Abstract
Background
Platelet-rich plasma (PRP) has been increasingly used in sports medicine applications. Platelets are thought to release growth factors important in wound healing, including transforming growth factor (TGF-β1), platelet-derived growth factor (PDGF-AB), and vascular endothelial growth factor (VEGF). However, little is known about the effect of platelet activator choice on growth factor release kinetics.
Hypothesis
The choice of platelet activator would affect the timing and level of growth factor release from PRP.
Study Design
Controlled laboratory study.
Methods
Platelet-rich plasma aliquots were activated with either thrombin or collagen. A control group of whole blood aliquots was clotted with thrombin. Supernatant containing the released growth factors was collected daily for 1 week. Levels of TGF-β1, PDGF-AB, and VEGF were measured using enzyme-linked immunosorbent assay (ELISA).
Results
The use of thrombin as an activator resulted in immediate release of TGF-β1 and PDGF-AB, while the collagen-activated PRP clots released similar amounts each day for 5 days. The use of collagen as an activator resulted in an 80% greater cumulative release of TGF-β1 from the PRP aliquots over 7 days (P < .001). Concentrating platelets to 3 times the systemic blood level resulted in a 3-fold higher release of TGF-β1, 2.5-fold greater release of PDGF, and 5-fold greater release of VEGF (all P < .0001) when compared with whole blood control clots, but no significant differences in the timing of release were noted.
Conclusion
These experiments demonstrated that the choice of platelet activator can significantly influence the release kinetics of cytokines from PRP, with thrombin resulting in an immediate release and collagen having a more sustained release pattern.
Clinical Relevance
The level and rate of growth factor release depends on the selected platelet activator, a factor that should be considered when selecting a PRP system for a given application.
Keywords: blood clot, growth factor, platelet activation, release kinetics
Platelet-rich plasma (PRP) has been of interest recently as a biological treatment for sports medicine injuries.15,21,33 It has been shown to have success in the clinical treatment of lateral epicondylitis,29 but other applications have had less success.6 Even within the same application, for example, as an adjunct to rotator cuff repair or anterior cruciate ligament (ACL) injury, some studies show efficacy,22,25,30,31 while others do not.34 One of the reasons for the variability in the outcomes when PRP is used may be related to the wide variation in PRP preparation methods currently in use. Some PRP techniques use bovine thrombin as an activator,31,34,37 while others use collagen13,25 or other activators.30 The concentration of platelets in the PRP also varies from system to system.23 What effect these differences might have on the efficacy of the PRP product is as yet relatively understudied.
It is understood that activated platelets release anabolic cytokines from their granules into the surrounding environment. As part of the wound healing process, these growth factors serve as mitogens, cell proliferation stimulants, and cell migration chemoattractants. Growth factors that have been shown to encourage wound healing include, but are not limited to, transforming growth factor (TGF-β1), platelet-derived growth factor (PDGF-AB), and vascular endothelial growth factor (VEGF).6,39 However, little information is available regarding the kinetics of growth factor release from a whole blood clot or PRP clot formed either spontaneously or through activation by exogenous thrombin. There is also little known about whether the release of growth factors from a clot over time is affected by the platelet concentration or presence of platelet activators, such as thrombin or collagen.
It is also of interest to determine whether increasing the concentration of platelets over the levels found in whole blood (ie, by creating PRP) results in a similar fold increase in growth factor release over time. Several previous studies have addressed this question, and the results have been inconsistent. In a quantitative analysis, Eppley et al8 found that the release of TGF-β1, PDGF-AB, and VEGF was significantly greater from PRP than from whole blood when both were induced to clot with bovine thrombin in vitro. In contrast, Weibrich et al38 and Mazzucco et al23 did not find any correlation between platelet concentration and growth factor levels in PRP.
Several negative side effects exist with the use of thrombin, potentially limiting the application of this additive for clinical use. Some such limitations include undesirable immune responses in humans19 and inhibition of cell proliferation and viability in vitro.25 As a result, identifying a safer platelet activator is beneficial. Collagen is an attractive alternative to thrombin due to its native involvement in the intrinsic clotting cascade and its wide use as a biomaterial.20,28 In addition, Fufa et al13 explored the use of type I collagen as a clotting agent and platelet activator in PRP instead of bovine thrombin, measuring clinically relevant levels of TGF-β1, PDGF-AB, and VEGF from both types of clots over several days.
In this study, we hypothesized that both the choice of platelet activator and a higher concentration of platelets, as seen in PRP compared with whole blood, would significantly affect the release of growth factors important in sports medicine applications. Specifically, we hypothesized that the choice of platelet activator would affect the timing and level of growth factor release and that the higher concentration of platelets in PRP would result in a greater level of growth factor release. While other factors such as centrifugation protocols, choice of anticoagulant, platelet integrity, and the presence of other cell types may all influence the efficacy of PRP, proving or disproving these hypotheses would begin to provide us with one possible rationale by which to understand why some studies using PRP may show more efficacy than others for a given clinical situation. While PRP releases a (still mostly unknown) plethora of growth factors, TGF-β1, PDGF-AB, and VEGF are well known for their association with PRP and their effects on wound healing. These 3 are chosen as a representative of PRP growth factors, and because they are used in most PRP studies, this choice allows for comparison of our results with earlier findings.
MATERIALS AND METHODS
Blood Preparation
Human blood was collected from 9 donors (150 mL each; 8 men, 1 woman; age, 28.4 ± 9.1 years). Blood collection was through a blood collection facility holding institutional review board approvals for the collection and distribution of human blood for scientific purposes. For each donor, 30 mL was reserved with no anticoagulant for the spontaneous blood clot control group. The remaining 120 mL of blood was mixed with 10% acid-citrate-dextrose (ACD) as an anticoagulant. The anticoagulated blood was separated into 2 aliquots, one for the whole blood group (40 mL per donor) and one for the PRP groups (80 mL per donor). The PRP was made using the HarvestSmartPreP2 System (Harvest Technologies, Plymouth, Massachusetts), which typically yields a platelet concentration of approximately 3 times over baseline using the selected protocol. Complete blood counts (CBCs) were recorded and reported for the whole blood and PRP samples.
For each of the 9 donors, 28 borosilicate glass tubes (Fisher Scientific, Waltham, Massachusetts) were prepared. Seven tubes (one for each time point) were filled for each of the following groups: (1) whole blood with bovine thrombin (reconstituted with 10% calcium chloride) at a 50:1 ratio, (2) PRP with bovine thrombin (reconstituted with 10% calcium chloride) at a 50:1 ratio, (3) PRP with a collagen sponge, and (4) whole blood with no anticoagulant. The collagen sponges were prepared by lyophilizing an atelocollagen solution10 into a cylindrical sponge 14 mm in diameter and then cutting this into discs 2 mm in width. All samples were allowed to clot and were stored at room temperature (25° C).
Supernatant Extraction
Samples of supernatant exuded from each clot were collected at 2 hours and 1, 2, 3, 4, 5, and 6 days after sample preparation. At each time point, one tube from each type of clot was centrifuged (Sorvall RC3 Centrifuge, Thermo Scientific, Waltham, Massachusetts) at 2500 rpm for 10 minutes, while the remaining tubes were left at room temperature. The supernatant was removed and transferred to a 4-mL cryogenic vial for storage at −80° C. Care was taken to not disturb the clot at the bottom of each tube. The amount of volume removed at each time point was recorded. Tubes containing the clots were discarded after the supernatant was removed.
ELISA
To measure growth factor release in the supernatant, 3 enzyme-linked immunosorbent assays (ELISAs) were run with each sample, transforming growth factor beta (TGF-β1), platelet-derived growth factor (PDGF-AB), and vascular endothelial growth factor (VEGF). Assays were performed according to protocol in commercially available Quantikine colorimetric sandwich ELISA kits (R&D Systems, Minneapolis, Minnesota). As directed by the protocol, all TGF-β1 in the samples was activated before completion of the assay.
Statistical Analysis
Levels of TGF-β1, PDGF-AB, and VEGF were compared between naturally clotted whole blood (WB) and enhanced clotting groups (WB + BT, PRP + BT, PRP + COL) to determine differences in release per volume between groups and over the 7-day time course using mixed-model repeated-measures analysis of variance (ANOVA) with F tests for assessing significant overall differences and a compound symmetry covariance structure to handle the repeated measurements of each growth factor at different time points and different clots for the same donor.9 Means and standard deviations were used to summarize the results because the variables followed a normal Gaussian-shaped distribution closely as tested by the Kolmogorov-Smirnov goodness-of-fit test.2 Statistical analysis was performed using the mixed procedure in the SPSS software package (version 18.0, SPSS Inc, an IBM Company, Chicago, Illinois). Two-tailed values of P < .05 were considered statistically significant.
RESULTS
Cell Counts in Whole Blood and PRP Aliquots
The volume of cumulative released supernatant was approximately 2 mL at every retrieval point for all 4 groups. The erythrocyte, leukocyte, and platelet counts for the whole blood and WB + BT samples were identical because whole blood was used in both. The PRP used in the PRP + BT and PRP + COL samples had a slightly lower hematocrit level and elevated white blood cell and platelet counts, approximately 2 and 3 times higher than the whole blood, respectively (Table 1).
TABLE 1.
Mean ± Standard Deviation of Hematocrit and Platelet, White Blood Cell, Neutrophil, and Monocyte and Lymphocyte Count in the 2 Types of Blood Fractions Used to Form Clots
| Whole Blood | Platelet-Rich Plasma | |
|---|---|---|
| Hematocrit (%) | 37.9 ± 3.9 | 23.1 ± 8.7 |
| Platelets (× 103/μL) | 238.3 ± 44.7 | 721.4 ± 147.4 |
| White blood cells (× 103/μL) | 5.36 ± 1.27 | 11.4 ± 1.69 |
| Neutrophils (× 103/μL) | 3.46 ± 1.40 | 3.68 ± 1.53 |
| Monocytes (× 103/μL) | 0.26 ± 0.12 | 0.83 ± 0.31 |
| Lymphocytes (× 103/μL) | 1.63 ± 0.46 | 6.90 ± 1.94 |
Effect of Activator Choice
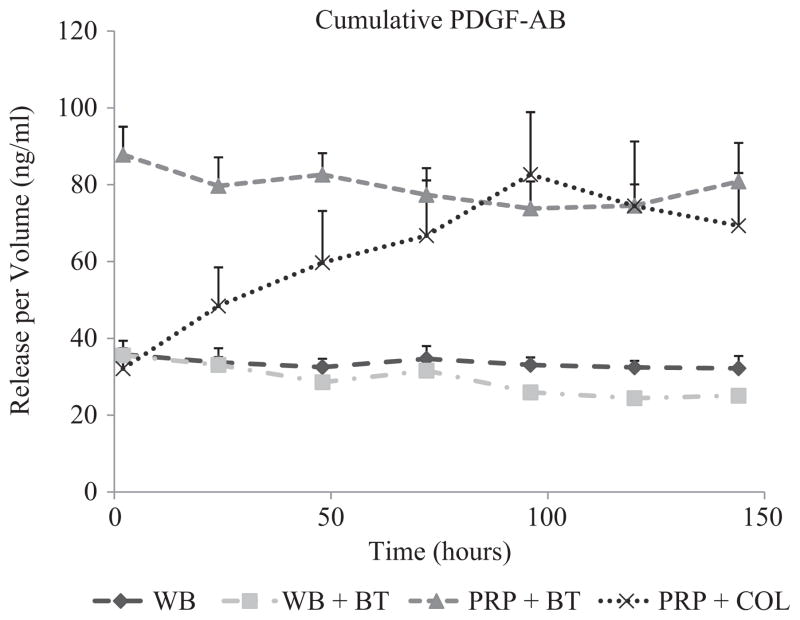
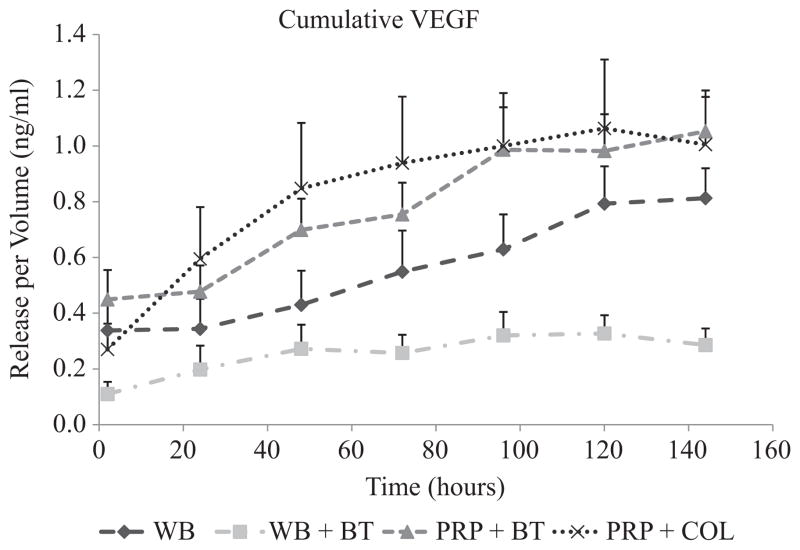
The aliquots of PRP clotted with thrombin had an immediate release of TGF-β1 and PDGF, with all the growth factor released in the first 2 hours and no subsequent release (Figures 1 and 2). In contrast, the aliquots of PRP clotted with collagen released a relatively small amount of TGF-β1 and PDGF at the 2-hour time point and showed an increase in the cumulative TGF-β1 and PDGF levels in the supernatant each subsequent day through day 5, after which the concentration remained constant. This time-dependent release of all growth factors from PRP clotted with collagen was statistically significant by repeated-measures ANOVA (P < .0001). Release of VEGF continued over the week-long study from both groups of PRP clots, with no significant difference in release kinetics or cumulative amount (Figure 3). The cumulative release of TGF-β1 was 80% greater in the collagen group (P < .001) (Figure 1), although there were no significant differences in cumulative release of PDGF (P = .96) or VEGF (P = .55) in the 2 activator groups (Figure 1).
Figure 1.
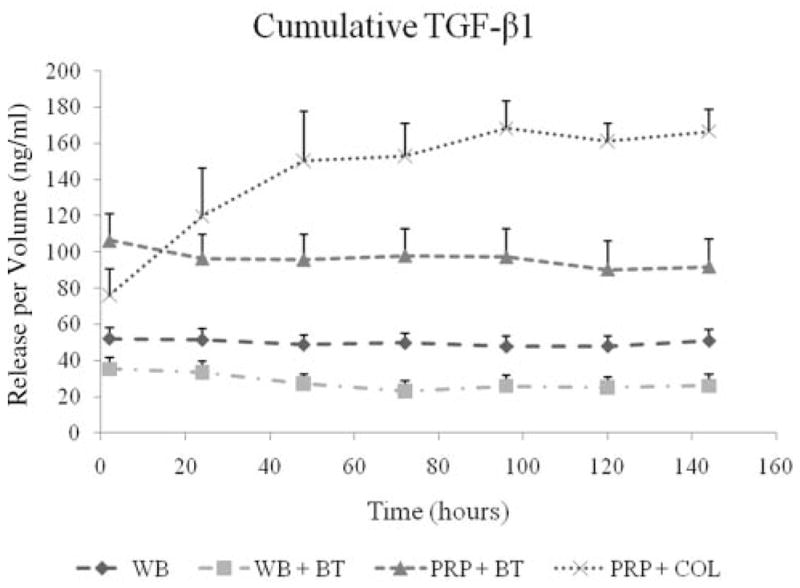
Cumulative TGF-β1 release over time measured in the supernatant surrounding 4 types of blood clots. The naturally clotting whole blood (WB), thrombin-activated whole blood (WB + BT), and thrombin-activated platelet-rich plasma (PRP + BT) clots released TGF-β1 at the 2-hour time point and maintained the same level of cumulative TGF-β1 on the remaining 6 days. In contrast, the collagen-activated platelet-rich plasma (PRP + COL) clots released a relatively small amount of TGF-β1 at the 2-hour time point and showed an increase in the cumulative TGF-β1 levels in the supernatant between day 1 and 5, after which the concentration remained constant. This time-dependent release for PRP + COL was statistically significant by repeated-measures ANOVA (P < .0001) but not significant for WB (P = .999), WB + BT (P = .895), or PRP + BT (P = .532). The shapes at each time point represent average values (n = 9) of growth factor released, and error bars are standard errors of the mean (SEMs).
Figure 2.
Cumulative PDGF-AB release over time in hours measured in the supernatant surrounding 4 types of blood clots. The PDGF-AB release trends were similar to those observed for TGF-β1 in Figure 1. The naturally clotted whole blood (WB) and thrombin-activated clots released a large amount of PDGF-AB at the 2-hour time point and maintained the same cumulative level of PDGF-AB in the supernatant on the remaining 6 days. In contrast, the collagen-activated platelet-rich plasma (PRP) released an increasing total amount of PDGF-AB in the supernatant between days 1 and 5. Repeated-measures ANOVA confirmed a statistically significant time-dependent PDGF-AB release for PRP + COL (P <.0001) but not for WB (P = .999), WB + BT (P = .864), or PRP + BT (P = .800). While the profile of release was different for the collagen and thrombin-activated release of PDGF-AB, there was no significant difference between the cumulative release of PDGF-AB by the fifth day after clot activation. The shapes at each time point represent average values (n = 9) of growth factor released, and error bars are standard errors of the mean (SEMs).
Figure 3.
Cumulative VEGF release over time measured in the supernatant surrounding 4 types of blood clots (refer to Figure 1 for sample descriptions). The majority of the release of VEGF from the thrombin-activated whole blood clot was over the first 2 hours, while that from the collagen-activated platelet-rich plasma (PRP) clot occurred over a longer period of time. The release of VEGF from the naturally clotted whole blood (WB) and thrombin-activated PRP groups also occurred over 5 days. These trends were supported by repeated-measures ANOVA for PRP + COL (P < .0001) and PRP + BT (P < .0001) and WB (P <.0001) but not significant for WB + BT (P = .124). The shapes at each time point represent average values (n = 9) of growth factor released, and error bars are standard errors of the mean (SEMs).
Effect of Platelet Concentration
The PRP aliquots released a 3-fold greater amount of TGF-β1 than the whole blood at all 7 time points (P < .0001) (Figure 1). In addition, the PRP clots released a 2.5-fold greater amount of PDGF-AB than the whole blood clots on day 1 and maintained the same level for 1 week (all P < .0001). The PRP clots released a 5-fold greater amount of VEGF than the whole blood clots at day 1 (P < .0001), and this difference increased through day 7 (P < .0001). There were no significant differences in PDGF release between whole blood naturally clotted or induced to clot with thrombin on any of the 7 days (all P > .40); however, TGF-β1 and VEGF release were suppressed from the whole blood when it was induced to clot with thrombin (P <.031 and P <.007, respectively).
DISCUSSION
In this study, we hypothesized that release kinetics of growth factors released from activated platelets is affected by both platelet activator and platelet concentration. Our results show that both factors have significant influence on growth factor release. We studied 3 different growth factors: TGF-β1, PDGF-AB, and VEGF. While there is a plethora of growth factors released from platelets, these 3 are best described for both their association with platelets and their effect in the wound healing cascade and will therefore serve as surrogates for growth factor release for the purpose of this study. TGF-β1 is released from platelets in a latent form and later becomes activated by any number of molecules involved in extracellular matrix perturbations.24 To enable measurement of TGF-β1 in vitro, an activation step is required during the ELISA procedure. As a result, the state of the TGF-β1 (active or latent) released into the supernatant was not specifically determined. However, this limitation is somewhat mitigated by the prior work reporting that in the in vivo environment where platelets are present, latent TGF-β1 released from the clot is activated by a furin-like enzyme, which is simultaneously released by the platelets.4 Thus, for evaluation of the performance of the 2 platelet activators, collagen and thrombin, the determination of latent versus activated TGF-β1 in vitro may have less of an effect on what occurs in vivo.
Our first hypothesis was that activator choice would significantly affect growth factor release. This hypothesis was found to be true when comparing thrombin and collagen activation of PRP. Thrombin activation resulted in an almost immediate release of PDGF-AB and TGF-β1 from the clots, whereas the collagen activator resulted in a gradual accumulation of the growth factors in the surrounding supernatant. For PDGF-AB, the activator changed only the release profile over time but not the final concentration. In contrast, the cumulative release of TGF-β1 from PRP was higher when collagen was used as the activator, suggesting a sustained release of growth factor. In this case, both release time and total amount were influenced by the activator choice.
Prior studies have reported almost complete release of growth factors from thrombin-activated PRP within the first few hours of activation.32,35 Our results in the thrombin group parallel this observation. However, the collagen group had a sustained release of cytokines over the first several days. This difference in timing of release may be due to the mechanisms by which thrombin and collagen activate platelets. For collagen to activate platelets, the platelets must first adhere to the collagen and then subsequently be activated by it through a second receptor.5 This may require a lengthier mechanism for platelet activation than the enzymatic cleavage process of thrombin-mediated platelet activation. In addition, in normal wound healing, collagen is often the initial activator of platelets, with a platelet monolayer forming over the exposed collagen. Once the initial platelet layer is formed, there is a secondary accumulation of additional platelets via the action of thrombin. Thus, collagen activation of platelets occurs earlier in wound healing, and thus, a delayed activation may be functionally helpful so that the release of growth factors does not occur prematurely before full formation of a provisional scaffold.
Furthermore, the cumulative release of growth factors was less from whole blood when thrombin was used as an activator than when the whole blood was allowed to clot without use of an activator. This effect is most evident in the release of VEGF but also present for PDGF-AB and TGF-β1. This is consistent with prior studies that have shown decreased levels of fibroblast growth factor activity due to cleavage by thrombin.36 Serine proteases, including thrombin, actively degrade growth factors in chronic wound fluid.18 As such, we hypothesize that the decreased levels of growth factors found in whole blood with thrombin compared with whole blood without thrombin are due to proteolytic cleavage, and further studies to validate this hypothesis are planned. In contrast to the influence on release time provided by collagen activation, thrombin activation of whole blood results in a reduction in total growth factor concentrations, suggesting an interaction between growth factor activator choice and platelet concentration.
An activated platelet delivery system that provides for the sustained release of growth factors from platelets could help stimulate a functional healing process similar to that seen in tissues that heal spontaneously.3,17 To date, bovine thrombin has been a commonly used activator of PRP and fibrin sealant.1,16 While bovine thrombin is a potent platelet activator,11 it also causes the development of antibodies against thrombin, prothrombin, factor V, and cardiolipin with resultant clinical problems that include severe postoperative bleeding to bypass graft thrombosis and an auto-immune syndrome similar to lupus in animal studies.19 The use of thrombin also results in impaired migration of anterior cruciate ligament (ACL) cells through collagen-PRP hydrogels as well as impaired strength of the hydrogels24 so alternatives to use of this material are desirable.
The release of VEGF did not appear to be affected significantly by activator choice and was incremental over the 7-day period for both activators. One possible reason for the delayed release of VEGF in all groups and the different response in the VEGF profile from the TGF-β1 and PDGF-AB profiles may be due to the other cell types present in the PRP. Not only were platelets concentrated in the PRP groups, but the number of white blood cells was doubled as well, due principally to a 5-fold increase in lymphocyte concentration (Table 1). Vascular endothelial growth factor has been shown to accumulate during blood storage in a time-dependent manner in nonleukodepleted whole blood.26,27 The accumulation was shown to occur for up to 35 days, including and extending beyond the 1-week time frame for this study. One suggested hypothesis for the accumulation is a gradual release of presynthesized VEGF from white blood cell granules.26 Synthesis of VEGF has been verified in both lymphocytes and neutrophils.12,14 Thus, if the overall VEGF release were affected by the concentration of white blood cells rather than only platelet activation, we might expect similar profiles in both the thrombin- and collagen-activated PRP groups as seen in this experiment. The gradual release of VEGF from the whole blood groups suggests a contribution from neutrophils as well because they accounted for the majority of white blood cells in whole blood, whereas lymphocytes were most populous in the PRP. It is unclear whether this white blood cell effect dominated the VEGF release profile or if it was simply an accessory to the release from platelets observed for the other 2 growth factors. Further studies evaluating the effect of lymphocyte concentration on cytokine release from PRP are planned.
We also hypothesized that concentrating platelets as PRP would result in increased cytokine release from a clot when compared with whole blood. This hypothesis was proven when comparing whole blood and PRP activated by the same activator, thrombin. We found increases in PDGF-AB and TGF-β1 that paralleled the increase in platelet count (approximately 3 times) and an increase in VEGF release that was 5-fold greater than the systemic blood clot. Our results are in line with previous findings that increasing platelet counts in blood may result in higher levels of growth factor release but not necessarily on through a linear correlation for every individual.8,23,38 The PRP used in this study contained 3 times more platelets than the baseline whole blood, which is similar to the increase in PDGF and TGF-β1. The higher release of VEGF may again be due to the increased concentration not only of platelets but of leukocytes as well within the PRP.12,26,27
There were several limitations of this study. First, we only measured the growth factor release in the supernatant. There may have been additional growth factors released from the platelets but bound to the collagen or other extracellular matrix proteins and thus not available in the supernatant. Further work examining the growth factor adhesion to the multiple extracellular matrix proteins would be required to define this. However, the majority of the global biological activity of a blood clot in the first week after injury is to emit cytokines and growth factors that stimulate cells within the surrounding tissues (macrophages, stem cells, and fibroblasts) rather than cells within the scaffold itself (red blood cells, small numbers of terminally differentiated white blood cells).7 Thus, the growth factors released into the surrounding milieu of the wound are also of some importance in understanding the activity of PRP placed in a wound site. Second, this is an in vitro study, and how the release kinetics would be different in vivo remains to be studied. A final limitation is that in this study, we used a PRP preparation method in which the PRP contains not only platelets but also red blood cells and white blood cells. The inclusion of these other cell types may have confounded results to some extent, even though the same numbers of all cell types were used in each PRP group. While the use of a platelet-only preparation would certainly have been of interest on a basic science level, it may be less useful for the clinician trying to understand how his or her PRP preparation method will perform in the operating room.
In summary, the use of thrombin to activate platelet clots results in an almost immediate release of all the platelet-associated anabolic growth factors studied here, while the use of collagen as an activator results in a more sustained release over a week of 2 of the 3 cytokines studied. In addition, using a higher concentration of platelets does result in a higher average growth factor release, but the increase in growth factor release may not correlate exactly with the increase in platelet count for every individual. The use of thrombin as an activator should be considered carefully, and the optimal concentration of platelets for any given clinical application also deserves further study. In applications where a more sustained growth factor release is desired, a collagen activator may be of some benefit.
Acknowledgments
One or more of the authors has declared the following potential conflict of interest or source of funding: Support for this study was received from the National Institutes of Health (NIAMS grant numbers AR052772 and AR054099). Supplies for platelet-rich plasma preparation were provided by Harvest Technologies. Sherwin Kevy is a consultant for Harvest Technologies.
Footnotes
For reprints and permission queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav
References
- 1.Albala DM. Fibrin sealants in clinical practice. Cardiovasc Surg. 2003;11 (Suppl 1):5–11. doi: 10.1016/S0967-2109(03)00065-6. [DOI] [PubMed] [Google Scholar]
- 2.Altman D. Practical Statistics for Medical Research. Boca Raton, Florida: Chapman and Hall; 1991. [Google Scholar]
- 3.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 4.Blakytny R, Ludlow A, Martin GE, et al. Latent TGF-beta1 activation by platelets. J Cell Physiol. 2004;199(1):67–76. doi: 10.1002/jcp.10454. [DOI] [PubMed] [Google Scholar]
- 5.Brass LF. Thrombin and platelet activation. Chest. 2003;124(3 Suppl):18S–25S. doi: 10.1378/chest.124.3_suppl.18s. [DOI] [PubMed] [Google Scholar]
- 6.de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144–149. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 7.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 8.Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114(6):1502–1508. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 9.Fahrmeir L, Tutz G. Multivariate Statistical Modelling Based on Generalized Linear Models. 2. New York: Springer; 2001. [Google Scholar]
- 10.Fleming BC, Magarian EM, Harrison SL, Paller DJ, Murray MM. Collagen scaffold supplementation does not improve the functional properties of the repaired anterior cruciate ligament. J Orthop Res. 2010;28(6):703–709. doi: 10.1002/jor.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frechette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84(5):434–439. doi: 10.1177/154405910508400507. [DOI] [PubMed] [Google Scholar]
- 12.Freeman MR, Schneck FX, Gagnon ML, et al. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res. 1995;55(18):4140–4145. [PubMed] [Google Scholar]
- 13.Fufa D, Shealy B, Jacobson M, Kevy S, Murray MM. Activation of platelet-rich plasma using soluble type I collagen. J Oral Maxillofac Surg. 2008;66(4):684–690. doi: 10.1016/j.joms.2007.06.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudry M, Bregerie O, Andrieu V, et al. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood. 1997;90(10):4153–4161. [PubMed] [Google Scholar]
- 15.Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602–608. doi: 10.5435/00124635-200910000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Jackson MR. Fibrin sealants in surgical practice: an overview. Am J Surg. 2001;182(2 Suppl):1S–7S. doi: 10.1016/s0002-9610(01)00770-x. [DOI] [PubMed] [Google Scholar]
- 17.Jurk K, Kehrel BE. Platelets: physiology and biochemistry. Semin Thromb Hemost. 2005;31(4):381–392. doi: 10.1055/s-2005-916671. [DOI] [PubMed] [Google Scholar]
- 18.Lauer G, Sollberg S, Cole M, et al. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol. 2000;115(1):12–18. doi: 10.1046/j.1523-1747.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- 19.Lawson JH. The clinical use and immunologic impact of thrombin in surgery. Semin Thromb Hemost. 2006;32 (Suppl 1):98–110. doi: 10.1055/s-2006-939559. [DOI] [PubMed] [Google Scholar]
- 20.Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221(1–2):1–22. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26(2):269–278. doi: 10.1016/j.arthro.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Maniscalco P, Gambera D, Lunati A, et al. The “Cascade”: membrane: a new PRP device for tendon ruptures. Description and case report on rotator cuff tendon. Acta Biomed. 2008;79(3):223–226. [PubMed] [Google Scholar]
- 23.Mazzucco L, Balbo V, Cattana E, Guaschino R, Borzini P. Not every PRP-gel is born equal. Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang. 2009;97(2):110–118. doi: 10.1111/j.1423-0410.2009.01188.x. [DOI] [PubMed] [Google Scholar]
- 24.Murray MM, Forsythe B, Chen F, et al. The effect of thrombin on ACL fibroblast interactions with collagen hydrogels. J Orthop Res. 2006;24(3):508–515. doi: 10.1002/jor.20054. [DOI] [PubMed] [Google Scholar]
- 25.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25(1):81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen HJ, Werther K, Mynster T, Brunner N. Soluble vascular endothelial growth factor in various blood transfusion components. Transfusion. 1999;39(10):1078–1083. doi: 10.1046/j.1537-2995.1999.39101078.x. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen HJ, Werther K, Mynster T, et al. Bacteria-induced release of white cell- and platelet-derived vascular endothelial growth factor in vitro. Vox Sang. 2001;80(3):170–178. doi: 10.1046/j.1423-0410.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- 28.Pachence JM. Collagen-based devices for soft tissue repair. J Biomed Mater Res. 1996;33(1):35–40. doi: 10.1002/(SICI)1097-4636(199621)33:1<35::AID-JBM6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 29.Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255–262. doi: 10.1177/0363546509355445. [DOI] [PubMed] [Google Scholar]
- 30.Radice F, Yanez R, Gutierrez V, et al. Comparison of magnetic resonance imaging findings in anterior cruciate ligament grafts with and without autologous platelet-derived growth factors. Arthroscopy. 2010;26(1):50–57. doi: 10.1016/j.arthro.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 31.Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair: a pilot study. Disabil Rehabil. 2008;30(20–22):1584–1589. doi: 10.1080/09638280801906081. [DOI] [PubMed] [Google Scholar]
- 32.Roussy Y, Bertrand Duchesne MP, Gagnon G. Activation of human platelet-rich plasmas: effect on growth factors release, cell division and in vivo bone formation. Clin Oral Implants Res. 2007;18(5):639–648. doi: 10.1111/j.1600-0501.2007.01385.x. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez M, Anitua E, Orive G, et al. Platelet-rich therapies in the treatment of orthopaedic sport injuries. Sports Med. 2009;39(5):345–354. doi: 10.2165/00007256-200939050-00002. [DOI] [PubMed] [Google Scholar]
- 34.Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing? Knee Surg Sports Traumatol Arthrosc. 2009;17(6):676–682. doi: 10.1007/s00167-009-0762-8. [DOI] [PubMed] [Google Scholar]
- 35.Su CY, Kuo YP, Nieh HL, Tseng YH, Burnouf T. Quantitative assessment of the kinetics of growth factors release from platelet gel. Transfusion. 2008;48(11):2414–2420. doi: 10.1111/j.1537-2995.2008.01862.x. [DOI] [PubMed] [Google Scholar]
- 36.Totta P, De Cristofaro R, Giampietri C, et al. Thrombin-mediated impairment of fibroblast growth factor-2 activity. FEBS J. 2009;276(12):3277–3289. doi: 10.1111/j.1742-4658.2009.07042.x. [DOI] [PubMed] [Google Scholar]
- 37.Virchenko O, Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop. 2006;77(5):806–812. doi: 10.1080/17453670610013033. [DOI] [PubMed] [Google Scholar]
- 38.Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 39.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]