Abstract
Objectives
Our earlier post hoc analysis suggested possible benefits of statins in reducing risk of Alzheimer’s disease (AD) in younger participants (< age 80 years) with the APOE ε4 allele. We further investigated these findings with more follow-up time and more recently enrolled participants.
Design
A cohort of cognitively intact elderly was assessed biennially for dementia and AD.
Setting
Community based.
Participants
3,392 non-demented member of a health maintenance organization (HMO) aged ≥ 65 years.
Measurements
We identified statin use from the HMO pharmacy database and applied proportional hazards models with statin use as a time-dependent covariate to assess the statin-AD association and the modifying effects of age and the APOE ε4 allele.
Results
Over an average of 6.1 years of follow-up of 3099 participants, 263 participants developed probable AD. The adjusted hazard ratio (aHR) for statin use was 0.62 (95% confidence interval [CI], 0.40 – 0.97) for AD in models including demographic characteristics and vascular risk factors as covariates. The strength of the statin-AD association diminished with age (statin × age-at-entry interaction p = 0.04); the aHR in those younger than 80 was 0.44 (CI 0.25 – 0.78) vs. 1.22 (CI 0.61 – 2.42) for those older than 80. The interaction term for statin use × APOE ε4 was not significant (p = 0.65).
Conclusion
This enlarged study confirms earlier findings that statin therapy in early old age, but not in late age, may be associated with reduced risk of AD. The relationship between statin use and AD was consistent across APOE genotypes.
Keywords: Statin, Old age, APOE genotype, Alzheimer disease
INTRODUCTION
The effect of statin therapy in reducing the risk of dementia is still controversial. Alzheimer’s disease (AD) may start decades prior to clinical symptoms of dementia. Epidemiological studies showed that hypertension and hypercholesterolemia in mid-life increased the risk of dementia 20–30 years later, but similar effects were not evident in late-life.1 Both our earlier study2 and the Canadian Study on Health and Aging3 that examined the association between statin exposure and risk of AD in large cohorts suggested that the association between statin use and AD risk may differ by age at treatment initiation, with an apparent benefit in individuals younger than age 80.2, 3 We hypothesize that statin therapy initiated in relatively early old ages, before profound neuropathological changes of AD occur, may delay dementia onset more than therapy initiated later. We used our Adult Changes in Thought (ACT) cohort, a large community based longitudinal study of dementia with computerized pharmacy data, to investigate the modifying effect of age on the association between statin use and risk of AD. Statins were not widely used before the mid-1990’s when the ACT study began and were not available for most of our older subjects in their middle age or early old age. Thus, ACT provides a natural laboratory for a study which would otherwise be difficult to conduct due to ethical concerns.
The APOE ε4 allele is a well-known genetic risk factor for AD.4 Statins appear to be especially beneficial in reducing mortality for those with the APOE ε4 allele compared with those without this allele.5 Our earlier exploratory analysis suggested a trend toward AD risk reduction with statin use in younger participants (age-at-entry 65–79 years) who carry the APOE ε4 allele, but not in those younger participants without the allele nor in older participants (age-at-entry ≥ 80).2 Here, we address two specific hypotheses related to the APOE ε4 allele: 1) APOE ε4 status modifies the association between statin use and risk of probable AD, 2) statin use reduces the risk of AD in individuals who carry the ε4 allele and therefore who are more vulnerable to the early development of AD.
METHODS
Participants
The ACT study has been described elsewhere.6 Briefly, this community-based prospective cohort study drew participants from Seattle area members of the Group Health Cooperative (GHC) health maintenance organization. 2581 participants aged 65 years and older were enrolled in 1994–96 (Original cohort) and 811 participants were enrolled in 2000–02 (Expansion cohort). The criteria for enrollment were a score of ≥ 86 out of 100 on the Cognitive Abilities Screening Instrument 7 (CASI, a brief cognitive screen test) or lack of evidence of dementia after additional examination. Demographic characteristics, medical history, and suspected AD risk factors were obtained at time of entry to study. The Expansion cohort participants tended to be slightly older at ACT study entry (mean ± standard deviation [SD], 75.2 ± 6.1 vs 76.2 ± 6.7 years), more non-White (13% vs 9%), and were more likely to be taking statins (29% vs 21%) at a relatively younger age (73.2 ± 7.3 vs 76.3 ± 5.9) than Original cohort participants; they were otherwise similar with respect to gender, education, and baseline CASI score.
Exposure Measurement
GHC patients receive prescriptions through the GHC pharmacy at no or nominal cost. The GHC pharmacy database was established in January 1977. Its data files contain information on drug, dosage, quantity dispensed, prescription date, and instructions. We defined use of statin medications (simvastatin, lovastatin, pravastatin, and atorvastatin) and other lipid lowering agents (LLAs), including niacin, cholestyramine, colestipol, gemfibrozil, and clofibrate, by at least three filled prescriptions for statins and/or LLAs numbering ≥15 tablets. Subjects who did not use statins consistently with average daily dose (cumulative dose/duration) < 0.5 and total number of refills < 12) were considered non-users. To quantify statin use, we used an approximation of one “statin equivalent dose” as 10 mg of simvastatin, 20mg of lovastatin or pravastatin, or 5mg of atorvastatin. Duration of statin use was defined as the time elapsed from the date of first statin prescription to the date of last prescription. APOE genotype was assessed8 on 2,755 (89%) participants who had at least one follow-up, but was unavailable for 344 participants (11%) for reasons such as refusal or lab failure in genotyping.
Outcome measurement
After enrollment, participants were re-screened every two years with the CASI. Those whose CASI scores were below 86 underwent a standardized dementia diagnostic evaluation that included examination by a study physician and neuropsychological tests. Relevant laboratory tests and neuroimaging studies were performed or results were obtained from GHC records. Diagnoses were assigned at consensus diagnostic conferences using DSM-IV criteria for dementia9 and the National Institute of Neurological and Communicative Disorders and Stroke – Alzheimer Disease and Related Disorders Association (NINCDS-ADRDA) criteria for AD.10 Those with new onset dementia underwent at least one annual follow-up examination for verification of dementia status and subtype. Dementia onset was defined by convention as halfway between the date of diagnosis and the date of the prior ACT study examination that showed no dementia. Our primary outcome was NINCDS-ADRDA probable AD. Participants who developed other types of dementia were censored at the time of estimated onset.
Statistical Analyses
Data from both statin users and non-users were censored at the estimated onset date of dementia or last observation. Bivariate differences in sample characteristics were assessed using two sample t-tests or chi square tests. We used Cox proportional hazards regression models to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the risk of probable AD associated with statin use.11 The time axis was participant age. Statin use was modeled as a time-dependent variable in that participants were not considered users until after the initiation of statin use, and were analyzed as exposed to statins thereafter.
To examine the modifying effects of age and APOE genotype on the association between statin use and risk of AD, we estimated interaction terms for age-at-entry (categorized into 5-year categories) × statin exposure and APOE ε4 status (unknown status excluded) × statin exposure. We performed a sensitivity analysis in which we assigned all of those with missing APOE genotypes to ε4 present, and another in which we assigned all of those with missing APOE genotypes to ε4 absent. We also constructed models stratified by age-at-entry (65–69 years, 70–74 years, 75–79 years, 80–84 years, and 85+ years), and by APOE ε4 status to examine the association between statin exposure and AD risk within each group.
To control for confounding by indication and other potential bias, adjusted HRs (aHR) for the association between statin and AD were estimated using stratified Cox models with five category age-at-onset and APOE ε4 status as the strata over which separate baseline hazard functions were estimated, where appropriate. Additional model covariates included cohort (Original vs. Expansion cohort), gender, self-reported race/ethnicity (white vs. non-white), years of education, other LLA use (modeled as time-dependent similar to statins) and study entry characteristics including CASI score (to control for pre-clinical dementia), body mass index (BMI), cigarette smoking history and presence of self-reported co-morbid vascular diseases (cardiovascular disease, cerebrovascular disease, diabetes mellitus, and hypertension). There were 68 participants (2%) with missing covariates: these participants were excluded from the adjusted analyses. Furthermore, 2848 participants (92%) had total cholesterol (TC) and high density lipoprotein (HDL) measurement available through a computerized laboratory database. Average TC and HDL levels prior to statin treatment or prior to entry into study (for non-statin users) were added as covariates in an additional set of models. As statin users began treatment an average 1.2 years prior to study entry, the average time of assessment of untreated cholesterol levels for statin users was close to study entry. Schoenfeld residual plots and statistical tests were used to test the proportional hazards assumption.12 Results from the Cox models are presented as adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs). All statistical analyses were performed using R.13
RESULTS
Of 3,392 enrolled participants, 3,099 (91.4%) had at least one follow-up examination and contributed to the present analyses. Compared with participants remaining in the study, those who dropped out or died prior to the first follow-up visit after initial enrollment were slightly older (78 vs. 75 years, p < 0.01), were more likely to be male (46% vs. 41%, p = 0.05), and had slightly lower educational attainment (13 vs. 14 years of education, p < 0.01). During follow-up 263 participants developed probable AD. Mean age at onset of AD was 82.9 (SD 5.1) years. The completeness of follow-up rate of 92% was excellent.14
Of 3,099 participants, 711 had been exposed to statins with duration of exposure of 5.4 ± 3.6 years. Compared with non-users, statin users were younger, had higher CASI scores at study entry and were followed longer (Table 1). Not surprisingly, statin users had higher rates of self-reported co-morbid vascular conditions and history of cigarette smoking along with higher serum TC, lower HDL and higher BMI. However, there were no differences in race, education or presence of APOE ε4 allele between statin users and non-users.
Table 1.
Characteristics of Participants by Statin Therapy
| Statin users (n=711) mean ± SD or n (%) |
Statin non users (n=2388) mean ± SD or n (%) |
|
|---|---|---|
| Age-at-entry, years | 74.2 ± 5.5 | 75.8 ± 6.4* |
| Age at time of censoring | 80.7 ± 5.4 | 81.8 ± 6.3* |
| Duration of follow-up, year | 6.6 ± 3.3 | 6.0 ± 3.2* |
| Sex, male | 355 (50) | 901 (38)* |
| Non-white | 74 (10) | 228 (10) |
| Education, years | 14.1 ± 3.2 | 14.0 ± 3.1 |
| CASI score at entry | 93.3 ± 4.6 | 92.8 ± 5.2* |
| Presence of APOE ε4, % | 166 (27) | 536 (25) |
| Comorbid conditions at entry | ||
| Cardiovascular disease | 315 (44) | 293 (12)* |
| Cerebrovascular disease | 92 (13) | 221 (9)* |
| Diabetes mellitus | 140 (20) | 166 (7)* |
| Hypertension | 357 (50) | 845 (36)* |
| Current cigarette smoking | 37 (5) | 145 (6) |
| History of cigarette smoking | 405 (57) | 1211 (51)* |
| BMI | 28.5 ± 4.7 | 27.0 ± 4.8* |
| TC, mg/dl | 240.3 ± 40.9 | 227.0 ± 37.0* |
| HDL, mg/dl | 48.9 ± 12.7 | 56.1 ± 16.0* |
| Other LLA use | 211 (30) | 133 (6)* |
p < 0.05 by 2-sample t-test or χ2 between cohorts.
Information missing for CASI (n=1), Education (n=3), Cardiovascular disease (n=4), Cerebrovascular disease (n=3), Diabetes mellitus (n=2), Hypertension (n=12), BMI (n=49), APOE ε4 (n=344), Total cholesterol (n=237) and HDL (n=251).
SD: standard deviation; CASI: Cognitive Abilities Screening Instrument (score range 0 – 100); BMI: body mass index; TC: total cholesterol; HDL: high density lipoprotein; and LLA: other lipid lowering agent.
Table 2 shows participant characteristics by age and presence of APOE ε4 allele. Compared with younger participants (< 80 years), older participants were more likely to be female and white, had less education, poorer baseline cognitive performance and shorter duration of follow-up, and were more likely to develop dementia and AD during the follow-up period. Although they were more likely to have co-morbid cardiovascular and cerebrovascular disease, older participants had lower BMI and higher serum HDL levels, and were less likely to smoke and use statins or other LLAs than younger participants. Baseline characteristics were similar in those with and without the APOE ε4 allele except that participants with the APOE ε4 allele were more likely to be female and have higher serum TC levels.
Table 2.
Characteristics of Participants by Age-at-study Entry and APOE ε4 status
| Age-at-entry < 80 years mean ± SD or n (%) |
Age-at-entry ≥ 80 years mean ± SD or n (%) |
|||||
|---|---|---|---|---|---|---|
| ε4 + (n=553) |
ε4 − (n=1526) |
total‡ (n=2343) |
ε4 + (n=149) |
ε4 − (n=527) |
total‡ (n=756) |
|
| Age-at-entry, years | 72.4 ± 3.8 | 72.7 ± 3.8 | 72.6 ± 3.8* | 84.0 ± 3.9 | 84.1 ± 3.5 | 84.2 ± 3.6 |
| Age at time of censoring | 78.8 ± 4.7† | 79.5 ± 4.6 | 79.2 ± 4.7* | 88.3 ± 4.7 | 88.8 ± 4.1 | 88.7 ± 4.2 |
| Duration of follow-up, year | 6.4 ± 3.2† | 6.8 ± 3.1 | 6.6 ± 3.2* | 4.3 ± 2.7 | 4.7 ± 2.9 | 4.5 ± 2.8 |
| Sex, male | 206 (37.3) † | 688 (45.1) | 994 (42.4)* | 56 (37.6) | 184 (34.9) | 262 (34.7) |
| Non-white | 62 (11.2) | 151 (9.9) | 256 (10.9)* | 8 (5.4) | 26 (4.9) | 46 (6.1) |
| Education, years | 14.3 ± 3.0 | 14.2 ± 3.0 | 14.3 ± 3.0* | 13.7 ± 3.0 | 13.3 ± 3.2 | 13.3 ± 3.2 |
| CASI score at entry | 93.7 ± 4.5 | 93.8 ± 4.5 | 93.6 ± 4.6* | 90.3 ± 5.9 | 91.0 ± 6.0 | 90.7 ± 5.9 |
| Comorbid conditions at entry | ||||||
| Cardiovascular disease | 113 (20.4) | 268 (17.6) | 433 (18.5)* | 37 (25.0) | 121 (23.0) | 175 (23.2) |
| Cerebrovascular disease | 60 (10.8) | 122 (8.0) | 215 (9.2)* | 20 (13.5) | 67 (12.7) | 98 (13.0) |
| Diabetes mellitus | 56 (10.1) | 168 (11.0) | 259 (11.1)* | 7 (4.7) | 36 (6.8) | 47 (6.2) |
| Hypertension | 207(37.4) | 574 (37.8) | 895 (38.3) | 55 (37.2) | 215 (41.0) | 307 (40.8) |
| Current cigarette smoking | 41 (7.4) | 101 (6.6) | 158 (6.7)* | 5 (3.4) | 17 (3.2) | 24 (3.2) |
| History of cigarette smoking | 294 (53.3) | 833 (54.6) | 1262 (53.9)* | 73 (49.0) | 244 (46.3) | 354 (46.8) |
| BMI | 27.7 ± 5.1 | 27.6 ± 4.9 | 27.7 ± 5.0* | 26.1 ± 4.2 | 26.1 ± 4.0 | 26.1 ± 4.1 |
| TC, mg/dl | 235.3 ± 35.6† | 229.0 ± 39.7 | 230.6 ± 38.8 | 237.1 ± 36.2† | 225.9 ± 37.1 | 229.3 ± 37.3 |
| HDL, mg/dl | 53.4 ± 14.7 | 54.1 ± 15.8 | 53.9 ± 15.7* | 55.3 ± 15.4 | 55.4 ± 15.1 | 55.8 ± 15.2 |
| Statin therapy | ||||||
| Frequency (%) | 140 (25.3) | 379 (24.8) | 596 (25.4)* | 26 (17.4) | 75 (14.2) | 115 (15.2) |
| Daily equivalent dose | 2.1 ± 1.3 | 2.2 ± 1.4 | 2.1 ± 2.0 | 2.0 ± 0.9 | 2.0 ± 1.0 | 2.0 ± 0.9 |
| Duration, years | 4.9 ± 3.3 | 4.8 ± 3.6 | 4.8 ± 3.5 | 4.8 ± 3.5 | 4.4 ± 3.3 | 4.6 ± 3.4 |
| Other LLA use | 76 (13.7) | 178 (11.7) | 292 (12.5)* | 9 (6.0) | 39 (7.4) | 52(6.9) |
| Dementia | 105 (19.0) † | 141 (9.2) | 281 (12.0)* | 50 (33.6) † | 128 (24.3) | 201 (26.6) |
| Probable AD | 65 (11.8) † | 72 (4.7) | 157 (6.7)* | 29 (19.5) † | 65 (12.3) | 106 (14.0) |
Information missing for CASI (n=1), education (n=3), Cardiovascular disease (n=4), Cerebrovascular disease (n=3), Diabetes mellitus (n=2), Hypertension (n=12) and BMI (n=49).
p< 0.05 for difference between age groups (age < 80 vs age ≥ 80 years) in either t-test or χ2 test.
p <0.05 for difference between those with and without ε4 allele.
includes participants with missing APOE.
SD: standard deviation; CASI: Cognitive Abilities Screening Instrument (score range 0 – 100); BMI: body mass index; TC: total cholesterol; HDL: high density lipoprotein; LLA: other lipid lowering agent; and AD: Alzheimer disease.
Table 3 presents frequencies of AD and both crude and adjusted hazard ratios for probable AD by statin use for all subjects, and within age and APOE genotype groups. The overall aHR for probable AD with statin use was 0.62 (CI 0.40 – 0.97). There was no significant association between other LLA use and risk of probable AD (aHR = 1.23, CI 0.79 –1.91). Further adjustment for average TC and HDL levels did not change these findings (data not shown).
Table 3.
HR of Statin Use for Risk of Probable AD by Age and APOE ε4 Status
| Number of probable AD/ person-years |
HR (95% CI) | ||
|---|---|---|---|
| Basic Model | Adjusted Model | ||
| All subjects | 263/18933 | 0.69 (0.46 – 1.02) | 0.62 (0.40 – 0.97) |
| Age-at-entry, years | |||
| < 80 years | 157/15529 | 0.52 (0.31 – 0.88) | 0.44 (0.25 – 0.78) |
| ≥ 80 years | 106/3404 | 1.12 (0.61 – 2.06) | 1.22 (0.61 – 2.42) |
| APOE ε4 status | |||
| ε4 + | 94/4200 | 0.61 (0.32 – 1.18) | 0.42 (0.20 – 0.91) |
| ε4 − | 137/12894 | 0.73 (0.42 – 1.27) | 0.77 (0.42 – 1.40) |
| Unknown | 32/1839 | 0.59 (0.20 – 1.70) | 0.84 (0.22 – 3.15) |
Basic Model: adjusted for cohort (Original vs Expansion cohort) only. Adjusted Model: Based on stratified Cox models adjusted for cohort, gender, race, years of education, CASI score at baseline, comorbid vascular diseases, BMI, history of cigarette smoking and other LLA use, with strata defined by 5 category age (except for models on individual age groups) and APOE ε4 status (except for models of individual APOE ε4 categories). Adjusted model estimates were based on 68 fewer participants due to participants with missing covariates.
HR: hazard ration; AD: Alzheimer’s disease; CASI: Cognitive Abilities Screening Instrument (score range 0 – 100); BMI: body mass index; LLA: other lipid lowering agent.
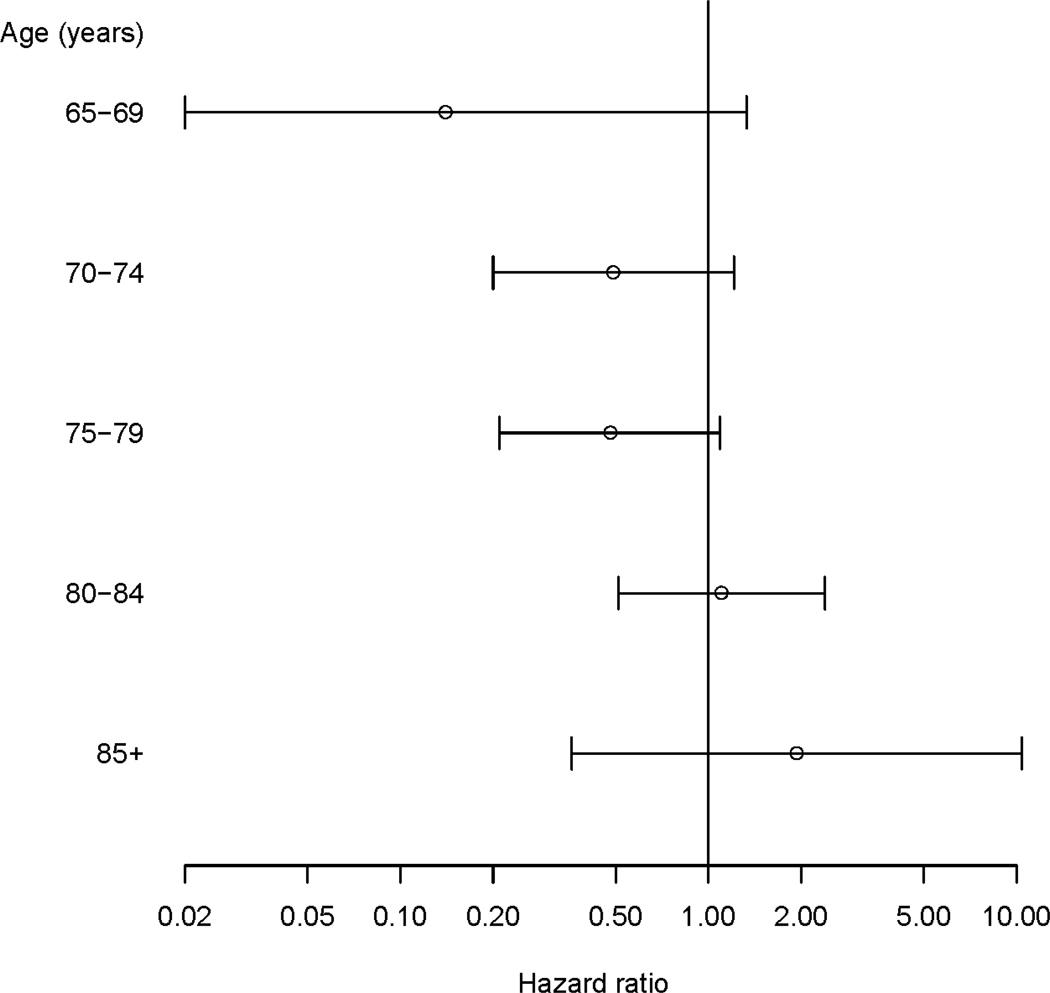
The association between statin use and AD varied by age-at-entry (statin × 5-year age interaction p = 0.04) in the fully adjusted model. The aHRs for the association between statin use and risk for probable AD decreased as age increased Figure 1). Statin exposure was associated with reduced risk for probable AD in younger study participants (those less than age 80 at study entry; aHR 0.44, 95% CI 0.25–0.78, Table 3). Statin exposure was not associated with risk for probable AD in those age 80 or older (aHR =1.22, 95% CI 0.61 – 2.42). By contrast, there was no association between exposure to other LLAs and risk for probable AD in either the younger (aHR = 1.26, CI 0.75 – 2.13) or the older (aHR = 1.33, CI 0.58 – 3.06) age group.
Figure 1.
Adjusted HR and 95% confidence interval of statin therapy for AD by age-at-entry based on Cox models adjusted for cohort, gender, race, years of education, CASI score at baseline, comorbid vascular diseases, BMI, history of cigarette smoking and other LLA use.
HR: hazard ration; AD: Alzheimer’s disease; CASI: Cognitive Abilities Screening Instrument (score range 0 – 100); BMI: body mass index; LLA: other lipid lowering agent.
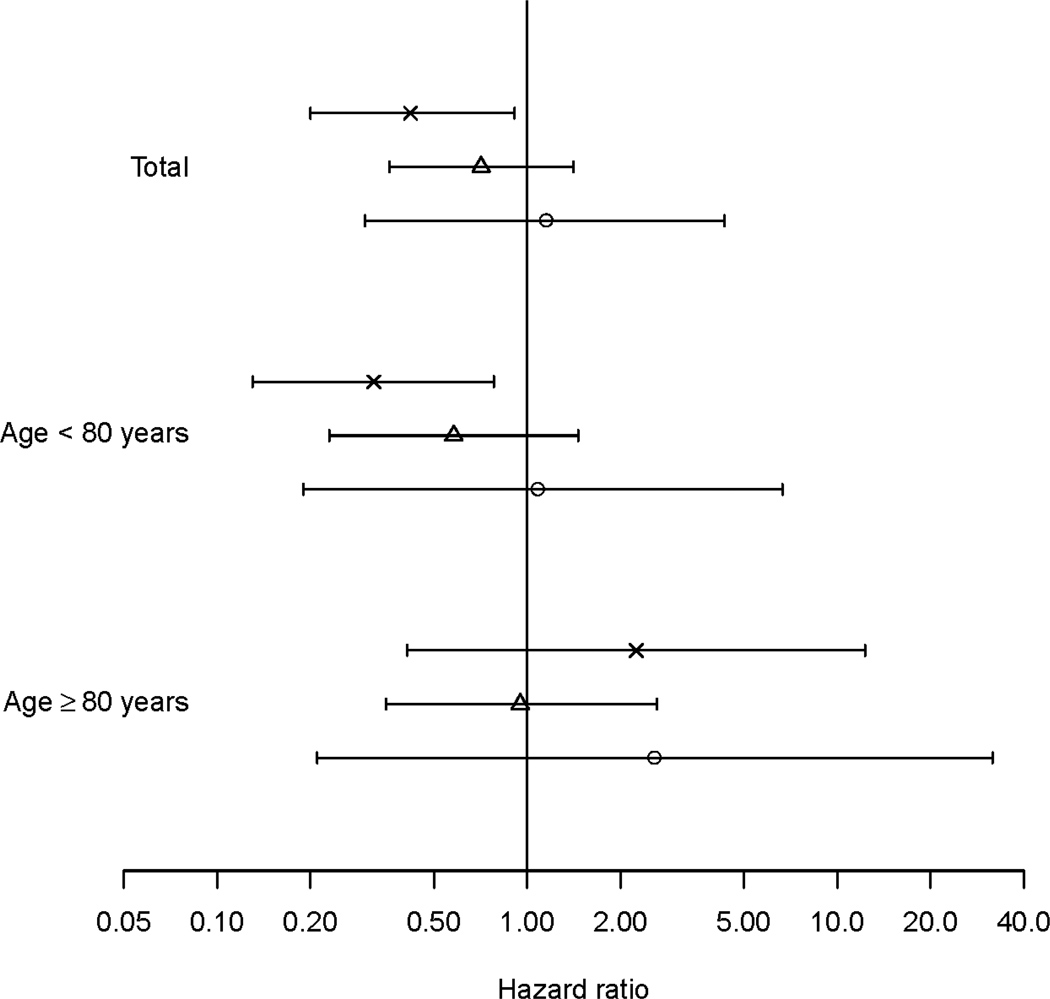
Figure 2 presents adjusted HRs by APOE genotype. The interaction between statin use and APOE ε4 status was not significant (p = 0.65) in the adjusted stratified Cox model with five category age-at-entry defining the strata. This result was consistent whether assigning all of those with missing APOE ε4 status to “absent” or “present” or in those under age 80 or those aged 80 and older (p > 0.67). Statin use was associated with a significantly reduced risk of AD among those with an ε4 allele (aHR = 0.42, CI 0.20–0.91; including those with missing APOE ε4 genotype, aHR = 0.43, CI 0.22–0.84) but not among those without (aHR = 0.77, CI 0.42–1.40; including missing APOE ε4, aHR = 0.82, CI 0.48–1.41). This pattern was seen among the younger participants but not in older participants where statin use showed no association with AD risk regardless of APOE ε4 status (Figure 2) All of the main findings reported remained unchanged with the addition of the baseline cholesterol measures TC and HDL to the model (data not shown).
Figure 2.
Adjusted HR and 95% confidence interval of statin therapy for AD by age-at-entry and APOE allele ε2/ε2 or ε2/ε3 (circle), ε3/ε3 (triangle) and ε2/ε4, ε3/ε4, or ε4/ε4 ('x') based on stratified Cox models adjusted for cohort, gender, race, years of education, CASI score at baseline, comorbid vascular diseases, BMI, history of cigarette smoking and other LLA use, with strata defined by 5 category age.
HR: hazard ration; AD: Alzheimer’s disease; CASI: Cognitive Abilities Screening Instrument (score range 0 – 100); BMI: body mass index; LLA: other lipid lowering agent.
DISCUSSION
This study, with an additional three more years of follow-up of the original cohort and an 2,006 person-years from a new cohort enrolled in 2000–2002, confirmed our earlier finding that the potential protective effect of statin for AD appears to diminish with advancing age. This result is also consistent with the results from the Canadian Study of Health and Aging, which found a lower risk of AD in statin users younger than 80 years but no reduction in risk in older individuals.3 The lack of association between statin use and AD in very old participants may be explained in part by survival bias. Participants who survived to old age without dying or becoming demented may have biological characteristics not shared by those who did not survive to a similar age without dementia. For example, those with the most severe cardiovascular disease, diabetes or cerebrovascular disease, who would likely have been at highest risk for dementia, were also more likely to have died from vascular diseases or to have developed dementia at earlier ages and, thus, to have been unavailable or ineligible to be enrolled in the ACT study. If statins protect against AD by lowering the risk of vascular disease, then the statin-AD association could be weakened in the surviving older participants. Another potential bias may be due to older participants having different indication for statin therapy and receiving a combination of slightly lower dosages with relatively shorter durations of exposure than the younger participants, although the difference in duration or average daily dose of statin therapy between the old and young group was not statistically significant (Table 2). Alternatively, an age-dependent association between statins and risk of AD might suggest the existence of a critical time window for the use of statins to protect against AD. The neurodegenerative processes of AD begin years, probably decades, before the onset of dementia.15, 16 Although animal and epidemiological studies provide fairly strong evidence of a potential protective effect of statins against AD, randomized controlled clinical trials of statins for treatment of AD and vascular care in AD patients with cerebrovascular lesions have been largely negative.17–19 Together, these findings suggest that statin use might prevent or delay pathogenic neurodegenerative process(es) early in the course of AD but may not reverse profound neuronal damage seen later.
Where our previous analyses had failed to demonstrate a significant relationship between statin use and incident AD (aHR = 0.82, 95% CI: 0.46 – 1.46),2 analyses in this enlarged cohort that included younger statin users showed that statin exposure was associated with a lower risk of AD (aHR=0.58). The magnitude of this risk reduction is comparable to the recently reported HR of 0.52 (0.34 – 0.80) for dementia and cognitive impairment in the Sacramento Area Latino Study on Aging (SALSA) study20 and to the HR of 0.57 (0.37– 0.90) for AD from the Rotterdam study21 with both of these studies enrolling relatively younger participants. The current positive finding may reflect the addition of the Expansion cohort in which participants had a greater opportunity to be treated with statins in relatively earlier life than those in the Original ACT cohort, because these individuals were younger when statins came on the market.
The lack of a modifying effect of APOE ε4 is consistent with other reports.22–25 Adjusted HRs and 95% confidence intervals reported from the Multi-Institutional Research in Alzheimer Genetic Epidemiology (MIRAGE) study (with ε4: 0.55, 0.28 – 1.1; without ε4: 0.56, 0.19 –1.7)25 and those reported from the Rotterdam study (with ε4: 0.50, 0.26–0.94; without ε4: 0.61, 0.32– 1.18)21 also demonstrated no differences in the association between statin use and risk of AD by presence of the APOE ε4 allele. While the statin-AD association did not differ significantly by presence of APOE ε4, we found a significant statin-AD association in those with an ε4 allele (aHR = 0.37, 0.17 – 0.81) but not in those without (0.71, 0.39 – 1.29), similar to the Rotterdam results.21 The absence of a significant association for those without an APOE ε4 allele may reflect the heterogeneous nature of these individuals as a group with respect to the pathogenesis of AD. The aHR of 0.71 (0.39 – 1.29) leaves open the possibility of a weaker association between statins and AD risk that might be demonstrable with a larger sample. For now we conclude that the statin use among APOE ε4 carriers, who are particularly vulnerable to AD, may be associated with reduced risk of AD. Whether the potential protective effect of statins on risk of AD occurs in those without ε4 requires further study.
Because the present study is observational in nature, and statin therapy was not randomly assigned, our results are subject to the potential for confounding by indication and other potential sources of bias. For example, participants who used statins were more likely to have multiple cardiovascular risk factors beyond elevated cholesterol. Because these vascular risk factors are likely associated with increased risk of AD, our estimates of the association between statin exposure and risk of AD may have been biased toward the null, i.e., with diminished ability to detect a potential protective effect. Indeed, after adjusting for baseline comorbid vascular disease, the inverse association between statin use and risk of AD appeared to be stronger (Table 3). We have demonstrated a similar finding in an earlier study of the relationship between statin use and AD-related neuropathological changes.26 In that study, the association between statin therapy with reduced neurofibrillary tangles burden became stronger after adjustment for brain microvascular lesions.26 However, the model adjustments in our current study may not accomplish full control of confounding due to indication. Frailty, variation in the type of statin and/or dosage, adherence to medication, and other unmeasured factors (such as serum low density lipoprotein-cholesterol) could affect the relationship between statin exposure and risk of AD. Given these considerations, our findings should be interpreted cautiously, especially in their application to clinical practice. Risks and benefits need to be weighed carefully in light of these limitations of observational data.
Ideally, a randomized controlled trial will eliminate indication bias and provide a more definitive finding concerning the effect of statins for protection against AD. However, such a primary prevention trial faces great challenges due to the current lack of tools to assess disease-modifying effects in the absence of clinical signs of dementia and requires the recruitment and retention of a large cohort of subjects over a prolonged follow up period. The present observational study capitalizes on pharmacy dispensing exposure data, longer duration of follow-up and the recent addition of the Expansion cohort that includes participants who were exposed to statin at relatively earlier ages. Our findings now suggest that statin treatment in early old age may prevent or delay the neurodegenerative process in AD, especially in those who carry APOE ε4 allele. At what age does statin therapy cease to be beneficial and whether those without APOE ε4 benefit from statin therapy are open and important questions and need to be further investigated. As obstacles to doing a randomized controlled trial are resolved, future clinical trials should target preclinical stages of disease in middle age or early old age populations, especially those with APOE ε4 allele as these individuals may especially benefit from statin therapy.
ACKNOWLEDGMENT
The authors thank the named and unnamed faculty and staff who have worked on this study and made this article possible. Specifically, they thank Darlene White, BA, Meredith Pfanschmidt, RN, Sheila O’Connell, BA, Lisa Millspaugh, MS, Malia Oliver, BA, Duryah Mohamath, and Mary Jacka.
This study is supported by grants AG20020, AG06781, AG16976, and AG05136 from the National Institute of Aging, by the Department of Veterans Affairs, and by the Friends of Alzheimer’s Research.
Role of the funding source. The study sponsors had no role in the design or conduct of this study. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Study concept and design (G Li, WA Kukull, ER Peskind, and EB Larson), acquisition of study participants and data (JD Bowen, W McCormick, EB Larson and GD Shellenberg), analysis and interpretation of data (JB Shofer, IC Rhew, JCS Breitner, and PK Crane), preparation of manuscript (G Li), and critical review of the manuscript (JCS Breitner, PK Crane, WA Kukull, ER Peskind, EB Larson, JB Shofer, IC Rhew, W McCormick, JD Bowen, and GD Schellenberg).
REFERENCES
- 1.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer's disease in later life: Longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li G, Higdon R, Kukull WA, et al. Statin therapy and risk of dementia in the elderly: A community-based prospective cohort study. Neurology. 2004;63:1624–1628. doi: 10.1212/01.wnl.0000142963.90204.58. [DOI] [PubMed] [Google Scholar]
- 3.Rockwood K, Kirkland S, Hogan DB, et al. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol. 2002;59:223–227. doi: 10.1001/archneur.59.2.223. [DOI] [PubMed] [Google Scholar]
- 4.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 5.Gerdes LU, Gerdes C, Kervinen K, et al. The apolipoprotein epsilon4 allele determines prognosis and the effect on prognosis of simvastatin in survivors of myocardial infarction: A substudy of the Scandinavian simvastatin survival study. Circulation. 2000;101:1366–1371. doi: 10.1161/01.cir.101.12.1366. [DOI] [PubMed] [Google Scholar]
- 6.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: A prospective cohort study. Arch Neurol. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 7.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): A practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–62. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- 8.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. Wshington DC: American Psychiatric Association; 1994. [Google Scholar]
- 10.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 11.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145–157. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- 12.Therneau TM, Grambsch PM. Modelling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 13.R: A language and environment for statistical computing. [computer program]. Version 2006. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 14.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359:1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 15.Snowdon DA, Kemper SJ, Mortimer JA, et al. Linguistic ability in early life and cognitive function and Alzheimer's disease in late life. Findings from the Nun Study. JAMA. 1996;275:528–532. [PubMed] [Google Scholar]
- 16.Peskind ER, Li G, Shofer J, et al. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid beta-amyloid 42 in adults with normal cognition. Arch Neurol. 2006;63:936–939. doi: 10.1001/archneur.63.7.936. [DOI] [PubMed] [Google Scholar]
- 17.Simons M, Schwarzler F, Lutjohann D, et al. Treatment with simvastatin in normocholesterolemic patients with Alzheimer's disease: A 26-week randomized, placebo-controlled, double-blind trial. Ann Neurol. 2002;52:346–350. doi: 10.1002/ana.10292. [DOI] [PubMed] [Google Scholar]
- 18.Sparks DL, Sabbagh MN, Connor DJ, et al. Atorvastatin for the treatment of mild to moderate Alzheimer disease: Preliminary results. Arch Neurol. 2005;62:753–757. doi: 10.1001/archneur.62.5.753. [DOI] [PubMed] [Google Scholar]
- 19.Richard E, Kuiper R, Dijkgraaf MGW, et al. Vascular care in patients with Alzheimer’s disease with cerebrovascular lesions. A Randomized Clinical Trial. J Am Geriatr Soc. 2009;57:797–805. doi: 10.1111/j.1532-5415.2009.02217.x. [DOI] [PubMed] [Google Scholar]
- 20.Cramer C, Haan MN, Galea S, et al. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology. 2008;71:344–350. doi: 10.1212/01.wnl.0000319647.15752.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haag MD, Hofman A, Koudstaal PJ, et al. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2009;80:13–17. doi: 10.1136/jnnp.2008.150433. Epub 2008 Oct 17.. [DOI] [PubMed] [Google Scholar]
- 22.Zandi PP, Sparks DL, Khachaturian AS, et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry. 2005;62:217–224. doi: 10.1001/archpsyc.62.2.217. [DOI] [PubMed] [Google Scholar]
- 23.Rea TD, Breitner JC, Psaty BM, et al. Statin use and the risk of incident dementia: The Cardiovascular Health Study. Arch Neurol. 2005;62:1047–1051. doi: 10.1001/archneur.62.7.1047. [DOI] [PubMed] [Google Scholar]
- 24.Dufouil C, Richard F, Fievet N, et al. APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: The Three-City Study. Neurology. 2005;64:1531–1538. doi: 10.1212/01.WNL.0000160114.42643.31. [DOI] [PubMed] [Google Scholar]
- 25.Green RC, McNagny SE, Jayakumar P, et al. Statin use and risk of Alzheimer's disease: The MIRAGE Study. Alzheimer's Dement. 2006;2:96–103. doi: 10.1016/j.jalz.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Larson EB, Sonnen JA, et al. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007;69:878–885. doi: 10.1212/01.wnl.0000277657.95487.1c. [DOI] [PubMed] [Google Scholar]