Abstract
Background
Lymphangioleiomyomatosis (LAM) is a disorder that affects women and is characterized by cystic lung destruction, chylous effusions, lymphangioleiomyomas, and angiomyolipomas. It is caused by proliferation of abnormal smooth muscle–like cells. Sirolimus is a mammalian target of rapamycin inhibitor that has been reported to decrease the size of neoplastic growths in animal models of tuberous sclerosis complex and to reduce the size of angiomyolipomas and stabilize lung function in humans.
Objective
To assess whether sirolimus therapy is associated with improvement in lung function and a decrease in the size of chylous effusions and lymphangioleiomyomas in patients with LAM.
Design
Observational study.
Setting
The National Institutes of Health Clinical Center.
Patients
19 patients with rapidly progressing LAM or chylous effusions.
Intervention
Treatment with sirolimus.
Measurements
Lung function and the size of chylous effusions and lymphangioleiomyomas before and during sirolimus therapy.
Results
Over a mean of 2.5 years before beginning sirolimus therapy, the mean (±SE) FEV1 decreased by 2.8% ± 0.8% predicted and diffusing capacity of the lung for carbon monoxide (DLCO) decreased by 4.8% ± 0.9% predicted per year. In contrast, over a mean of 2.6 years of sirolimus therapy, the mean (± SE) FEV1 increased by 1.8% ± 0.5% predicted and DLCO increased by 0.8% ± 0.5% predicted per year (P < 0.001). After beginning sirolimus therapy, 12 patients with chylous effusions and 11 patients with lymphangioleiomyomas experienced almost complete resolution of these conditions. In 2 of the 12 patients, sirolimus therapy enabled discontinuation of pleural fluid drainage.
Limitations
This was an observational study. The resolution of effusions may have affected improvements in lung function.
Conclusion
Sirolimus therapy is associated with improvement or stabilization of lung function and reduction in the size of chylous effusions and lymphangioleiomyomas in patients with LAM.
Primary Funding Source
Intramural Research Program, National Heart, Lung, and Blood Institute, National Institutes of Health.
Lymphangioleiomyomatosis (LAM) is a multisystem disorder that affects primarily women and is characterized by proliferation of abnormal smooth muscle–like cells (LAM cells) that lead to cystic lung destruction, lymphatic masses (for example, lymphangioleiomyomas), and abdominal angiomyolipomas (1, 2). Lymphangioleiomyomatosis that occurs in patients with no evidence of genetic disease is known as sporadic LAM. Lymphangioleiomyomatosis also occurs in approximately one third of women with tuberous sclerosis complex (TSC) (3–5), an autosomal dominant syndrome that is caused by mutations in the TSC1 or TSC2 gene and is characterized by hamartomatous growths in various organs (6, 7). Mutations in the TSC2 gene and loss of heterozygosity of TSC2 have been reported in LAM-related lung lesions from patients with sporadic LAM, which suggests that mutations in the TSC genes may cause sporadic LAM as well as LAM associated with TSC (8, 9).
Persons with LAM may present with dyspnea, pneumothorax, thoracoabdominal lymphangioleiomyomas (1, 2, 10, 11), chylous effusions (12–17), or abdominal hemorrhage caused by angiomyolipomas (18, 19). Lymphangioleiomyomas occur in approximately 21% of persons with LAM and may cause abdominal pain, obstipation, the Horner syndrome, a malabsorption syndrome, and bladder obstruction (10, 14–17). The clinical course of LAM is highly variable (20). This condition was originally described as a fatal disease affecting women of child-bearing age, but LAM also occurs in postmenopausal women and can be a chronic disease associated with a life expectancy spanning decades (21). Although younger, premenopausal patients seem to have more aggressive lung disease (20), accurate predictors of disease course or severity are unknown.
Recommended therapies for LAM have included oophorectomy and antiestrogenic agents, such as progesterone and gonadotropin-releasing hormone analogues, but no evidence has shown that these treatments are effective (20). Effective treatments also are lacking for patients with rapidly progressing lung disease or morbid symptoms caused by chylous effusions or lymphangioleiomyomas associated with LAM.
Lymphangioleiomyomatosis is caused by a deficiency of hamartin and tuberin, 2 proteins encoded by TSC1 and TSC2 genes, respectively. These proteins regulate the mammalian target of rapamycin (mTOR) through a guanine nucleotide–binding protein called Ras homolog enriched in brain (Rheb) (22–27). Deficiency of hamartin or tuberin due to mutations of the TSC1 or TSC2 gene leads to increased activation of Rheb, upregulation of mTOR, and abnormal cellular growth (22–26).
The immunosuppressant sirolimus inhibits mTOR and has been shown to decrease the size of neoplastic growths in animal models of TSC (28–30). Sirolimus therapy was reported to be associated with decreased size of angiomyolipomas and improved or stabilized lung function in humans with LAM or TSC (31, 32). Case reports also describe resolution of chylous effusions and lymphangioleiomyomas after sirolimus therapy (33–35). In addition, therapy with everolimus, another mTOR inhibitor, was associated with reductions in the size of giant cell astrocytomas and in the frequency of seizures in patients with TSC (36).
The MILES (Multicenter International LAM Efficacy of Sirolimus) Trial, a recently completed double-blind, placebo-controlled trial, showed that sirolimus stabilized lung function and was associated with a reduction in symptoms and improvement in quality of life in patients with LAM (37). However, patients with pleural effusions were excluded from this study because of the potential effects on pulmonary function. TESSTAL (Trial of Efficacy and Safety in Sirolimus) is an ongoing study in the United Kingdom that is focused on changes in angiomyolipomas. However, because the planned enrollment for this trial is only 14 participants and includes participants of both sexes, the trial is unlikely to definitively evaluate lung function or to include a substantial number of patients with pleural effusions and lymphatic involvement.
We sought to evaluate the effect of sirolimus in patients with LAM who had rapidly progressive or severe lung disease and in those with chylous effusions and lymphangioleiomyomas, a population in whom sirolimus has not been tested. Because these patients were participating in a natural history study at the National Institutes of Health (NIH), we had their physiologic and radiologic data for several years before initiation of sirolimus therapy and were able to compare data obtained before and after therapy.
Methods
Patients
Our study included 19 patients with LAM who were participating in a natural history protocol at the NIH Clinical Center (National Heart, Lung, and Blood Institute [NHLBI] protocol 95-H-0186) and had received off-label sirolimus therapy. The natural history protocol had been designed to define the clinical course of LAM, elucidate the pathogenesis of the disease at the cellular and molecular levels, and develop more effective therapy for this condition. Participants underwent a tissue biopsy that was diagnostic of LAM in 13 patients, whereas 6 patients had a history of dyspnea or pneumothorax with evidence of cystic lung lesions on axial computed tomography (CT) of the chest plus extrapulmonary disease, such as angiomyolipoma, chylous effusion, or lymphangioleiomyomas. All 19 patients participating in the drug trial had either progressive disease or chylous effusions.
We included all patients who received sirolimus therapy in our analysis. Patients decided to receive off-label sirolimus therapy after consultation with their local physicians. Five patients began off-label sirolimus therapy before enrollment in the MILES Trial was available, 11 patients had pleural effusions that excluded them from this trial, and 3 patients chose not to participate.
Local physicians prescribed sirolimus therapy to patients in our trial and monitored serum sirolimus levels and potential toxicity. Every 3 to 12 months, patients underwent blood work, measurement of serum sirolimus levels, pulmonary function tests, and chest radiography. Computed tomography of the thorax and abdomen were performed once a year or when medically indicated. This study was approved by the institutional review board of the NHLBI, NIH.
Chest Radiography and CT
A General Electric HiSpeed Advantage Unit (GE Medical Systems, Milwaukee, Wisconsin) was used to perform CT of the lungs with 8.0- to 10.0-mm collimation at 1.0-cm intervals. In addition, all patients underwent CT with 1.0-mm collimation (high-resolution CT) at 3.0-cm intervals. Computed tomography of the abdomen was performed as reported elsewhere (11).
Pulmonary Function Tests
Lung volumes, flow rates, and single-breath DLCO were measured (SensorMedics Vmax 229, Yorba Linda, California) according to the standards of the American Thoracic Society and European Respiratory Society (38, 39).
Study End Points
End points were changes in lung function, chylous effusions, and lymphangioleiomyomas.
Statistical Analysis
We evaluated changes in lung function associated with sirolimus therapy by using mixed-effects models. A time-dependent group indicator and adjustment for baseline results of pulmonary function tests were used in all models. The nonparametric sign test was used to evaluate changes in the size of lymphangioleiomyomas associated with sirolimus therapy. All reported P values are 2-sided, and data are reported as means (±SEs). Age data are reported as means (SDs).
Role of the Funding Source
This study was supported by the Intramural Research Program, NHLBI, NIH. The funding source had no role in the design, conduct, and analysis of the study or in the decision to submit the manuscript for publication.
Results
Study Population
Mean patient age at the time of enrollment in the NHLBI natural history protocol was 36.9 years (SD, 9.8). Mean age at presentation of LAM-related symptoms was 34.6 years (SD, 9.4); a definite diagnosis was established at age 36.6 years (SD, 8.7). Treatment with sirolimus was started at age 41.0 years (SD, 9.0). The mean interval between diagnosis to initiation of sirolimus therapy was 4.4 years (SD, 3.7), and the average patient age at the last physician visit during the study was 43.6 years (SD, 8.8).
At enrollment, 13 patients received oxygen therapy. Twelve patients had chylous effusions, and 11 patients had lymphangioleiomyomas. Five patients had renal angiomyolipomas. Initial presentation before diagnosis of LAM was pneumothorax in 9 patients, dyspnea in 5 patients, and abdominal masses in 5 patients. No patient had TSC.
Five patients had previous pregnancies, 3 patients were menopausal, 5 patients had received progesterone therapy, and 2 patients had received leuprolide therapy. No patient had previously undergone oophorectomy. Five patients were former smokers, and the rest were nonsmokers. All patients had progressive signs or symptoms that were attributable to LAM.
Changes in Study Outcomes With Sirolimus Therapy
Lung Function
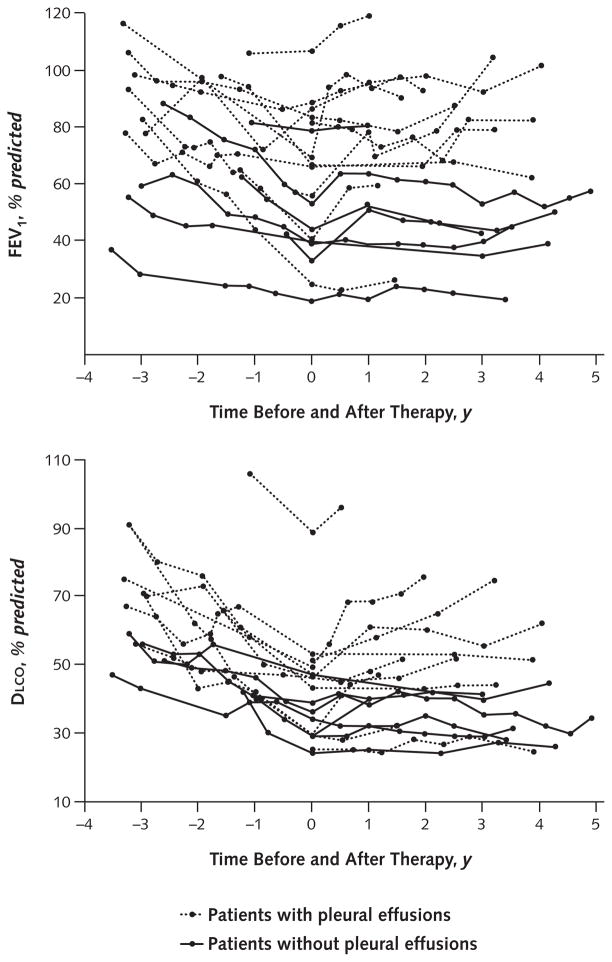
Figure 1 shows the percent predicted FEV1 and DLCO at each NIH visit before and after beginning sirolimus therapy in 18 patients (1 patient could not undergo these tests because of chest tube drainage). Results of the adjusted analysis obtained by using mixed-effects models showed statistically significant differences in the annual rate of change in FVC, FEV1, and DLCO before and after sirolimus therapy.
Figure 1. FEV1 (top) and DLCO (bottom) measurements obtained at each visit before and after sirolimus therapy.
Data at 0 y were obtained just before starting sirolimus therapy. One of the 19 patients with chylothorax and continuous pleural drainage could not undergo pulmonary function tests. DLCO = diffusing capacity of the lung for carbon monoxide.
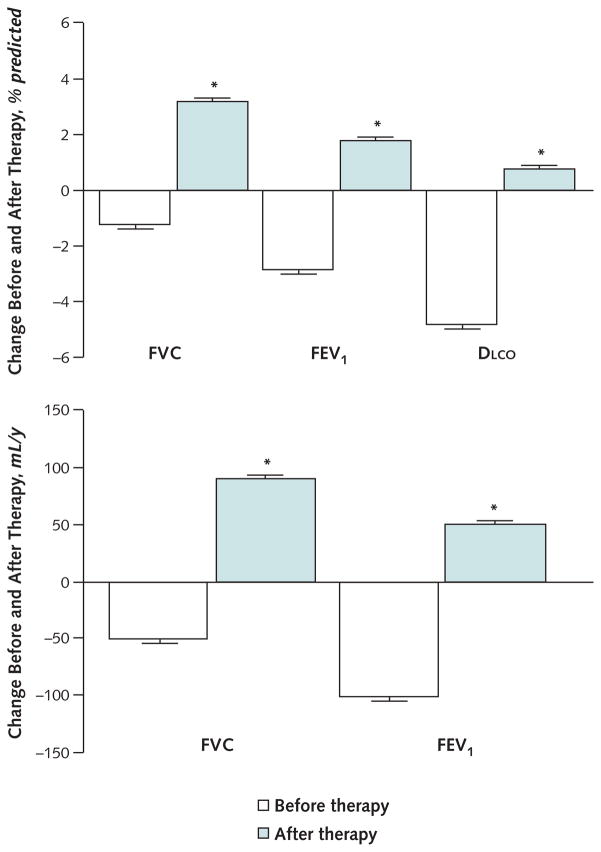
Over a mean duration of 2.5 ± 1.2 years before initiation of sirolimus therapy, FVC decreased by 50 ± 30 mL per year (1.2% ± 0.6% predicted), FEV1 decreased by 100 ± 30 mL per year (2.8% ± 0.8% predicted), and DLCO decreased by 1.1 ± 0.1 mL/min per mm Hg (4.8% ± 0.9% predicted) per year (Figure 2). In contrast, over a mean of 2.6 ± 1.2 years of sirolimus therapy, FVC increased by 90 ± 20 mL per year (3.2% ± 0.5% predicted; P < 0.001), FEV1 increased by 50 ± 20 mL per year (1.8% ± 0.5% predicted; P < 0.001), and DLCO increased by 0.2 ± 0.1 mL/min per mm Hg (0.8% ± 0.5% predicted; P < 0.001) per year (Figure 2). Statistically significant increases in FVC (80% ± 5% predicted to 88% ± 5% predicted; P < 0.001), FEV1 (62% ± 7% predicted to 69% ± 7% predicted; P < 0.010), and DLCO (40% ± 4% predicted to 45% ± 5% predicted; P < 0.026) were observed within 6 months (0.4 ± 0.1 y) after initiation of sirolimus therapy in 14 of 18 patients for whom early lung function data were available.
Figure 2. Mean annual changes in FVC, FEV1, and DLCO before and after sirolimus therapy in patients with lymphangioleiomyomatosis.
Data were obtained by using mixed-effects models. During sirolimus therapy, the FVC, FEV1, and DLCO increased instead of decreasing. DLCO = diffusing capacity of the lung for carbon monoxide.
* Eighteen patients were included in these analyses because 1 patient could not undergo pulmonary function tests.
To determine whether lung function improvement was independent of the resolution of chylous effusions, data from 7 patients without effusions were analyzed further. In this subgroup, FEV1 decreased by 110 ± 10 mL per year before initiation of sirolimus therapy compared with a decrease of 10 ± 10 mL per year after beginning sirolimus therapy (P = 0.002). The DLCO decreased by 0.5 ± 0.2 mL/min per mm Hg per year before initiation of sirolimus therapy compared with an increase of 0.04 ± 0.1 mL/min per mm Hg per year after sirolimus therapy was started (P = 0.020). Forced vital capacity increased by 50 ± 40 mL per year before initiation of sirolimus therapy compared with an increase of 110 ± 30 mL per year after sirolimus therapy was started (P = 0.162).
The Appendix Table (available at www.annals.org) shows complete pulmonary function data obtained at study enrollment for 18 patients immediately before initiation of sirolimus therapy and at the most recent follow-up visit.
Appendix Table.
Age and Pulmonary Function Test Results in Patients With Lymphangioleiomyomatosis Before and After Sirolimus Therapy*
| Characteristic | First Test at Enrollment in Natural History Study | Just Before Sirolimus Therapy | At Most Recent Follow-up Visit |
|---|---|---|---|
| Mean age (SD), y | 39.6 (9.8) | 41.0 (9.0) | 43.6 (8.8) |
|
| |||
| Mean time before and after therapy (SD), y | −2.6 (1.3) | 0 | 2.6 (1.1) |
| Median pulmonary function study results (IQR) | |||
|
| |||
| TLC, % predicted | 94 (84–103) | 90 (69–96) | 96 (86–107) |
|
| |||
| FRC, % predicted | 102 (92–110) | 100 (78–108) | 109 (90–112) |
|
| |||
| RV, % predicted | 103 (90–118) | 99 (86–130) | 99 (86–130) |
|
| |||
| RV–TLC ratio, % | 35 (33–41) | 38 (32–46) | 34 (30–41) |
|
| |||
| FVC, % predicted | 95 (77–100) | 79 (61–95) | 92 (69–113) |
|
| |||
| FEV1, % predicted | 81 (59–98) | 61 (40–81) | 70 (42–87) |
|
| |||
| FEV1–FVC ratio, % | 71 (60–80) | 58 (46–72) | 54 (39–73) |
| DLCO, % predicted | 58 (47–71) | 41 (29–47) | 43 (30–52) |
DLCO = diffusing capacity of the lung for carbon monoxide; FRC = functional residual capacity; IQR = interquartile range; RV = residual volume; TLC = total lung capacity.
Eighteen patients were included in these analyses because 1 patient could not undergo pulmonary function tests.
Chylous Effusions
Twelve patients had chylous effusions. Eleven patients had pleural effusions, 7 of whom also had ascites (Table). One patient had only ascites. Of the 11 patients with pleural effusions, 8 patients had them at the time of enrollment in the natural history protocol. In the remaining 3 patients, chylous effusions were noted 3 to 12 months after enrollment. Chylous effusions had been present for a minimum of 1.5 ± 0.4 years before initiation of sirolimus therapy. Before sirolimus therapy, all patients had undergone thoracentesis, and 2 patients had required chest tube drainage. Pleurodesis had been performed in 12 of the 19 patients, but this intervention prevented recurrence of pleural effusions in only 1 patient.
Table 1.
Changes in Chylous Effusions and Lymphangioleiomyomas During Treatment With Sirolimus
| Patient | Pleural Effusions | Ascites | Lymphangioleiomyomas | Mean Serum Sirolimus Level (±SE), ng/mL | Response to Therapy | Adverse Events |
|---|---|---|---|---|---|---|
| 1 | No | No | No | 7.7 ± 1.3 | No lymphatic involvement | Hypertension, acne |
| 2 | No | No | Yes | 9.7 ± 1.6 | Reduction in size of lymphangioleiomyomas | None |
| 3 | Yes | Yes | Yes | 4.6 ± 0.7 | Resolution of effusions and lymphangioleiomyomas | Oral ulcers, diarrhea, hyperlipidemia, acne, hypertension |
| 4 | No | No | No | 7.0 ± 0.3 | No lymphatic involvement | None |
| 5 | No | No | No | 7.4 ± 1.8 | No lymphatic involvement | Oral ulcers, rash, hyperlipidemia, diarrhea, hypertension, neutropenia |
| 6 | No | Yes | Yes | 7.7 ± 0.6 | Resolution of lymphangioleiomyomas | Oral ulcers, acne, hyperlipidemia, rash |
| 7 | Yes | No | Yes | 10.4 ± 1.6 | Resolution of effusions and lymphangioleiomyomas | Oral ulcers |
| 8 | No | No | No | 5.7 ± 0.8 | No lymphatic involvement | Hyperlipidemia, acne |
| 9 | Yes | No | Yes | 5.8 ± 0.2 | Resolution of effusions and lymphangioleiomyomas | None |
| 10 | No | No | No | 11.3 ± 0.3 | No lymphatic involvement | Hyperlipidemia, acne, diarrhea |
| 11 | Yes | No | Yes | 5.8 ± 0.5 | Decrease in size of effusions; resolution of lymphangioleiomyomas | Hyperlipidemia, bruising |
| 12 | Yes | No | Yes | 9.4 ± 1.9 | Resolution of effusions and lymphangioleiomyomas | None |
| 13 | Yes | No | Yes | 7.4 ± 1.6 | Resolution of effusions and lymphangioleiomyomas | Oral ulcers, diarrhea, hyperlipidemia, irregular menses |
| 14 | Yes | Yes | Yes | 6.2 ± 1.8 | Resolution of effusions and lymphangioleiomyomas; discontinuation of pleural drainage | Oral ulcers, acne |
| 15 | No | No | No | 6.6 ± 1.3 | No lymphatic involvement | Acne of the face and edema of the legs |
| 16 | Yes | Yes | No | 5.6 ± 0.3 | Resolution of effusions | Oral ulcers, hyperlipidemia |
| 17 | Yes | Yes | Yes | 9.9 ± 0.6 | Resolution of effusions | Oral ulcers, menorrhagia |
| 18 | Yes | Yes | No | 5.0 ± 0.2 | Resolution of effusions | Skin infection |
| 19 | Yes | Yes | Yes | 7.7 ± 1.7 | Marked resolution of effusions and lymphangioleiomyomas; discontinuation of pleural drainage | None |
Before sirolimus therapy, the sizes of the effusions had not substantially decreased except on drainage by thoracentesis or the placement of a chest tube. During sirolimus therapy, 9 patients had complete resolution of the pleural effusions (Figure 3, A and B). Four of these effusions were large, 3 were moderate in size, and 2 were small.
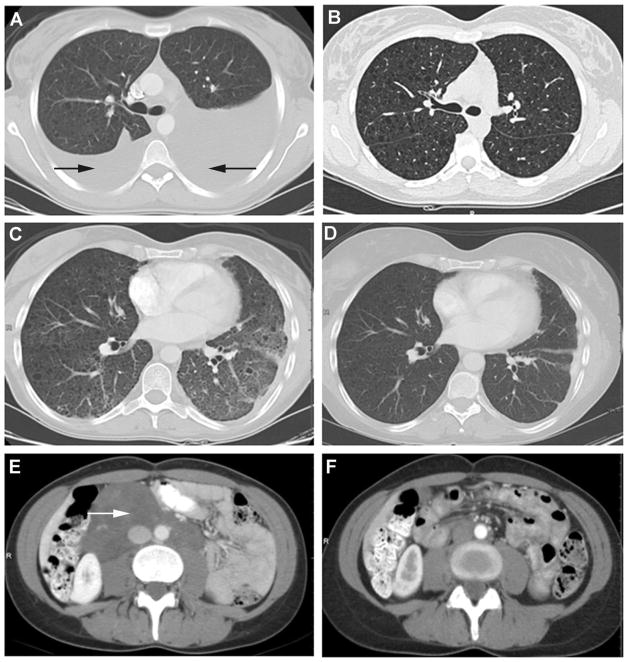
Figure 3. CT scans in 3 patients with lymphangioleiomyomatosis before and after sirolimus therapy.
CT = computed tomography. A. CT scan of a 29-year-old woman with bilateral chylothorax (black arrows). B. Repeated CT scan of the same patient 30 months after starting sirolimus therapy that shows complete resolution of the pleural effusions. C. CT scan of a 45-year-old woman with bilateral lung infiltrates. D. Repeated CT scan of the same patient performed after 2.5 years of sirolimus therapy that shows complete clearing of the infiltrates; her lung function also had improved. E. CT scan of a 39-year-old woman with a large lymphangioleiomyoma (white arrow). F. Repeated CT scan of the same patient after 11 months of sirolimus therapy that shows the lymphangioleiomyoma completely resolved.
Complete resolution of effusions was noted after 410 ± 111 days of therapy; in 6 patients, effusions resolved after 131 ± 61 days of therapy. Of the 2 patients with partially resolved effusions, 1 patient had large effusions that required frequent drainage before beginning sirolimus therapy and the other patient had a small effusion. The average duration of sirolimus therapy for all patients with effusions was 2.4 ± 1.0 years. All 8 patients with ascites had complete resolution.
Two patients had dramatic responses to sirolimus therapy. A 29-year-old woman with recurrent chylous pleural effusions despite chest tube drainage and talc pleurodesis had required thoracentesis as frequently as once every week for 7 months; this intervention was associated with a total weight loss of 11 kg. After 6 months of sirolimus therapy, further thoracentesis was not required (Figure 3, A and B). After 30 months of therapy, the effusions resolved completely, and the patient’s body mass index increased from 21 to 25 kg/m2.
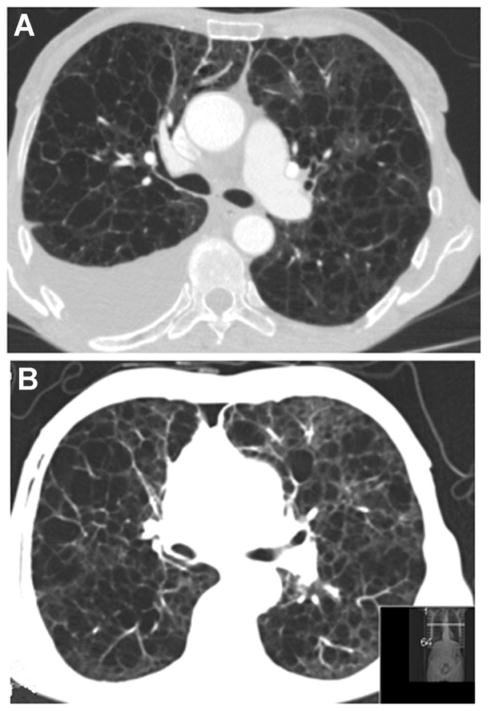
A 62 year-old woman in whom chemical pleurodesis was unsuccessful had persistent pleural fluid drainage averaging 1500 to 3000 mL/d despite a low-fat diet and administration of octreotide. After 4 weeks of sirolimus therapy, a substantial decrease in drainage volume to less than <200 mL/d and in the size of the lymphangioleiomyomas was noted and the chest tube was removed. Six months after initiation of sirolimus therapy, the pleural effusion had resolved almost completely (Appendix Figure, available at www.annals.org).
Appendix Figure. Follow-up Visit CT scan in a patient with lymphangioleiomyomatosis with pleural effusion requiring persistent drainage.
CT = computed tomography. A. CT scan of a 62-year-old woman who had undergone unsuccessful chemical pleurodesis and required daily drainage of substantial amounts of pleural fluid. B. Repeated CT scan after 6 months of sirolimus therapy showing nearly complete resolution of pleural effusion.
Lymphangioleiomyomas
Eleven patients had lymphangioleiomyomas averaging 114 ± 50 mL in volume (Table). During sirolimus therapy, the tumors resolved completely in 9 patients. In the remaining 2 patients, the volume of the tumors decreased from 44 ± 22 mL to 17 ± 13 mL (P < 0.001, nonparametric sign test) (Table and Figure 3, E and F). Lymphangioleiomyomas and chylous effusions tended to resolve concurrently.
Angiomyolipomas
Of the 5 patients with angiomyolipomas, 2 had a substantial decrease in tumor size during sirolimus therapy. The tumor decreased from 12 to 8 mL in 1 patient and from 12 to 4 mL in another patient. In a third patient, tumor size could not be assessed because the tumor was intermixed with the kidney parenchyma. A fourth patient had angiomyolipomas that were too small for measurement, and the fifth patient did not undergo follow-up abdominal CT.
Dose of Sirolimus
The mean dosage of sirolimus was 2.6 ± 0.9 mg/d (range, 1 to 5 mg/d), and the average duration of therapy was 2.6 ± 1.2 years (range, 0.7 to 5.4 y). The sirolimus dose was adjusted by the patient’s local physician to achieve serum levels between 5 and 15 ng/mL. The Table shows the mean serum sirolimus level for each patient. The mean total dose of sirolimus was 2447 ± 1663 mg (range, 532 to 6425 mg).
Adverse Events Potentially Related to Sirolimus
Adverse events that were probably related to sirolimus therapy included mouth ulcers in 8 patients, hyperlipidemia in 8 patients, acne in 6 patients, 3 cases of worsening hypertension, 3 cases of diarrhea, and 1 case of persistent mild neutropenia (Table). The mean leukocyte count and total neutrophil count at the patients’ last physician visit were 5.4 ± 0.3 × 109 cells/L (range, 2.8 to 4.8 × 109 cells/L) and 3.4 ± 0.3 × 109 cells/L (range, 1.4 to 5.8 × 109 cells/L), respectively. Mean total and low-density lipoprotein cholesterol levels were, respectively, 4.95 ± 0.23 mmol/L (range, 3.85 to 7.30 mmol/L) (191 ± 9 mg/dL [range, 149 to 282 mg/dL]) and 3.11 ± 0.21 mmol/L (range, 1.94 to 5.08 mmol/L) (120 ± 8 mg/dL [range, 75 to 196 mg/dL]). Six of the 8 patients with hyperlipidemia received statin therapy.
One patient had a major skin infection secondary to pneumococcal vaccination that required temporary discontinuation of sirolimus therapy and administration of antibiotics. No other patient had to discontinue sirolimus therapy because of adverse events. Local physicians managed all adverse events.
Discussion
We found that treatment with sirolimus was associated with improvement or stabilization of lung function and decrease in the size of chylous effusions and lymphangioleiomyomas in a selected population of patients with LAM. Of note, adverse events associated with sirolimus therapy were manageable and, as of now, all patients continue to receive this therapy and have sustained improvement or stabilization of lung function and continued resolution of effusions and lymphangioleiomyomas.
Although previous reports have described improvement (31) or stabilization (32, 37) of lung function associated with sirolimus therapy, we believe that our study is the first to systematically evaluate the effect of sirolimus in patients with chylous effusions and lymphangioleiomyomas. In this subgroup of patients, sirolimus therapy was associated with reduction or resolution of the effusions and lymphangioleiomyomas, with consequent dramatic improvement in lung function. Patients with uncontrolled pleural effusions that required frequent pleural drainage no longer required repeated drainage after beginning sirolimus therapy. Another strength of our study is the body of both radiologic and physiologic information spanning several years preceding treatment with sirolimus, which provides a valuable basis for comparison with posttherapy data.
In patients without baseline pleural effusions, lung function during sirolimus therapy stabilized and, in contrast to the characteristic progressive nature of LAM (20, 40–42), no substantial decline in lung function was observed. This finding suggests that the beneficial effects of sirolimus therapy in patients with LAM may extend to those without involvement of the lymphatic system, although our small sample limits robust conclusions. Overall, however, our data are consistent with those of the recently completed MILES Trial (37), which showed that 1 year of sirolimus therapy was associated with stabilized lung function and reported changes in FEV1 in the placebo and sirolimus groups that were similar to those in our study (Figure 2).
Our hypothesis was that suppression of LAM cell proliferation and growth by the sirolimus-mediated inhibition of the mTOR pathway would improve lung function and reduce the size of chylous effusions and lymphangioleiomyomas. This hypothesis was based on previous studies showing that sirolimus reduced the size of neoplastic growths in animal models of TSC (28–30). A literature search of the PubMed database for all articles related to sirolimus therapy in human patients with LAM through 2010 revealed individual case reports of reduction in the size of chylous effusions and lymphangioleiomyomas (33–35) and improved lung function in a series of patients treated for 1 year (31). Reductions in the size of angiomyolipomas (31) and giant cell astrocytomas in patients with LAM or TSC have also been reported (36). Now, the MILES Trial (37) and our data show that sirolimus therapy is associated with stabilization of lung function. Our study, however, extends these observations to patients with LAM and lymphatic involvement.
During the observation year of the MILES Trial, when patients did not receive sirolimus therapy, changes in FEV1 and FVC in the sirolimus and placebo groups did not significantly differ, which indicates that the beneficial effects of sirolimus ceased after sirolimus therapy was discontinued (37). Our study shows that the benefits of sirolimus seem to be sustained for more than 2 years of therapy as long as patients continue to receive the drug. In addition, as in the MILES Trial, the adverse events associated with sirolimus in our study were manageable.
The MILES Trial (37) also showed that levels of serum vascular endothelial growth factor-D (VEGF-D), a lymphangiogenic growth factor implicated in the pathophysiology of LAM, were reduced in response to sirolimus, and that a decrease in mean VEGF-D levels even after discontinuation of the drug was consistent with a sustained effect on the lymphatic component of the disease. Because VEGF-D levels are reported to be higher in patients with LAM and lymphatic involvement than in those with cystic disease limited to the lung (14), the data from the MILES Trial (37) are consistent with our finding that lymphatic involvement in LAM seems to be highly responsive to sirolimus therapy.
Our study has limitations. The selected LAM population in our study consisted of patients with progressive disease, most of whom had lymphatic involvement and chylous effusions. The rates of decline in pulmonary function observed in our patients before sirolimus therapy were higher than those previously reported (20, 40–42). In other studies, reported rates of decline in FEV1 have ranged from 75 ± 9 mL per year to 118 ± 21 mL per year (20, 40, 41) and rates of decline in DLCO have ranged from 0.69 ± 0.07 mL/min per mm Hg to 0.90 ± 0.26 mL/min per mm Hg per year (20, 40); each of these values is less than one half of the unadjusted rates of decline in pulmonary function that our patients experienced.
In a large previous study, the rates of decline in percentage of predicted FEV1 and DLCO were 1.7% ± 0.4% predicted and 2.4% ± 0.4% predicted per year, respectively (20). In our study, the average unadjusted rates of decline in FEV1 and DLCO were approximately 9% predicted per year, which reflects rapidly progressing severe disease.
A large difference in the rates of decline in FEV1 between patients who present with dyspnea and those who present with pneumothorax has been reported (41). This finding suggests that LAM can be a very aggressive disease in some patients and can cause declines in FEV1 or DLCO ranging from 5% to 10% predicted per year, whereas in other patients, lung disease progresses slowly and interferes little with activities of daily living. Therefore, our findings cannot be generalized to patients with LAM who have milder disease without chylous effusions or lymphangioleiomyomas.
In addition, our study was not controlled, and it is possible that the pleural effusions may have resolved spontaneously. However, we have never observed spontaneous resolution of large chylous effusions in the NHLBI natural history protocol cohort.
Overall, our data suggest that sirolimus should be evaluated as a treatment of chylous effusions and symptomatic lymphangioleiomyomas in patients with LAM. As our study demonstrates, chylous effusions in LAM are difficult to treat and repeated thoracentesis may lead to severe weight loss. Other therapies, such as pleuroperitoneal shunts and octreotide, have been tried, but there is little experience with these therapeutic methods in patients with LAM (43–45). Although lymphangioleiomyomas are usually asymptomatic, compression of the bladder, bowel, pelvic veins, nerves, and other organs may cause pain, obstipation, frequent urination, and peripheral edema. Surgery has been performed but can lead to persistent lymphatic leakage, chylothorax, and ascites.
In conclusion, we found that sirolimus therapy was associated with improved or stabilized lung function and reductions in the size of chylous effusions and lymphangioleiomyomas in patients with aggressive LAM. Our findings suggest a potential role for sirolimus in the management of patients with LAM, especially for those with lymphatic involvement. The adverse events associated with sirolimus therapy were manageable in our patients. However, the long-term risks of sirolimus therapy must be weighed against its benefits, as we lack information regarding the optimal duration of therapy and when cessation should be considered. Lifelong therapy may be required, or resistance to sirolimus may eventually develop. Despite these possibilities, sirolimus therapy may be a reasonable consideration for patients with intractable chylous effusions and lymphangioleiomyomas.
Acknowledgments
The authors thank Drs. Martha Vaughan, Wendy Steagall, and Gustavo Pacheco-Rodriguez for critical review of the manuscript.
Grant Support: By the Intramural Research Program, NHLBI, NIH (protocol 95-H-0186).
Footnotes
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M10-1139.
Reproducible Research Statement: Study protocol: Available from the Cardiovascular and Pulmonary Branch, NHLBI. Data set: Available from the Cardiovascular and Pulmonary Branch, NHLBI, after approval by the NHLBI, institutional review board, and the institutional review board of the requesting investigator and completion of a Material Transfer Agreement. Statistical code: Not available.
Author Contributions: Conception and design: A.M. Taveira-DaSilva, M. Stylianou, J. Moss.
Analysis and interpretation of the data: A.M. Taveira-DaSilva, M. Stylianou, J. Moss.
Drafting of the article: A.M. Taveira-DaSilva, M. Stylianou, J. Moss.
Critical revision for important intellectual content: M. Stylianou, J. Moss.
Final approval of the article: M. Stylianou, J. Moss.
Provision of study materials or patients: J. Moss.
Statistical expertise: M. Stylianou.
Collection and assembly of data: A.M. Taveira-DaSilva, O. Hathaway, J. Moss.
References
- 1.Ryu JH, Moss J, Beck GJ, Lee JC, Brown KK, Chapman JT, et al. NHLBI LAM Registry Group. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173:105–11. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest. 2008;133:507–16. doi: 10.1378/chest.07-0898. [DOI] [PubMed] [Google Scholar]
- 3.Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc. 2000;75:591–4. doi: 10.4065/75.6.591. [DOI] [PubMed] [Google Scholar]
- 4.Moss J, Avila NA, Barnes PM, Litzenberger RA, Bechtle J, Brooks PG, et al. Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am J Respir Crit Care Med. 2001;164:669–71. doi: 10.1164/ajrccm.164.4.2101154. [DOI] [PubMed] [Google Scholar]
- 5.Franz DN, Brody A, Meyer C, Leonard J, Chuck G, Dabora S, et al. Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis. Am J Respir Crit Care Med. 2001;164:661–8. doi: 10.1164/ajrccm.164.4.2011025. [DOI] [PubMed] [Google Scholar]
- 6.Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:125–7. doi: 10.1111/j.1749-6632.1991.tb37754.x. [DOI] [PubMed] [Google Scholar]
- 7.European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–15. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 8.Smolarek TA, Wessner LL, McCormack FX, Mylet JC, Menon AG, Henske EP. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet. 1998;62:810–5. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2000;97:6085–90. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsui K, Tatsuguchi A, Valencia J, Yu Z, Bechtle J, Beasley MB, et al. Extrapulmonary lymphangioleiomyomatosis (LAM): clinicopathologic features in 22 cases. Hum Pathol. 2000;31:1242–8. doi: 10.1053/hupa.2000.18500. [DOI] [PubMed] [Google Scholar]
- 11.Avila NA, Kelly JA, Chu SC, Dwyer AJ, Moss J. Lymphangioleiomyomatosis: abdominopelvic CT and US findings. Radiology. 2000;216:147–53. doi: 10.1148/radiology.216.1.r00jl42147. [DOI] [PubMed] [Google Scholar]
- 12.Ryu JH, Doerr CH, Fisher SD, Olson EJ, Sahn SA. Chylothorax in lymphangioleiomyomatosis. Chest. 2003;123:623–7. doi: 10.1378/chest.123.2.623. [DOI] [PubMed] [Google Scholar]
- 13.Almoosa KF, McCormack FX, Sahn SA. Pleural disease in lymphangioleiomyomatosis. Clin Chest Med. 2006;27:355–68. doi: 10.1016/j.ccm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Glasgow CG, Taveira-DaSilva A, Pacheco-Rodriguez G, Steagall WK, Tsukada K, Cai X, et al. Involvement of lymphatics in lymphangioleiomyomatosis. Lymphat Res Biol. 2009;7:221–8. doi: 10.1089/lrb.2009.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaiswal VR, Baird J, Fleming J, Miller DS, Sharma S, Molberg K. Localized retroperitoneal lymphangioleiomyomatosis mimicking malignancy. A case report and review of the literature. Arch Pathol Lab Med. 2003;127:879–82. doi: 10.5858/2003-127-879-LRLMM. [DOI] [PubMed] [Google Scholar]
- 16.Lu HC, Wang J, Tsang YM, Lin MC, Li YW. Lymphangioleiomyomatosis initially presenting with abdominal pain: a case report. Clin Imaging. 2003;27:166–70. doi: 10.1016/s0899-7071(01)00349-7. [DOI] [PubMed] [Google Scholar]
- 17.Wong YY, Yeung TK, Chu WC. Atypical presentation of lymphangioleiomyomatosis as acute abdomen: CT diagnosis. AJR Am J Roentgenol. 2003;181:284–5. doi: 10.2214/ajr.181.1.1810284. [DOI] [PubMed] [Google Scholar]
- 18.Bissler JJ, Kingswood JC. Renal angiomyolipomata. Kidney Int. 2004;66:924–34. doi: 10.1111/j.1523-1755.2004.00838.x. [DOI] [PubMed] [Google Scholar]
- 19.Sooriakumaran P, Gibbs P, Coughlin G, Attard V, Elmslie F, Kingswood C, et al. Angiomyolipomata: challenges, solutions, and future prospects based on over 100 cases treated. BJU Int. 2010;105:101–6. doi: 10.1111/j.1464-410X.2009.08649.x. [DOI] [PubMed] [Google Scholar]
- 20.Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Hathaway O, Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126:1867–74. doi: 10.1378/chest.126.6.1867. [DOI] [PubMed] [Google Scholar]
- 21.Cohen MM, Pollock-BarZiv S, Johnson SR. Emerging clinical picture of lymphangioleiomyomatosis. Thorax. 2005;60:875–9. doi: 10.1136/thx.2004.035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goncharova EA, Krymskaya VP. Pulmonary lymphangioleiomyomatosis (LAM): progress and current challenges. J Cell Biochem. 2008;103:369–82. doi: 10.1002/jcb.21419. [DOI] [PubMed] [Google Scholar]
- 23.Krymskaya VP, Goncharova EA. PI3K/mTORC1 activation in hamartoma syndromes: therapeutic prospects. Cell Cycle. 2009;8:403–13. doi: 10.4161/cc.8.3.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci U S A. 2002;99:13571–6. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–68. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 26.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002;277:30958–67. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 27.Lamb RF, Roy C, Diefenbach TJ, Vinters HV, Johnson MW, Jay DG, et al. The TSC1 tumour suppressor hamartin regulates cell adhesion through ERM proteins and the GTPase Rho. Nat Cell Biol. 2000;2:281–7. doi: 10.1038/35010550. [DOI] [PubMed] [Google Scholar]
- 28.El-Hashemite N, Zhang H, Walker V, Hoffmeister KM, Kwiatkowski DJ. Perturbed IFN-gamma-Jak-signal transducers and activators of transcription signaling in tuberous sclerosis mouse models: synergistic effects of rapamycin-IFN-gamma treatment. Cancer Res. 2004;64:3436–43. doi: 10.1158/0008-5472.CAN-03-3609. [DOI] [PubMed] [Google Scholar]
- 29.Kenerson H, Dundon TA, Yeung RS. Effects of rapamycin in the Eker rat model of tuberous sclerosis complex. Pediatr Res. 2005;57:67–75. doi: 10.1203/01.PDR.0000147727.78571.07. [DOI] [PubMed] [Google Scholar]
- 30.Lee L, Sudentas P, Donohue B, Asrican K, Worku A, Walker V, et al. Efficacy of a rapamycin analog (CCI-779) and IFN-gamma in tuberous sclerosis mouse models. Genes Chromosomes Cancer. 2005;42:213–27. doi: 10.1002/gcc.20118. [DOI] [PubMed] [Google Scholar]
- 31.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–51. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies DM, Johnson SR, Tattersfield AE, Kingswood JC, Cox JA, Mc-Cartney DL, et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis [Letter] N Engl J Med. 2008;358:200–3. doi: 10.1056/NEJMc072500. [DOI] [PubMed] [Google Scholar]
- 33.Taillé C, Debray MP, Crestani B. Sirolimus treatment for pulmonary lymphangioleiomyomatosis [Letter] Ann Intern Med. 2007;146:687–8. doi: 10.7326/0003-4819-146-9-200705010-00022. [DOI] [PubMed] [Google Scholar]
- 34.Sugimoto R, Nakao A, Yamane M, Toyooka S, Okazaki M, Aoe M, et al. Sirolimus amelioration of clinical symptoms of recurrent lymphangioleiomyomatosis after living-donor lobar lung transplantation. J Heart Lung Transplant. 2008;27:921–4. doi: 10.1016/j.healun.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Ohara T, Oto T, Miyoshi K, Tao H, Yamane M, Toyooka S, et al. Sirolimus ameliorated post lung transplant chylothorax in lymphangioleiomyomatosis. Ann Thorac Surg. 2008;86:e7–8. doi: 10.1016/j.athoracsur.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 36.Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–11. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 37.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, et al. National Institutes of Health Rare Lung Diseases Consortium. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 39.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–35. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 40.Johnson SR, Whale CI, Hubbard RB, Lewis SA, Tattersfield AE. Survival and disease progression in UK patients with lymphangioleiomyomatosis. Thorax. 2004;59:800–3. doi: 10.1136/thx.2004.023283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazor R, Valeyre D, Lacronique J, Wallaert B, Urban T, Cordier JF Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires. Low initial KCO predicts rapid FEV1 decline in pulmonary lymphangioleiomyomatosis. Respir Med. 2004;98:536–41. doi: 10.1016/j.rmed.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 42.Hayashida M, Seyama K, Inoue Y, Fujimoto K, Kubo K Respiratory Failure Research Group of the Japanese Ministry of Health, Labor, and Welfare. The epidemiology of lymphangioleiomyomatosis in Japan: a nationwide cross-sectional study of presenting features and prognostic factors. Respirology. 2007;12:523–30. doi: 10.1111/j.1440-1843.2007.01101.x. [DOI] [PubMed] [Google Scholar]
- 43.Gupta D, Ross K, Piacentino V, 3rd, Stepnowski D, McClurken JB, Furukawa S, et al. Use of LeVeen pleuroperitoneal shunt for refractory high-volume chylothorax. Ann Thorac Surg. 2004;78:e9–12. doi: 10.1016/j.athoracsur.2003.12.069. [DOI] [PubMed] [Google Scholar]
- 44.Mikroulis D, Didilis V, Bitzikas G, Bougioukas G. Octreotide in the treatment of chylothorax [Letter] Chest. 2002;121:2079–80. doi: 10.1378/chest.121.6.2079. [DOI] [PubMed] [Google Scholar]
- 45.Makrilakis K, Pavlatos S, Giannikopoulos G, Toubanakis C, Katsilambros N. Successful octreotide treatment of chylous pleural effusion and lymphedema in the yellow nail syndrome [Letter] Ann Intern Med. 2004;141:246–7. doi: 10.7326/0003-4819-141-3-200408030-00028. [DOI] [PubMed] [Google Scholar]