Abstract
The aetiology of idiopathic scoliosis (IS) remains unknown, but there is growing support for the possibility of an underlying neurological disorder. Functional magnetic resonance imaging (fMRI) can characterize the abnormal activation of the sensorimotor brain network in movement disorders and could provide further insights into the neuropathogenesis of IS. Twenty subjects were included in the study; 10 adolescents with IS (mean age of 15.2, 8 girls and 2 boys) and 10 age-matched healthy controls. The average Cobb angle of the primary curve in the IS patients was 35° (range 27°–55°). All participants underwent a block-design fMRI experiment in a 1.5-Tesla MRI scanner to explore cortical activation following a simple motor task. Rest periods alternated with activation periods during which participants were required to open and close their hand at an internally paced rate of approximately 1 Hz. Data were analyzed with Statistical Parametric Mapping (SPM5) including age, sex and laterality as nuisance variables to minimise the presence of bias in the results. Compared to controls, IS patients showed significant increases in blood oxygenation level dependent (BOLD) activity in contralateral supplementary motor area when performing the motor task with either hand. No significant differences were observed when testing between groups in the functional activation in the primary motor cortex, premotor cortex and somatosensory cortex. Additionally, the IS group showed a greater interhemispheric asymmetry index than the control group (0.30 vs. 0.13, p < 0.001). This study demonstrates an abnormal pattern of brain activation in secondary motor areas during movement execution in patients with IS. These findings support the hypothesis that a sensorimotor integration disorder underlies the pathogenesis of IS.
Keywords: Idiopathic scoliosis, Functional magnetic resonance imaging, Aetiology, Sensorimotor cortex
Introduction
The cause and pathophysiology of adolescent Idiopathic scoliosis (IS) is not well understood despite decades of intensive research. A subclinical central nervous system alteration has been proposed as a causative factor given the fact that IS patients show abnormalities in equilibrium [1–4], asymmetries in the ability to reproduce articular angles [5, 6] and proprioception [7–9]. As compared to control subjects, patients with IS show abnormal static and dynamic balance control [10–14].
Although an increasing number of studies suggest that an anomalous sensorimotor integration critically contributes to the cause of IS, there is uncertainty about the level of the central nervous system accounting for the dysfunction. Postural tone, equilibrium and proprioception are mediated by the posterior columns, vestibular nuclei and visual afferences, and ultimately require a correct integration at subcortical and cortical level. Some studies have failed to find alteration in somatosensory evoked potentials (SSEP), which assess posterior column function [15, 16]. However, others did find SSEP anomalies in a variable percentage of IS patients [11, 17, 18]. Alterations of the postural and ocular motor control found in IS have been suggested to support abnormal processing of sensory afferences at cerebral cortex, rather than being due to conduction disturbances [3, 16, 17, 19]. Early in the 1980s, Herman [3] proposed that scoliosis may represent a specific impairment of higher cortical function. Moreover, the presence of electroencephalographic disturbances [20–22], cognitive vestibular integration impairment [23], and dichotic listening asymmetries support the notion of higher cortical processing alteration in IS patients [24]. Machida [17] found that the latency of cortical potential N37 after stimulation of tibial nerve was longer in IS patients than in controls, suggesting a dysfunction at cortical level. A recent study that explored the facilitatory and excitatory intracortical motor circuits using paired pulse transcranial magnetic stimulation, found an abnormally increased motor cortical excitability in IS as compared to healthy controls and patients with congenital scoliosis [25].
In the present study, we used functional magnetic resonance imaging (fMRI) to further evaluate cortical and subcortical function in patients with IS. Functional MRI has been widely employed to study the hemodynamic changes of the human cortex during physiological activation, including motor, somatosensory, visual and verbal tasks. This technique permits to detect brain activation based on blood oxygenation level dependent (BOLD) effects, which depend upon the increased paramagnetic properties of deoxyhemoglobin compared with oxyhemoglobin [26]. Neural activity is linearly correlated with an increase in regional cerebral blood flow and neural oxygen intake. During neural activation there is an increase in oxygen consumption but there is also a greater increase in regional blood flow, consequently the relative concentration of capillary and venous deoxyhemoglobin in activated brain areas is reduced [26, 27]. Thus, changes in oxygen consumption and regional cerebral blood flow lead to an increase in fMRI BOLD signals, providing an indirect measure of neuronal activity. Functional MRI is a very powerful method to map brain functions with relatively high spatial and temporal resolution and has contributed significantly to the noninvasive elucidation of physiological mechanism associated with human motor control. Motor control depends on multiple cortical brain regions that are interconnected to provide adequate sensorimotor integration and coordination in a networked or cooperative manner. Some studies using fMRI have found abnormal activation of the motor cortical network in some forms of idiopathic dystonia [28–30], where a defective sensorimotor integration has been related to its pathogenesis [31–33].
Therefore, we hypothesized that an abnormal sensorimotor integration disorder at cortical level may underlie in the pathogenesis of IS, and that fMRI might be able to provide critical support for such a notion, as in the case in idiopathic dystonia. To our knowledge this issue has not been previously investigated using such a neuroimaging approach. The objective of the present study was to evaluate the pattern of brain motor activation in patients with IS, as shown by fMRI, and contrast the findings with those in age-matched healthy controls.
Methods
Subjects
Twenty subjects were included in the study: 10 adolescents with IS and 10 healthy age-matched controls. The IS Group comprised 8 girls and 2 boys with a mean age of 15.2 years (14–16). In all of them the neurological examination was normal. The age of detection of the spinal deformity was 11 years or later. None of the patients had been operated on and all of them wore orthopaedic corsets. The level of spinal deformity was right thoracic in six subjects, right thoracolumbar in two, left thoracic in one, and lumbar in one. The average deformity was 35° Cobb (27°–55°). As part of the standard clinical management, spine MRI exams were done in the subject with left thoracic curve and in three subjects with rapid progression. The spine MRIs failed to reveal any further pathologies in all four instances.
Healthy teenage volunteers were recruited from several educational centres as healthy controls. In the control group there were 7 girls and 3 boys with a mean age of 14.7 years (14–16). Prior to inclusion in the study, an orthopaedic exam failed to detect any spine deformities in any of these adolescents. Medical and neurological exams were similarly unremarkable and none had a history of significant illnesses or previous neurological disease. All had a negative family history for neurological disorders, particularly dystonia.
None of the participants in either groups, IS patients and controls, were taking any chronic or neuroactive medications. Handedness was assessed with the Oldfield Questionnaire [34]. Of the subjects in the IS group, eight were right-handed and two left-handed; while in the control group one subject was left handed. Prior to the inclusion in the study, the participants and their parents gave written informed consent to the study, which had been approved by the local Ethics Committee.
Activation paradigm
Previous to the exam, the participants were instructed to open and close one fist for 60 s approximately at a rate of 1 time per second (1 Hz), while the other hand remained at rest. Participants practiced this movement condition once with each of their hands before the experiment started. During the experiment the participants lay supine and were requested to stay at rest (rest blocks) or to perform the motor task (movement blocks) at an internally self-paced rate, without any external pacing or visual trigger. Their head was accommodated in a soft-padded frame to minimize head movements. Movement blocks with the right or left hand were performed in random order. The protocol consisted in eight blocks of 60 s alternating rest with movement blocks, thus each participant completed four movement blocks with each hand intercalated with four rest blocks. An independent examiner observed the participant during the entire procedure to ascertain a proper execution of motor task and rest periods. The radiology team was blinded to the allocation group of each participant.
Functional MRI
All the experiments were performed on a 1.5-Tesla MRI scanner (Philips Intera, Best, The Netherlands) equipped with a circularly polarized volume head coil. At the beginning of each fMRI session a high-resolution T1-weighted anatomical MRI study was acquired, which was then used to correctly localize the areas of brain activation.
Eighty dynamics were acquired in 8 blocks (4 rest blocks and 4 active blocks interleaved) of 60 s duration each. Both the right and left hands were evaluated in different fMRI runs. Data were acquired with an EPI T2*-weighted sequence (TR = 59 ms, TE = 40 ms, voxel size 1.72 × 1.72 × 5.00, no inter-slice gap, FA = 55º, 12 slices). Due to temporal and spatial resolution requirements the dynamic series covered only the supracallosal brain (60 mm coverture in the craniocaudal direction).
Data were analyzed with Statistical Parametric Mapping (SPM5). Statistical measurements were made under the General Linear Model (GLM) framework [35]. The constructed GLM included a group condition (patients vs. controls) and three nuisance variables (age, sex and laterality) to minimize the presence of bias in the results.
Using SPM one-tailed contrasts, statistical parametric maps were obtained by performing t tests across voxels, in order to measure differences among groups. Significance criteria were established as a p < 0.005, after correction for multiple comparisons. In order to minimize the presence of false positives, a spatial filter was applied to consider only the clusters with a minimum size of 35 voxels (expected number of voxels per cluster, k ≥ 35).
Activation differences between rest and movement conditions were quantified in subtraction images. The signal intensity increase (expressed as a percentage signal change) was measured using 3.44 mm2 regions of interest (ROIs) placed on sites where greater changes were observed, and specifically in the primary sensorimotor cortex (PMC), premotor area (PMA), and anterior cingulate cortex.
To define a region of interest (ROI) around the selected areas, Pickatlas utility [36] was employed. This tool is an automated software for generating ROI masks in the Talairach space. The binary ROI masks were overlaid with the GLM maps to define the areas to study.
Results were labelled with the Automated Anatomical Labelling (AAL) software [37]. Areas-identifying coordinates in Talairach space were determined by the maximum student t value in the corresponding brain area. To evaluate the possible difference of activation produced by the movement of the dominant and the non-dominant hand, we used the asymmetry index. The asymmetry index for each group was calculated as abs [voxels (left − right)]/sum [voxels (left + right)] [38]. The value of asymmetry ranges from 0 (low asymmetry) to 1 (high asymmetry). Only the areas consistently activated were compared and two tailed t test was used for comparisons of continuous variables between IS patients and controls. To assess the interaction of the different associative sensorimotor areas involved in movement execution, the Pearson correlation coefficient was calculated between the different ROI.
Results
Within group analysis
No abnormalities in sulcal pattern or macroanatomical structures were noted in the high-resolution MRI studies in any of the patients or controls.
The activation (movement) paradigm, compared to rest, produced in both controls (Fig. 1a; Table 1) and patients (Fig. 1b; Table 2) a significant activation in the contralateral primary sensorimotor area and a variable amount of activation in premotor cortex areas known to be involved in preparing and executing movements (Table 1).
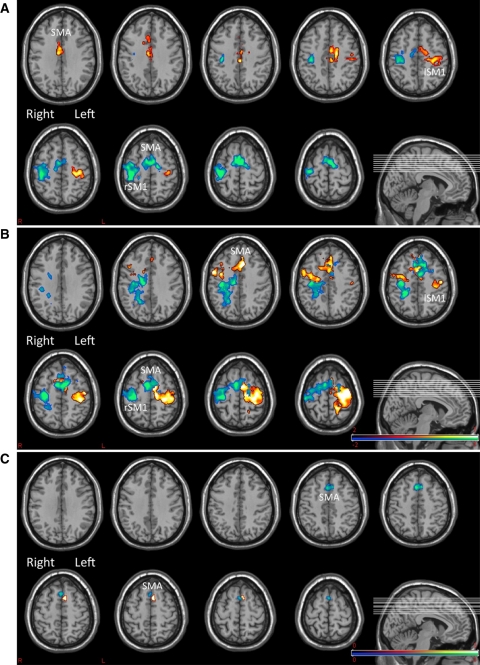
Fig. 1.
Areas of significant activation in control subjects (a), and patients with IS (b). c Shows the conjunction analysis revealing the differences in motor task activation across the two groups. In all figures red-yellow reveals activation for right hand movement and blue-green for left hand. In all figures p < 0.005 FDR, k = 35. SMA supplementary motor area, SM1 primary sensory-motor area on the left (lSM1) or the right hemisphere (rSM1). Note in c the significantly differential activation of SMA across the groups (greater in patients with IS than in controls)
Table 1.
Significant areas with functional activation in healthy subjects
| Talairach coordinates (mm) | Area | Hemisphere | t value | p value | Brodmann area | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Left hand | |||||||
| 37 | −20 | 53 | Postcentral gyrus | R | 7.86 | 0.0001 | 3 |
| 5 | −6 | 53 | Supplementary motor area | R | 7.18 | 0.0016 | 6 |
| −6 | −2 | 57 | Supplementary motor area | L | 7.08 | 0.0039 | 6 |
| 44 | −14 | 54 | Precentral gyrus | R | 6.89 | 0.0035 | 4 |
| Right hand | |||||||
| −5 | −11 | 32 | Anterior cingulated gyrus | L | 7.51 | 0.0003 | 23 |
| −35 | −25 | 52 | Precentral gyrus | L | 7.50 | 0.0013 | 4 |
| −3 | −2 | 64 | Supplementary motor area | L | 6.89 | 0.0025 | 6 |
| 9 | −5 | 56 | Supplementary motor area | R | 6.87 | 0.0026 | 6 |
p < 0.005 FDR, k ≥ 35
Table 2.
Significant areas with functional activation in patients with scoliosis
| Talairach coordinates (mm) | Area | Hemisphere | t value | p value | Brodmann area | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Left hand | |||||||
| −8 | 1 | 49 | Supplementary motor area | L | 8.89 | 0.0000 | 6 |
| 6 | 2 | 57 | Supplementary motor area | R | 8.45 | 0.0001 | 6 |
| 33 | −18 | 47 | Precentral gyrus | R | 8.12 | 0.0001 | 4 |
| 10 | −16 | 43 | Middle cingulated gyrus | R | 6.55 | 0.0031 | – |
| Right hand | |||||||
| 6 | 4 | 54 | Supplementary motor area | R | 8.16 | 0.0001 | 6 |
| −34 | −18 | 66 | Precentral gyrus | L | 8.08 | 0.0002 | 6 |
| −30 | −28 | 56 | Precentral | L | 7.48 | 0.0021 | 4 |
| −9 | −3 | 60 | Supplementary motor area | L | 7.31 | 0.0022 | 6 |
| −2 | 64 | 27 | Frontal superior medial | L | 7.11 | 0.0046 | 10 |
p < 0.005 FDR, k ≥ 35
Between-group analysis
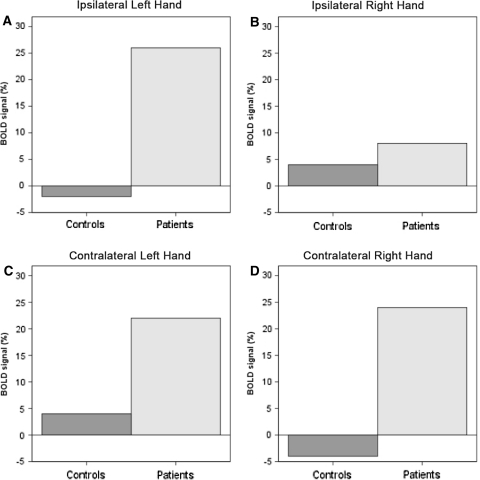
No significant differences were observed when testing between groups in the functional activation in the primary motor cortex, premotor cortex and somatosensory cortex. However, the supplementary motor area (SMA) showed a significant increase in activation in patients with IS as compared with control subjects when performing both right (p = 0.03) and left hand motor task (p = 0.04) (Figs. 1c, 2). This increased SMA activation appeared lateralized to the hemisphere contralateral to the moving hand.
Fig. 2.
Changes in BOLD signal (%) when comparing patients and controls in the SMA. a Left SMA during left hand movement (ipsilateral to left hand movement) (p < 0.03). b Right SMA during right hand movement (ipsilateral to right hand movement) (p < 0.13). c Right SMA during left hand movement (contralateral to left hand movement) (p < 0.04). d Left SMA during right hand movement (contralateral to right hand movement) (p < 0.03)
Interhemispheric asymmetries
Detailed assessment of the activation patterns revealed that nine control subjects activated the SMA ipsilateral to the moving hand and six activated the contralateral SMA, while all ten patients with IS activated the ipsilateral SMA and five of them in addition activated the contralateral SMA. Overall, the IS group showed a greater asymmetry index than the control group [asymmetry index IS group: 0.30; asymmetry index control group: 0.13 (p < 0.001)], for details in the different ROI see Table 3. The laterality index for activation during left hand movement was 0.52 for the IS group and 0.32 for the control group (p < 0.001). For the right hand the laterality index was 0.22 for the IS group and 0.27 for the control group (p < 0.001).
Table 3.
Asymmetry indexes between left and right hand for both groups
| Controls | Scoliosis | Sig | |
|---|---|---|---|
| Primary motor cortex | 0.18 | 0.29 | t 11.056 p < 0.001 |
| Premotor cortex | 0.17 | 0.30 | t 13.169 p < 0.000 |
| Supplementary motor area | 0.22 | 0.37 | t 16.102 p < 0.000 |
| Sensorial cortex | 0.11 | 0.16 | t 7.231 p < 0.009 |
Motor network correlations
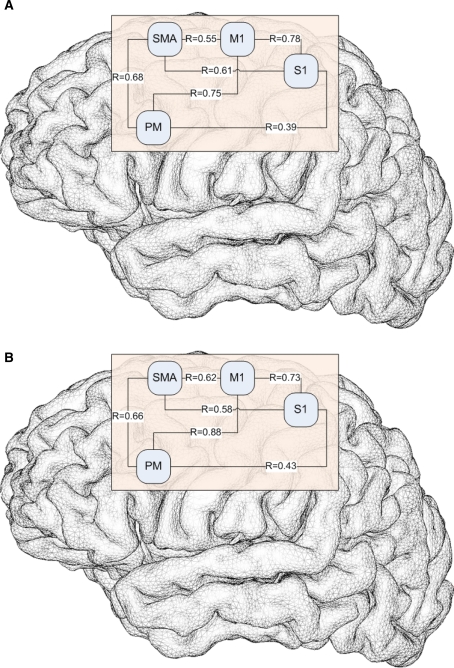
In order to assess the distributed dynamics of activity across the main nodes of the motor network, we evaluated the correlations in temporal time courses across various ROIs. The correlation study between the temporal time courses in different areas of interest showed very similar Pearson coefficients for both groups of participants (Fig. 3).
Fig. 3.
Pearson coefficients for control subjects (a) and patients with scoliosis (b) between the areas of interest. M1 primary motor cortex, S1 somatosensory cortex, PM premotor cortex, SMA supplementary motor area
Discussion
This study demonstrates for the first time an abnormal pattern of brain activation in the bihemispheric motor network during movement execution in patients with IS. This finding provides strong evidence supporting the notion that a central sensorimotor integration disorder underlies the pathogenesis of IS. The main finding of this study is a significant over activation of different secondary motor areas in IS patients as compared to healthy controls. Patients with IS showed a significant overactivation of SMA while performing the motor task as compared to healthy controls. SMA has a major role in selection, preparation, initiation and execution of voluntary movements, and also in posture control [39, 40]. This cortical area is a main target of basal ganglia motor output projections [41] and sends direct projections to the primary motor cortex [42] and spinal cord [43]. Furthermore, the SMA is not only a motor area, but is also involved in sensory integration, receiving dense sensory inputs from S1 [44].
Activation studies using PET and fMRI in different motor tasks show contradictory results regarding the level of activation of SMA in some forms of idiopathic dystonia [45–47]. The inappropriate and increased muscular activity that is characteristic of dystonic patients has been related to the abnormal modulation of SMA activity. Some studies have found overactivity in SMA in torsion dystonia supporting the idea of this kind of dystonia as being a disease of movement preparation driven by a disruption of sensorimotor integration [28–30]. A study using a model of secondary dystonia in monkeys showed that the SMA was overexcitable in dystonic animals, and that proprioceptive inputs processed by SMA showed a mismatch between sensory inputs and motor outputs, suggesting that abnormal sensory inputs impinging upon SMA neurons play a critical role in the pathophysiology of dystonia [48].
The reason for the overactivity of SMA in IS patients is not revealed by the results of our study, but it might be related to the abnormal sensorimotor integration disturbances found in IS patients. Subjects with IS have normal neurological exams (aside from findings directly related to the skeletal deformity). However, several findings point to a subclinical neurological impairment as a causative factor in the development of the spinal deformity. A sensorimotor integration disorder has been proposed as the result of alterations found in studies that examine proprioception [5, 6], vestibular integration [1, 23], balance control and postural sway [2, 6, 10, 13], visuospatial processing and perceptual cognitive functions [24, 49]. Motor commands and postural tone depend on a proper integration of proprioceptive, visual and vestibular information at central level [50]. Simoneau et al. [49] found that IS patients, as compared to controls, do not scale appropriately their balance control commands during sensory integration after a transient proprioceptive and visual deprivation. Using tibial SSEPs, Machida et al. [17] demonstrated a delay in N37 that arises at cortical level in patients with IS suggesting a cortical dysfunction. In summary, a defective sensorimotor integration at cortical level leading to improper motor commands appears to play a critical role in the pathogenesis of IS.
The novelty of our study lies in the demonstration of an objective abnormal motor cortical activation using fMRI in patients with IS during the performance of a simple hand motor task. In this context, it is important to emphasize that subjects with IS performed the motor task as well as the control subjects. Therefore, the differences in brain activation cannot be accounted for by behavioural differences.
A recent study, Lee et al. [51] explored cerebral glucose metabolism using brain positron emission tomography (PET) and failed to find differences between IS patients and to controls in any of the brain areas explored. In another study of the same group, Park et al. [52] studied PET glucose metabolism at rest in a group of 14 IS patients and 12 congenital scoliosis patients (with scoliosis produced by osseous malformations at the spine). They found that IS patients demonstrated higher metabolism in left prefrontal cortex (BA 10), left dorsolateral prefrontal cortex (BA 9), and left anterior cingulated cortex (BA24) than subjects with congenital scoliosis. Our study is not in contradiction with these findings. Both of these PET studies examined brain metabolism at rest, while we show abnormalities during the performance of a motor task. Additionally, Lee et al. compared patients with IS with a mean age of 14.6 years with older controls of an average age of 27.0 years. Age influences patterns of activity on brain imaging studies [53–55] so that age-matched controls are important when assessing brain activity in patients with IS.
It is noteworthy that significant difference was found in the asymmetry index (corrected for handedness) between healthy controls and IS patients. The control group showed a slight asymmetry in the cortex activation in accordance with previous reports in adults and children. Guzzetta et al. [54] studied with fMRI a sample of 10 healthy children aged 7–15 years, and 6 healthy adults while performing a motor task similar to the one used in our study. Consistent with our findings, they found an asymmetry index near to 0.1 in the healthy controls, slightly, but non-significantly lower than in adults. However, in our study, IS patients revealed a greater asymmetry index than healthy age-matched controls, reflecting an increased lateralization of motor activation in IS patients. Goldberg et al. [24] studied dichotic listening, which tests higher cortical cognitive processing, and found that scoliosis patients are significantly more lateralized for linguistic processing than the control group. A previous study using paired pulse transcranial magnetic brain stimulation found a significant interhemispheric asymmetry in cortico-cortical inhibitory circuits in IS patients as compared to healthy controls [25]. It is possible that the asymmetrical motor cortical activation pattern found in the present fMRI study is related to asymmetrical impairment of inhibitory intracortical motor circuits, and constitutes a generalized alteration of hemispheric balance in IS.
A delay in neurological maturation has been suggested to be related to the aetiology of IS [56]. The age of the participants in both groups was matched and within the range of 14–16 years. However, the pattern of fMRI activation during a motor task may well be related to brain maturation. For example, Mall et al. investigated the age dependency of motor cortex activation using fMRI in children (range 6–10 years), adolescents (range 11–15 years), and adults (range 23–42 years) performing a repetitive motor task [55]. Compared to children, adults showed significantly increased activation of bilateral sensorimotor cortex, parietal areas, the supplementary motor area, and the cerebellum suggesting that the change in fMRI activation patterns may reflect a maturation process of primary and secondary motor areas.
Our results provide new data that strongly support the hypothesis that a dystonic-like disorder might underlie the pathogenesis of idiopathic scoliosis. A deregulation in the modulation of the motor activity controlling spine posture at cortical level and leading to an interhemispheric imbalance could be the cause of progressive scoliotic deformity. fMRI cannot specify whether abnormal motor cortical activation reflects changes in the activity of excitatory or inhibitory structures within the motor or supplementary motor cortex. However, our prior study using transcranial magnetic stimulation did provide evidence of altered intracortical inhibition [25]. This is reminiscent of findings in dystonia. The relationship between scoliosis and dystonia is further reinforced by their clinical association. Scoliosis is present in 39% of adults diagnosed of cervical dystonia [57] and also scoliosis is more frequently developed in children and adolescents with idiopathic cervical dystonia [58]. In severe forms such as dystonia musculorum deformans, the presence of scoliosis is unfailing. Furthermore, scoliosis may be the first symptom of a dystonia [59] and the deformity can be corrected after treating the dystonia [60–62].
One limitation of our study is that the patients had different types of scoliosis. This is an exploratory study, so in the design we included the spectrum of the most common types of IS, not only the quite typical right thoracic curves. IS patients showed a uniform pattern of activation of contralateral SMA and an increased asymmetry index despite the type of curve. Human motor secondary cortices, including SMA, do not have a well-defined somatotopy of trunk muscles [40, 41] so it seems unlikely that the findings would differ by the type of spine curve. This is similar to the alterations in motor cortical areas demonstrated in different forms of focal dystonia, which do not show somatotopy of the target muscles affected but rather an abnormal activation of secondary motor areas [28–30, 45–47].
In conclusion, our study demonstrates, using fMRI, an abnormally increased activation in motor-related cortical areas in patients with IS during a simple, internally paced motor task. The results show a similar activation pattern to that found in patients with DYT1-linked torsion dystonia and a significantly increased asymmetry index in cortical activation following a simple motor task. These results represent an objective finding of a neurologic dysfunction in cortical sensorimotor integration in IS that could help understand the aetiology of IS. In addition, these abnormal fMRI findings may represent a valuable biomarker of IS disease progression and offers novel therapeutic targets.
Conflict of interest
None.
Footnotes
This is the article winner of the EuroSpine Full Paper Awards 2010.
References
- 1.Sahlstrand T, Örtengren R, Nachemson A. Postural equilibrium in adolescent idiopathic scoliosis. Acta Orthop Scand. 1978;49:354–365. doi: 10.3109/17453677809050088. [DOI] [PubMed] [Google Scholar]
- 2.Adler N, Bleck EE, Rinsky IA, Young W. Balance reactions and eye hand coordination in idiopathic scoliosis. J Orthop Res. 1986;4:102–107. doi: 10.1002/jor.1100040113. [DOI] [PubMed] [Google Scholar]
- 3.Herman R, Mixon J, Fisher A, Maulucci R, Stuyck J. Idiopathic scoliosis and the central nervous system: a motor control problem. Spine. 1985;10:1–14. doi: 10.1097/00007632-198501000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Keessen W, Crowe A, Hearn M. Proprioceptive accuracy in idiopathic scoliosis. Spine. 1992;17:149–155. doi: 10.1097/00007632-199202000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Barrack RL, Withecloud TS, Burke SW, Cook SD, Harding AS. Propriocepcion in idiopathic scoliosis. Spine. 1984;9:681–685. doi: 10.1097/00007632-198410000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Cook SD, Harding Af, Burke SW, et al. Upper extremity proprioception in idiopathic scoliosis. Clin Orthop. 1986;213:118–124. [PubMed] [Google Scholar]
- 7.Barrack RL, Wyatt MP, Whitecloud TS, Burke SW, Roberts JM, Brinker MR. Vibratory hypersensitivity in idiopathic scoliosis. J Pediatr Orthop. 1988;8:389–395. doi: 10.1097/01241398-198807000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Wyatt MP, Barrack RL, Mubarack SJ, Whitecloud TS, Burke SW. Vibratory response in idiopathic scoliosis. J Bone Jt Surg. 1986;68B:714–718. doi: 10.1302/0301-620X.68B5.3782230. [DOI] [PubMed] [Google Scholar]
- 9.McInnes E, Hill DL, Raso VJ, Chetner B, Greenhill BJ, Moreau MJ. Vibratory response in adolescents who have idiopathic scoliosis. J Bone Jt Surg. 1991;73:1208–1212. [PubMed] [Google Scholar]
- 10.Byl NN, Gray JM. Complex balance reactions in different sensory conditions: adolescents with and without idiopathic scoliosis. J Orthop Res. 1993;11:215–227. doi: 10.1002/jor.1100110209. [DOI] [PubMed] [Google Scholar]
- 11.Lao MLM, Chow DHK, Guo X, Cheng JC, Holmes AD. Impaired dynamic balance in adolescent with idiopathic scoliosis and abnormal somatosensory evoked potential. J Pediatr Orthop. 2008;28:846–849. doi: 10.1097/BPO.0b013e31818e1bc9. [DOI] [PubMed] [Google Scholar]
- 12.Nault ML, Allard P, Hinse S, Le Blanc R, Caron O, Labelle H, Sadeghi H. Relations between standing stability and body posture parameters in adolescent idiopathic scoliosis. Spine. 2002;27:1911–1917. doi: 10.1097/00007632-200209010-00018. [DOI] [PubMed] [Google Scholar]
- 13.Silferi V, Rougier P, Labelle H, Allard P. Postural control in idiopathic scoliosis: comparison between healthy and scoliotic subjects. Rev Chir Orthop Reparatrice Appar Mot. 2004;90:215–225. doi: 10.1016/s0035-1040(04)70097-5. [DOI] [PubMed] [Google Scholar]
- 14.Beaulieu M, Toulotte C, Gatto L, Rivard CH, Teasdale N, Simoneau M, Allard P. Postural imbalance in non-treated adolescent idiopathic scoliosis at different periods of progression. Eur Spine J. 2009;18:38–44. doi: 10.1007/s00586-008-0831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Bermejo E, García-Jimenez MA, Fernandez-Palomeque C, Munuera L. Adolescent idiopathic scoliosis and joint laxity. Spine. 1993;18:918–922. doi: 10.1097/00007632-199306000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Brinker MR, Willis JK, Cook SD, Whitecloud TS, Bennett JT, Barrck RL, Ellman MG. Neurologic testing with somatosensory evoked potentials in idiopathic scoliosis. Spine. 1992;17:277–279. doi: 10.1097/00007632-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J, Toriyama S. Pathogenesis of idiopathic scoliosis: SEPs in chicken with experimentally induced scoliosis and in patients with idiopathic scoliosis. J Pediatr Orthop. 1994;14:329–335. doi: 10.1097/01241398-199405000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Hausmann ON, Boni T, Pfirrmann CW, Curt A, Min K. Preoperative radiological and electrophysiological evaluation in 100 adolescent idiopathic scoliosis patients. Eur Spine J. 2003;12:501–506. doi: 10.1007/s00586-003-0568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowling F, Goldberg C (1988) Idiopathic scoliosis as a function of the cerebral cortex. In: 23rd annual meeting of the Scoliosis Research Society, Baltimore, MD
- 20.Lukeschitsch G, Meznik F, Feldner-Bustin H. Cerebral dysfunction in patients with idiopathic scoliosis. Z Orthop Ihre Grenzgeb. 1980;118:372–375. doi: 10.1055/s-2008-1053519. [DOI] [PubMed] [Google Scholar]
- 21.Petersén I, Sahlstrand T, Selldén U. Electroencephalographic investigation of patients with adolescent idiopathic scoliosis. Acta Orthop Scand. 1979;50:283–293. doi: 10.3109/17453677908989769. [DOI] [PubMed] [Google Scholar]
- 22.Dretakis EK, Paraskevaidis CH, Zarkadoulas V, Christodoulou N. Electroencephalographic study of schoolchildren with adolescent idiopathic scoliosis. Spine. 1988;13:143–145. doi: 10.1097/00007632-198802000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Simoneau M, Lamothe V, Hutin E, Mercier P, Teasdale N, Blouin J. Evidence for cognitive vestibular integration impairment in idiopathic scoliosis patients. BMC Neurosci. 2009;10:102. doi: 10.1186/1471-2202-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg CJ, Dowling FE, Fogarty EE, Moore DP. Adolescent idiopathic scoliosis and cerebral asymmetry. An examination of a nonspinal perceptual system. Spine. 1995;20:1685–1691. doi: 10.1097/00007632-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Doménech J, Tormos JM, Barrios C, Pascual-Leone A. Motor cortical hyperexcitability in idiopathic scoliosis: could focal dystonia be a subclinical etiological factor? Eur Spine J. 2010;19:223–230. doi: 10.1007/s00586-009-1243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 27.Jueptner M, Weiller C. Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage. 1995;2:148–156. doi: 10.1006/nimg.1995.1017. [DOI] [PubMed] [Google Scholar]
- 28.Hallett M. Disorder of movement preparation in dystonia. Brain. 2000;123:1765–1766. doi: 10.1093/brain/123.9.1765. [DOI] [PubMed] [Google Scholar]
- 29.Carbon M, Su S, Dhawan V, Raymond D, Bressman S, Eidelberg D. Regional metabolism in primary torsion dystonia. Effects of penetrance and genotype. Neurology. 2004;62:1384–1390. doi: 10.1212/01.wnl.0000120541.97467.fe. [DOI] [PubMed] [Google Scholar]
- 30.Playford ED, Passingham RE, Marsden CD, Brooks DJ. Increased activation of frontal areas during arm movement in idiopathic torsion dystonia. Mov Disord. 1998;13:309–318. doi: 10.1002/mds.870130218. [DOI] [PubMed] [Google Scholar]
- 31.Hallett M (1995) Editorial. Is dystonia a sensory disorder? Ann Neurol 38:139–140 [DOI] [PubMed]
- 32.Defazio G, Berardelli A, Hallett M. Do primary adult-onset focal dystonias share aetiological factors? Brain. 2007;130:1183–1193. doi: 10.1093/brain/awl355. [DOI] [PubMed] [Google Scholar]
- 33.Tinazzi M, Rosso T, Fiaschi A. Role of the somatosensory system in primary dystonia. Mov Disord. 2003;18:605–622. doi: 10.1002/mds.10398. [DOI] [PubMed] [Google Scholar]
- 34.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 35.Friston LJ, Holmes AP, Worsley LJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging. A general linear approach. Hum Brain Mapp. 1995;2:189–210. doi: 10.1002/hbm.460020402. [DOI] [Google Scholar]
- 36.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 37.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2001;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 38.Chee M, Tan E, Thiel T. Mandarin and English single word processing studies with functional magnetic resonance imaging. J Neurosci. 1999;19:3050–3056. doi: 10.1523/JNEUROSCI.19-08-03050.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature. 1994;371:4413–4416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- 40.Deiber MP, Honda M, Ibañez V, Sadato N, Hallett M. Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol. 1999;8:3065–3077. doi: 10.1152/jn.1999.81.6.3065. [DOI] [PubMed] [Google Scholar]
- 41.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-L. [DOI] [PubMed] [Google Scholar]
- 42.Muakkassa KF, Strick PL. Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized ‘premotor’ areas. Brain Res. 1979;177:176–182. doi: 10.1016/0006-8993(79)90928-4. [DOI] [PubMed] [Google Scholar]
- 43.Maier MA, Armond J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex. 2002;12:281–296. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]
- 44.Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 45.Ceballos-Baumann AO, Sheean G, Passingham RE, Marsden CD, Brooks DJ. Botulinum toxin does not reverse the cortical dysfunction associated with writer’s cramp. A PET study. Brain. 1997;120:571–582. doi: 10.1093/brain/120.4.571. [DOI] [PubMed] [Google Scholar]
- 46.Ibáñez V, Sadato N, Karp B, Deiber MP, Hallett M. Deficient activation of the motor cortical network in patients with writer’s cramp. Neurology. 1999;53:96–105. doi: 10.1212/wnl.53.1.96. [DOI] [PubMed] [Google Scholar]
- 47.Dresel C, Haslinger B, Castrop F, Wohlschlaeger AM, Ceballos-Baumann AO. Silent event-related fMRI reveals deficient motor and enhanced somatosensory activation in orofacial dystonia. Brain. 2006;129:36–46. doi: 10.1093/brain/awh665. [DOI] [PubMed] [Google Scholar]
- 48.Cuny E, Ghorayeb I, Guehl D, Escola L, Bioulac B, Burbaud P. Sensory motor mismatch within the supplementary motor area in the dystonic monkey. Neurobiol Dis. 2008;30:151–161. doi: 10.1016/j.nbd.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Simoneau M, Mercier P, Blouin J, Allard P, Teasdale N. Idiopathic scoliosis changes the sensory-weighting mechanisms involved in balance control. BMC Neurosci. 2006;7:68. doi: 10.1186/1471-2202-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asanuma H, Pavlides (1998) Neurobiological basis of motor learning in humans. Neuroreport 8:1–6 [PubMed]
- 51.Lee JS, Kim SJ, Suh KT, Kim IJ, Kim YK. Adolescent idiopathic scoliosis may not be associated with brain abnormalities. Acta Radiol. 2009;50:941–946. doi: 10.1080/02841850903104161. [DOI] [PubMed] [Google Scholar]
- 52.Park WW, Suh KT, Kim JI, Ku JG, Lee HS, Kim SJ, Kim IJ, Kim YK, Lee JS. Cerebral glucose metabolic abnormality in patients with congenital scoliosis. Eur Spine J. 2008;17:948–955. doi: 10.1007/s00586-008-0682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–635. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- 54.Guzzetta A, Staudt M, Petacchi E, Ehlers J, Erb M, Wilke M, Krägeloh-Mann I, Cioni G. Brain representation of active and passive hand movements in children. Ped Res. 2007;61:485–490. doi: 10.1203/pdr.0b013e3180332c2e. [DOI] [PubMed] [Google Scholar]
- 55.Mall V, Linder M, Herpers M, Schelle A, Mendez-Mendez J, Korinthenberg R, Schumacher M, Spreer J. Recruitment of the sensorimotor cortex: a developmental FMRI study. Neuropediatrics. 2005;36:373–379. doi: 10.1055/s-2005-873077. [DOI] [PubMed] [Google Scholar]
- 56.Burwell RG, Freeman BJ, Dangerfield PH, Aujla RK, Cole AA, Kirby AS, Polak F, Pratt RK, Webb JK, Moulton A. Etiologic theories of idiopathic scoliosis: neurodevelopmental concept of maturational delay of the CNS body schema (“body-in-the-brain”) Stud Health Technol Inform. 2006;123:72–79. [PubMed] [Google Scholar]
- 57.Jankovic J, Leder S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology. 1991;41:1088–1091. doi: 10.1212/wnl.41.7.1088. [DOI] [PubMed] [Google Scholar]
- 58.Defazio G, Abbruzzese G, Girlanda P, Buccafusca M, Curra A, Marchese R, Martino D, Masi G, Mazzella L, Vacca L, Livrea P, Berardelli A. Primary cervical dystonia and scoliosis: a multicenter case-control study. Neurology. 2003;60:1012–1015. doi: 10.1212/01.wnl.0000049932.22065.60. [DOI] [PubMed] [Google Scholar]
- 59.Duane DD. Familiar cervical dystonia, head tremor and scoliosis: a case report. Adv Neurol. 1998;78:117–120. [PubMed] [Google Scholar]
- 60.Furukawa Y, Kish SJ, Lang AE. Scoliosis in a Dopa responsive dystonia family with mutation of of the GTP cyclohydrolase I gene. Neurology. 2000;54:2187. doi: 10.1212/wnl.54.11.2187. [DOI] [PubMed] [Google Scholar]
- 61.Micheli F, Pardal MF, Gatto E, Paradiso G. Dopa responsive dystonia masquerading as idiopathic kyphoscoliosis. Clin Neuropharmacol. 1991;14:367–371. doi: 10.1097/00002826-199108000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Narayan RK, Loubser PG, Jankovic J, Donovan WH, Bontke CF. Intrathecal baclofen for intractable axial dystonia. Neurology. 1991;41:1141–1142. doi: 10.1212/wnl.41.7.1141. [DOI] [PubMed] [Google Scholar]