Abstract
Some studies report differences in opioid withdrawal between racial/ethnic groups. However, it is not known if these differences are reflected in differential treatment response. Data from NIDA Clinical Trials Network-003 were used to examine racial/ethnic differences before and during stabilization with buprenorphine. At induction non-Hispanic Caucasians had higher objective and subjective withdrawal scores and greater opioid craving than minority participants. No significant between-groups differences were observed on these scales following buprenorphine. Non-Hispanic Caucasians and Hispanics reported more adverse events than African Americans. Although ethnic and racial differences were observed prior to buprenorphine treatment, scores following buprenorphine were similar between groups.
Keywords: opioid, buprenorphine, race, ethnicity
Introduction
Opioid use is a significant public health concern. The National Epidemiologic Survey on Alcohol and Related Conditions reported lifetime rates of nonmedical prescription opioid use of 4.7% (Huang, et al., 2006) and rates of opioid abuse or dependence of 1.4% (Compton, Conway, Stinson, Colliver, & Grant, 2005). Treatment of opioid and other substance use-disorders is challenging due, in part, to high rates of attrition and relapse. Past year heroin use rates are 0.2% among Caucasians and Hispanics and 0.4% among African-Americans (Substance Abuse and Mental Health Services Administration Office of Applied Studies) comprising 28% and 26% respectively of persons admitted to public-funded treatment programs for heroin abuse or dependence (Administration, 2008).
Despite high rates of opioid dependence in racial and ethnic minorities, little is known about the impact of racial and ethnic differences on opioid treatment. Members of minority racial and ethnic groups reportedly have higher rates of attrition than Caucasians during substance abuse treatment (Booth, Blow, Cook, Bunn, & Fortney, 1992) (McCaul, Svikis, & Moore, 2001) (Mertens & Weisner, 2000). A factor that could lead to attrition during treatment for opioid-related disorders is the presence of withdrawal symptoms. Kosten and Rayford reported that African-American and Hispanic persons, relative to Caucasians, had more severe opioid physical dependence (as assessed by the naloxone challenge test) but reported similar severity of objective withdrawal symptoms and significantly lower levels of subjective withdrawal symptoms during buprenorphine stabilization and maintenance (Kosten & Rayford, 1995). The authors suggested that this difference between objective and subjective measures may be due to hesitancy by minority participants to report personal information during structured interviews (Kosten & Rayford, 1995). In this report, minority participants had similar rates of opioid use during buprenorphine therapy and similar rates of attrition as Caucasian participants. These findings are potentially consistent with an underreporting of subjective withdrawal symptoms by minority participants despite a greater severity of physical dependence and objective opioid withdrawal.
In order to clarify potential differences in withdrawal symptoms between various ethnic and racial groups, this report assessed participants in a NIDA Clinical Trials Network (CTN) 003 trial before and after buprenorphine stabilization. The CTN 003 study found no difference in opiate use following 28 day vs. 7 day buprenorphine tapers (Ling et al., 2009). The current report examines racial and ethnic differences on outcomes during the buprenorphine stabilization phase of this study prior to beginning the taper. Clinician-rated and self-reported opioid withdrawal symptoms, opioid craving and use, attrition, and adverse events were determined in Non-Hispanic Caucasian, African-American and Hispanic participants. Based on findings from the literature discussed above, our hypotheses were that African-American and Hispanic participants would 1) have higher scores on the objective Clinical Opiate Withdrawal Scale (COWS) (Wesson & Ling, 2003) and lower scores on the subjective self-report Adjective Rating Scale for Withdrawal (ARSW) (Bickel, et al., 1988) at baseline and after buprenorphine treatment (primary outcome) than non-Hispanic Caucasians; and 2 ) would report fewer adverse events than non-Hispanic Caucasian participants. We also explored attrition, and opioid use and craving in the three groups.
Methods
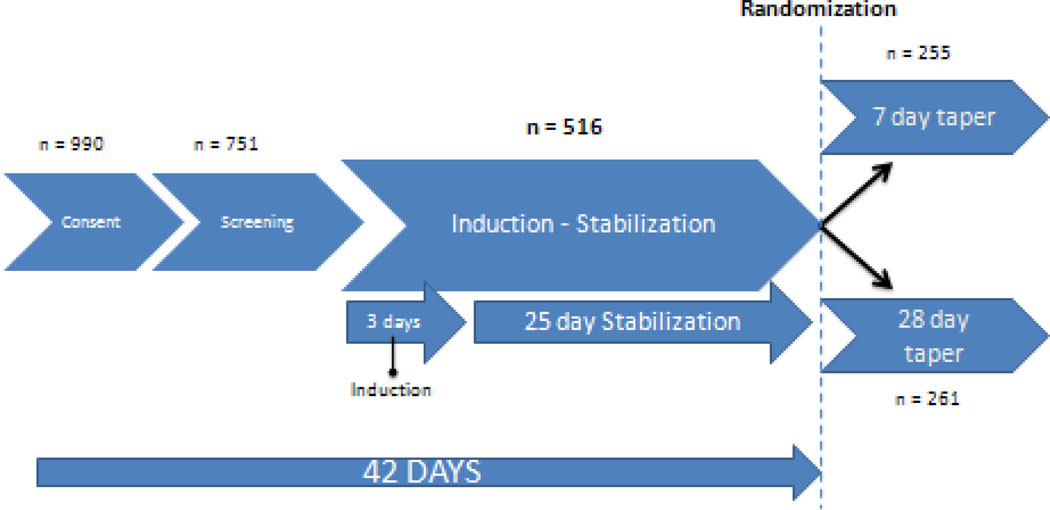
This is a secondary analysis of data from the NIDA CTN 0003 study, Suboxone (buprenorphine/naloxone) taper: a comparison of two schedules (Ling, et al., 2009). CTN 0003 was conducted at eleven sites in the United States. A total of 990 participants provided IRB-approved informed consent to participate in the trial, and 751 participants completed the screening phase and initiated medication. A total of 516 participants were randomized into parallel groups to compare a 7 or 28 day outpatient buprenorphine/naloxone taper. Participants underwent a three day buprenorphine/naloxone induction and 25 day stabilization phase prior to randomization. At the end of the stabilization phase, all subjects on a stable dose of 8, 16 or 24 mg buprenorphine were randomized to a 7 or 28 day tapering regime (Figure 1). Participants provided IRB-approved written informed consent. Results of the primary outcome analysis and a detailed description of the experimental methods were published elsewhere (Ling, et al., 2009).
Figure 1.
Study Phases
The current analysis focused on the period during buprenorphine stabilization, between induction and randomization, and compared racial and ethnic differences across four domains: 1) opiate withdrawal as measured by the COWS and ARSW; 2) attrition during the induction and stabilization phase; 3) medication tolerability as measured by frequency of reported adverse events; and 4) therapeutic response to medication as measured by urine drug screens and a Visual Analog Scale (VAS) for craving.
The COWS is an 11-item clinician administered questionnaire designed to measure active signs and symptoms of opiate withdrawal (Wesson & Ling, 2003). The ARSW is a 16-item self report that requires subjective rating of current signs and symptoms of opioid withdrawal (Bickel, et al., 1988). The VAS is a self-report questionnaire in which participants rate craving for opiates along a linear scale from 0 (no symptoms) to 100 (severe symptoms).
Subjects were divided into four racial/ethnic groups: Caucasian, African-American, Hispanic and Other (Asian, American Indian). Due to the very low number of participants in the “Other” group (n=22, 3%), this group was excluded from the analysis reducing the sample size for analysis from 751 to 729. Of these 729, four had missing COWS/ARSW scores and one had missing race information. Hence, the baseline comparison was performed using 724 subjects. Between-race comparisons for categorical variables were done using chi-square test whereas comparisons for continuous variables were performed using one-way analysis of variance (ANOVA) or analysis of covariance (ANCOVA), as appropriate. Following significant F-tests, pairwise comparisons were performed using Fisher’s protected t-tests. All hypothesis tests were conducted at alpha = 0.05.
Results
A total of 558 Non-Hispanic Caucasian, 93 African-American, and 73 Hispanic participants (n=724 total) were assessed at induction and 501 of these 724 completed the buprenorphine stabilization phase. Demographic characteristics of the three ethnic/racial groups are provided in Table 1. Since the three racial/ethnic groups are found to be significantly different in terms of mean age at induction (p < 0.001) and percent female (p=0.003), heroin and other opioids use in the past 30 days as well as lifetime use comparisons were adjusted for age at induction and gender. It should be noted here that, even though mean years of education is significantly different among the racial/ethnic groups (p < 0.001), it was not used as a covariate as the observed mean education levels are not clinically substantial (13.1 years for non-Hispanic Caucasian, 12.1 for African American, and 12.4 for Hispanic group). Significant between-groups differences were found in opioid use with higher lifetime heroin use in minority participants after adjusting for age at induction and gender (p < 0.001). However, heroin use in the past 30 days (p = 0.166) as well as lifetime (p = 0.755) and past 30 day use of other (i.e. prescription) opioids (p = 0.580) among the three racial/ethnic groups were not found to be statistically significantly different, after adjusting for age at induction and gender.
Table 1.
Demographic information on Caucasian, African-American and Hispanic participants
| Demographic Data | Caucasian (n = 558) |
African- American (n = 93) |
Hispanic (n = 73) |
(p value) |
|---|---|---|---|---|
| Age (mean years ± SD) | 34.2 ± 9.9 | 42.5 ± 9.3 | 39.9 ± 10.4 | < 0.001 |
| Gender, Female, n (%) | 199 (35.7) | 18 (19.4) | 18 (24.7) | 0.003 |
| Education (mean years ± SD) | 13.1 ± 2.2 | 12.1 ± 2.1 | 12.4 ± 1.6 | < 0.001 |
| Heroin in the past 30 days (mean days ± SD)* | 22.5 ± 11.9 | 26.7 ± 8.0 | 28.4 ± 5.4 | 0.166 |
| Heroin in lifetime (mean years ± SD)* | 4.9 ± 6.3 | 13.0 ± 10.3 | 12.2 ± 11.3 | < 0.001 |
| Other opioids in the past 30 (mean days ± SD)* | 7.0 ± 11.4 | 2.3 ± 7.1 | 1.4 ± 5.3 | 0.755 |
| Other opioids in lifetime (mean years ± SD)* | 2.3 ± 4.2 | 0.72 ± 2.6 | 0.34 ± 0.8 | 0.580 |
| Employment in past 30 days, n(%) | 0.001 | |||
| Full/part-time/service | 308 (55.2) | 41 (44.1) | 29 (39.7) | |
| Student/homemaker | 38 (6.8) | 0 (0.0) | 4 (5.5) | |
| Unemployed/disabled | 212 (38.0) | 52 (55.9) | 40 (54.8) | |
| Marital Status, n(%) | 0.869 | |||
| Married/living with a partner | 160 (28.7) | 29 (31.2) | 22 (30.1) | |
| Divorced/never married/widowed | 398 (71.3) | 64 (68.8) | 51 (69.9) |
Adjusted for age and gender.
Significant between-race differences at induction were observed on the COWS (p < 0.001), ARSW (p < 0.001), and VAS craving (p < 0.001) (Table 2) suggesting higher craving and withdrawal symptoms scores in non-Hispanic Caucasians and Hispanics as compared to African-Americans. However, adjusted for buprenorphine dose , there is no significant difference between non-Hispanic Caucasians and Hispanic participants in terms of mean COWS, ARSW, and VAS scores at the end of the buprenorphine stabilization phase (0.406 < p < 0.760). In addition, following 28 days of buprenorphine administration, no differences were observed between racial/ethnic groups on percentage of positive urines for opioids (p = 0.520) or all drugs (p = 0.862) (Table 3). Attrition during the buprenorphine induction and maintenance phase did not differ significantly between groups (p = 0.415).
Table 2.
Withdrawal symptoms and craving in Caucasian, African-American and Hispanic participants
| Race/ Ethnic Group | Caucasian n = 558A |
African- American n = 93B |
Hispanic n = 73C |
p-value |
|---|---|---|---|---|
| COWS, mean score ± SD | ||||
| At Induction | 5.3 ± 3.7 | 3.1 ± 2.9 | 5.4 ± 5.7 | <0.001 |
| At RandomizationD | 1.0 ± 1.3 | 0.8 ± 1.3 | 0.8 ± 1.2 | 0.704 |
| ARSW, mean score ± SD | ||||
| At Induction | 40.7 ± 31.3 | 28.0 ± 28.9 | 46.2 ± 36.5 | <0.001 |
| At RandomizationD | 11.0 ± 15.3 | 7.1 ± 17.6 | 9.4 ± 13.2 | 0.406 |
| VAS, mean score ± SD | ||||
| At Induction | 49.4 ± 29.7 | 31.6 ± 28.2 | 49.8 ± 27.6 | < 0.001 |
| At RandomizationD | 13.2 ± 20.5 | 9.1 ± 16.1 | 9.4 ± 14.7 | 0.760 |
At randomization, the sample size is reduced to 391for COWS, and 392 for ARSW and VAS.
At Randomization, the sample size is reduced to 60 for COWS, ARSW, and VAS.
At randomization, the sample size is reduced to 48 for COWS, ARSW, and VAS.
Adjusted for buprenorphine dose at randomization.
Table 3.
Adverse events, drug use and attrition during 28 day buprenorphine administration in Caucasian, African-American and Hispanic participants
| Race/ Ethnic Group | Caucasian n = 558A |
African- American n = 93B |
Hispanic n = 73C |
p-value |
|---|---|---|---|---|
| Total Adverse Events,Mean # of events ± SD | 5.2 ± 4.0 | 2.9 ± 2.0 | 4.9 ± 3.0 | < 0.001 |
| Adverse Events Definitely Not Study Drug Related, Mean # of events ± SD | 3.7 ± 3.3 | 1.5 ± 1.4 | 3.8 ± 3.0 | < 0.001 |
| Opioid-positive Urine Test at Randomization, n(%) | 144 (36.7%) | 18 (31.0%) | 20 (41.7%) | 0.520 |
| Any Drug-positive Urine Test at Randomization, n(%) | 260 (66.3%) | 37 (63.8%) | 33 (68.8%) | 0.862 |
| Attrition during Buprenorphine administration, n(%) | 165 (29.6%) | 33 (35.5%) | 25 (34.3%) | 0.415 |
| Buprenorphine dose at randomization, mean score ± SD | 20.4 ± 5.2 | 18.8 ± 5.9 | 21.2 ± 5.1 | 0.045 |
At randomization, the sample size is reduced to 392 for Urine test, 393 for buprenorphine dose.
At Randomization, the sample size is reduced to 58 for Urine test, 60 for buprenorphine dose.
At randomization, the sample size is reduced to 48 for Urine test, and buprenorphine dose.
Mean levels of total number of adverse events reported during buprenorphine administration period were significantly different among the racial/ethnic groups (p = 0.001) with non-Hispanic Caucasians (p = 0.070) and Hispanics (p < 0.001) reporting more total adverse events than African-Americans. The racial/ethnic groups were also significantly different in the mean total numbers of adverse events that were definitely not related to the study drug. Non-Hispanic Caucasians (p <0.001) and Hispanics (p < 0.004) reported significantly higher number of definitely non-study related adverse events as compared to African-Americans. Mean doses of buprenorphine at the end of 28 days were different (p ≤ 0.045) in the three racial/ethnic groups with African-Americans having significantly lower mean dose level as compared to non-Hispanic Caucasians (p = 0.021) and Hispanics (p = 0.030).
Discussion
This study examined acute withdrawal symptoms and persistent withdrawal symptoms following four weeks of buprenorphine treatment in non-Hispanic Caucasian, African-American and Hispanic participants. We hypothesized that objective withdrawal symptoms would be higher in minority participants and subjective withdrawal symptoms would be higher in non-Hispanic Caucasians. The data do not support this hypothesis. During induction, Non-Hispanic Caucasians tended to have higher levels of both objective and subjective withdrawal symptoms, but the three groups had similar scores after completing the buprenorphine stabilization.
We also hypothesized that minority participants would report fewer adverse events than non-Hispanic Caucasians during buprenorphine administration. The data partially support this hypothesis with similar rates of adverse event reported in non-Hispanic Caucasian and Hispanic participants but lower rates of adverse events in African-Americans. The racial-ethnic differences in adverse events appear to be related to adverse events that were not study related. Thus, the data may support the idea suggested by (Kosten & Rayford, 1995) that African-Americans may be hesitant to report adverse events. However, given the data, we cannot rule out the possibility that factors other than underreporting resulted in the between-group differences.
Some prior studies have reported higher attrition in minority participants during substance abuse treatment (Booth, et al., 1992) (McCaul, et al., 2001) (Mertens & Weisner, 2000). We found similar rates of study attrition in the three groups. This finding is consistent with the report by (Kosten & Rayford, 1995) that, as with the current report, examined attrition during buprenorphine therapy. Since buprenorphine effectively relieves uncomfortable withdrawal symptoms and decreases opioid craving, it is associated with relatively high treatment retention across racial and ethnic groups.
Opioid use differed between-groups in the 30 days prior to study entry. Minority participants reported more days of heroin use while non-Hispanic Caucasian participants reported more days of other opioid use (e.g. prescription opioids) than did minority participants. However, we did not find significant between-group differences in opioid use at the end of the buprenorphine stabilization period. About one third of participants in each group used opioids and two thirds used some drug following four weeks of buprenorphine administration. Similarly drug craving while higher in non-Hispanic Caucasians at induction, was similar following buprenorphine administration in the three groups. Thus, drug use was common during buprenorphine therapy but racial and ethnic differences were not found.
A major strength of the study is the large sample size that included many minority participants suggesting that negative findings are not due to Type II error. However, the study also has limitations. We omitted the “Other race” group which excluded a small number of participants. Hispanic, as defined for the analysis, includes both non-Hispanic Caucasian and African-American Hispanics. Mean withdrawal symptoms, both at induction and following buprenorphine, were low in all three groups. This is a secondary data analysis, so the study was not designed with the present analyses in mind. The study did not conduct Spanish-language withdrawal assessments which potentially led to reporting differences in some Hispanic participants who were not fluent in English.
In summary, racial/ethnic differences were found during a four week trial of buprenorphine administration. Non-Hispanic Caucasians tended to use more opioids other than heroin prior to study participation and had higher levels of withdrawal symptoms. However, drug use, craving, and withdrawal symptoms were similar in the three groups following buprenorphine administration. Thus, although the groups were different at induction, they showed similar symptomatology following the four week buprenorphine maintenance phase. African-Americans reported fewer adverse events during buprenorphine therapy suggesting either a more favorable course of treatment or reluctance to report adverse events to clinicians. Additional research is needed to identify factors responsible for racial differences in adverse event reporting during buprenorphine administration. The findings suggest an overall favorable treatment outcome for all three racial/ethnic groups during buprenorphine administration and suggest race/ethnicity may not play a major role in buprenorphine response and treatment retention.
References
- Administration, S. A. a. M. H. S. Analysis of Substance Abuse and Treatment Need Issues, Table of Contents (TOC) 2008 2008b from http://www.drugabusestatistics.samhsa.gov/treatan/toc.htm.
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. A clinical trial of buprenorphine: comparison with methadone in the detoxification of heroin addicts. Clin Pharmacol Ther. 1988;43(1):72–78. doi: 10.1038/clpt.1988.13. [DOI] [PubMed] [Google Scholar]
- Booth BM, Blow FC, Cook CAL, Bunn JY, Fortney JC. Age and ethnicity among hospitalized alcoholics: A nationwide study. Alcoholism: Clinical and Experimental Research. 1992;16(6):1029–1034. doi: 10.1111/j.1530-0277.1992.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Compton WM, Conway KP, Stinson FS, Colliver JD, Grant BF. Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2005;66(6):677–685. doi: 10.4088/jcp.v66n0602. [DOI] [PubMed] [Google Scholar]
- Huang B, Dawson DA, Stinson FS, Hasin DS, Ruan WJ, Saha TD, et al. Prevalence, correlates, and comorbidity of nonmedical prescription drug use and drug use disorders in the United States: Results of the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67(7):1062–1073. doi: 10.4088/jcp.v67n0708. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rayford BS. Effects of ethnicity on low-dose opiate stabilization. J Subst Abuse Treat. 1995;12(2):111–116. doi: 10.1016/0740-5472(94)00069-4. [DOI] [PubMed] [Google Scholar]
- Ling W, Hillhouse M, Domier C, Doraimani G, Hunter J, Thomas C, et al. Buprenorphine tapering schedule and illicit opioid use. Addiction. 2009;104(2):256–265. doi: 10.1111/j.1360-0443.2008.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul ME, Svikis DS, Moore RD. Predictors of outpatient treatment retention: patient versus substance use characteristics. Drug Alcohol Depend. 2001;62(1):9–17. doi: 10.1016/s0376-8716(00)00155-1. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Weisner CM. Predictors of substance abuse treatment retention among women and men in an HMO. Alcohol Clin Exp Res. 2000;24(10):1525–1533. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (Office of Applied Studies) 6. Prescription Drug Dependence, Abuse, and Treatment. Misuse of Prescription Drugs. 2008 June 03; Retrieved mm/dd, 2009, from http://www.oas.samhsa.gov/prescription/ch6.htm.
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J Psychoactive Drugs. 2003;35(2):253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]