kyphosis being 60.8 and 53.4%, respectively. The mean follow-up duration was 32.2 months. No iatrogenic neurological deterioration had been encountered during the operation procedure and follow-up. After vertebral column resection, neurological dysfunctions such as relaxation of anal sphincter or hypermyotonia that occurred in three patients preoperatively improved gradually. In summary, suboccipital decompression prior to correction of spine deformity may not always be necessary for adolescent patients with scoliosis associated with Chiari I malformation. Particularly in patients with a severe and rigid curve or with significant neurological deficits, posterior approach total vertebral column resection is likely a good option, which could not only result in satisfactory correction of deformity, but also decrease the risk of neurological injury secondary to surgical intervention by shortening spine and reducing the tension of spinal cord.
Keywords: Scoliosis, Kyphosis, Chiari malformation, Adolescent, Corrective surgery
Introduction
With the widespread use of magnetic resonance imaging (MRI) since the early 1990s, Chiari type I malformation (CM I) and syringomyelia have been discovered with increasing frequency in patients who suffered from spinal deformities. It was reported that 50–90% patients with CM were associated with scoliosis, whereas scoliosis occurred in 15–65% of patients with CM I [11, 16]. The mechanism by which patients with CM or syringomyelia develop scoliosis remains unclear. It is postulated to be related with abnormal tension of spinal cord, abnormal or imbalanced proprioceptive sensory, or injury to dorsomedial and ventromedial nuclei of the spinal cord by syringomyelia, and denervation of paravertebral muscles [24]. Intriguingly, literatures have noted cases of developmental scoliosis in patients with only CM I and no syringomyelia [23].
Regardless of the various pathogenesis factors, when scoliosis in adolescents presents large curves or significant progression, surgical intervention must be undertaken. It is clear now that bracing for scoliosis with associated CM I or syringomyelia is ineffective [8]. In addition, most surgeons agree that spinal deformity treatment should always be preceded by a full neurosurgical evaluation and, if warranted, treatment of the neural axis malformation, because of the increased risk of neurologic deterioration during deformity correction [1, 7, 9]. In recent years, some authors have investigated the feasibility of directly posterior or circumferential approach for spinal deformity correction without neurosurgery, in patients of syringomyelia-associated scoliosis [17].
As a technical procedure, posterior approach total vertebral column resection (P-VCR) was firstly reported by Suk et al. [21] and became increasingly used in the treatment of rigid and severe spinal deformity. The procedure offered a significantly better Cobb correction and global balance when compared to traditional posterior spinal fusion techniques. Actually, the frequently used term P-VCR indicated resection of total anterior and posterior vertebral column elements on apical level through a single posterior approach, thereby creating an artificial spinal gap needing additional reconstruction. Based on our experiences, we believed that in scoliosis-presented preoperative spinal cord stretching from associated CM I, vertebral column shorting and indirect untethering of the spinal cord would be achieved though appropriate correction force utilized on the artificial gap in P-VCR procedures.
Thus, we undertook a retrospective study that involved 13 adolescents with CM I and thoracolumbar scoliosis who underwent single posterior approach instrumentation/P-VCR correction but had no neurosurgical decompression, at the authors’ unit from October 2004 to October 2007. The focus of the present study was on the operative maneuvers, correction efficiencies, and neurological prognosis of patients with scoliosis and CM I.
Materials and methods
Thirteen patients (six boys and seven girls) in this series were referred to the doctors for initial symptom of hump deformity caused by scoliosis. The protocol and consent forms were approved by the institutional appropriate review board of Kunming Medical University and written informed consent was obtained from all participants and their parents. None of the patients had symptoms of obvious headache, neck pain, or weakness. Eight of 13 patients presented with asymmetric abdominal reflex or tendon reflex of lower extremities, two patients with dissociation of superficial and deep sensory, two patients with hypermyotonia of lower extremities and tendon hyperreflexia, and one patient with relaxation of anal sphincter. Cervical and lumbosacral MRI was undertaken for all of the patients in whom CM I was diagnosed. The criteria to diagnose CM I were that the caudal edge of the cerebellar tonsils was found to extend 3 mm or more below the foramen magnum. Details of each patient are shown in Table 1. Tethering spinal cord syndrome on the lumbosacral area, trauma, infection, or other congenital craniocervical abnormalities were excluded. Configuration of scoliosis was assessed on standing anterior–posterior radiograph, lateral radiograph, and lateral side bending radiograph, with flexibility of scoliosis, trunk balance, and kyphosis associated with scoliosis evaluated concomitantly. Three-dimensional computer tomography (CT) scan of the curves was performed to rule out congenital vertebral deformity. Prior to the surgical procedures, all the patients were evaluated for their neurological functions by neurosurgeons and spine surgeons and individualized treatment plans were discussed.
Table 1.
Demographic data of the 13 patients
| Patients | Operation condition | |||||||
|---|---|---|---|---|---|---|---|---|
| Serial no. | Age (years) | Sex | Neural deficits preoperation | Follow-up (months) | Subgroups | Operation time (min) | Estimated blood loss (ml) | Complication |
| 1 | 16 | M | Hypermyotonia | 30 | VR | 340 | 5,100 | Pleura perforated |
| 2 | 13 | F | – | 40 | VR | 430 | 4,100 | Pleura perforated |
| 3 | 13 | M | – | 36 | VR | 410 | 6,500 | Pneumonia postoperation |
| 4 | 13 | M | – | 24 | VR | 490 | 4,100 | – |
| 5 | 17 | M | Anal sphincter relaxation | 36 | VR | 600 | 7,600 | Abnormal coagulation during operation |
| 6 | 13 | F | – | 29 | VR | 320 | 4,500 | – |
| 7 | 18 | F | Hypermyotonia | 24 | VR | 650 | 4,400 | – |
| 8 | 15 | F | – | 48 | CA | 280 | 1,050 | – |
| 9 | 12 | F | – | 30 | CA | 310 | 1,400 | – |
| 10 | 17 | M | – | 36 | CA | 250 | 1,000 | – |
| 11 | 19 | F | – | 31 | CA | 210 | 2,100 | – |
| 12 | 15 | M | – | 30 | CA | 186 | 1,400 | – |
| 13 | 12 | F | – | 24 | CA | 240 | 1,750 | – |
| Average | 14.8 | – | – | 32.2 | – | 362.8 | 3,461.5 | – |
In patients with severe deformity or obvious neurological dysfunctions such as hypermyotonia or relaxation of anal sphincter, P-VCR and pedicle instrumentation were planned. Before the operation, the physical situation including lung ventilation function and nutritional status of the patients were fully evaluated. The remaining patients underwent posterior pedicle instrumentation correction alone. Methylprednisolone (1,000 mg) was used as routine during the operative procedure, and wake-up tests were done 2–3 times. Somatosensory evoked potential (SSEP) or magnetic motor evoked potential (MEP) was not used.
P-VCR and deformity correction
The operative procedure was a modified method based on the technique reported by Suk and Lenke [14, 21]. The patients were placed in prone position under general anesthesia and a posterior incision was made. Following insertion of the pedicle screws, the P-VCR began with a complete exposure of the vertebral column elements at the apex of the deformity. First, bilateral transverse processes were removed, and lateral wall of the pedicle and vertebral body were exposed under meticulous subperiosteal dissection on each side though the costotransverse approach. Parts of the corresponding ribs were removed and intercostal vessels were ligated, respectively. Second, posterior elements include lamina, spinous process, and bilateral superior and inferior articular processes were removed one by one. Most of the anterior portion of the vertebral body could be removed before the dural canal was exposed to reduce blood loss during the procedure. Under visual control, the pedicle on one side and the thin shell of bony posterior vertebral wall beneath the dural sac were removed by using rongeurs, curette, and pituitary forceps, and then the pedicle screws were connected on the working side of the vertebral column with a temporary rod contoured to the shape of the deformity without any attempt at correction. Subsequently, the same surgical process was performed on the opposite side and the adjacent intervertebral discs were eradicated.
At the completion of resection, an artificial gap for further correction was created and the stability of vertebral column was maintained completely by the temporary fixation. Compression under the cephalad and caudad sides to the gap could reduce its height and shorten the vertebral column. Then, based on the exchanged-rods technique [21], in situ rod bending, opening, closing, additional compression forces, and derotation maneuver that relied on long arm screws were combined to correct the deformity.
After deformity correction, the height of the anterior interbody gap was measured. If the residual gap from the vertebral column resection was >1 cm, a titanium mesh filled with bone harvested from incised vertebral body was inserted from the outer side of the rod, and when it was <1 cm, impacted cancellous autograft was used to fill in the gap.
Posterior pedicle instrumentation correction alone
In case of pedicle screw-rod correction procedures applied alone, when apex region of the curve was completely released, the convex side of the curve was compressed to achieve mainly correction of the deformity and the rod was then locked into place on concave side. Other 3-D correction forces used for the treatment of idiopathic scoliosis were then applied.
Postoperation care
A–P radiograph and lateral radiograph of spine were performed routinely in order to get the message of deformity correction. Check-up of neural system was documented 3 days and 3 weeks after operation. Follow-up was performed to examine the neurological functions as well as implant and fusion on radiograph 3, 6, 12 and 24 or more months after operation.
Results
The average age of the patients was 14.8 years (range 12–19 years). Only one patient with CM I was not associated with syringomyelia. The remaining 12 patients harbored syrinxes that were located at the cervical spine or cervicothoracic spine. No posterior craniocervical decompression but one-stage posterior correction of spinal deformity was performed. The fixation and fusion spanned from the upper thoracic spine to the lumber spine, with three patients caudally ended at L2, two at L3, seven at L4, and one at L5. The correction rate is shown in Table 2.
Table 2.
Radiographic data on 13 patients
| Serial no. | Syrinx involved | Apex site | Curve direct | Deformity on coronal plane | Deformity on sagittal plane | Fusion involved | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preoperation (°) | Postoperation (°) | Correction (%) | Preoperation (°) | Postoperation (°) | Correction (%) | |||||
| 1 | C3–C7 | T9 | L | 105 | 40 | 61.9 | 94 | 28 | 70.2 | T3–L3 |
| 2 | C3–C6 | T9 | R | 110 | 44 | 60.0 | 95 | 36 | 62.1 | T2–L4 |
| 3 | C2–T3 | T7 | R | 108 | 42 | 61.1 | 93 | 24 | 71.2 | T2–L2 |
| 4 | – | T9 | R | 105 | 32 | 69.5 | 87 | 28 | 67.8 | T2–L2 |
| 5 | C2–C6 | T8 | R | 82 | 34 | 61.0 | 115 | 37 | 67.8 | T2–L4 |
| 6 | C5–C7/T3–T6 | T9 | L | 98 | 27 | 72.4 | 68 | 18 | 73.5 | T3–L3 |
| 7 | C5–C6 | T8 | R | 93 | 38 | 61.2 | 128 | 26 | 79.7 | T2–L5 |
| 8 | C4–T6 | T10 | R | 83 | 32 | 61.4 | 65 | 22 | 66.2 | T4–L4 |
| 9 | C6–T2 | T10 | R | 85 | 47 | 44.7 | 52 | 30 | 42.3 | T2–L2 |
| 10 | C3–C7 | T12 | L | 98 | 55 | 43.9 | 65 | 35 | 46.2 | T2–L4 |
| 11 | C4–T10 | T8 | R | 56 | 12 | 78.6 | 0 | 21 | – | T3–L4 |
| 12 | C7–T6 | T12 | L | 90 | 25 | 72.2 | 45 | 8 | 82.2 | T2–L4 |
| 13 | C2–T1 | T10 | R | 52 | 11 | 78.8 | 37 | 28 | 24.3 | T3–L4 |
| Average | – | – | 89.6 | 33.8 | 62.2 | 78.7 | 26.7 | 66.1 | – | |
Mean operating time and amount of intraoperative hemorrhage in seven patients undergoing P-VCR (group VR, vertebral resection) was 463 min and 5,190 ml, respectively, whereas that for single-pedicle instrumentation correction (group CA, correction alone) was 246 min and 1,450 ml, respectively.
Neural physical examination of each patient was not worse than the preoperative situation. No symptoms of nerve root palsy or pain/numbness were found. Pleural membrane perforation occurred in two patients of group VR during operation and thoracic drainage was performed. Coagulation mechanism disorder was noticed during operation in two cases of group VR, and pneumonia after operation in one case of group VR. No superficial or deep wound infection occurred.
All of the patients in present study were followed-up, with a mean follow-up period of 32.2 months (range 24–48 months). At last follow-up, none of the patients demonstrated failure of instrumentations. Solid bone fusion was achieved in all patients with P-VCR (Fig. 1a–n). The scoliotic Cobb angle was lost by 4.8% (range 1.2–8.3%) and kyphotic Cobb angle decreased by 1.2% (range 0–3.7%) contrasted between that at postoperation and that at final follow-up. At the follow-up, compared with preoperative situation, there was no difference in sensory disturbance or asymmetric superficial reflex, however, two cases with hypermyotonia experienced a significant decrease of muscle tension after P-VCR and one case with relaxation of anal sphincter manifested recovery at 6 months after operation.
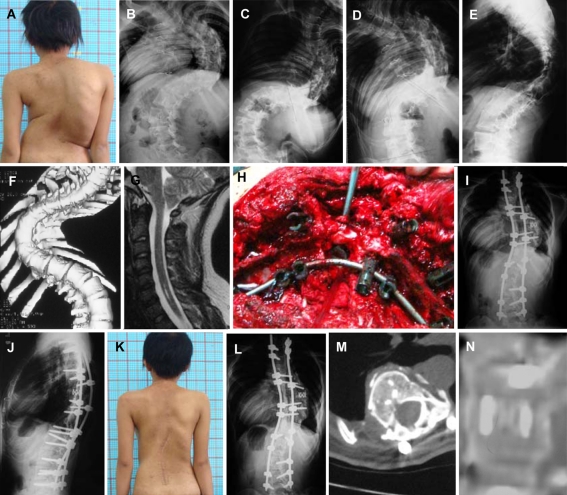
Fig. 1.
An 18-year-old girl who was operated on for congenital heart disease (case 7, group VR). a Preoperative clinical feature. b–f Preoperaitve radiographs showed severe and rigid curves on coronal and sagittal planes. g CM I with cervical syringomyelia. h Posterior approach to resect the vertebral of T8 with pedicle screw fixation. i, j Deformity correction was shown on the radiographs postoperative, with titanium mesh and PSF T2–L5. k 24-month follow-up outlook image. l Good maintenance of correction effect was shown at the final 24-months follow-up. m, n Computer tomography images showed solid bone fusion in the gap
Discussion
There is evidence in the literature that neurosurgery alone could stabilize or improve the condition of spinal deformity in children with mild scoliosis associated CM I [19]. However, the results are not nearly as good in adolescents with a large curve. Certain prognostic factors indicated that curve progression after neurosurgery may guide the surgeon in determining treatment options. Brockmeyer and colleagues stated that patients older than 12 years or with a curve greater than 50° before decompression could hardly have improvement of the scoliosis during the follow-up [5]. Flynn and colleagues demonstrated that further progression of scoliosis at follow-up after neurosurgical management was associated with greater age (>11 years), presence of neurological symptoms, rotation of the vertebral body, double scoliosis curve patterns, and larger curve (>50° kyphosis or >40° curve) at presentation [10]. In addition, Bhangoo et al. studied 13 patients with symptomatic CM I and clinically detected scoliosis who underwent primary cranio-vertebral decompression. The patients requiring scoliosis corrective surgery had a mean age of 158 months and a 76° Cobb angle [2]. All adolescent patients in our series presented large and predictable progressed scoliotic curves, therefore proper spinal deformity corrections and fusions were essential.
In general, correction procedures for patients with scoliosis associated with CM I or syringomyelia are often considered to have higher risks of iatrogenic neural structure injuries for the following reasons: (1) cerebellar tonsilar descent causes hypertension of the spinal cord, and more traction on spinal cord seems unavoidable in correction procedure; (2) the expanded syrinx compressed spinal cord and reduced its blood supply; and (3) pressure of the cerebrospinal fluid pathways significantly changed intraoperatively. Therefore, traditional theories advocated that decompression of craniocervical bone and fossa elements and shunting of the syrinx should be performed prior to spinal fusion in those cases, and 3–6 months later, correction procedures be undertaken to improve neurologic safety [7].
Qiu et al. [17] reported a series of patients with syringomyelia-associated scoliosis in whom direct spinal deformity correction was performed without neurosurgical decompression. Ultimate safety correction extent had been determined though Halo traction or fulcrum pressurized radiograph preoperatively, and posterior or combined anterior–posterior approach spinal surgery results in satisfactory correction outcomes. However, the clinical cases presented in the report only corresponded to patients with no neurological deficits and mild to moderate spinal curves. In addition, the operative strategies were single pedicle instrumentation and deformity correction but without osteotomy or VCR.
In the present study, group CA included patients with a curve <90° (average 77.3° scoliotic and 44.0° kyphotic curves) and no neurological deficits. The postoperative correction rates in this group for coronal and sagittal plane were 60.8 and 53.4%, respectively. In contrast, Bradley et al. reported 13 cases that underwent initial neurosurgical decompression and involved adolescents. Based on their research, the average thoracic scoliosis and kyphosis measured 46° (29–69°) and 71° (31–119°), and average correction from the deformity surgery was 48% (6–83%) [3].
Neurological decompression was obviated in our series, based on the following considerations. First of all, scoliosis progression was obviously predictable in those patients, whether they received neurosurgery previously or not. In our series, it could be observed that adolescents with CM I and scoliosis experienced significant progression curves, which became the imperative point of surgical care. Also, many neurosurgeons advocated cautious observation for asymptomatic syringomyelia rather than prophylactic neurological operation [12]. Moreover, despite the increased neurologic complications from scoliosis surgery that have been referred in the literature [7, 18, 22], the reports described included undiagnosed syrinx patients, and varying correction procedures. Tomlinson et al. once reported a case of transient neurologic deficit. Two weeks following spinal fusion, the patient developed headaches, dysphagia, and neck pain. An MRI revealed syringomyelia [22]. Schlesinger et al. included a patient who became acutely paraplegic after scoliosis surgery. The patient underwent a laminectomy with no improvement [18]. There was no neurological deterioration encountered in the group. Before operation, detailed neurosurgical evaluation was warranted. During the procedure, gradual correction of deformities primarily resulted from convexity segmental compression. Additionally, the residual angle of the main curve in preoperative convexity bending radiography often indicated the ultimate correction destination (Fig. 2a–i), and it played a positive role in the protection of spinal cord.
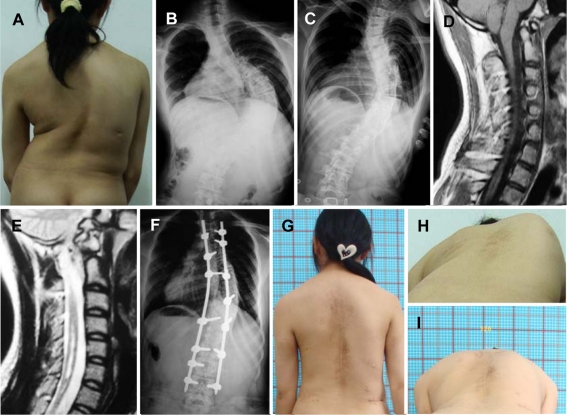
Fig. 2.
A 15-year-old girl with thoracic scoliosis (case 8, group CA). a Preoperative clinical feature. b, c Right scoliosis of 83° and bending film showing a flexible curve. d, e The cerebellar tonsils herniation with syringomyelia ranged C4–T6 can be noticed. f Posterior correction with instrumentation alone (T4–L4) was performed, and scoliosis was corrected to 32°. g Good maintenance of correction effect was shown at 24-month follow-up. h, i Clinical correction of hump deformity was shown on pre- and postoperative photos
The mean preoperative Cobb angle of scoliosis and kyphosis in group VR was more than 90° (average 100.1° scoliotic and 97.1° kyphotic curves), whereas the correction rates for scoliosis and kyphosis were 63.3 and 71.1%, respectively. Based on our review of current literature, we have not found any previous report involving the same procedure and treatment of severe spinal deformity with CM deformity.
We attributed the optimal surgical outcome to several factors concerning the procedure. The key points of P-VCR included removing anterior and posterior vertebral column elements and then creating a correction gap that divided rigid spine into cranial and caudal portions, and reconstructed the new spinal geometry by pedicle instrumentation. First, the pre-contoured rod for temporary fixation was required to match the curvature of the deformity to avoid any tension of spinal cord from anterior or lateral direction. In addition, at the primal step of the correction procedure, compressing the correction gap could significantly decrease the risk of spinal cord tension. Meanwhile, excessive displacement and overlapping of the dural sac could be observed and prompt adjustment could be performed during the whole procedure. The clinical observation and animal experiences by Kawahara et al. [13] also confirmed that the spine shortened within one-third of the height of the vertebral would not lead to the functional change of the spinal cord. In our opinion, an observed wrinkle or kink of the dura sac no greater than 2–3 cm was admissible and would not lead to positive neurological impact. After pedicel screw insertion and correction procedures were completed, a routine wake-up test must be undertaken to confirm that there was no neurological deterioration.
During spinal surgery, monitoring MEP is a means of assessing the intraoperative integrity of corticospinal pathways [15]. Due to the limitation of the situations, intra-operative SSEP or MEPs was not monitored in our study. Although Bridwell et al. [4] also suggested that potentials of the spinal cord could not monitor all the neurologic deficits, and routine wake-up tests were conducted by the end of their spinal correction procedure, it should be admitted that the lack of real-time and continuous monitoring decreased the maximum efficiency of the deformity correction. However, P-VCR provided the opportunity to directly observe and palpate the tension of dural sac circumferentially, and taking appropriate compression if necessary, to obtain a balanced spine with controllable translation of the spinal cord.
Tethered cords must be treated by releasing the affected cord and this offers the best opportunity to stabilize or improve the scoliosis [6]. The present study indicated that P-VCR can effectively avoid spinal cord supertension. Meanwhile, we noticed that the length of the whole spine can be shortened during the procedure, thus indirectly reducing neural tension on the suboccipital level. It should be noted that in patients with preoperatively presented hypermyotonia or relaxation of anal sphincter whose neural deficits may result from tethered cord due to CM I, neurological function improved dramatically during follow-up. Lenke et al. recently reported a series of 35 consecutive patients undergoing P-VCR for severe pediatric spinal deformity. Analogously, there were no spinal cord-related neurologic deficits in any of these patients. They also mentioned that circumferential access makes the posterior VCR procedure safer from a neurologic prospective [14].
P-VCR is an effective alternative for severe rigid scoliosis. However, it is a highly technical procedure and should only be performed by an experienced surgical team [20]. In the present study, mean operating time and amount of hemorrhage in group VR were considerably higher than that in group CA. What needs to be emphasized is that the crucial factors that are disadvantageous to the P-VCR procedure were great bleeding volume and frequent non-neurologic complications.
Lastly, concerning extensional details, we suggest that surgeons should be more cautions with respect to end vertebrae selection in those non-idiopathic scoliosis. We advocate that confirmation of lower-end vertebra of scoliosis must meet three criteria: that the vertebra are bisected by the sacral midline, that they show adequate flexibility at the bending position, and almost complete derotation. Surgeons are not supposed to try selective fusion; otherwise, patients may be susceptible to postoperative decompensation. This opinion is similar to that of Bradley and colleagues [3].
The main limitation of this study was the exclusion of patients or procedures from statistical analyses. However, this was obligatory because of the diversity of presentation and treatment protocols in the small number of patients. In addition, our mean follow-up of 32.2 months was still not enough to observe the outcome of all procedures, as subsequent additional evaluation, include MRI scan of craniocervical district and evolution of fused spinal curve may continue to be required throughout the longer period of their lives.
In conclusion, suboccipital decompression prior to correction of spine deformity may not always be necessary for adolescent patients with scoliosis associated with CM I. Particularly in patients with severe and rigid curve or with significant neurological deficits, P-VCR is likely a good option. It could not only result in satisfactory correction of deformity but could also decrease the risk of neurological injury secondary to surgical intervention by shortening the spine and reducing the tension of the spinal cord during the procedures.
Conflict of interest
No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1.Akhtar OH, Rowe DE. Syringomyelia-associated scoliosis with and without the Chiari I malformation. J Am Acad Orthop Surg. 2008;16:407–417. doi: 10.5435/00124635-200807000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Bhangoo R, Sgouros S. Scoliosis in children with Chiari I-related syringomyelia. Childs Nerv Syst. 2006;22:1154–1157. doi: 10.1007/s00381-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 3.Bradley LJ, Ratahi ED, Crawford HA, Barnes MJ. The outcomes of scoliosis surgery in patients with syringomyelia. Spine (Phila Pa 1976) 2007;32:2327–2333. doi: 10.1097/BRS.0b013e3181557989. [DOI] [PubMed] [Google Scholar]
- 4.Bridwell KH, Lewis S, Rinella A, Lenke LG, Baldus C, Blanke K. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. J Bone Joint Surg Am. 2004;86(Suppl 1):44–50. doi: 10.2106/00004623-200403001-00007. [DOI] [PubMed] [Google Scholar]
- 5.Brockmeyer D, Gollogly S, Smith JT. Scoliosis associated with Chiari I malformations: the effect of suboccipital decompression on scoliosis curve progression: a preliminary study. Spine. 2003;28:2505–2509. doi: 10.1097/01.BRS.0000092381.05229.87. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso M, Keating RF. Neurosurgical management of spinal dysraphism and neurogenic scoliosis. Spine (Phila Pa 1976) 2009;34:1775–1782. doi: 10.1097/BRS.0b013e3181b07914. [DOI] [PubMed] [Google Scholar]
- 7.Charry O, Koop S, Winter R, Lonstein J, Denis F, Bailey W. Syringomyelia and scoliosis: a review of twenty-five pediatric patients. J Pediatr Othop. 1994;14:309–317. doi: 10.1097/01241398-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Eule JM, Erickson MA, O’Brien MF, Handler M. Chiari I malformation associated with syringomyelia and scoliosis: a twenty-year review of surgical and nonsurgical treatment in a pediatric population. Spine (Phila Pa 1976) 2002;27:1451–1455. doi: 10.1097/00007632-200207010-00015. [DOI] [PubMed] [Google Scholar]
- 9.Farley FA, Song KM, Birch JG, Browne R. Syringomyelia and scoliosis in children. J Pediatr Orthop. 1995;15:187–192. [PubMed] [Google Scholar]
- 10.Flynn JM, Sodha S, Lou JE, Adams SB, Jr, Whitfield B, Ecker ML, Sutton L, Dormans JP, Drummond DS. Predictors of progression of scoliosis after decompression of an Arnold Chiari I malformation. Spine (Phila Pa 1976) 2004;29:286–292. doi: 10.1097/01.BRS.0000109884.05548.68. [DOI] [PubMed] [Google Scholar]
- 11.Hankinson TC, Klimo P, Jr, Feldstein NA, Anderson RC, Brockmeyer D. Chiari malformations, syringohydromyelia and scoliosis. Neurosurg Clin N Am. 2007;18:549–568. doi: 10.1016/j.nec.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Haroun RI, Guarnieri M, Meadow JJ, Kraut M, Carson BS. Current opinions for the treatment of syringomyelia and Chiari malformations: survey of the pediatric section of the American Association of Neurological Surgeons. Pediatr Neurosurg. 2000;33:311–317. doi: 10.1159/000055977. [DOI] [PubMed] [Google Scholar]
- 13.Kawahara N, Tomita K, Kobayashi T, Abdel-Wanis ME, Murakami H, Akamaru T. Influence of acute shortening on the spinal cord: an experimental study. Spine (Phila Pa 1976) 2005;30:613–620. doi: 10.1097/01.brs.0000155407.87439.a2. [DOI] [PubMed] [Google Scholar]
- 14.Lenke LG, O’Leary PT, Bridwell KH, Sides BA, Koester LA, Blanke KM. Posterior vertebral column resection for severe pediatric deformity: minimum two-year follow-up of thirty-five consecutive patients. Spine (Phila Pa 1976) 2009;34:2213–2221. doi: 10.1097/BRS.0b013e3181b53cba. [DOI] [PubMed] [Google Scholar]
- 15.Lo YL, Dan YF, Tan YE, Nurjannah S, Tan SB, Tan CT, Raman S. Intraoperative motor-evoked potential monitoring in scoliosis surgery: comparison of desflurane/nitrous oxide with propofol total intravenous anesthetic regimens. J Neurosurg Anesthesiol. 2006;18:211–214. doi: 10.1097/01.ana.0000211007.94269.50. [DOI] [PubMed] [Google Scholar]
- 16.Ono A, Ueyama K, Okada A, Echigoya N, Yokoyama T, Harata S. Adult scoliosis in syringomyelia associated with Chiari I malformation. Spine (Phila Pa 1976) 2002;27:E23–E28. doi: 10.1097/00007632-200201150-00011. [DOI] [PubMed] [Google Scholar]
- 17.Qiu Y, Wang B, Zhu ZZ, Lv JY, Yu Y, Zhu LH. Clinical manifestation and treatment strategy of scoliosis associated with Chiari malformation and/or syringomyelia. Chin J of Orthopaedics. 2003;23:564–567. [Google Scholar]
- 18.Schlesinger EB, Antunes JL, Michelsen WJ, Louis KM. Hydromyelia: clinical presentation and comparison of modalities of treatment. Neurosurgery. 1981;9:356–365. doi: 10.1227/00006123-198110000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Sengupta DK, Dorgan J, Findlay GF. Can hindbrain decompression for syringomyelia lead to regression of scoliosis? Eur Spine J. 2000;9:198–201. doi: 10.1007/s005860000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suk SI, Chung ER, Kim JH, Kim SS, Lee JS, Choi WK. Posterior vertebral column resection for severe rigid scoliosis. Spine (Phila Pa 1976) 2005;30:1682–1687. doi: 10.1097/01.brs.0000170590.21071.c1. [DOI] [PubMed] [Google Scholar]
- 21.Suk SI, Kim JH, Kim WJ, Lee SM, Chung ER, Nah KH. Posterior vertebral column resection for severe spinal deformities. Spine (Phila Pa 1976) 2002;27:2374–2382. doi: 10.1097/00007632-200211010-00012. [DOI] [PubMed] [Google Scholar]
- 22.Tomlinson RJ, Jr, Wolfe MW, Nadall JM, Bennett JT, MacEwen GD. Syringomyelia and developmental scoliosis. J Pediatr Othop. 1994;14:580–585. doi: 10.1097/01241398-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Tubbs RS, Doyle S, Conclin M, Oakes WJ. Scoliosis in a child with Chiari I malformation and the absence of syringomyelia: case report and a review of the literature. Childs Nerv Syst. 2006;22:1351–1354. doi: 10.1007/s00381-006-0079-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhu ZZ, Qiu Y, Wang B, Yu Y, Qian B, Zhu F. Abnormal spreading and subunit expression of junctional acetylcholine receptors of paraspinal muscles in scoliosis associated with syringomyelia. Spine (Phila Pa 1976) 2007;32:2449–2454. doi: 10.1097/BRS.0b013e3181573d01. [DOI] [PubMed] [Google Scholar]