Abstract
Varicella zoster virus (VZV) is a neurotropic herpesvirus that infects nearly all humans. Primary infection usually causes chickenpox (varicella), after which virus becomes latent in cranial nerve ganglia, dorsal root ganglia and autonomic ganglia along the entire neuraxis. Although VZV cannot be isolated from human ganglia, nucleic acid hybridization and, later, polymerase chain reaction proved that VZV is latent in ganglia. Declining VZV-specific host immunity decades after primary infection allows virus to reactivate spontaneously, resulting in shingles (zoster) characterized by pain and rash restricted to 1-3 dermatomes. Multiple other serious neurological and ocular disorders also result from VZV reactivation. This review summarizes the current state of knowledge of the clinical and pathological complications of neurological and ocular disease produced by VZV reactivation, molecular aspects of VZV latency, VZV virology and VZV-specific immunity, the role of apoptosis in VZV-induced cell death, and the development of an animal model provided by simian varicella virus infection of monkeys.
Keywords: VZV, neurological disease, latency, apoptosis, animal model
Herpes zoster
As cell-mediated immunity to VZV declines with age or immunosuppression (as in organ transplant recipients and patients with cancer or AIDS), VZV reactivates from its latent state to cause zoster (shingles) characterized by dermatomal distribution pain and rash. Nearly 1,000,000 individuals in the U.S. are affected annually. Unlike varicella, which occurs primarily in the spring, there is no seasonal predilection for zoster. Zoster in young adults may be the first manifestation of HIV infection [1]. Varicella (chickenpox) in infancy predisposes to zoster earlier in life [2]. Like varicella, zoster skin lesions begin to resolve in less than a week, although pain normally lasts 4-6 weeks. Unfortunately, many patients experience chronic pain or postherpetic neuralgia (PHN). In chronic active VZV infection of ganglia, magnetic resonance imaging (MRI) has shown enhancement of ganglia and affected nerve roots [3]. Because VZV becomes latent in ganglia along the entire neuraxis, zoster can develop anywhere on the body. Zoster paresis (zoster with lower motor neuron type weakness) occurs in the arm, leg [4,5], diaphragm [6] or abdominal muscles [7].
The pathological features of zoster are characterized by inflammation and haemorrhagic necrosis with associated neuritis, localized leptomeningitis, unilateral segmental poliomyelitis, and degeneration of related motor and sensory roots [8,9]. Demyelination is seen in areas with mononuclear cell (MNC) infiltration and microglial proliferation. Intranuclear inclusions, viral antigen and herpesvirus particles have been found in acutely infected ganglia [10-13]. Antiviral drugs (e.g., valacyclovir, 1 g three times daily for 7-10 days) speed healing of rash and shorten the duration of acute pain. In immunocompromised patients, intravenous acyclovir (5-10 mg/kg three times daily for 5-7 days) is recommended.
Neurological complications of VZV reactivation
VZV reactivation causing shingles is often followed by chronic pain (PHN) as well as vasculopathy, meningitis/meningoencephalitis, myelopathy, cerebellitis and various ocular disorders, the most serious of which is retinal necrosis (Figure 1). VZV reactivation can also produce radicular pain without rash (zoster sine herpete). Importantly, all the neurological and ocular complications of VZV reactivation identified above may develop in the absence of rash. In addition, subclinical VZV reactivation (without pain or rash) has been demonstrated in astronauts [14], with shedding of infectious virus [15].
Figure 1.
Neurological complications of varicella zoster virus reactivation.
Postherpetic neuralgia (PHN)
PHN, operationally defined as pain that persists for at least 3 months and sometimes years after resolution of zoster rash, occurs in about 40% of patients over age 60 [16,17]. While the cause and pathogenesis of PHN are unknown, two non-mutually exclusive theories are that: (1) the excitability of ganglionic or even spinal cord neurons is altered after VZV-mediated damage; and (2) persistent or low-grade productive virus infection exists in ganglia.
Smith’s [18] analysis of ganglia from an early case of PHN of 2.5 months’ duration revealed diffuse and focal infiltration by chronic inflammatory cells (Figure 2A), an observation confirmed by Watson et al. [19] who found prominent collections of lymphocytes in ganglia from a patient with PHN of 2 years’ duration (Figure 2B). The inflammatory response in ganglia of these subjects raised the possibility of prolonged viral infection. Further evidence that PHN may be produced by low-level ganglionitis comes from the detection of VZV DNA and proteins in blood MNCs of many patients with PHN [20-22], and from the favourable response of some PHN patients to antiviral treatment [23,24]. In a prospective, open-label phase I/II clinical trial, 15 patients with moderate to severe PHN were treated with intravenous acyclovir for 2 weeks, followed by oral valacyclovir for 1 month; 8 of 15 (53%) patients reported improvement of pain [25], warranting further investigation of antiviral therapy in a larger, randomized, double-blind, placebo-controlled trial.
Figure 2.
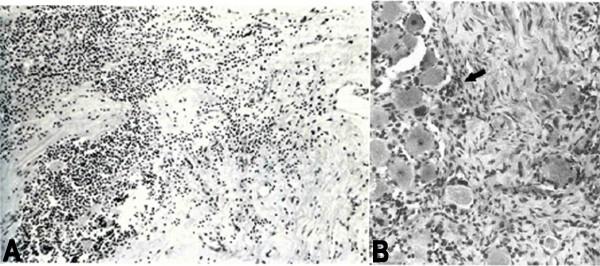
Haematoxylin and eosin-stained sections of dorsal root ganglia from patients with postherpetic neuralgia, revealing diffuse and focal infiltration by chronic inflammatory cells (A). Arrow in panel B points to a prominent collection of lymphocytes. Figure 2 previously published in Surg Neurol, 1978; 10:50-3; Smith FP; Pathological studies of spinal nerve ganglia in relation to intractable intercostal pain. Reprinted with permission of Elsevier, Copyright 1978.
VZV vasculopathy
VZV vasculopathy or stroke caused by productive virus infection of cerebral arteries is one of the most serious complications of VZV infection. VZV vasculopathy has also been referred to as granulomatous angiitis, VZV vasculitis/encephalitis, post-varicella arteriopathy, and herpes zoster ophthalmicus with delayed contralateral hemiparesis in a subset of patients with ocular and motor findings. Patients can present with fever, altered mental status, headaches and focal neurological deficits. In a study of 30 patients with VZV vasculopathy [26], 37% did not have rash preceding stroke and 33% had no cerebrospinal fluid (CSF) pleocytosis, making diagnosis difficult. In addition, 97% of patients had imaging abnormalities, often with characteristic lesions at grey-white matter junctions on MRI. Angiography in 23 of the 30 (70%) patients revealed focal arterial stenosis, occlusion, aneurysm or haemorrhage. Both large and small arteries were involved in 50%, small arteries in 37%, and large arteries alone in only 13% of 30 patients. CSF of 30% of patients contained VZV DNA while 93% had anti-VZV IgG antibody in CSF, supporting previous studies that the detection of anti-VZV IgG antibody in CSF is a better test for diagnosing VZV vasculopathy than the detection of VZV DNA in CSF [27]. When CSF pleocytosis is present, cells are predominantly mononuclear and oligoclonal bands are present; the oligoclonal IgG is antibody directed against VZV [28].
The prevalence of VZV vasculopathy is unknown since stroke in the elderly is often attributed to atherosclerotic disease and the CSF is not examined in those patients. Even among immunocompromised patients with strokes, CSF is not routinely examined for anti-VZV IgG antibody. Two recent studies revealed an increased risk of stroke after zoster. An analysis of 7760 patients who had been treated for zoster and 23,380 controls found that the risk of stroke was 31% higher within one year after zoster and approximately 4-fold higher in patients with herpes zoster ophthalmicus (HZO) versus the comparison cohort [29]. Similarly, in a study of 658 HZO patients and 1974 controls, the risk of stroke was 4.52-fold higher in the patients than in controls [30]. To put this in perspective, the more than 500,000 annual cases of zoster expected even if all individuals over age 60 years in the U.S. received zoster vaccine would be at increased risk of stroke within the following year.
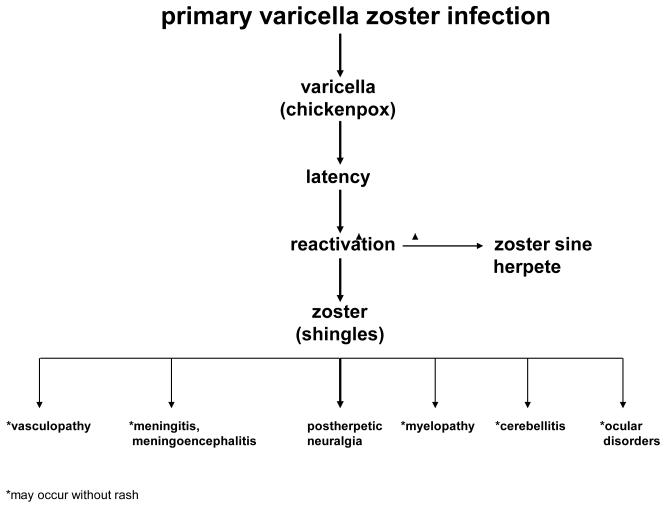
Studies of the pathogenesis of VZV vasculopathy have been limited since there is no animal model to study stroke caused by VZV. Case reports of VZV vasculopathy in which cerebral arteries were obtained at autopsy revealed virus in affected cerebral arteries, as evidenced by the presence of multinucleated giant cells, Cowdry A inclusion bodies, herpesvirus particles by electron microscopy, VZV DNA, and VZV antigen [31]. In chronic cases, virus is also found in areas of infarction, usually close to arteries and veins. Depending on the time elapsed since the onset of virus-mediated damage and on other possible unknown factors, a wide range of vascular involvement exists, from necrotizing arteritis to modest, chronic vascular inflammation, to thrombosis without inflammation, and remote vascular occlusion resembling atherosclerosis [32]. Recently, Salazar et al. [33] described an 80-year-old man who developed left ophthalmic distribution zoster and left-sided vision loss from ophthalmic artery occlusion. Before the diagnosis of VZV vasculopathy was established, giant cell arteritis was considered and a left temporal artery biopsy was performed. In contrast to a normal cerebral artery with no VZV antigen (Figure 3A), the asymptomatic left temporal artery contained VZV antigen in the adventitia (Figure 3B, red color). The presence of most VZV antigen in the adventitia of this early case of VZV vasculopathy supports the notion that VZV spreads transmurally from the adventitia to the intima, presumably after transaxonal spread to the artery via ganglionic afferent fibres [34,35]. Although it remains unknown why virtually all cases of VZV vasculopathy involve cerebral arteries rather than systemic arteries, it is possible that the absence of an external elastic lamina in cerebral arteries, unlike systemic arteries [36], facilitates transmural travel of the virus in cerebral arteries with continued virus production in a thickened intima.
Figure 3.
Virological analysis of an asymptomatic left temporal artery from a patient with VZV vasculopathy. Sections of the temporal artery were deparaffinized and incubated with 10% normal sheep serum (NSS) in phosphate-buffered saline (PBS) for 1 hour at room temperature. To prevent non-specific binding, primary antibodies were adsorbed with normal human liver powder for 30 minutes and again for 20 hours at 4°C. Sections were then incubated with polyclonal antibodies raised against VZV ORF 63 protein (1:1000 dilution) or normal rabbit serum (1:1000 dilution), rinsed with PBS and incubated with a 1:300 dilution of biotinylated goat anti-rabbit IgG in PBS containing 5% NSS, washed 3 times in PBS, incubated with alkaline phosphatase-conjugated streptavidin (1:100 dilution) and washed three times with PBS. The color reaction was developed for 5-30 minutes with fresh fuchsin substrate system. Levimasole was added to the colour reaction to block endogenous phosphatase. Uninfected and VZV-infected BSC-1 cells were used as controls (not shown). The normal cerebral artery does not contain VZV antigen (A), whereas the temporal artery contains abundant VZV antigen in the arterial adventitia (B, red). Panels A, B 600X.
VZV meningitis, meningoencephalitis, meningoradiculitis and cerebellitis
Acute VZV infection may also present as meningitis or a meningoencephalitis. Many reported cases of VZV encephalitis may actually be VZV vasculopathy [37]. Recent reports of VZV meningitis [38,39], meningoradiculitis [40] and cerebellitis (gait ataxia and tremor predominated) [41,42], all in the absence of rash and confirmed by the detection of VZV DNA and anti-VZV antibody in CSF, revealed that VZV is not an uncommon cause of aseptic meningitis.
VZV myelopathy
VZV myelopathy presents in various ways. One form is a self-limiting, monophasic spastic paraparesis, with or without sensory features and sphincter problems. This so-called post-infectious myelitis usually occurs in immunocompetent patients days to weeks after acute varicella or zoster. Its pathogenesis is unknown. The CSF usually contains a mild mononuclear pleocytosis, with a normal or slightly elevated protein. Steroids are used to treat these patients [43], although some improve spontaneously [44]. Rarely, VZV myelitis recurs, even in immunocompetent patients [45].
VZV myelopathy may also present as an insidious, progressive and sometimes fatal myelitis, mostly in immunocompromised individuals. Indeed, AIDS has commonly and increasingly become associated with VZV myelitis. MRI reveals longitudinal serpiginous enhancing lesions. Diagnosis is confirmed by the presence of VZV DNA or anti-VZV IgG or both in CSF [45]. Pathological and virological analyses of the spinal cord from fatal cases have shown frank invasion of VZV in the parenchyma [32] and in some instances, spread of virus to adjacent nerve roots [46]. Not surprisingly, some patients respond favourably to antiviral therapy [47-49]. Importantly, VZV myelitis may develop without rash. Early diagnosis and aggressive treatment with intravenous acyclovir has been helpful, even in immunocompromised patients [47]. The benefit of steroids in addition to antiviral agents is unknown. VZV can also produce spinal cord infarction identified by diffusion-weighted MRI and confirmed virologically [50]. Thus, VZV vasculopathy can cause stroke in the spinal cord as well as in the brain.
VZV retinal necrosis
VZV produces multiple ocular disorders, including both acute retinal necrosis (ARN) and progressive outer retinal necrosis (PORN). ARN develops in both immunocompetent and immunocompromised hosts. Patients present with periorbital pain and floaters with hazy vision and loss of peripheral vision. Treatment is typically intravenous acyclovir, steroids and aspirin followed by oral acyclovir [51]. Intravitreal injections of foscarnet and oral acyclovir have been used in early, milder cases. Although PORN can be caused by herpes simplex virus and cytomegalovirus, most cases are produced by VZV, primarily in AIDS patients with CD4+ counts typically less than 10 cells/mm3 of blood [52], but also in other immunosuppressed individuals [53]. PORN may be preceded by retrobulbar optic neuritis and aseptic meningitis [54], central retinal artery occlusion or ophthalmic-distribution zoster [55], and may occur together with multifocal vasculopathy or myelitis. Patients present with sudden painless loss of vision, floaters and constricted visual fields with resultant retinal detachment. Multifocal, discrete opacified lesions begin in the outer retinal layers peripherally and/or posterior pole; only late in disease are inner retinal layers involved. Diffuse retinal haemorrhages and whitening with macular involvement bilaterally are characteristic findings. Treatment with intravenous acyclovir has given poor or inconsistent results [56], and even when acyclovir helped, VZV retinopathy recurred when drug was tapered or stopped. PORN patients treated with ganciclovir alone or in combination foscarnet had a better final visual acuity than those treated with acyclovir or foscarnet [57]. The best treatment for PORN in AIDS patients may be prevention with HAART, which appears to decrease its incidence [58].
Zoster sine herpete
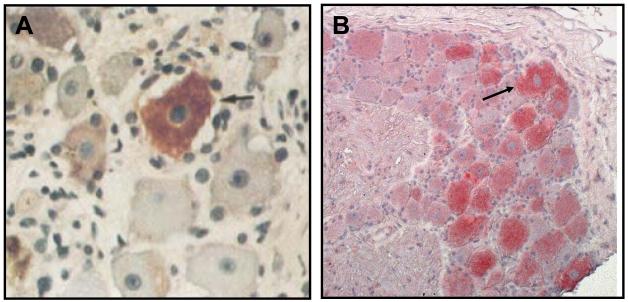
Lewis [59] originally described multiple patients with dermatomal distribution radicular pain in areas distinct from pain with rash in zoster patients. Currently, most clinicians regard zoster sine herpete exclusively as the occurrence of chronic radicular pain without rash. The first two virologically confirmed cases of zoster sine herpete were verified by detection of VZV DNA in CSF [60]. A third case of thoracic-distribution zoster sine herpete, in which electromyography of paraspinal muscles demonstrated frequent fibrillation potentials restricted to chronically painful thoracic root segments, was confirmed by detection of VZV DNA in blood MNCs and anti-VZV IgG antibody in CSF [61]. Recently, in a patient with zoster sine herpete, the CSF did not contain amplifiable VZV DNA, but did contain anti-VZV IgG with reduced serum/CSF ratios of anti-VZV IgG indicative of intrathecal synthesis [62]. Finally, the most compelling evidence that persistent radicular pain without rash can be caused by a chronic active VZV ganglionitis came from our analysis of a trigeminal ganglionic mass removed from an immunocompetent adult who had experienced relentless trigeminal distribution pain for more than a year. Pathological and virological analyses (Figure 4) revealed that the patient’s zoster sine herpete was caused by an active VZV ganglionitis [63].
Figure 4.
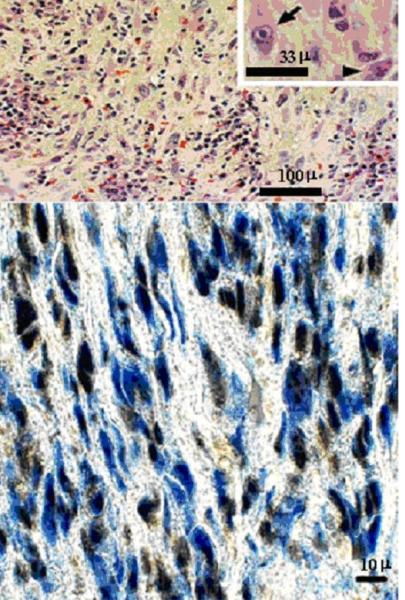
Ganglionitis and intranuclear inclusions in zoster sine herpete. Haematoxylin and eosin staining of the trigeminal ganglion (top panel) shows widespread chronic inflammation with fibrosis and loss of neurons. Cells in some foci contain Cowdry type A intranuclear inclusions (arrow, inset) indicative of virus infection; the inflammatory cells are mainly lymphocytes with some plasma cells (arrowhead, inset). Immunohistochemical staining of the same ganglion (bottom panel) with mouse monoclonal antibody directed against VZV gene 63 protein indicates VZV antigen (brown staining) in multiple cells throughout the ganglion. Adjacent sections stained with antibody directed against HSV or with normal rabbit serum were negative (not shown).
In recent years, the detection of VZV DNA and anti-VZV IgG antibody in patients with meningoencephalitis, vasculopathy, myelitis, cerebellar ataxia and polyneuritis cranialis, all without rash, has expanded the spectrum of neurological disease produced by VZV in the absence of rash. An illustrative example is provided by the report of an immunocompetent 45-year-old-woman who had multiple episodes of neurological disease (meningoencephalitis, multifocal vasculopathy, recurrent myelitis and inflammatory brain stem disease) over an 11-month period produced by VZV in which the CSF contained anti-VZV IgG antibody, but not VZV DNA, throughout her illness [64]. Overall, if the diagnosis of zoster sine herpete is suspected, the CSF and blood MNCs should be studied for VZV DNA, the CSF for the presence of anti-VZV IgG and IgM antibody, and the serum for anti-VZV IgM antibody.
VZV genome
The VZV genome was the first herpesvirus genome to be sequenced completely [65]. Currently, the complete DNA sequence of 24 independently obtained VZV isolates is available in the National Institutes of Health public access data base (http://www.ncbi.nlm.nih.gov/pubmed/). The VZV genome contains 71 open reading frames (ORFs) with the potential to encode 68 proteins (ORFs 42 and 45 are predicted exons from the same primary transcript, and ORFs 62, 63 and 64 are repeated as ORFs 71, 70 and 69, respectively). In addition to the predicted genes, three VZV genes have been experimentally detected: gene 33.5 [66], 9A [67], and ORF (S/L) [68]. Thus, VZV has the smallest alphaherpesvirus genome (125 Kb) with the coding capacity of 70 unique genes. The VZV genome is remarkably stable. Analysis of VZV propagated over 1206 passages in tissue culture cells reveals only three point mutations (E184G, N221D, and one silent) within the 834-bp ORF 63 [69]. Worldwide, VZV shows little geographic variation [70], although DNA sequencing has identified five VZV regional clades [71].
VZV pathogenesis
Upon initial exposure to aerosolized VZV, infected Langerhans cells along with mucosal and plasmacytoid dendritic cells carry the virus to resident T cells located within draining lymph nodes [72,73]. Infected T cells are induced to express skin homing factors and transport the virus to dermal fibroblasts and keratinocytes which in turn produce proinflammatory cytokines resulting in the vesicles characteristic of varicella [74-76]. Since afferent fibres of the sensory nervous system terminate in the skin, cell-free VZV present in varicella vesicles have direct access to dorsal root ganglia, cranial nerve ganglia and autonomic nervous system ganglia. Thus VZV, like herpes simplex virus (HSV)-1, the prototype neurotropic human alphaherpesvirus, can be transported along sensory nerve axons to the neuron, where latency is established [77,78]. Since varicella vesicles can develop on any dermatome, all sensory ganglia can become latently infected, as evidenced by the detection of latent VZV DNA in cranial nerve, cervical, thoracic, lumbar and sacral root ganglia [79-81].
Along with axonal retrograde transport, neurons might be infected haematogenously, given the high virus load in blood during varicella (200-5000 copies of VZV DNA per 150,000 peripheral blood MNCs, 100-1000 VZV DNA copies per ml whole blood and 100-10,000 VZV DNA copies per ml serum [82,83]. Evidence for such a route of neuronal infection comes from the isolation of genotypically distinguishable strains of VZV from vesicles of a single individual during two separate episodes of virus reactivation despite only one episode of varicella [84]. Experimentally, the haematogenous route of neuronal infection is observed in monkeys infected with simian varicella virus (SVV), the primate counterpart of human VZV, with SVV DNA detected in ganglia even before the development of skin rash [85].
VZV-induced apoptosis in non-neuronal and neuronal cells
Apoptosis is a regulated form of cell death characterized by DNA fragmentation, membrane blebbing and cell shrinkage. This pathway proceeds through sequential proteolytic activation of caspases, a family of cysteine proteases. Two major pathways of apoptosis have been described. The extrinsic pathway is initiated by ligand interaction with death receptors resulting in activation of caspase-8. The intrinsic pathway is initiated when cytochrome c, released from damaged mitochondria, which activates caspase-9. Both caspases activate caspase-3, a biochemical marker for apoptosis, resulting in cell death.
VZV-induced apoptosis in vitro and in vivo has been reported in several cell types [86-88]. Analysis of the mechanism of varicella-induced apoptosis in SVV-infected monkey kidney (Vero) cells and in VZV-infected human melanoma (MeWo) cells showed that the induced apoptosis proceeds through the intrinsic pathway in both cases [89.90]. The intrinsic cell death pathway is determined by a balance between the proapoptotic (e.g., Bak and Bax) and anti-apoptotic (e.g., Bcl-2, Bcl-xL) mitochondrial Bcl-2 family of proteins. In addition, BH3-only proteins (e.g., Bad, Bim, Bid, Noxa and Puma) induce apoptosis by activating proapoptotic proteins or by neutralizing anti-apoptotic proteins. Real-time PCR and Western blot analyses revealed downregulation of Bcl-2 in varicella-infected cells, leading to release of cytochrome c from mitochondria and activation of caspase-9, a marker of the intrinsic apoptotic pathway.
Earlier, Hood et al. [91] reported that VZV induces apoptosis in human foreskin fibroblasts, but not in human sensory neurons. Recently, we developed an in vitro cell culture model to study the virus-neuron relationship. Neural stem cells (NSC) in the subgranular layer of the dentate gyrus of the hippocampus and subventricular zone of the lateral ventricle support neurogenesis in the adult brain. NSCs isolated from human fetal brain and cultured in suspension in the presence of epidermal growth factor produce spherical clusters known as ‘neurospheres’. Depending on culture conditions, these self-renewing multipotent cells can be induced to differentiate into neurons, astrocytes and oligodendrocytes after adhesion to specific substrata. In fact, we obtained cultures containing more than 90% neurons, as confirmed by immunofluorescence staining for MAP2a, by inducing differentiation of NSCs in the presence of retinoic acid, dibutyryl cyclic AMP and neurotrophic growth factors (nerve growth factor and BDNF). Infection of these neurons with cell-free VZV did not lead to a cytopathic effect (CPE) even after 3 weeks, whereas a CPE developed within a week in human fetal lung fibroblasts infected with the same amount of VZV. VZV DNA and VZV-specific transcripts and protein were found in healthy-appearing neurons. Furthermore, the apoptotic markers TUNEL staining and caspase-3 activation were detected in VZV-infected fibroblasts, but not in neurons. The relationship between inhibition of apoptosis and the establishment of VZV latency in neurons awaits further analysis.
VZV latency
In human ganglia, VZV establishes latency in the neuron [92-97]. The prevalence of latent VZV in the normal population has been variously reported as 63% [98], 79% [99], 87% [100], 91% [80], and 100% [101,102]. The largest study to date found latent VZV DNA in 94% of 414 trigeminal ganglia removed from 207 cadavers [103]. The VZV DNA burden during latency has also been variously reported as 6-31 [104], 258 ±38 [100], and 9,046 ±13,225 [101] copies per 100,000 ganglionic cells. The latter two studies are interesting since the same technique (real-time PCR) was used, and both studies detected similar amounts of latent HSV-1 DNA: 2902 ±1082 copies [100] and 3042 ± 3274 copies [101] per 100,000 ganglionic cells. The large range in VZV DNA burden during latency may reflect analysis of autopsy tissue collected many decades after primary infection and after multiple episodes of re-exposure to virus normally circulating in the population. Nonetheless, the VZV DNA copy number per latently infected neuron is too low to be detected with in situ technologies unless supplemented with prior in situ PCR amplification [94,105].
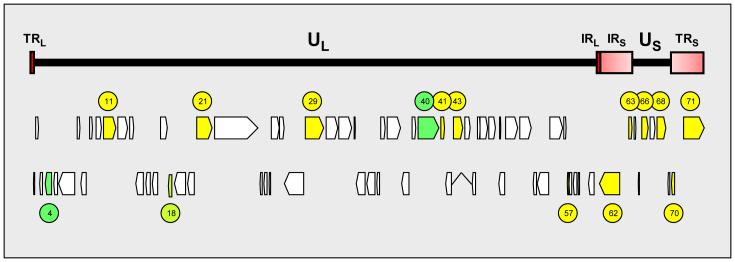
VZV gene expression is restricted during latency. Transcripts mapping to VZV ORFs 4, 18, 21, 29, 40, 62, 63 and 66 have been detected in latently infected human trigeminal ganglia [101,106-110]. The copy number of latently transcribed transcripts is extremely low. Among the VZV transcripts quantified by real-time PCR, ORF 63 transcripts are the most abundant and are present at ~3.7 ×103 copies per μg mRNA [109]. Considering that the average cell mRNA is 1.4 kb in length [111], the ratio of VZV ORF 63 to cell mRNA is, on average, 1:3.5 ×108. This low level of latent VZV gene transcription has hindered the global search of the VZV genome for active regions of transcription. The genome expression system (GeXP) is a new technical advancement which incorporates unbiased PCR amplification with fluorescence-labeled universal primers to simultaneously amplify multiple targets that are subsequently size-separated by gel electrophoresis on capillary tubes and detected after laser excitation [112,113]. The use of GeXP revealed multiple transcripts mapping to the entire VZV genome (the VZV transcriptome) in five independent PCR assays [114] and detected mRNA for VZV ORFs 4, 11, 29, 40, 41, 43, 57, 62, 63 and 68 in the VZV transcriptome of 26 latently infected human trigeminal ganglia [115]. Consistent with previous findings, VZV ORF 63 transcripts were the most frequent [101,109,115]; however, it should be noted that a distinct variability of VZV gene expression in latently infected ganglia exists (Figure 5) and not all VZV ORF transcripts are present in all ganglia. The detection of multiple transcripts corresponding to all stages of the virus life cycle clearly indicates that VZV latency differs from HSV-1 latency, where only a single latency-associated gene appears to be transcribed [116], although GeXP has not yet been used to study HSV-1 transcription in latently infected ganglia.
Figure 5.
VZV genes transcribed during latency. The VZV genome consists of ~125,000 base pairs of double-stranded DNA arranged in two units. The unique long segment (UL) and unique short (US) segments are each bounded by internal and terminal invert repeat sequences (TRL, terminal repeat of unique long, IRL, internal repeat of unique long; IRS, internal repeat of unique short; TRS, terminal repeat of short [red shaded boxes]). The VZV genome contains 71 predicted open reading frames (ORFs) consecutively numbered from the leftward end of the virus genome. The entire VZV transcriptome has been identified in productively infected cells. In latently infected ganglia, VZV genes that have been sequence-verified include ORFs 11, 21, 29, 41, 43, 57, 62, 63, 66, 68, 70 and 71 (yellow). VZV ORF 4 and 40 transcripts have been detected by two independent techniques (PCR and in situ hybridization [dark green]), while VZV ORF 18 transcripts have been detected by only one technique (in situ hybridization [light green]).
Identification of VZV proteins present during latency is hindered by the lack of suitable antibodies with sufficient specificity and sensitivity to detect low amounts of virus proteins in rare latently infected neuronal cells. Nonetheless, proteins encoded by VZV ORFs 4, 21, 29, 62, 63, and 66 have been detected in latently infected neurons [102,117-121]. Transcripts encoding these proteins have also been detected in latently infected ganglia [117,118,121]. However, only the one technique of immunohistochemistry has successfully detected latently expressed VZV proteins. Furthermore, a recent report suggested that the detection of VZV proteins during latency in human ganglia is a rare event confounded by the presence of lipofuscin, a potential source of non-specific staining [122]. Currently, all VZV proteins detected during latency are localized in the neuronal cytoplasm, including proteins localized to the nucleus during productive virus infection. This intriguing finding may provide a clue to the maintenance of latency. Current efforts of numerous laboratories are exploring the mechanism of intracellular VZV protein localization and its outcome on productive virus infection.
VZV gene transcription is regulated at the epigenetic level during latency. The promoters for ORFs 62 and 63, VZV genes that are transcribed during latency, are associated with the histone protein H3 containing post-translational acetylation indicative of the euchromatic (transcriptionally active) state. Conversely, promoters for ORF 36 (thymidine kinase) and ORF 14 (glycoprotein C), VZV genes that are repressed during latency, lack enrichment in euchromatic modified histones [123]. That report remains the only confirmation in human ganglia of the concept of epigenetic regulation of neurotropic alphaherpesvirus gene transcription first described in animal models of HSV-1 latency [124]. Whereas latent HSV-1 gene regulation also involves microRNA silencing [125], no such microRNAs are present in human ganglia latently infected with VZV [126,127].
VZV-specific immunity
Primary VZV infection that induces varicella is followed by the production of VZV-specific antibody and VZV-specific T cell-mediated immunity [128,129]. Antibodies to VZV are detected throughout life, with the serum of individuals 60 to 94 years of age containing a variable presence of VZV-specific antibodies to VZV glycoproteins I-IV and to three non-glycosylated proteins; antibodies to VZV are also present in some elderly individuals with no history of varicella or zoster, indicative of subclinical infection [20]. Increased levels of antibody to VZV do not confer protection against zoster or PHN. In fact, increased levels of antibody to VZV after the onset of zoster are associated with more severe disease and a greater risk of PHN, perhaps because they reflect more extensive VZV replication [130].
T cell immunity to VZV is more important than the antibody response, as evidenced in studies of natural VZV infection in agammaglobulinaemic humans, who are unable to produce VZV-specific antibodies yet are protected against second episodes of varicella because of their ability to mount a VZV-specific T cell-mediated immune response [131]. Individuals with T cell-immune deficiency disorders have more severe disease than normal hosts [132], and a significant increase in the incidence of zoster is associated with immune suppression [133]. Even in human stem cell transplant recipients who received inactivated VZV vaccine, protection was correlated with VZV-specific T cell immunity, not with anti-VZV antibody [134].
VZV-specific T cell-mediated immunity maintains latent VZV in ganglia. The immune response can be subsequently boosted by subclinical reactivation of latent virus or environmental exposure to virus. Most importantly, the incidence of zoster increases with age as VZV-specific T cell-mediated immunity declines. For example, the frequency of VZV-specific memory CD4 T cells is significantly influenced by age as evidenced by their decrease during the first three years after varicella [135]. After mid-adult life, the intensity and quality of antigenic stimulation provided by re-exposure and asymptomatic reactivation are not sufficient to maintain VZV-specific T cell immunity. Furthermore, a comparison of the cell-mediated immune response to VZV antigen in vitro in young adults and individuals over age 60 revealed 5-fold fewer CD4 cells producing interferon-gamma or interleukin 4 and 5, as well as fewer CD4 early effectors and CD8 effector memory cells in the over age 60 group [136].
While reduced cell-mediated immunity with age or after exposure to immunosuppressive regimens in cancer patients or bone marrow transplant recipients results in VZV reactivation [137,138], virus-specific T cells are rarely seen in human ganglia latently infected with VZV [139]. Analysis of human ganglia from donors who had zoster 1-5 months before death revealed VZV glycoprotein E (gE) in neurons and infiltration of non-cytolytic CD8+ T cells, but neurons that were positive for VZV gE were neither positive for MHC class I nor surrounded by T cells, suggesting that immune control of virus reactivation may not be dependent on direct contact with T cells [140]. Since VZV has been shown to down-regulate MHC I surface expression, virus latency is probably regulated by an innate immune response involving cytokines or chemokines [72,141,142]. CXCL10 has been proposed as a potential driver of T cell recruitment, based on its detection along with its receptor (CXCR3) in human ganglia from zoster patients [143].
Recognition of the essential role of cell-mediated immunity to VZV for protection against and recovery from varicella and zoster has led to studies designed to boost the cell-mediated immune response to VZV by immunization of elderly adults. Demonstration of the ability of zoster vaccine to reverse VZV-specific T cell deficiencies that were present before immunization was followed by the Shingles Prevention Study (see below).
VZV vaccine
There are two VZV vaccines, both of which contain live, attenuated virus. The first to be developed was the varicella (Oka strain) vaccine (Varivax, Merck) which has been used to vaccinate children to prevent varicella (chickenpox) in Japan since 1975 and in the US since 1996. The second vaccine (Zostavax, Merck) is used to prevent zoster in elderly individuals. The only difference between the two vaccines is that Zostavax contains 19,400 pfu per dose, 14-fold more virions than the varicella vaccine. Varivax generates VZV-specific humoral and cell-mediated immune responses, particularly CD8+ T lymphocytes [144]. The memory cell response that occurs after vaccination protects from infection during re-exposure to VZV.
Zostavax is a live, attenuated virus vaccine indicated for the prevention of herpes zoster in individuals 60 years and older. The most widely used measure of the cell-mediated immune response to VZV in elderly individuals before and after zoster vaccination has been the responder cell frequency [145]. Indeed, zoster vaccine administered to people over 60 years of age resulted in increased numbers of CD4 and CD8 cells, CD4 and CD8 effector memory T cells, and CD8 early-effector T cells; the half-life of the boost in T cell immunity to VZV is at least 5 years [146]. Zoster vaccine also boosts VZV-specific immunity in adults with a history of zoster before vaccination or with chronic illness.
The critical trial (Shingles Prevention Study, SPS) of the licensed zoster vaccine was a placebo-controlled, double-blind study of more than 38,000 adults over age 60 years and randomized to receive either zoster vaccine or placebo. All subjects were monitored for zoster. Endpoints included the burden of illness due to zoster and zoster-associated pain as well as the incidence of clinically significant PHN. Subjects received a single dose of Zostavax (n = 19,270) or placebo (n = 19,276). Racial distribution across both vaccination groups was similar: White (95%), Black (2%), Hispanic (1%) and other (1%). The gender distribution was 59% male and 41% female in both groups. The most common side effects reported by participants after zoster vaccination were redness, pain, itching, swelling, warmth or bruising at the injection site and sometimes headache. Varicella-like rashes at the injection site were more common in zoster vaccine than in placebo recipients (0.1% v. 6.4%; p < 0.05).
After a mean follow-up of 3 years, the SPS found that use of the Zostavax vaccine reduced the incidence of zoster by 51%. Subjects in the immunization group who developed zoster reported significantly less pain and discomfort than those in the placebo group, and PHN was less frequent (an overall 61% lower burden of disease). While the vaccine group had a significantly greater risk of a serious adverse event (1.9% v. 1.3%) and experienced more adverse events at the injection site (48.3% v. 16.6%) than the placebo group during the first 42 days after vaccination, no significant differences were seen between zoster vaccine and placebo groups in the incidence of vaccine-related serious adverse events (both <0.1%) at the end of the study.
Baseline immunological measurements from the SPS confirmed that VZV-specific T cell immunity measured by responder cell frequency, as well as by ELISPOT assay, declined continuously with advancing age [147]. ELISPOT responses peaked at 2 weeks after immunization, with responses at 6 weeks post-immunization approximately 2-fold higher in immunized than in placebo recipients [148]. All values fell during the first year after immunization, but remained approximately 50% higher than pre-immunization levels for the 3-year study period. Importantly, the boost in VZV-specific T cell-mediated immunity was similar to that developing after naturally occurring zoster [130]. The magnitude of the boost in cell-medicated immunity to VZV was greatest in younger subjects, consistent with the efficacy of vaccine in preventing zoster which was greater in adults of age 60 to 69 years compared to those more than 70 years of age. The memory responses elicited in older subjects was similar to that seen after vaccination of the elderly with pneumococcal and influenza vaccines. Zoster vaccine was well-tolerated when administered concomitantly or sequentially with an inactivated influenza vaccine or pneumococcal vaccine.
Importantly, in immunized individuals who developed zoster, high VZV-specific cell-mediated immune responses were associated with reduced zoster severity and a lower frequency of PHN than in participants with lower VZV-specific cell-mediated responses, whereas a high humoral response was associated with increased severity of zoster and a higher frequency of PHN than in participants with a lower humoral immune response [130].
In the United States, the Center for Disease Control and Prevention Advisory Committee on Immunization Practices recommends zoster vaccine for all persons over the age of 60 years with no prior indications and in persons reporting a previous episode of zoster or who have chronic medical conditions. Although zoster vaccine is not recommended for immunocompromised individuals, it appears that the current zoster vaccine could be safely administered to several groups of moderately immunocompromised adult patients, such as VZV-seropositive HIV patients with CD4 T cell counts greater than 200 cells/ml, or even to patients with rheumatoid arthritis or psoriasis who are receiving moderate doses of methotrexate, steroids or tumour necrosis factor inhibitors [149]. In addition to the need to test the currently licensed zoster vaccine in moderately immunocompromised patients, higher titre zoster vaccines should be also tested for safety and immunogenicity, especially in light of reports of the safe administration of heat-inactivated VZV vaccine to autologous bone marrow transplant recipients and their accelerated recovery of cell-mediated immunity to VZV and reduced incidence of zoster [134,150].
By 2008, three years after zoster vaccine was licensed and recommended by the Advisory Committee on Immunization Practices for persons age 60 and older, less than 7% of the age group in the US was vaccinated [151]. This was due to a combination of lack of patient awareness regarding the availability of a vaccine, physicians’ uncertainty about the duration of protection, and different cost-sharing plans for immunization. This is disappointing. Zoster vaccine should be universally administered to all individuals over age 60. Routine immunization is not currently recommended for people age 50-59 because of lack of efficacy data and cost-effectiveness information in this group, but consideration should be given to immunization of this population since 15% of zoster occurs between ages 50 and 59 years.
In the United Kingdom, the live attenuated vaccine against herpes zoster was approved for use in 2006. In England and Wales in people aged 60 or over, there were 88,650 cases and an estimated 18,200 people were still in pain after 3 months. In this age group an estimated 1750 people are hospitalized each year, and herpes zoster is given as the cause of death in 55 cases each year. Zoster generated an overall annual cost to the healthcare system of £17.3m (€20m; $24m), of which £11.5m was related to general practice and the remainder of secondary care. Almost 50% of the total costs related to disease in people aged over 80, because of the higher incidence and likelihood of complications in this group. Vaccination of 65-year-olds at 73.5% coverage was estimated to reduce the lifetime risk of zoster from 15% to 12%, or nearly 11,200 cases. It was also estimated that 1500 fewer cases of postherpetic neuralgia would occur each year, and that vaccination would reduce the annual number of hospitalizations by nearly 150. Vaccination of the 65-year olds was calculated to cost about £23.7m, but would result in savings to the health service of around £1.3m over the lifespan of the cohort, most of which would be saved in primary care. Results from this analysis suggest that immunization is likely to represent a cost-effective intervention for England and Wales, although there is a lost of uncertainty around the duration and range of vaccine-induced protection and the quality adjusted life year loss due to long-term pain. The most cost-effective age to vaccinate is 70 years, or 65 years if the vaccine does not offer additional protection against the severity of disease [152].
Several important questions regarding zoster vaccines remain. How many years will the current zoster vaccine maintain immunity to prevent zoster? Is zoster vaccine safe for immunocompromised individuals? Will a killed VZV vaccine produce a significant increase in cell-mediated immunity to VZV? Should multiple vaccinations of the elderly every decade of life after age 60 be considered? Should zoster vaccine be refined to include epitopes that induce cell-mediated immunity to VZV?
Attempts to produce varicella in animals
VZV produces disease only in humans. Experimental infection of small animals including mice [153], rats [154] and guinea pigs [155-159] by different routes resulted in seroconversion without productive infection (Table 1). Establishment of latent infection after corneal inoculation of mice could not be confirmed because VZV DNA was detected not only in ganglionic neurons, but also in non-neuronal cells in ganglia as well as in non-ganglionic tissue [160]. Nine months after foot-pad inoculation of rats, VZV nucleic acids as well as proteins were found in dissociated dorsal root ganglionic neurons in culture in the absence of clinical signs [161]. Because the ganglia were cultured 3-12 days before analysis, virus reactivation can not be ruled out. VZV gene 63 RNA and its encoded protein have been detected in sections containing ganglionic neurons from rats 18 months after inoculation [154,162]. However, virus DNA was found in both neurons and non-neuronal cells of rat ganglia 1-3 months after inoculation [163], unlike what is seen in humans. Thus the problem with the rat model is difficulty in distinguishing between a persistent and a latent infection or both. It is also possible that the pathogenesis of VZV infection in rats is different than in humans. Although viraemia and mild rash have been demonstrated in chimpanzees after subcutaneous inoculation of the vaccine strain of VZV (Oka) into the breast, ganglionic and non-ganglionic tissues were not studied [164]. In severely immunodeficient (SCID) mice implanted under the kidney capsule with human thymus or liver tissue followed by subcutaneous implantation of human skin or ganglia, virus infection and limited virus transcription in the ganglionic implants were detected after either skin or ganglia implantation [165,166]. Finally, reactivation of VZV has not been demonstrated in any of the above models.
Table 1.
Results of attempts to produce VZV-induced disease in animals
| Species | Route of inoculation |
Sero- conversion |
Rash (dpi) | Viremia | Time of sacrifice |
Detection of SVV nucleic acids in tissue |
Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ganglionic |
Lung/ liver | ||||||||||
| hyb1 | PCR | Neuronal | Non-neuronal | ||||||||
| Chimpanzee | Subcutaneous | + | 10 | + | ND2 | ND | ND | ND | ND | ND | [164] |
| Guinea pig | Intranasal/corneal | + | 4 | ND | ND | ND | ND | ND | ND | ND | [156] |
| Guinea pig | Intramuscular | + | 9-25 | + | 23 d | +3 | ND | ND | ND | + | [198] |
| Guinea pig | Intratracheal | + | − | ND | 60 d | ND | ND | ND | ND | ND | [157] |
| Guinea pig | Ocular | ND | − | ND | 21 d | ND | ND | ND | ND | ND | [199] |
| Guinea pig | Intramuscular | + | 4-7 | + | nd | NA4 | NA | NA | NA | NA | [200] |
| Guinea pig | Subcutaneous | ND | +5 | + | 80 d | +3 | + | ND | ND | ND | [159] |
| Guinea pig | Corneal | ND | NR6 | ND | 35 d | +7 | + | − | ND | [158] | |
| Rat | Subcutaneous | + | None | ND | 9 m | +7 | ND | + | ND | ND | [161] |
| Rat | Subcutaneous | ND | NR | ND | 30 d | ND | ND | + | + | ND | [154] |
| Rat | Subcutaneous | ND | NR | ND | 18 m | +7 | + | + | + | ND | [162] |
| Rat | Subcutaneous | + | None | − | 1-3 m | +7 | + | + | ND | [163] | |
| Rat | Intramuscular | ND | NR | ND | 1 m | + | ND | ND | ND | [201] | |
| Mouse | Subcutaneous | + | NR | ND | 4-9 d | ND | ND | ND | ND | ND | [153] |
| Mouse | Corneal | + | None | ND | 33 d | +7 | + | + | + | ND | [160] |
| SCID-hu-mouse | Skin implants | NA | NA | ND | nd | ND | ND | ND | ND | ND | [165] |
| SCID-hu-mouse | Ganglia implants | NA | NA | NA | 14 d | ND | + | + | − | ND | [166] |
hybridization
not done
dot blot
not applicable
dpi not reported
not reported
in situ.
Table reproduced from Mahalingam et al. [ref. 182] with permission from Springer.
Simian varicella virus (SVV) infection of primates as a model for human VZV infection
Virological and pathological features of SVV infection of non-human primates closely resemble those of VZV infection in humans [167-170]. Both SVV and VZV produce varicella, become latent and reactivate decades later to produce shingles (zoster) in their respective hosts. SVV and VZV belong to the alphaherpesvirus family and share 75% DNA homology. The genetic organization of the two virus genomes is similar [171]. Like VZV, SVV ORF 63 is translated in latently infected monkey ganglia [172].
VZV and SVV ORF 63 are present as duplicate copies of ORF 70 on their respective virus genomes. There are conflicting reports regarding the requirement of VZV ORF 63/70 expression for virus replication in culture [173,174]. Recently, we constructed a recombinant bacmid clone containing the complete SVV genome in which stop codons were inserted into both SVV ORFs 63 and 70. In contrast to the CPE that develops 3 days after transfection of Vero cells with wild-type SVV, a CPE did not develop until 10 days after transfection with the double mutant. Slow growth of the double mutant virus in culture indicated that while SVV ORF 63 is not required for replication in Vero cells in culture, its expression helps to produce robust virus replication (Mahalingham, unpublished observations).
Pathogenesis of SVV infection
Primary SVV infection in monkeys results in viraemia, as confirmed by the detection of virus DNA in peripheral blood MNCs [175-177]. Like disseminated VZV infection in humans, SVV infection is often disseminated, with liver and lung as the most affected organs [178]. Inflammation, haemorrhagic necrosis and eosinophilic intranuclear inclusions are seen in affected skin and viscera [175,177]. SVV also establishes latent infection in sensory ganglionic neurons at multiple levels of the neuraxis [179,180]. Natural infection of monkeys with SVV leads to establishment of latency in ganglia and reactivation after social and environmental stress [181] and after immunosuppression [182,183]. SVV is readily isolated from zoster rash [181].
Models of SVV infection in monkeys
Various models of experimental infection of monkeys with SVV have been established: (1) intratracheal inoculation of SVV into African green and Cynomologous monkeys [184,185]; (2) intrabronchial inoculation of rhesus macaques with SVV [172,186]; and (3) natural SVV infection by exposure of African green and Cynomologous monkeys to monkeys previously inoculated intratracheally [172,179,183].
In the first model, Cynomologous or African green monkeys inoculated intratracheally with SVV developed varicella rash at 7-10 days post-inoculation (dpi). SVV DNA was detected in blood MNCs at 3-11 dpi. Anti-SVV antibodies were detected 12 dpi, correlating with the resolution of varicella, and antibody titres remained stable for 4 months pi [170,176,187-191]. SVV was shown to infect ganglia before the appearance of varicella rash [85]. SVV DNA persisted in blood MNCs 7 days to 23 months pi, exclusively in T cells (CD4+, CD8+) at 23 months pi; however, virus could not recovered by cocultivation of blood MNCs with indicator cells [185]. SVV DNA and transcripts corresponding to putative immediate early (IE), early (E) and late (L) SVV genes were detected in liver, lung and ganglia at multiple intervals for 12 months [184].
Transcription of SVV ORFs 21, 29, 61 and 63 as well as antisense RNA with respect to ORF 61 was found in Vervet monkeys inoculated intratracheally multiple times with SVV [192].
In the second model, intrabronchial inoculation of rhesus macaques with SVV resulted in varicella rash followed by establishment of latency in ganglia along the entire neuraxis. Virological and immunological features of primary SVV infection and latency in these monkeys were similar, if not identical to, those of VZV infection in humans [172]. A proliferative burst of T cells was seen starting at 7 dpi and peaking at 14 dpi. Virus-specific CD4+ T cells also peaked at 14 dpi and reached stable levels at 73 dpi. Granzyme B-expressing CD4+ and CD8+ memory T cells, indicative of cytotoxic potential, appeared at 7 dpi and remained at high levels at least 28 dpi before decreasing to pre-infection levels. At 2 to 3 months after the resolution of varicella rash, SVV-specific DNA sequences were detected in ganglia, but not in liver or lung. RNA corresponding to SVV ORFs 21, 61, 62, 63 and 66 as well as antisense transcripts specific for SVV ORF 61, but not 40 (a late gene), was found in ganglia latently infected with SVV. Finally, like VZV, SVV ORF 63 protein was detected exclusively in the cytoplasm of neurons in latently infected ganglia (Figure 6).
Figure 6.
Detection of VZV and SVV ORF 63 proteins in the cytoplasm of neurons in ganglia of a human latently infected with VZV and a rhesus macaque latently infected with SVV. Paraformaldehyde-fixed, paraffin-embedded sections of thoracic ganglia from a VZV-seropositive 46-year-old man (A) and from a rhesus macaque latently infected with SVV (B) were analyzed by immunohistochemistry using rabbit anti-VZV ORF 63. Both VZV and SVV ORF 63 proteins (arrows) are located exclusively in the cytoplasm of neurons in the respective ganglia.
The latency model above has been used to examine the potential reactivation of latent varicella due to delay in sampling ganglia after death. Two rhesus macaques, inoculated intrabronchially with SVV, were euthanized 12 weeks later and tissues were processed at different times following euthanasia. Analysis of SVV-specific DNA, RNA and ORF 63 protein in ganglionic and non-ganglionic tissues from the two monkeys showed that a 30-hour delay in time after death did not affect virus gene expression or the cytoplasmic localization of SVV ORF 63 [193].
In the third model, seronegative African green and Cynomologous monkeys were exposed to cage-mates previously inoculated intratracheally with SVV. Mild varicella developed at 10-12 dpi and virus DNA was detected by PCR in skin scrapings [179,183]. SVV DNA was only occasionally detected in blood MNCs. The establishment of latent infection was confirmed by detection of virus DNA in ganglia along the neuraxis and not in lung or liver. Furthermore, latent SVV DNA was localized exclusively in ganglionic neurons [180].
Experimental reactivation of latent SVV in monkeys
Using the third model described above, latent SVV infection was established in Cynomologous monkeys after which they were exposed once to X-irradiation and treated with tacrolimus and prednisone for 4 months. In one monkey, thoracic zoster was seen at 2 weeks after immunosuppression; the presence of virus antigen in biopsies of zoster rash was confirmed by immunohistochemistry, while the detection of transcripts specific for SVV capsid proteins in ganglia as well as the detection of virus glycoproteins in both ganglia and lung confirmed the presence of reactivated SVV [183].
Recently, SVV-latently infected African green and Cynomologous monkeys were treated with tacrolimus and prednisone as well as tacrolimus with or without exposure to X-irradiation (Table 2). None of 4 African green monkeys treated with tacrolimus and prednisone developed zoster, while 3 of 4 Cynomologous monkeys developed rash at 7-14 days after treatment with tacrolimus alone; zoster also developed in 1 of 4 African green monkeys at 42 days after X-irradiation and in all 4 African green monkeys at 10-14 days after X-irradiation and beginning of tacrolimus treatment. Thus, latent SVV was experimentally reactivated after immunosuppression with tacrolimus with or without X-irradiation as confirmed by immunohistochemical detection of virus glycoproteins in punch biopsies of zoster rash, in sections of lungs, and in multiple ganglia of most of the immunosuppressed monkeys but not in control monkey tissues [186]. Virus glycoproteins were found to be better markers than nucleic acids to confirm virus reactivation in ganglia.
Table 2.
Experimental reactivation of SVV in monkeys
Subclinical SVV reactivation after irradiation of rhesus macaques latently infected with SVV resulted in disseminated varicella in another irradiated SVV-seronegative monkey in the same colony [194]. There are other reports of SVV reactivation in irradiated rhesus [195] and pig-tailed macaques [196,197]. Clearly, irradiation is an important contributing factor for SVV reactivation in non-human primates.
Monkey ganglia containing reactivated SVV await analyses of relevant components of the immune system to better understand virus pathogenesis and to develop strategies to counter disease produced by virus reactivation.
Acknowledgements
The authors’ studies cited in this Review were funded by NIH grants AG006127 to DG, AG032958 to DG, SP, RJC, and NS067070 to MAN; and Merit Review grant NEUD-004-07F from the Veterans Administration to SP.
We thank Marina Hoffman for editorial assistance and Cathy Allen for manuscript preparation.
Abbreviations
- ARN
acute retinal necrosis
- CPE
cytopathic effect
- CSF
cerebrospinal fluid
- dpi
days post-inoculation
- GeXP
genome expression system
- HSV
herpes simplex virus
- HZO
herpes zoster ophthalmicus
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- MNC
mononuclear cell
- MRI
magnetic resonance imaging
- NSC
neural stem cells
- ORF
open reading frame
- PHN
postherpetic neuralgia
- PORN
progressive outer retinal necrosis
- PCR
polymerase chain reaction
- SVV
simian varicella virus
- VZV
varicella zoster virus
Footnotes
Conflict of Interest: All authors report no conflict of interest.
References
- 1.Leppard B, Naburi AE. Herpes zoster: an early manifestation of HIV infection. Afr Health. 1998;21:5–6. [PubMed] [Google Scholar]
- 2.Kakourou T, Theodoridou M, Mostrou G, Syriopoulou V, Papadogeorgaki H, Constantopoulos A. Herpes zoster in children. J Am Acad Dermatol. 1998;39:207–10. doi: 10.1016/s0190-9622(98)70076-3. [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal DT, Salzman KL, Baringer JR, Forghani B, Gilden DH. MRI abnormalities in chronic active varicella zoster infection. Neurology. 2004;63:1538–9. doi: 10.1212/01.wnl.0000141855.67420.73. [DOI] [PubMed] [Google Scholar]
- 4.Merchet MP, Gruener G. Segmental zoster paresis of limbs. Electromyogr Clin Neurophysiol. 1996;36:369–75. [PubMed] [Google Scholar]
- 5.Yoleri O, Olmez N, Oztura I, Sengül I, Günaydin R, Memis A. Segmental zoster paresis of the upper extremity: a case report. Arch Phys Med Rehabil. 2005;86:1492–4. doi: 10.1016/j.apmr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JP, Keal EE. Cervical herpes zoster and diaphragmatic paralysis. Br J Dis Chest. 1969;63:222–6. doi: 10.1016/s0007-0971(69)80022-7. [DOI] [PubMed] [Google Scholar]
- 7.Tjandra J, Mansel RE. Segmental abdominal herpes zoster paresis. Aust N Z J Surg. 1986;56:807–8. doi: 10.1111/j.1445-2197.1986.tb02331.x. [DOI] [PubMed] [Google Scholar]
- 8.Head H, Campbell AW. The pathology of herpes zoster and its bearing on sensory localization. Brain. 1900;23:353–523. doi: 10.1002/(sici)1099-1654(199709)7:3<131::aid-rmv198>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Denny-Brown D, Adams RD, Fitzgerald PJ. Pathologic features of herpes zoster: A note on “geniculate herpes.”. Arch Neurol Psychiatry. 1944;51:216–31. [Google Scholar]
- 10.Cheatham WJ, Dolan TF, Jr, Dower JC, Weller TH. Varicella: report on two fatal cases with necropsy, virus isolation, and serologic studies. Am J Pathol. 1956;32:1015–35. [PMC free article] [PubMed] [Google Scholar]
- 11.Esiri MM, Tomlinson AH. Herpes zoster: demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J Neurol Sci. 1972;15:35–48. doi: 10.1016/0022-510x(72)90120-7. [DOI] [PubMed] [Google Scholar]
- 12.Ghatak NR, Zimmerman HM. Spinal ganglion in herpes zoster. Arch Pathol. 1973;95:411–5. [PubMed] [Google Scholar]
- 13.Nagashima K, Nakazawa M, Endo H. Pathology of the human spinal ganglia in varicella-zoster virus infection. Acta Neuropathol. 1975;33:105–17. doi: 10.1007/BF00687537. [DOI] [PubMed] [Google Scholar]
- 14.Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–9. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- 15.Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol. 2008;80:1116–22. doi: 10.1002/jmv.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers RS, III, Tindall JP. Herpes zoster in the elderly. Postgrad Med. 1971;50:153–7. doi: 10.1080/00325481.1971.11697705. [DOI] [PubMed] [Google Scholar]
- 17.Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327–31. doi: 10.1016/s0885-3924(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 18.Smith FP. Pathological studies of spinal nerve ganglia in relation to intractable intercostal pain. Surg Neurol. 1978;10:50–3. [PubMed] [Google Scholar]
- 19.Watson CPN, Deck JH, Morshead C, Van der Kooy D, Evans RJ. Postherpetic neuralgia: further post-mortem studies of cases with and without pain. Pain. 1991;44:105–17. doi: 10.1016/0304-3959(91)90124-G. [DOI] [PubMed] [Google Scholar]
- 20.Vafai A, Wellish M, Gilden DH. Expression of varicella-zoster virus in blood mononuclear cells of patients with postherpetic neuralgia. Proc Natl Acad Sci USA. 1988;85:2767–70. doi: 10.1073/pnas.85.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devlin ME, Gilden DH, Mahalingam R, Dueland AN, Cohrs R. Peripheral blood mononuclear cells of the elderly contain varicella-zoster virus DNA. J Infect Dis. 1992;165:619–22. doi: 10.1093/infdis/165.4.619. [DOI] [PubMed] [Google Scholar]
- 22.Mahalingam R, Wellish M, Brucklier J, Gilden DH. Persistence of varicella-zoster virus DNA in elderly patients with postherpetic neuralgia. J Neurovirol. 1995;1:130–3. doi: 10.3109/13550289509111018. [DOI] [PubMed] [Google Scholar]
- 23.Terada K, Niizuma T, Kawano S, Kataoka N, Akisada T, Orita Y. Detection of varicella-zoster virus DNA in peripheral mononuclear cells from patients with Ramsay Hunt syndrome or zoster sine herpete. J Med Virol. 1998;56:359–63. [PubMed] [Google Scholar]
- 24.Gilden DH, Cohrs RJ, Hayward AR, Wellish M, Mahalingam R. Chronic varicella zoster virus ganglionitis – a possible cause of postherpetic neuralgia. J Neurovirol. 2003;9:404–7. doi: 10.1080/13550280390201722. [DOI] [PubMed] [Google Scholar]
- 25.Quan D, Hammack BN, Kittelson J, Gilden DH. Improvement of postherpetic neuralgia after treatment with intravenous acyclovir followed by oral valacyclovir. Arch Neurol. 2006;63:940–2. doi: 10.1001/archneur.63.7.noc60049. [DOI] [PubMed] [Google Scholar]
- 26.Nagel MA, Cohrs RJ, Mahalingam R, Wellish MC, Forghani F, Schiller A, Safdieh JE, Kamenkovich E, Ostrow LW, Levy M, Greenberg B, Russman AN, Katzan I, Gardner CJ, Häusler M, Nau R, Saraya T, Wada H, Goto H, de Martino M, Ueno M, Brown WD, Terborg C, Gilden DH. The varicella zoster vasculopathies: clinical, CSF, imaging, and virologic features. Neurology. 2008;70:853–60. doi: 10.1212/01.wnl.0000304747.38502.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagel MA, Forghani B, Mahalingam R, Wellish MC, Cohrs RJ, Russman AN, Katzan I, Lin R, Gardner CJ, Gilden DH. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology. 2007;68:1069–73. doi: 10.1212/01.wnl.0000258549.13334.16. [DOI] [PubMed] [Google Scholar]
- 28.Burgoon MP, Hammack BN, Owens GP, Maybach AL, Eikelenboom MJ, Gilden DH. Oligoclonal immunoglobulins in cerebrospinal fluid during varicella zoster virus (VZV) vasculopathy are directed against VZV. Ann Neurol. 2003;54:459–63. doi: 10.1002/ana.10685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang JH, Ho JD, Chen YH, Lin HC. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009;40:3443–8. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 30.Lin H-C, Chien C-W, Ho J-D. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology. 2010;74:792–7. doi: 10.1212/WNL.0b013e3181d31e5c. [DOI] [PubMed] [Google Scholar]
- 31.Gilden DR, Kleinschmidt-DeMasters BK, Wellish M, Hedley-Whyte ET, Rentier B, Mahalingam R. Varicella zoster virus, a cause of waxing and waning vasculitis: the New England Journal of Medicine case 5-1995 revisited. Neurology. 1996;47:1441–6. doi: 10.1212/wnl.47.6.1441. [DOI] [PubMed] [Google Scholar]
- 32.Kleinschmidt-DeMasters BK, Gilden DH. Varicella-zoster virus infections of the nervous system: clinical and pathologic correlates. Arch Pathol Lab Med. 2001;125:770–80. doi: 10.5858/2001-125-0770-VZVIOT. [DOI] [PubMed] [Google Scholar]
- 33.Salazar R, Russman AN, Nagel MA, Cohrs RJ, Mahalingam R, Schmid DS, Kleinschmidt-DeMasters BK, VanEgmond EM, Gilden D. VZV ischemic optic neuropathy and subclinical temporal artery involvement. Arch Neurol. 2011 doi: 10.1001/archneurol.2011.64. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayberg MR, Langer RS, Zervas NT, Moskowitz MA. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science. 1981;213:228–30. doi: 10.1126/science.6166046. [DOI] [PubMed] [Google Scholar]
- 35.Mayberg MR, Zervas NT, Moscowitz MA. Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J Comp Neurol. 1984;223:46–56. doi: 10.1002/cne.902230105. [DOI] [PubMed] [Google Scholar]
- 36.Lee RM. Morphology of cerebral arteries. Pharmacol Ther. 1995;66:149–73. doi: 10.1016/0163-7258(94)00071-a. [DOI] [PubMed] [Google Scholar]
- 37.Gilden DH. Varicella zoster virus vasculopathy and disseminated encephalomyelitis. J Neurol Sci. 2002;195:99–101. doi: 10.1016/s0022-510x(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 38.Habib AA, Gilden D, Schmid DS, Safdieh JE. Varicella zoster virus meningitis with hypoglycorrhachia in the absence of rash and in an immunocompetent woman. J Neurovirol. 2009;15:206–8. doi: 10.1080/13550280902725550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein NC, McDermott B, Cunha BA. Varicella-zoster virus meningoencephalitis in an immunocompetent patient without a rash. Scand J Infect Dis. 2010;42:631–3. doi: 10.3109/00365540903510716. [DOI] [PubMed] [Google Scholar]
- 40.Gunson RN, Aitken C, Gilden D. A woman with acute headache and sacral dermatomal numbness. J Clin Virol. 2011;50:191–3. doi: 10.1016/j.jcv.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moses H, Nagel MA, Gilden DH. Acute cerebellar ataxia in a 41 year old woman. Lancet Neurol. 2006;5:984–8. doi: 10.1016/S1474-4422(06)70601-9. [DOI] [PubMed] [Google Scholar]
- 42.Ratzka P, Schlachetzki JC, Bähr M, Nau R. Varicella zoster virus cerebellitis in a 66-year-old patient without herpes zoster. Lancet. 2006;367:182. doi: 10.1016/S0140-6736(06)67967-1. [DOI] [PubMed] [Google Scholar]
- 43.Pina MA, Ara JR, Capablo JL, Omeñaca M. Myelitis and optic neuritis caused by varicella. Rev Neurol. 1997;25:1575–6. [PubMed] [Google Scholar]
- 44.Celik Y, Tabak F, Mert A, Celik AD, Aktuğlu Y. Transverse myelitis caused by varicella. Clin Neurol Neurosurg. 2001;103:260–1. doi: 10.1016/s0303-8467(01)00166-4. [DOI] [PubMed] [Google Scholar]
- 45.Gilden DH, Beinlich BR, Rubinstien EM, Stommel E, Swenson R, Rubinstein D, Mahalingam R. Varicella-zoster virus myelitis: an expanding spectrum. Neurology. 1994;44:1818–23. doi: 10.1212/wnl.44.10.1818. [DOI] [PubMed] [Google Scholar]
- 46.Devinsky O, Cho ES, Petito CK, Price RW. Herpes zoster myelitis. Brain. 1991;114:1181–96. doi: 10.1093/brain/114.3.1181. [DOI] [PubMed] [Google Scholar]
- 47.de Silva SM, Mark AS, Gilden DH, Mahalingam R, Balish M, Sandbrink F, Houff S. Zoster myelitis: improvement with antiviral therapy in two cases. Neurology. 1996;47:929–31. doi: 10.1212/wnl.47.4.929. [DOI] [PubMed] [Google Scholar]
- 48.Chua HC, Tjia H, Sitoh YY. Concurrent myelitis and Guillain-Barre syndrome after varicella infection. Int J Clin Pract. 2001;55:643–4. [PubMed] [Google Scholar]
- 49.Schvoerer E, Frechin V, Warter A, Gasser B, Jouin H, Gut JP, Stoll-Keller F. Persistent multiple pulmonary nodules in a nonimmunocompromised woman after varicella-related myelitis treated with acyclovir. J Clin Microbiol. 2003;41:4904–5. doi: 10.1128/JCM.41.10.4904-4905.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orme HT, Smith G, Nagel MA, Bert RJ, Mickelson TS, Gilden DH. VZV spinal cord infarction identified by diffusion-weighted magnetic resonance imaging (DWI) Neurology. 2007;69:398–400. doi: 10.1212/01.wnl.0000266390.27177.7b. [DOI] [PubMed] [Google Scholar]
- 51.Bonfioli AA, Eller AW. Acute retinal necrosis. Semin Ophthalmol. 2005;20:155–60. doi: 10.1080/08820530500232027. [DOI] [PubMed] [Google Scholar]
- 52.Guex-Crosier Y, Rochat C, Herbort CP. Necrotizing herpetic retinopathies. A spectrum of herpes virus-induced diseases determined by the immune state of the host. Ocul Immunol Inflamm. 1997;5:259–65. doi: 10.3109/09273949709085066. [DOI] [PubMed] [Google Scholar]
- 53.Lewis JM, Nagae Y, Tano Y. Progressive outer retinal necrosis after bone marrow transplantation. Am J Ophthalmol. 1996;122:892–5. doi: 10.1016/s0002-9394(14)70391-5. [DOI] [PubMed] [Google Scholar]
- 54.Franco-Paredes C, Bellehemeur T, Merchant A, Sanghi P, DiazGranados C, Rimland D. Aseptic meningitis and optic neuritis preceding varicella-zoster progressive outer retinal necrosis in a patient with AIDS. AIDS. 2002;16:1045–9. doi: 10.1097/00002030-200205030-00011. [DOI] [PubMed] [Google Scholar]
- 55.Menerath JM, Gerard M, Laurichesse H, Goldschmidt P, Peigue-Lafeuille H, Rozenberg F, Beytout J. Bilateral acute retinal necrosis in a patient with acquired immunodeficiency syndrome. J Fr Ophtalmol. 1995;18:625–33. [PubMed] [Google Scholar]
- 56.Johnston WH, Holland GN, Engstrom RE, Jr, Rimmer S. Recurrence of presumed varicella-zoster virus retinopathy in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993;116:42–50. doi: 10.1016/s0002-9394(14)71742-8. [DOI] [PubMed] [Google Scholar]
- 57.Moorthy RS, Weinberg DV, Teich SA, Berger BB, Mintum JT, Kumar S, Rao NA, Fowell SM, Loose IA, Jampol LM. Management of varicella zoster virus retinitis in AIDS. Br J Ophthalmol. 1997;81:189–94. doi: 10.1136/bjo.81.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Austin RB. Progressive outer retinal necrosis syndrome: a comprehensive review of its clinical presentation, relationship to immune system status, and management. Clin Eye Vis Care. 2000;12:119–29. doi: 10.1016/s0953-4431(00)00052-7. [DOI] [PubMed] [Google Scholar]
- 59.Lewis GW. Zoster sine herpete. Br Med J. 1958;2:418–21. doi: 10.1136/bmj.2.5093.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilden DH, Wright RR, Schneck SA, Gwaltney JM, Jr, Mahalingam R. Zoster sine herpete, a clinical variant. Ann Neurol. 1994;35:530–3. doi: 10.1002/ana.410350505. [DOI] [PubMed] [Google Scholar]
- 61.Amlie-Lefond C, Mackin GA, Ferguson M, Wright RR, Mahalingam R, Gilden DH. Another case of virologically confirmed zoster sine herpete, with electrophysiologic correlation. J NeuroVirol. 1996;2:136–8. doi: 10.3109/13550289609146547. [DOI] [PubMed] [Google Scholar]
- 62.Blumenthal DT, Shacham-Shmueli E, Bokstein F, Schmid DS, Cohrs RJ, Nagel MA, Mahalingam R, Gilden D. Zoster sine herpete: virological verification by detection of anti-VZV IgG antibody in CSF. Neurology. 2011;76:484–5. doi: 10.1212/WNL.0b013e31820a0d28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hevner RF, Vilela MD, Rostomily RC, Cohrs RJ, Mahalingam R, Wellish M, Gilden DH. An unusual cause of trigeminal-distribution pain and tumour. Lancet Neurol. 2003;2:567–72. doi: 10.1016/s1474-4422(03)00506-4. [DOI] [PubMed] [Google Scholar]
- 64.Haug A, Mahalingam R, Cohrs RJ, Schmid DS, Corboy JR, Gilden D. Recurrent polymorphonuclear pleocytosis with increased red blood cells caused by varicella zoster virus infection of the central nervous system. J Neurol Sci. 2010;292:85–8. doi: 10.1016/j.jns.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davison AJ, Scott JE. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 66.Preston VG, Kennard J, Rixon FJ, Logan AJ, Mansfield RW, McDougall IM. Efficient herpes simplex virus type 1 (HSV-1) capsid formation directed by the varicella-zoster virus scaffolding protein requires the carboxy-terminal sequences from the HSV-1 homologue. J Gen Virol. 1997;78:1633–46. doi: 10.1099/0022-1317-78-7-1633. [DOI] [PubMed] [Google Scholar]
- 67.Ross J, Williams M, Cohen JI. Disruption of the varicella-zoster virus dUTPase and the adjacent ORF9A gene results in impaired growth and reduced syncytia formation in vitro. Virology. 1997;234:186–95. doi: 10.1006/viro.1997.8652. [DOI] [PubMed] [Google Scholar]
- 68.Kemble GW, Annuziato P, Lungu O, Winter RE, Cha TA, Silverstein SJ, Spaete RR. Open reading frame S/L of varicella-zoster virus encodes a cytoplasmic protein expressed in infected cells. J Virol. 2000;74:11311–21. doi: 10.1128/jvi.74.23.11311-11321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu M, Vafai N, Liu A, Hart J, Liu H, He J, Tang X, Wang D, Vafai A. Stability of varicella-zoster virus open reading frame 63. Arch Virol. 2008;153:1943–7. doi: 10.1007/s00705-008-0197-4. [DOI] [PubMed] [Google Scholar]
- 70.Hawrami K, Harper D, Breuer J. Typing of varicella zoster virus by amplification of DNA polymorphisms. J Virol Methods. 1996;57:169–74. doi: 10.1016/0166-0934(95)01981-2. [DOI] [PubMed] [Google Scholar]
- 71.Breuer J. VZV molecular epidemiology. Curr Top Microbiol Immunol. 2010;342:15–42. doi: 10.1007/82_2010_9. [DOI] [PubMed] [Google Scholar]
- 72.Abendroth A, Lin I, Slobedman B, Ploegh H, Arvin AM. Varicella-zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells. J Virol. 2001;75:4878–88. doi: 10.1128/JVI.75.10.4878-4888.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morrow G, Slobedman B, Cunningham AL, Abendroth A. Varicella-zoster virus productively infects mature dendritic cells and alters their immune function. J Virol. 2003;77:4950–9. doi: 10.1128/JVI.77.8.4950-4959.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor SL, Moffat JF. Replication of varicella-zoster virus in human skin organ culture. J Virol. 2005;79:11501–6. doi: 10.1128/JVI.79.17.11501-11506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Desloges N, Schubert C, Wolff MH, Rahaus M. Varicella-zoster virus infection induces the secretion of interleukin-8. Med Microbiol Immunol. 2008;197:277–84. doi: 10.1007/s00430-007-0060-3. [DOI] [PubMed] [Google Scholar]
- 76.Huch JH, Cunningham AL, Arvin AM, Nasr N, Santegoets SJ, Slobedman E, Slobedman B, Abendroth A. Impact of varicella-zoster virus on dendritic cell subsets in human skin during natural infection. J Virol. 2010;84:4060–72. doi: 10.1128/JVI.01450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bearer EL, Breakefield XO, Schuback D, Reese TS, LaVail JH. Retrograde axonal transport of herpes simplex virus: evidence for a single mechanism and a role for tegument. Proc Natl Acad Sci USA. 2000;97:8146–50. doi: 10.1073/pnas.97.14.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antinone SE, Smith GA. Retrograde axon transport of herpes simplex virus and pseudorabies virus: a live-cell comparative analysis. J Virol. 2010;84:1504–12. doi: 10.1128/JVI.02029-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gilden DH, Vafai A, Shtram Y, Becker Y, Devlin M, Wellish M. Varicella-zoster virus DNA in human sensory ganglia. Nature. 1983;306:478–80. doi: 10.1038/306478a0. [DOI] [PubMed] [Google Scholar]
- 80.Mahalingam R, Wellish MC, Dueland AN, Cohrs RJ, Gilden DH. Localization of herpes simplex virus and varicella zoster virus DNA in human ganglia. Ann Neurol. 1992;31:444–8. doi: 10.1002/ana.410310417. [DOI] [PubMed] [Google Scholar]
- 81.Richter ER, Dias JK, Gilbert JE, Atherton SS. Distribution of herpes simplex virus type 1 and varicella zoster virus in ganglia of the human head and neck. J Infect Dis. 2009;200:1901–6. doi: 10.1086/648474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mainka C, Fuss B, Geiger H, Hofelmayr H, Wolff MH. Characterization of viremia at different stages of varicella-zoster virus infection. J Med Virol. 1998;56:9198. doi: 10.1002/(sici)1096-9071(199809)56:1<91::aid-jmv15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 83.de Jong MD, Weel JF, Schuurman T, Wertheim-van Dillen PM, Boom R. Quantitation of varicella-zoster virus DNA in whole blood, plasma, and serum by PCR and electrochemiluminescence. J Clin Microbiol. 2000;38:2568–73. doi: 10.1128/jcm.38.7.2568-2573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taha Y, Scott FT, Parker SP, Court D Syndercombe, Quinlivan ML, Breuer J. Reactivation of 2 genetically distinct varicella-zoster viruses in the same individual. Clin Infect Dis. 2006;43:1301–2. doi: 10.1086/508539. [DOI] [PubMed] [Google Scholar]
- 85.Mahalingam R, Wellish M, Soike K, White T, Kleinschmidt-DeMasters BK, Gilden DH. Simian varicella virus infects ganglia before rash in experimentally infected monkeys. Virology. 2001;279:339–42. doi: 10.1006/viro.2000.0700. [DOI] [PubMed] [Google Scholar]
- 86.Sadzot-Delvaux C, Thonard P, Schoonbroodt S, Piette J, Rentier B. Varicella-zoster virus induces apoptosis in cell culture. J. Gen. Virol. 1995;76:2875–9. doi: 10.1099/0022-1317-76-11-2875. [DOI] [PubMed] [Google Scholar]
- 87.Pignata C, Fiore M, de Filippo S, Cavalcanti M, Gaetaniello L, Scotese I. Apoptosis as a mechanism of peripheral blood mononuclear cell death after measles and varicella-zoster virus infections in children. Pediatr Res. 1998;43:77–83. doi: 10.1203/00006450-199801000-00012. [DOI] [PubMed] [Google Scholar]
- 88.König A, Hömme C, Hauröder B, Dietrich A, Wolff MH. The varicella-zoster virus induces apoptosis in vitro in subpopulations of primary human peripheral blood mononuclear cells. Microbes Infect. 2003;4:879–89. doi: 10.1016/s1286-4579(03)00177-1. [DOI] [PubMed] [Google Scholar]
- 89.Pugazhenthi S, Gilden DH, Nair S, McAdoo A, Wellish M, Brazeau E, Mahalingam R. Simian varicella virus induces apoptosis in monkey kidney cells by the intrinsic pathway and involves downregulation of Bcl-2 expression. J Virol. 2009;83:9273–82. doi: 10.1128/JVI.00768-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brazeau E, Mahalingam R, Gilden D, Wellish M, Kaufer BB, Osterrieder N, Pugazhenthi S. Varicella-zoster virus–induced apoptosis in MeWo cells is accompanied by down-regulation of Bcl-2 expression. J Neurovirol. 2010;16:133–140. doi: 10.3109/13550281003682547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hood C, Cunningham AL, Slobedman B, Boadle RA, Abendroth A. Varicella-zoster virus-infected human sensory neurons are resistant to apoptosis, yet human foreskin fibroblasts are susceptible: evidence for a cell-type-specific apoptotic response. J Virol. 2003;77:12852–64. doi: 10.1128/JVI.77.23.12852-12864.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hyman RW, Ecker JR, Tenser RB. Varicella-zoster virus RNA in human trigeminal ganglia. Lancet. 1983;2:814–6. doi: 10.1016/s0140-6736(83)90736-5. [DOI] [PubMed] [Google Scholar]
- 93.Gilden DH, Rozenman Y, Murray R, Devlin M, Vafai A. Detection of varicella-zoster virus nucleic acid in neurons of normal human thoracic ganglia. Ann Neurol. 1987;22:377–80. doi: 10.1002/ana.410220315. [DOI] [PubMed] [Google Scholar]
- 94.Kennedy PG, Grinfeld E, Gow JW. Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc Natl Acad Sci USA. 1998;95:4658–62. doi: 10.1073/pnas.95.8.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.LaGuardia JJ, Cohrs RJ, Gilden DH. Prevalence of varicella-zoster virus DNA in dissociated human trigeminal ganglion neurons and nonneuronal cells. J Virol. 1999;73:8571–7. doi: 10.1128/jvi.73.10.8571-8577.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Levin MJ, Cai GY, Manchak MD, Pizer LI. Varicella-zoster virus DNA in cells isolated from human trigeminal ganglia. J Virol. 2003;77:6979–87. doi: 10.1128/JVI.77.12.6979-6987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang K, Lau TY, Morales M, Mont EK, Straus SE. Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal ganglia at the single-cell level. J Virol. 2005;79:14079–87. doi: 10.1128/JVI.79.22.14079-14087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liedtke W, Opalka B, Zimmermann CW, Lignitz E. Age distribution of latent herpes simplex virus 1 and varicella-zoster virus genome in human nervous tissue. J Neurol Sci. 1993;116:6–11. doi: 10.1016/0022-510x(93)90082-a. [DOI] [PubMed] [Google Scholar]
- 99.Furuta Y, Takasu T, Fukuda S, Sato-Matsumura KC, Inuyama Y, Hondo R, Nagashima K. Detection of varicella-zoster virus DNA in human geniculate ganglia by polymerase chain reaction. J Infect Dis. 1992;166:11579–9. doi: 10.1093/infdis/166.5.1157. [DOI] [PubMed] [Google Scholar]
- 100.Pevenstein SR, Williams RK, McChesney D, Mont EK, Smialek JE, Straus SE. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol. 1999;73:10514–8. doi: 10.1128/jvi.73.12.10514-10518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cohrs RJ, Randall J, Smith J, Gilden DH, Dabrowski C, van der Keyl H, Tal-Singer R. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J Virol. 2000;74:11464–71. doi: 10.1128/jvi.74.24.11464-11471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hufner K, Derfuss T, Herberger S, Sunami K, Russell S, Sinicina I, Arbusow V, Strupp M, Brandt T, Theil D. Latency of alpha-herpes viruses is accompanied by a chronic inflammation in human trigeminal ganglia but not in dorsal root ganglia. J Neuropathol Exp Neurol. 2006;65:1022–30. doi: 10.1097/01.jnen.0000235852.92963.bf. [DOI] [PubMed] [Google Scholar]
- 103.Inoue H, Motani-Saitoh H, Sakurada K, Ikegaya H, Yajima D, Jayakawa M, Sato Y, Otsuka K, Kobayashi K, Nagasawa S, Iwase H. Detection of varicella-zoster virus DNA in 414 human trigeminal ganglia from cadavers by the polymerase chain reaction: a comparison of the detection rate of varicella-zoster virus and herpes simplex virus type 1. J Med Virol. 2010;82:345–9. doi: 10.1002/jmv.21687. [DOI] [PubMed] [Google Scholar]
- 104.Mahalingam R, Wellish M, Lederer D, Forghani B, Cohrs R, Gilden DH. Quantitation of latent varicella-zoster virus DNA in human trigeminal ganglia by polymerase chain reaction. J Virol. 1993;67:2381–4. doi: 10.1128/jvi.67.4.2381-2384.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dueland AN, Ranneberg-Nilsen T, Degré M. Detection of latent varicella zoster virus DNA and human gene sequences in human trigeminal ganglia by in situ amplification combined with in situ hybridization. Arch Virol. 1995;140:2055–66. doi: 10.1007/BF01322692. [DOI] [PubMed] [Google Scholar]
- 106.Cohrs RJ, Srock K, Barbour MB, Owens G, Mahalingam R, Devlin ME, Wellish M, Gilden DH. Varicella-zoster virus (VZV) transcription during latency in human ganglia: construction of a cDNA library from latently infected human trigeminal ganglia and detection of a VZV transcript. J Virol. 1994;68:7900–7908. doi: 10.1128/jvi.68.12.7900-7908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cohrs RJ, Barbour M, Gilden DH. Varicella-zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in cDNA library enriched for VZV RNA. J Virol. 1996;70:2789–96. doi: 10.1128/jvi.70.5.2789-2796.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cohrs RJ, Gilden DH, Kinchington PR, Grinfeld E, Kennedy PGE. Varicella-zoster virus gene 66 transcription and translation in latently infected human ganglia. J Virol. 2003;77:6660–5. doi: 10.1128/JVI.77.12.6660-6665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cohrs RJ, Gilden DH. Prevalence and abundance of latently transcribed varicella zoster virus genes in human ganglia. J Virol. 2007;81:2950–6. doi: 10.1128/JVI.02745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kennedy PGE, Grinfeld E, Bell JE. Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J Virol. 2000;74:11893–8. doi: 10.1128/jvi.74.24.11893-11898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sommer SS, Cohen JE. The size distributions of proteins, mRNA, and nuclear RNA. J Mol Evol. 1980;15:37–57. doi: 10.1007/BF01732582. [DOI] [PubMed] [Google Scholar]
- 112.Vansant G, Pezzoli P, Saiz R, Birch A, Duffy C, Ferre F, Monforte J. Gene expression analysis of troglitazone reveals its impact on multiple pathways in cell culture: a case for in vitro platforms combined with gene expression analysis for early (idiosyncratic) toxicity screening. Int J Toxicol. 2006;25:85–94. doi: 10.1080/10915810600605690. [DOI] [PubMed] [Google Scholar]
- 113.Rai AJ, Kamath RM, Gerald W, Fleisher M. Analytical validation of the GeXP analyzer and design of a workflow for cancer-biomarker discovery using multiplexed gene-expression profiling. Anal Bioanal Chem. 2009;393:1505–11. doi: 10.1007/s00216-008-2436-7. [DOI] [PubMed] [Google Scholar]
- 114.Nagel MA, Gilden D, Shade T, Gao B, Cohrs RJ. Rapid and sensitive detection of 68 unique varicella zoster virus gene transcripts in five multiplex reverse transcription-polymerase chain reactions. J Virol Methods. 2009;157:62–8. doi: 10.1016/j.jviromet.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nagel MA, Choe A, Traktinskiy I, Cordery-Cotter R, Gilden D, Cohrs RJ. Varicella-zoster virus transcriptome in latently infected human ganglia. J Virol. 2011;85:2276–8. doi: 10.1128/JVI.01862-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stevens JG, Wagner EK, vi-Rao GB, Cook ML, Feldman LT. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987;235:1056–9. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 117.Mahalingam R, Wellish M, Cohrs R, Debrus S, Piette J, Rentier B, Gilden DH. Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc Natl Acad Sci USA. 1996;93:2122–4. doi: 10.1073/pnas.93.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lungu O, Panagiotidis CA, Annunziato PW, Gershon AA, Silverstein SJ. Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc Natl Acad Sci USA. 1998;95:7080–5. doi: 10.1073/pnas.95.12.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cohrs RJ, Hurley MP, Gilden DH. Array analysis of viral gene transcription during lytic infection of cells in tissue culture with varicella zoster virus. J Virol. 2003;77:11718–32. doi: 10.1128/JVI.77.21.11718-11732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Theil D, Derfus T, Paripovic I, Herberger S, Meini E, Schueler O, Strupp M, Arbusow V, Brandt T. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol. 2003;163:2179–84. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grinfeld E, Kennedy PG. Translation of varicella-zoster virus genes during human ganglionic latency. Virus Genes. 2004;29:317–9. doi: 10.1007/s11262-004-7434-z. [DOI] [PubMed] [Google Scholar]
- 122.Zerboni L, Sobel RA, Ramachandran V, Rajamani J, Ruyechan W, Abendroth A, Arvin A. Expression of varicella-zoster virus immediate-early regulatory protein IE63 in neurons of latently infected human sensory ganglia. J Virol. 2010;84:3421–30. doi: 10.1128/JVI.02416-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gary L, Gilden DH, Cohrs RJ. Epigenetic regulation of varicella-zoster virus open reading frames 62 and 63 in latently infected human trigeminal ganglia. J Virol. 2006;80:4921–6. doi: 10.1128/JVI.80.10.4921-4926.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kubat NJ, Amelio AL, Giordani NV, Bloom DC. The herpes simplex virus type 1 latency-associated transcript (LAT) enhancer/rcr is hyperacetylated during latency independently of LAT transcription. J Virol. 2004;78:12508–18. doi: 10.1128/JVI.78.22.12508-12518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cui C, Griffiths A, Li G, Silva LM, Kramer MF, Gaasterland T, Wang XJ, Coen DM. Prediction and identification of herpes simplex virus 1-encoded microRNAs. J Virol. 2006;80:5499–508. doi: 10.1128/JVI.00200-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Umbach JL, Nagel MA, Cohrs RJ, Gilden DH, Cullen BR. Analysis of human alphaherpesvirus microRNA expression in latently infected human trigeminal ganglia. J Virol. 2009;83:10677–83. doi: 10.1128/JVI.01185-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Umbach JL, Wang K, Tang S, Krause PR, Mont EK, Cohen JI, Cullen BR. Identification of viral microRNAs expressed in human sacral ganglia latently infected with herpes simplex virus 2. J Virol. 2010;84:1189–92. doi: 10.1128/JVI.01712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kumagai T, Chiba Y, Wataya Y, Hanazono H, Chiba S, Nakao T. Development and characteristics of the cellular immune response to infection with varicella-zoster virus. J Infect Dis. 1980;141:7–13. doi: 10.1093/infdis/141.1.7. [DOI] [PubMed] [Google Scholar]
- 129.Arvin AM, Koropchak CM, Williams BR, Grumet FC, Foung SK. Early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J Infect Dis. 1986;154:422–9. doi: 10.1093/infdis/154.3.422. [DOI] [PubMed] [Google Scholar]
- 130.Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, Irwin MR, Clair J, Smith JG, Stanley H, Marchese RD, Harbecke R, Williams HM, Chan IS, Arbeit RD, Gershon AA, Schödel F, Morrison VA, Kauffman Ca, Straus SE, Schmader KE, Davis LE, Levin MJ, US Department of Veterans Affairs (VA) Cooperative Studies Program Shingles Prevention Study Investigators Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200:1068–77. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Good RA, Zak SJ. Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics. 1956;18:109–49. [PubMed] [Google Scholar]
- 132.Gershon AA, Steinberg SP. Cellular and humoral immune responses to varicella-zoster virus in immunocompromised patients during and after varicella-zoster infections. Infect Immun. 1979;26:170–4. doi: 10.1128/iai.25.1.170-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buchbinder SP, Katz MH, Hessol NA, Liu JY, O’Malley PM, Underwood R, Holmberg SD. Herpes oster and human immunodeficiency virus infection. J Infect Dis. 1992;166:1153–6. doi: 10.1093/infdis/166.5.1153. [DOI] [PubMed] [Google Scholar]
- 134.Hata A, Asanuma H, Rinki M, Sharp M, Wong RM, Blume K, Arvin AM. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med. 2002;347:26–34. doi: 10.1056/NEJMoa013441. [DOI] [PubMed] [Google Scholar]
- 135.Burke BL, Steele RW, Beard Ow, Wood JS, Cain TD, Marmer DJ. Immune responses to varicella-zoster in the aged. Arch Intern Med. 1982;142:291–3. [PubMed] [Google Scholar]
- 136.Patterson-Bartlett J, Levin MJ, Lang N, Schödel FP, Vessey R, Weinberg A. Phenotypic and functional characterization of ex vivo T cell responses to the live attenuated herpes zoster vaccine. Vaccine. 2007;25:7087–93. doi: 10.1016/j.vaccine.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 137.Dolin R, Reichman RC, Mazur MH, Whitley RJ. NIH conference. Herpes zoster-varicella infections in immunosuppressed patients. Ann Intern Med. 1978;89:375–88. doi: 10.7326/0003-4819-89-3-375. [DOI] [PubMed] [Google Scholar]
- 138.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW, Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL, Shingles Prevention Study Group A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 139.Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci USA. 2007;104:3496–501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gowrishankar K, Steain M, Cunningham AL, Rodriguez M, Blumbergs P, Slobedman B, Abendroth A. Characterization of the host immune response in human ganglia after herpes zoster. J Virol. 2010;84:8861–70. doi: 10.1128/JVI.01020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cohen JI. Infection of cells with varicella-zoster virus down-regulates surface expression of class I major histocompatibility complex antigens. J Infect Dis. 1998;177:1390–3. doi: 10.1086/517821. [DOI] [PubMed] [Google Scholar]
- 142.Eisfeld AJ, Yee MB, Erazo A, Abendroth A, Kinchington PR. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J Virol. 2007;81:9034–49. doi: 10.1128/JVI.00711-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Steain M, Gowrishankar K, Rodriguez M, Slobedman B, Abendroth A. Upregulation of CXCL10 in human dorsal root ganglia during experimental and natural varicella zoster virus infection. J Virol. 2010;85:626–31. doi: 10.1128/JVI.01816-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Frey CR, Sharp MA, Min AS, Schmid DS, Loparev V, Arvin AM. Identification of CD8+ T cell epitopes in the immediate early 62 protein (IE62) of varicella-zoster virus, and evaluation of frequency of CD8+ T cell response to IE62, by use of IE62 peptides after varicella vaccination. J Infect Dis. 2003;182:40–52. doi: 10.1086/375828. [DOI] [PubMed] [Google Scholar]
- 145.Hayward AR, Zerbe GO, Levin MJ. Clinical application of responder cell frequency estimates with four years of follow up. J Immunol Methods. 1994;170:27–36. doi: 10.1016/0022-1759(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 146.Levin MJ, Barber D, Goldblatt E, Jones M, LaFleur B, Chan C, Stinson D, Zerbe GO, Hayward AW. Use of a live attenuated varicella vaccine to boost varicella-specific immune responses in seropositive people 55 years of age and older: duration of booster effect. J Infect Dis. 1998;178(Suppl 1):S109–12. doi: 10.1086/514264. [DOI] [PubMed] [Google Scholar]
- 147.Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, Chan IS, Williams H, Harbecke R, Marchese R, Straus SE, Gershon A, Weinberg A, Veterans Affairs Cooperative Studies Program Shingles Prevention Study Investigators Varicella-zoster virus-specific immune responses in elderly recipients of herpes zoster vaccine. J Infect Dis. 2008;197:825–35. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341–57. doi: 10.1007/82_2010_31. [DOI] [PubMed] [Google Scholar]
- 149.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57(RR-5):1–30. etc. [PubMed] [Google Scholar]
- 150.Redman RL, Nader S, Zerboni L, Liu C, Wong RM, Brown BW, Arvin AM. Early reconstitution of immunity and decreased severity of herpes zoster in bone marrow transplant recipients immunized with inactivated varicella vaccine. J Infect Dis. 1997;176:578–85. doi: 10.1086/514077. [DOI] [PubMed] [Google Scholar]
- 151.Lu P-J, Euler GL, Harpaz R. Herpes zoster vaccination among adults aged 60 years or older, in the U.S., 2008. Am J Prev Med. 2011;40:e1–e6. doi: 10.1016/j.amepre.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 152.Dobson R. Shingles vaccination is likely to be cost effective at age 65 or 70. BMJ. 2009;338:b944. doi: 10.1136/bmj.b944. [DOI] [PubMed] [Google Scholar]
- 153.Wroblewska Z, Devlin M, Reilly K, van Trieste H, Wellish M, Gilden DH. The production of varicella zoster virus antiserum in laboratory animals. Arch Virol. 1982;74:233–8. doi: 10.1007/BF01314717. [DOI] [PubMed] [Google Scholar]
- 154.Debrus S, Sadzot-Delvaux C, Nikkels AF, Piette J, Rentier B. Varicella-zoster virus gene 63 encodes an immediate-early protein that is abundantly expressed during latency. J Virol. 1995;69:3240–5. doi: 10.1128/jvi.69.5.3240-3245.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Myers MG, Duer HL, Hausler CK. Experimental infection of guinea pigs with varicella-zoster virus. J Infect Dis. 1980;142:414–20. doi: 10.1093/infdis/142.3.414. [DOI] [PubMed] [Google Scholar]
- 156.Matsunaga Y, Yamanishi K, Takahashi M. Experimental infection and immune response of guinea pigs with varicella-zoster virus. Infect Immun. 1982;37:407–12. doi: 10.1128/iai.37.2.407-412.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Walz-Cicconi MA, Rose RM, Dammin GJ, Weller TH. Inoculation of guinea pigs with varicella-zoster virus via the respiratory route. Arch Virol. 1986;88:265–77. doi: 10.1007/BF01310880. [DOI] [PubMed] [Google Scholar]
- 158.Tenser RB, Hyman RW. Latent herpesvirus infections of neurons in guinea pigs and humans. Yale J Biol Med. 1987;60:159–67. [PMC free article] [PubMed] [Google Scholar]
- 159.Lowry PW, Sabella C, Koropchak CM, Watson BN, Thackray HM, Abbruzzi GM, Arvin AM. Investigation of the pathogenesis of varicella-zoster virus infection in guinea pigs by using polymerase chain reaction. J. Infect. Dis. 1993;167:78–83. doi: 10.1093/infdis/167.1.78. [DOI] [PubMed] [Google Scholar]
- 160.Wroblewska Z, Valyi-Nagy T, Otte J, Dillner A, Jackson A, Sole DP, Fraser NW. A mouse model for varicella-zoster virus latency. Microb Pathog. 1993;15:141–51. doi: 10.1006/mpat.1993.1064. [DOI] [PubMed] [Google Scholar]
- 161.Sadzot-Delvaux C, Merville-Louis MP, Delree P, Marc P, Piette J, Moonen G, Rentier B. An in vivo model of varicella-zoster virus latent infection of dorsal root ganglia. J Neurosci Res. 1990;26:83–9. doi: 10.1002/jnr.490260110. [DOI] [PubMed] [Google Scholar]
- 162.Kennedy PG, Grinfeld E, Bontems S, Sadzot-Delvaux C. Varicella-zoster virus gene expression in latently infected rat dorsal root ganglia. Virology. 2001;289:218–23. doi: 10.1006/viro.2001.1173. [DOI] [PubMed] [Google Scholar]
- 163.Annunziato P, LaRussa P, Lee P, Steinberg S, Lungu O, Gershon AA, Silverstein S. Evidence of latent varicella-zoster virus in rat dorsal root ganglia. J Infect Dis. 1998;178(Suppl 1):S48–51. doi: 10.1086/514261. [DOI] [PubMed] [Google Scholar]
- 164.Cohen JI, Moskal T, Shapiro M, Purcell RH. Varicella in chimpanzees. J Med Virol. 1996;50:289–92. doi: 10.1002/(SICI)1096-9071(199612)50:4<289::AID-JMV2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 165.Moffat JF, Stein MD, Kaneshima H, Arvin AM. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J Virol. 1995;69:5236–42. doi: 10.1128/jvi.69.9.5236-5242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zerboni L, Ku CC, Jones CD, Zehnder JL, Arvin AM. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc Natl Acad Sci USA. 2005;102:6490–5. doi: 10.1073/pnas.0501045102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Felsenfeld AD, Schmidt NJ. Antigenic relationships among several simian varicella-like viruses and varicella-zoster virus. Infect Immun. 1977;15:807–12. doi: 10.1128/iai.15.3.807-812.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Padovan D, Cantrell CA. Varicella-like herpesvirus infections of nonhuman primates. Lab Anim Sci. 1986;36:7–13. [PubMed] [Google Scholar]
- 169.Clarke P, Rabkin SD, Inman MV, Mahalingam R, Cohrs R, Wellish M, Gilden DH. Molecular analysis of simian varicella virus DNA. Virology. 1992;190:597–605. doi: 10.1016/0042-6822(92)90897-x. [DOI] [PubMed] [Google Scholar]
- 170.Dueland AN, Martin JR, Devlin ME, Wellish M, Mahalingam R, Cohrs R, Soike KF, Gilden DH. Acute simian varicella infection: Clinical, laboratory, pathologic, and virologic features. Lab Invest. 1992;66:762–73. [PubMed] [Google Scholar]
- 171.Gray WL, Starnes B, White MW, Mahalingam R. The DNA sequence of the simian varicella virus genome. Virology. 2001;284:123–30. doi: 10.1006/viro.2001.0912. [DOI] [PubMed] [Google Scholar]
- 172.Messaoudi I, Barron A, Wellish M, Engelmann F, Legasse A, Planer S, Gilden D, Nikolich-Zugich J, Mahalingam R. Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans. PLoS Pathog. 2009;5:e1000657. doi: 10.1371/journal.ppat.1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sommer MH, Zagha E, Serrano OK, Ku CC, Zerboni L, Baiker A, Santos R, Spengler M, Lynch J, Grose C, Ruyechan W, Hay J, Arvin AM. Mutational analysis of the repeated open reading frames, ORFs 63 and 70 and ORFs 64 and 69, of varicella-zoster virus. J Virol. 2001;75:8224–39. doi: 10.1128/JVI.75.17.8224-8239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cohen JI, Cox E, Pesnicak L, Srinivas S, Krogmann T. The varicella-zoster virus open reading frame 63 latency-associated protein is critical for establishment of latency. J Virol. 2004;78:11833–40. doi: 10.1128/JVI.78.21.11833-11840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Clarkson MJ, Thorpe E, McCarthy K. A virus disease of captive vervet monkeys (Cercopithecus aethiops) caused by a new herpesvirus. Arch Gesamte Virusforsch. 1967;22:219–34. doi: 10.1007/BF01240517. [DOI] [PubMed] [Google Scholar]
- 176.Soike KF, Baskin G, Cantrell C, Gerone P. Investigation of antiviral activity of 1-beta-D-arabinofuranosylthymine (ara-T) and 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl)uracil (BV-ara-U) in monkeys infected with simian varicella virus. Antiviral Res. 1984;4:245–57. doi: 10.1016/0166-3542(84)90030-5. [DOI] [PubMed] [Google Scholar]
- 177.Wolf RH, Smetana HF, Allen WP, Felsenfeld AD. Pathology and clinical history of Delta herpesvirus infection in patas monkeys. Lab Anim Sci. 1974;24:218–21. [PubMed] [Google Scholar]
- 178.Roberts ED, Baskin GB, Soike K, Gibson SV. Pathologic changes of experimental simian varicella (Delta herpesvirus) infection in African green monkeys (Cercopithecus aethiops) Am J Vet Res. 1984;45:523–30. [PubMed] [Google Scholar]
- 179.Mahalingam R, Traina-Dorge V, Wellish M, Smith J, Gilden DH. Naturally acquired simian varicella virus infection in African green monkeys. J Virol. 2002;76:8548–50. doi: 10.1128/JVI.76.17.8548-8550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kennedy PGE, Grinfeld E, Traina-Dorge V, Gilden DH, Mahalingam R. Neuronal localization of simian varicella virus DNA in ganglia of naturally infected African green monkeys. Virus Genes. 2004;28:273–6. doi: 10.1023/b:viru.0000025774.19557.39. [DOI] [PubMed] [Google Scholar]
- 181.Soike KF, Rangan SR, Gerone PJ. Viral disease models in primates. Adv Vet Sci Comp Med. 1984;28:151–99. doi: 10.1016/b978-0-12-039228-5.50011-5. [DOI] [PubMed] [Google Scholar]
- 182.Mahalingam R, Messaoudi I, Gilden D. Simian varicella virus pathogenesis. Curr Top Microbiol Immunol. 2010;342:309–21. doi: 10.1007/82_2009_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mahalingam R, Traina-Dorge V, Wellish M, Lorino R, Sanford R, Ribka EP, Alleman SJ, Brazeau E, Gilden DH. Simian varicella virus reactivation in Cynomologous monkeys. Virology. 2007;368:50–9. doi: 10.1016/j.virol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 184.White TM, Mahalingam R, Traina-Dorge V, Gilden DH. Simian varicella virus DNA is present and transcribed months after experimental infection of adult African green monkeys. J Neurovirol. 2002;8:191–203. doi: 10.1080/13550280290049705. [DOI] [PubMed] [Google Scholar]
- 185.White TM, Mahalingam R, Traina-Dorge V, Gilden DH. Persistence of simian varicella virus DNA in CD4(+) and CD8(+) blood mononuclear cells for years after intratracheal inoculation of African green monkeys. Virology. 2002;303:192–8. doi: 10.1006/viro.2002.1664. [DOI] [PubMed] [Google Scholar]
- 186.Mahalingam R, Traina-Dorge V, Wellish M, Deharo E, Singletary ML, Ribka EP, Sanford R, Gilden D. Latent simian varicella virus reactivates in monkeys treated with tacrolimus with or without exposure to irradiation. J Neurovirol. 2010;16:342–54. doi: 10.3109/13550284.2010.513031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wenner HA, Abel D, Barrick S, Seshumurty P. Clinical and pathogenetic studies of Medical Lake macaque virus infections in Cynomolgus monkeys (simian varicella) J Infect Dis. 1977;135:611–22. doi: 10.1093/infdis/135.4.611. [DOI] [PubMed] [Google Scholar]
- 188.Iltis JP, Arrons MC, Castellano GA, Madden DL, Sever JL, Curfman BL, London WT. Simian varicella virus (delta herpesvirus) infection of patas monkeys leading to pneumonia and encephalitis. Proc Soc Exp Biol Med. 1982;169:266–79. doi: 10.3181/00379727-169-41342. [DOI] [PubMed] [Google Scholar]
- 189.Gray WL, Gusick NJ, Fletcher TM, Soike KF. Simian varicella virus antibody response in experimental infection of African green monkeys. J Med Primatol. 1995;24:246–51. doi: 10.1111/j.1600-0684.1995.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 190.Gray WL, Williams RJ, Chang R, Soike KF. Experimental simian varicella virus infection of St. Kitts vervet monkeys. J Med Primatol. 1998;27:177–83. doi: 10.1111/j.1600-0684.1998.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 191.Gray WL. Pathogenesis of simian varicella virus. J Med Virol. 2003;70(Suppl 1):S4–8. doi: 10.1002/jmv.10312. [DOI] [PubMed] [Google Scholar]
- 192.Ou Y, Davis KA, Traina-Dorge V, Gray WL. Simian varicella virus expresses a latency-associated transcript that is antisense to open reading frame 61 (ICP0) mRNA in neural ganglia of latently infected monkeys. J Virol. 2007;81:8149–56. doi: 10.1128/JVI.00407-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mahalingam R, Traina-Dorge V, Wellish M, Deharo E, Golilve A, Messaoudi I, Gilden D. Effect of time delay after necropsy on analysis of simian varicella virus expression in latently infected ganglia of rhesus macaques. J Virol. 2010;84:12454–7. doi: 10.1128/JVI.01792-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kolappaswamy K, Mahalingam R, Traina-Dorge V, Shipley ST, Gilden DH, Kleinschmidt-DeMasters BK, McLeod CG, Jr, Hungeford LL, DeTolla LJ. Disseminated simian varicella virus infection in an irradiated rhesus macaque (Macaca mulatta) J Virol. 2007;81:411–5. doi: 10.1128/JVI.01825-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schoeb TR, Eberle R, Black DH, Parker RF, Cartner SC. Diagnostic exercise: papulovesicular dermatitis in rhesus macaques (Macaca mulatta) Vet Pathol. 2008;45:592–4. doi: 10.1354/vp.45-4-592. [DOI] [PubMed] [Google Scholar]
- 196.Hukkanen RR, Gillen M, Grant R, Liggitt HD, Kiem HP, Kelley ST. Simian varicella virus in pigtailed macaques (Macaca nemestrina): clinical, pathologic, and virologic features. Comp Med. 2009;59:482–7. [PMC free article] [PubMed] [Google Scholar]
- 197.Murnnane R, Zhang XB, Hukkanen RR, Vogel K, Kelley S, Klem HP. Myelodysplasia in 2 pig-trained macaques (Macaca nemestrina) associated with retroviral vector-mediated insertional mutagenesis and overexpression of HOXB4. Vet Pathol. 2010 doi: 10.1177/0300985810382673. [Epub ahead of print] doi: 10.1177/0300985810382673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Myers MG, Stanberry LR, Edmond BJ. Varicella-zoster virus infection of strain 2 guinea pigs. J Infect Dis. 1985;151:106–13. doi: 10.1093/infdis/151.1.106. [DOI] [PubMed] [Google Scholar]
- 199.Pavan-Langston D, Dunkel EC. Ocular varicella-zoster virus infection in the guinea pig: a new in vivo model. Arch Ophthalmol. 1989;107:1058–72. doi: 10.1001/archopht.1989.01070020130046. [DOI] [PubMed] [Google Scholar]
- 200.Myers MG, Connelly BL, Stanberry LR. Varicella in hairless guinea pigs. J Infect Dis. 1991;163:746–51. doi: 10.1093/infdis/163.4.746. [DOI] [PubMed] [Google Scholar]
- 201.Sato H, Pesnicak L, Cohen JI. Use of a rodent model to show that varicella-zoster virus ORF61 is dispensable for establishment of latency. J Med Virol. 2003;70(Suppl 1):S79–81. doi: 10.1002/jmv.10326. [DOI] [PubMed] [Google Scholar]