Abstract
Impaired gastric slow waves, frequent gastrointestinal (GI) symptoms and altered GI peptides have been reported in Scleroderma (SSc) patients. The aim of this study was to investigate the associations among these three important components in GI dysmotility. Seventeen fasted SSc patients underwent four channel surface electrogastrography, measuring % of normal gastric slow waves or dysrhythmia. Patients completed a questionnaire designed by us to assess demographics, upper and lower GI symptoms (symptom presence, frequency and impact on quality of life, QOL), by YES/NO, Likert Scales and Visual Analogue Scales 1–100 mm (called GI Dysmotility Questionnaire, GIDQ) and health-related QOL by SF-36. Fasting plasma vasoactive intestinal peptide (VIP) and motilin levels were measured by peptide immunoassays. There were significant correlations between percentages of gastric dysrhythmias (bradygastria or arrhythmia) and a number of major GI symptoms such as nausea, abdominal bloating and pain. The plasma level of VIP was correlated positively with % dysrhythmia but negatively with % normal slow waves. Motilin was positively correlated with slow wave coupling (coordination). No major differences were noted in the measured peptides or gastric slow waves between limited SSc and diffuse SSc. Correlations were noted between SF-36 domain scores and our GIDQ scores. In SSc patients, gastric dysrhythmias are correlated with certain GI symptoms. Correlations are also noted between plasma VIP/Motilin levels and gastric slow waves. Thus in SSc, gastric dysrhythmias may be predictive of development of certain dyspeptic symptoms. Plasma VIP may be involved in the development of dysrhythmias.
Keywords: electrogastrography, gastric slow waves, gastrointestinal hormones, gastrointestinal motility, scleroderma
INTRODUCTION
Systemic Sclerosis (SSc, Scleroderma) is a multisystemic autoimmune disease characterized by prominent widespread small vessel vasculopathy and damage with activation of fibrosis of skin and organs.1,2 The major types of generalized SSc are diffuse cutaneous and limited cutaneous SSc, defined by the distribution of cutaneous fibrosis of the patient.
Gastrointestinal (GI) involvement as a major visceral manifestation is seen in up to 90% of SSc patients.1,2 The primary GI abnormality in SSc is dysmotility that occurs as a spectrum of GI motility disorders ranging from asymptomatic to severe paresis which is prognostically significant in 15% of the patients.3–5 GI dysmotility in SSc is hypothesized to result from initial vascular damage, with subsequent neurogenic and myogenic dysfunctioning.6–8 Any part of the GI tract may be affected and may significantly impact patient quality of life.9–11 Severe GI involvement correlates to an increased mortality of 85% within 9 years of diagnosis.3 Limited cutaneous disease has been reported to have a more benign disease course.3,12 A recent study reported significantly higher stomach involvement in diffuse cutaneous SSc.13 The GI symptoms in SSc have not been consistently correlative to GI dysmotility or predictive of morbidity in published studies,7,14,15 underscoring the need for more definitive and prognostic assessment of GI disease severity and clinical progression.
Surface multi-channel electrogastrography (EGG) is able to, non-invasively, measure abnormalities of gastric myoelectrical activity (GMA) in patients with SSc, functional dyspepsia and diabetic gastroparesis.15–20 Correlations between the EGG and gastric emptying have been reported.18 We have previously reported GMA abnormalities in SSc patients, including decreased normal slow waves, increased bradygastria and impaired gastric slow wave propagation and coordination by EGG.19,20 A self-reported questionnaire limited to the frequency of upper GI symptoms determined that heartburn and abdominal bloating were the most frequently experienced upper GI symptoms and weakly correlated to changes in GMA in response to acupressure applied to the GI antiemetic acupuncture point PC6.20
To expand our previous work, we designed a GI Dysmotility Questionnaire (GIDQ) to include the impact of GI-related symptoms and issues on activities of daily living, as at the time of this study, there were no validated GI questionnaires for SSc patients. We hypothesized that GMA measured by EGG would correlate with symptoms of the upper and lower GI tract and that the diffuse cutaneous disease would have a greater degree of hypomotility and worse GI symptoms compared to the limited cutaneous involvement. In addition to GI symptoms, we also measured plasma vasoactive intestinal peptide (VIP) and motilin, two major inhibitory and excitatory GI peptides that influence GI motility.
Accordingly, the aim of this study was to investigate possible correlations among EGG parameters and GI symptoms, patient functioning, and plasma levels of VIP and motilin in patients with SSc. Correlations between GI-related symptoms and GMA might reflect ongoing GI-related morbidity, clinical progression and possibly, generate more appropriate responses to assess the efficacy of therapeutic interventions of GI dysmotility in SSc.
METHODS
Patient enrollment
The research protocol was approved by the University of Texas Medical Branch Institutional Review Board, and written informed consent was obtained from all subjects before study entry. Seventeen SSc patients who met the American College of Rheumatology Criteria for Scleroderma21 were enrolled in the study (14 females and 3 males). All patients abstained from prokinetic agents for 72 h and fasted for ≥6 h before the initiation of the study. Patients were excluded if they: (i) were unable to give informed consent; (ii) were currently taking prokinetic, anticholinergic or dopaminergic agents which could potentially modify gastric motility; (iii) were unable to recline with a head elevation of 30 degrees; (iv) had a history of abdominal surgery that could distort cutaneous landmarks for EGG lead placement; (v) were pregnant or preparing to conceive a child during the study period. One patient was mildly glucose intolerant, treated by diet alone; otherwise, no patient had a history of diabetes mellitus. As the major aim of this study was not to investigate the normality and abnormality of gastric motility but to investigate the correlations among gastric slow waves, GI symptoms and GI peptides, this particular patient was not excluded.
Skin scores
At the time of SSc patient enrollment, the extent of skin involvement was measured by two of the clinicians (TAM, DD) the total skin score (TSS), which assesses 17 body areas.22,23
Modified Medsger Severity Scale
The Modified Systemic Sclerosis Severity Scale proposed by Medsger et al. (modified Medsger Severity Scale, MSS,24) includes a clinical, laboratory and radiological assessment of nine organ systems including GI tract, with identified variables scoring absent to severe disease involvement from 0–4.
Multi-channel surface EGG
Non-invasive assessment of gastric slow waves by EGG has been established and validated. The rhythmicity of gastric slow waves is accurately measured in the electrogastrogram and represented in the spectral analysis of the EGG.15,25 Subjects reclined at 30 degrees head elevation and were required to remain motionless to avoid possible artefacts derived from body movements. Surface EGG was recorded for 30 min in the fasting state using a multi-channel recording device (Medtronic; Shoreview, MN, USA), which is an FDA-approved clinical instrument. The device consists of four identical amplifiers (channels 1–4) with cut-off frequencies of 1.8 and 16.0 cycles min−1 (cpm) that measure GMA.17,25 The GMA is composed of the rhythmic slow wave (also called electrical control activity, reflecting pacemaker potential) and spikes (electrical response activity or action potentials). The gastric slow wave determines the frequency and propagation of gastric contractions. The normal gastric slow wave propagates from the corpus of the stomach (roughly channels 1 and 2) distally towards the pylorus (channels 3 and 4), maintaining a basal rhythm of 2–4 cpm. The spikes (superimposed on normal waves) determine the presence or absence of contractions. Episodes of gastric slow waves that have different frequencies are called bradygastria (0.5–2 cpm), tachygastria (4–9 cpm) or arrhythmia, (no clear peak in the spectral analysis). The recorded EGG data were converted from analog to digital with a sampling frequency of 1 Hz on line and stored on an IBM-compatible computer. Computerized spectral analysis methods were applied to derive the following parameters from the 4-channel EGG: (i) dominant frequency of the EGG which reflects the frequency of the gastric slow wave, (ii) changes in dominant power of the EGG reflecting slow wave amplitude, (iii) % normal slow waves in the range of 2–4 cpm, (iv) % bradygastria, (v) % tachygastria, (vi) % gastric arrhythmia and (vii) % slow wave coupling, reflecting the coordination of slow waves between two gastric regions.17,25
Patient self-report questionnaires
Assessment of GI symptoms by GIDQ
At the time of this study, there were no validated GI-specific instruments for SSc patients. We designed the GIDQ for this study to assess GI-related symptoms and quality of life (QOL) in SSc patients and their possible correlations to EGG recordings. The first part of GIDQ was designed to be focused for upper and lower GI-related symptoms derived from published reports26–30 and based on predictions of what would be pertinent from our previous studies.19,20 The second part of the study was comprised of questions based on QOL issues in the context of GI involvement including daily personal, work and social activities. Patients’ disease ratings were also recorded by self-report. Patient responses included both ‘yes’ and ‘no’ answers, Likert Scale and Visual Analog Scales (VAS) for frequency of symptoms (days 1–7 week−1) and subjective intensity of the symptom (rated from 0–100). The GIDQ contained 48 questions and is presented in the Appendix 1.
SF-36 questionnaires
The SSc patients also completed Medical Outcome Study Short Form-36 (SF-36) questionnaires, a validated instrument to measure general disease-related QOL and previously shown to predict morbidity in SSc patients.30–33 The Medical Outcome Study Short Form-36, Version 1.0, (recall of the last 4 weeks) was used to evaluate self-perceived psychological and physical limitations due to an underlying illness or the patient’s health-related QOL34,35. The SF-36 instrument is not specific to any organ system and has a significant correlation to systemic involvement in SSc patients.28,32 The SF-36 contains 36 items and measures eight domains of health: Physical Functioning, role limitations due to physical health (Role-Physical), Bodily Pain, (perceptions of) General Health, Vitality, Social Functioning, role limitations due to emotional problems (Role-Emotional) and Mental Health. Higher values indicate higher (better) levels of perceived functioning (range 0–100). Raw SF-36 version 1.0 scores were normalized to version 2.0 and scores of mental component summary (MCS) and physical component summary (PCS) were calculated using a 2.0 conversion program (SF Health Outcomes Scoring Software; QualiMetrics incorporated, Lincoln, RI, USA). Scores converted to 2.0 scale (0–50) allow comparison to normative values (set at 50) and to other populations including other rheumatic diseases.
Fasting plasma gastric peptide determinations of VIP and motilin
Plasma samples were collected from the study subjects just before initiation of EGG testing. Aliquoted samples were stored at −80 °C and thawed only once for determination of VIP or motilin levels. Vasoactive intestinal peptide ELISA-based assays were performed on plasma samples in duplicate according to manufacturer ‘s protocol for a sensitivity detection range of 0–25 ng mL−1 (Bachem, San Carlos, CA, USA). Radioimmunoassays were performed in duplicate to determine fasting plasma motilin levels according to the manufacturer’s protocol for a sensitivity detection range of 0–25 pg mL−1, (Bachem). Sample runs were also performed on patient samples diluted 1 : 3 to assure that assay determinations were within acceptable range.
Statistical analysis
Correlations between multi-channel EGG parameters, questionnaire scores and plasma peptide levels are reported as mean values ± standard error. The percentage (%) of normal slow waves, % bradygastria, % arrhythmia and % slow wave coupling are presented in this study. Multivariate correlations were assessed by the Spearman rank order correlation using STATISTICA software (Statsoft, Tulsa, OK, USA) and included scores from GMA, TSS, MSS, GIDQ, SF-36, fasting plasma VIP and motilin levels. Assessment of the association of frequency of nausea and % bradygastria, channel 3 were performed by chi-square and Cochran –Mantel–Haentzel statistics. Analyses between limited and diffuse SSc groups were performed using unpaired Student’s t-tests. Demographic data from EGG or questionnaires or plasma peptide levels that are uncorrelated are presented as mean values ± standard error. A value of P < 0.05 was considered significant.
RESULTS
The average age of enrolled patients was 55.0 ± 2.28 years. Females enrolled (n = 14), comprised 82% of the SSc patients. The SSc patients diagnosed with diffuse cutaneous involvement (n = 9) was 53% of the SSc patients. The mean number of years diagnosed with SSc was 8.88 ± 1.1 years. The mean TSS and MSS were 10.18 ± 1.3 and 7.76 ± 0.81 respectively.
GI-related symptoms correlate with GMA in SSc patients
Table 1 shows the GMA results, GI symptoms scores and plasma VIP and motilin levels from fasting SSc patients. The mean scores of the SF-36 domains are shown in Table 2.
Table 1.
GMA and GI symptoms from SSc patients
| Variables | Rhythms, % Symptoms, % Concentrations | Range |
|---|---|---|
| ch 2 Nl | 73.1 ± 5.4 | 39–100 |
| ch 2 Br | 4.9 ± 1.1 | 0–18 |
| ch 2 Ar | 14.5 ± 3.5 | 0–43 |
| ch 3 Nl | 72.1 ± 5.3 | 40–100 |
| ch 3 Br | 5.3 ± 1.7 | 0–18 |
| ch 3 Ar | 16.8 ± 3.6 | 0–40 |
| ch 4 Ar | 13.0 ± 2.9 | 0–39 |
| Coupl 1,2 | 71.2 ± 5.9 | 34–100 |
| GIDQ | ||
| Oesophageal (5 items) | 20–80 | |
| Heartburn | 80 | |
| Gastric (5 items) | ||
| Bloating | 90 | 20–90 |
| Early satiety | 50 | |
| Intestine (8 items) | ||
| Constipation | 50 | 10–50 |
| Diarrhoea | 30 | |
| Anorectal (5 items) | ||
| Incomplete BM | 60 | 20–60 |
| Plasma VIP (ng mL−1) | 22.4 ± 0.8 | 15–29 |
| Plasma motilin (pg mL−1) | 25.0 ± 3.6 | 13–62 |
Data presented are percentages of rhythm, symptoms or concentrations (mean + SEM) and the range of percentages for that variable among the fasting SSc, systemic sclerosis, patients. GMA, gastric myoelectrical activity is shown for channels 2 and 3, slow wave and dysrhythmias of bradygastria and arrhythmia and channel 4, arrhythmia. GIDQ, Gastrointestinal Dysmotility Questionnaire, is presented in Appendix 1. GI regions depicted had 5–8 related questions, as noted. The most prominent symptoms are shown for each GI region. Incomplete BM, incomplete bowel movement. The GI peptide levels are plasma vasoactive intestinal peptide (VIP) and plasma motilin. ch: channel; Ar: % of arrhythmia; Br: % of bradygastria; N1: % of normal slow waves.
Table 2.
SF-36 scores of SSc patients
| SF-36 domains | Mean score ± SEM | Score range |
|---|---|---|
| Physical Functioning | 27.6 ± 2.2 | 15–43 |
| Role Physical | 28.0 ± 3.5 | 17–56 |
| Body Pain | 38.8 ± 2.2 | 24–53 |
| General Health | 37.1 ± 2.6 | 17–55 |
| Vitality | 39.2 ± 2.2 | 27–58 |
| Social Functioning | 43.1 ± 2.7 | 24–57 |
| Role-Emotional | 39.8 ± 3.7 | 23–55 |
| Mental Health | 46.4 ± 2.9 | 18–64 |
Perceived functioning by health-related SF-36, (4-week recall). Data presented are mean scores ± SEM for each domain and the range of scores for each domain. SSc: systemic sclerosis.
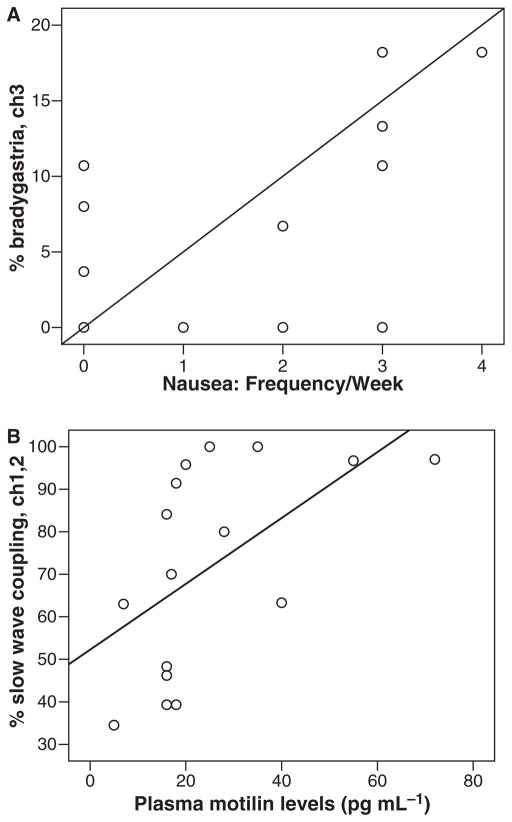
Table 3 demonstrates that the % mean slow wave dysrhythmia was positively and significantly correlated with some of GI symptoms throughout the GI tract. The % bradygastria and % arrhythmia in EGG channels 2 and 3 were the most frequent gastric dysrhythmia and regions that correlated with the GI symptoms (Spearman R range = 0.50–0.73, P ≤ 0.02), although other channels and other slow wave abnormalities also demonstrated significant correlations with GI symptoms. The gastric symptoms that were correlated with gastric dysrhythmia (bradygastria or arrhythmia) were nausea frequency and abdominal bloating. Fig. 1A shows the correlation between the frequency week−1 of nausea and % bradygastria in channel 3. In addition, the % normal slow waves was inversely correlated with awakening because of abdominal pain (Spearman R = −0.56, P < 0.02). The correlation of gastric arrhythmia and abdominal pain approached significance (Spearman R = 0.43, P = 0.06).
Table 3.
Correlation between % gastric dysrhythmia and GI-related symptoms
| GI region | GIDQ GI symptoms | GMA | Spearman R | P value |
|---|---|---|---|---|
| Oesophagus | Heartburn | ch 3 Ar | 0.79 | <0.01 |
| Stomach | Nausea frequency | ch 3 Br | 0.5 | 0.04 |
| Abdominal bloating | ch 2 Br | 0.6 | <0.01 | |
| Anorectal | Rectal fullness | ch 2 Ar | 0.59 | <0.01 |
| Abdominal pain | Abdominal pain | ch 4 Ar | 0.43 | 0.06 |
| Awakened by pain | ch 2 Nl | −0.56 | <0.02 |
GI-related symptoms derived from the GIDQ, Gastrointestinal Dysmotility Questionnaire, presented in Appendix 1. ch: channel; Ar: % of arrhythmia; Br: % of bradygastria; Nl: % of normal slow waves; GMA: gastric myoelectrical activity.
Figure 1.
Gastric waves with GI symptoms and peptides in 17 fasting SSc patients. (A) Correlation between percentage bradygastria, channel 3 with self reported frequency week−1 of nausea. (B) Correlation between percentage gastric wave coupling, channels 1 and 2 with plasma motilin levels, pg mL−1.
Detailed analysis of the associations of non-parametric data is presented in Table 4 for GI-related symptom ‘frequency of nausea’ (range 1–7 days) and % bradygastria, channel 3. SSc patients with % bradygastria scores <6 had lower values for frequency of nausea. SSc patients with % bradygastria scores >6 had higher values for frequency of nausea.
Table 4.
Association of frequency of nausea and ch 3, % bradygastria
| ch 3, % bradygastria | Nausea frequency week−1 |
Total | ||
|---|---|---|---|---|
| 0–1 | 1.1–2 | >2 | ||
| 0–6 | ||||
| Frequency | 7 | 2 | 1 | 10 |
| Percentage | 41.18 | 11.76 | 5.88 | 58.2 |
| Row percent | 70.00 | 20.00 | 10.00 | |
| Column percent | 77.78 | 66.67 | 20.00 | |
| >6 | ||||
| Frequency | 2 | 1 | 4 | 7 |
| Percentage | 11.26 | 5.88 | 23.53 | 41.18 |
| Row percent | 28.57 | 14.29 | 57.14 | |
| Column percent | 22.22 | 33.33 | 80.00 | |
| Total number (%) | 9 (52.94) | 3 (17.65) | 5 (29.41) | 17 (100) |
Association of frequency of nausea and percentage of bradygastria, channel 3. Analyses were performed by chi-square and Cochran– Mantel–Haentzel statistics. P < 0.05. Systemic sclerosis (SSc) patients with % bradygastria scores <6 had lower values for frequency of nausea. SSc patients with % bradygastria scores >6 had higher values for frequency of nausea.
Correlations between gastric slow waves and gastrointestinal peptides
The correlations between the gastric slow waves and the GI peptides are shown in Table 5. The plasma levels of VIP (inhibitory to GI motility) were signifi- cantly inversely correlated with the percentage of normal gastric slow waves (Spearman R = −0.56, P = 0.01) but positively correlated with the % bradygastria or % arrhythmia (Spearman R range = 0.55–0.66, P ≤ 0.02). Motilin, which is excitatory to GI motility, was significantly correlated with slow wave coupling (Spearman R = 0.55, P = 0.02). Fig. 1B shows the correlation between the plasma motilin level and the percentage of slow wave coupling between channels 1 and 2. A higher percentage of slow wave coupling was indicative of more robust coordination.
Table 5.
Correlations of gastric peptides with gastric rhythms
| Plasma gastric peptide levels | GMA parameters | Spearman R | P value |
|---|---|---|---|
| VIP (ng mL−1) | ch 3 % slow wave | −0.55 | 0.01 |
| ch 3 % bradygastria | 0.59 | <0.02 | |
| ch 3 % arrhythmia | 0.66 | <0.01 | |
| Motilin (pg mL−1) | ch 1, 2 % wave coupling | 0.55 | 0.02 |
ch: channel; GMA: gastric myoelectrical activity; VIP: vasoactive intestinal peptide.
Differences between limited and diffuse cutaneous scleroderma
Table 6 shows the differences in parameters between limited and diffuse SSc patient groups. Patient group percentage of females (75 vs 89%), mean age (57.38 ± 3.13 vs 53.11 ± 2.57 years) and patient disease rating (41 ± 9.23 vs 46.22 + 5.09) were matched between limited and diffuse SSc respectively. There were significant differences between groups in TSS (7 ± 0.59 vs 13 ± 1.97, P < 0.02), MSS-Skin component (1.0 vs 1.44 ± 0.18, P = 0.03). The only parameter of gastric slow waves that was significantly different between limited and diffuse cutaneous involvement was channel 1 (ch 1) arrhythmia (25.7 ± 5.77 vs 7.52 ± 3.26, P = 0.02). There were differences between the limited and diffuse SSc groups, respectively, in GI-related QOL questions of the GIDQ. (36) ‘How certain are you that you can manage your GI symptoms so that you can do the things you enjoy doing?’ (87.3 ± 6.4 vs 44.4 ± 10.3; P < 0.01), (46) ‘Have your GI problems limited your work at your place of employment?’ (5.75 ± 3.03 vs 45.44 ± 14.05, P < 0.01), (47) ‘Have your GI problems limited your social life?’ (10.63 ± 4.11 vs 48.22 ± 8.2, P < 0.01) and (45) ‘How effectively are you coping with your GI problems?’ (89 ± 3.27 vs 55.55 ± 9.5 P < 0.01). The limited and diffuse SSc groups had significantly different scores in the SF-36 social functioning domain (51.03 ± 1.9 vs 36.03 ± 3.3, P < 0.001) and mental health domain (52.43 ± 2.66 vs 41.10 ± 4.4, P = 0.02). Fasting plasma VIP and motilin levels were not significantly different between limited and diffuse SSc groups (23.8 ± 0.1 vs 21.23 ± 1.8, P = 0.1 and 19 ± 2.38 vs 30.44 ± 6, P = 0.11 respectively).
Table 6.
Limited vs diffuse SSc
| Variable | Limited SSc | Diffuse SSc | P value |
|---|---|---|---|
| Patient no. | 8 | 9 | ---- |
| % Female | 75% | 89% | 0.25 |
| Age, years | 57.38 ± 3.13 (40–66) | 53.11 ± 2.57 (42–62) | 0.31 |
| TSS (0–51) | 7 ± 0.59 (5–9) | 13 ± 1.97 (3–21) | <0.02 |
| MSS-Skin (0–4) | 1.0 (0–1) | 1.44 ± 0.18 (1–2) | 0.03 |
| MSS-Gen | 0.38 ± 0.2 (0–1) | 0.67 ± 0.37 (0–3) | 0.25 |
| MSS-GI | 1.3 ± 0.29 (0–3) | 1. 56 ± 0.29 (0–3) | 0.16 |
| GMA, ch 1 Ar | 25.7 ± 5.77 (0–46.2) | 7.52 ± 3.26 (0–28.6) | 0.02 |
| GIDQ, Certain (%) | 87.3 ± 6.4 (50–100) | 44.4 ± 10.3 (0–100) | <0.01 |
| Coping | 89 ± 3.27 (80–100) | 55.55 ± 9.5 (0–84) | <0.01 |
| Work limitation | 5.75 ± 3.03 (0–22) | 45.44 ± 14.05 (0–100) | 0.02 |
| Social limitation | 10.63 ± 4.11 (0–32) | 48.22 ± 8.2 (10–100) | <0.01 |
| Disease rating | 41 ± 9.23 (0–78) | 46.22 ± 5.09 (20–80) | 0.63 |
| SF-36: Social fcn | 51.03 ± 1.9 (46–57) | 36.03 ± 3.22 (19–46) | <0.01 |
| Mental Health | 52.43 ± 2.66 (39–64) | 41.10 ± 4.4 (18–64) | 0.02 |
| Plasma VIP (ng mL−1) | 23.8 ± 0.1 (18.7–26.2) | 21.23 ± 1.8 (15.66–26) | 0.11 |
| Plasma motilin (pg mL−1) | 19 ± 2.38 (13–25) | 30.44 ± 6 (16–60) | 0.11 |
Analysis between groups of limited compared to diffuse cutaneous systemic sclerosis (SSc) by Student’s t-test. Data are presented as percentages or as mean + SEM and (range of values). TSS: total skin score: (0–51). MSS: Skin, modified Medsger Severity Scale-Skin component; MSS-Gen: modified Medsger Severity Scale-General; MSS-GI: modified Medsger Severity Scale-GI tract; GMA: gastric myoelectrical activity, % gastric arrhythmia, channel 1. Gastrointestinal Dysmotility Questionnaire (GIDQ) quality of life questions. (36) How certain are you that you can manage your GI symptoms so that you can do the things you enjoy doing? (45) How effectively are you coping with your GI problems? (46). Have your GI problems limited your work at your place of employment? (47) Has your GI problems limited your social life? (48) Overall, considering pain, discomfort, limitations in your daily life and other changes in your body and life; how severe would you rate your disease today?’. Social fcn: Social functioning and Mental Health domains, Plasma VIP levels, vasoactive intestinal peptide, in ng mL−1, and plasma motilin levels, in pg mL−1.
Correlations between SF-36 and GIDQ
There were significant correlations between the SF-36 validated instrument and the GIDQ GI-related symptoms and GI-related QOL (See Appendix 2). The GI-related symptoms that most frequently correlated with the parts of the non-organ specific SF-36 were incomplete bowel movement, chest pain and being awakened by abdominal pain (not shown). The GI-related QOL questions with robust correlations to the SF-36 domain scores were pain control, stress effect and coping as shown. Also frequently correlated with SF-36 scores were work limitations, longer time to accomplish tasks and certainty of symptom management.
DISCUSSION
GMA has been studied non-invasively using single or multi-channel EGG in a number of previous studies in SSc patients4,9,10,14,19,20 with conflicting findings. Some of EGG studies revealed abnormal gastric slow waves or abnormal EGG parameters4,19,20 while others showed no difference in EGG parameters between SSc and controls.9,10,14 In our laboratory, one previous study investigated the difference in GMA assessed by multichannel EGG between SSc patients and healthy controls, and demonstrated impaired slow wave rhythms and propagations associated with SSc. In another study, we investigated the effects of acupressure on GMA and GI symptoms in SSc patients and reported a difference in acupressure-induced alterations in EGG parameters between SSc patients and control. The aim of this study was different from these previous studies and the findings on the correlations between GMA and GI symptoms/peptides and the correlations between the GIDQ scores and SF-36 domain scores were novel and never been reported previously.
In general, there is lack of correlation between gastric motility or GMA and GI symptoms in SSc patients,7,14,36 which is similar in patients with gastroparesis or functional dyspepsia. In this study, however, we identified correlations between GMA measured by EGG and general health-related and GI-related perceived functioning and plasma levels of gastric peptides. The findings of this study support our hypothesis that gastric dysrhythmia is associated with GI dyspeptic symptoms in patients with SSc. Significant correlations were seen between gastric dysrhythmia and the GIDQ, measuring organ specific GI-related symptoms and QOL issues. It should also be noted that although correlations were observed in this study between gastric dysrhythmias and certain GI symptoms, the causative relationship between these two measures could not be established in this study. Gastric dysrhythmias might be the cause of certain GI symptoms or both abnormal slow waves and symptoms could be manifestations of impaired functions of the gut. The GIDQ was designed to assess perceived presence and frequency of GI-related symptoms and the perceived impact of these symptoms on aspects contributing to quality of life. We developed this questionnaire to assess symptoms as at the time of this study, the recently published validated questionnaire for SSc was not available.37
Gastric dysrhythmias were associated with elevated plasma levels of VIP whereas slow wave coupling was correlated with plasma motilin. The list of GI hormones associated with gastric motility is lengthy, such as glucagon-like peptide-1, cholecystokinin, neurotensin, peptide YY and somatostatin. The major reason to study motilin and VIP in this study was because these two peptides are representatives of neurotransmitters that control/modulate gastric motility with motilin being excitatory and VIP being inhibitory.
Higher plasma VIP and motilin levels in SSc have been previously reported.38–41 VIP is known to inhibit gastric motility and elevated plasma levels of VIP have been reported in conditions related to gastric or intestinal dysmotility.42–45 Increased VIP levels are thought to reflect autonomic and GI abnormalities in SSc. In this study, the plasma level of VIP was found to be negatively correlated with the%normal slow waves and positively correlated with the % bradygastria/arrhythmia measured from channel 3 (in the antral area of the stomach). These correlations were in agreement with the inhibitory role VIP as bradygastria and arrhythmia are associated with gastric dysmotility and suggested that a high level of VIP might be associated with gastric dysrhythmia and lead to gastric dysmotility. In addition, we also found significantly elevated fasting plasma motilin levels in our SSc patients, compared with normal controls. Elevated plasma motilin levels have been associated with phase III of the migrating motor complex (MMC) mediated through the cholinergic pathway. 44 Motilin is one of major excitatory GI peptides and is known to regulate the MMC. Plasma motilin increases during phase III of the MMC. In this study, the level of motilin was significantly correlated with the percentage of slow wave coupling, which has not been previously reported. These data seem to suggest that a higher plasma concentration of motilin enhances slow wave propagation. Further studies assessing the physiologic relevance of plasma peptide levels in SSc patients are ongoing.
In this study, there were few significant differences between limited and diffuse cutaneous involvement in the context of GI dysrhythmias and GI symptoms. However, there were significantly worse scores in diffuse cutaneous SSc in GI-related perceived functioning, including coping and social and work limitations. In our study, diffuse cutaneous SSc patients also had significantly lower scores of social functioning and mental health domains on the SF-36 compared to the limited cutaneous SSc patients. In a previous study with the measurements of gastric emptying, EGG and symptoms in diffuse SSc and limited SSc, gastric emptying was found to be slower in diffuse SSc. In comparison with limited SSc, gastric slow waves were not different between the two groups of patients.14 Upper GI symptoms tended to be more abundant in diffuse SSc.13,14 The small number of patients with limited (n = 8) and diffuse (n = 9) cutaneous disease is a limitation of this study. Additional studies with higher patient numbers are needed to fully assess any distinct differences in GI functioning based on SSc subset.
SSc-related dysmotility is believed to stem from a primary neuropathic process, over time reflecting a vasculopathic, myopathic, and a consequently fibrotic process.6,7,45 Sympathetic activation in SSc has been consistently reported.6–8,46 However, the roles of sympathetic activation in the modulation of gastric slow waves and symptoms have not been established. While alterations have been reported in both the sympathetic activity and slow wave activity, it is not clear whether the slow wave abnormalities are caused by the impaired autonomic function. However, intuitively, we speculate that the altered GMA is attributed to myopathy as the slow waves are generated by smooth muscles and interstitial cells of Cajal. This could be similar to the neural influence on repolarization reported by Ciftci et al. in cardiac tissue.47 Differences in GI-related perceived functioning between limited and diffuse involvement may reflect distinctly evolving pathologies of vascular, fibrotic or immunologenic pathways.48–50 Significant correlations observed between GIDQ and SF-36 scores reflect associations in perceived functioning between organ specific and nonorgan specific items. The GI-related QOL questions on the GIDQ had acceptable to excellent correlations with the individual domains of the SF-36, especially MCS, PCS, Role-Physical and Physical Functioning domains.
To summarize, in SSc, gastric dysrhythmias are correlated with certain GI symptoms. Significant correlations are also noted between gastric slow waves and gastric peptide levels. These findings suggest that gastric dysrhythmia might be responsive for certain dyspeptic symptoms, and gastric peptides, particularly VIP may be involved in the development of gastric dysrhythmia.
Acknowledgments
The authors wish to thank Ms. Lynette Durant for secretarial assistance and Dr. Karin Westlund High, Mr. Julio Charles and Mr. Huaizhi Yin for technical support. The authors also wish to thank Dr. James Grady from the UTMB Office of Biostatistics for his assistance in the statistical analyses. Supported by NIH Specialized Center of Research (SCOR) Grant in Scleroderma P50AR44888 (TAM, SEH, MDM), NIH Centers for Research Translation (CORT) P50AR054144 (TAM, SEH, MDM), University Clinic Research Center Grant M01-RR00073 and R21 AG023951 NIH National Institute on Aging (TAM, SEH).
APPENDIX 1
The GIDQ presented here has not been validated. Patients respond by yes/no and VAS scoring 1. UPPER AND LOWER GI SYMPTOMS. Oesophageal: 1. Do you have difficulty swallowing liquids? 2. Do you have difficulty swallowing solid food? 3. Do you have any hoarseness? 4. Have you had any heartburn or acid reflux? 5. Have you had any pain or pressure behind the chest bone? 6. Are you taking any medication(s) as treatment for any of the above mentioned problems? Stomach and Small Intestine: 7. Do you experience a sensation of gas and/or bloating in the abdomen? 8. Have you increased a clothes size or adjusted your clothes for this problem? 9. Do you feel discomfort in your abdomen every day? 10. Do you have a sensation of fullness early in the meal (early satiety)? 11. Do you have abdominal pain? If YES to question 11, please indicate the frequency of pain by the number of days a week that you had pain on the scales provided below VAS (0–7 days). 12. How many days last week did you experience abdominal pain? 13. Have you been bothered by vomiting? a. How many days last week did you experience vomiting? 14. Have you been bothered by nausea? a. How many days last week did you experience nausea? Large Intestine and Anorectal Symptoms: In the last week…15. Have you been bothered by diarrhoea? a. How many days last week did you experience diarrhoea? 16. Have you been bothered by constipation? a. How many days last week did you experience constipation? 17. Have you been having both diarrhoea alternating with constipation? a. How many days last week did you experience diarrhoea/constipation? 18. Do you have bulky or foul smelling stools? a. How many days last week did you have foul smelling stools? 19. Have you been bothered by hard stools? a. How many days last week did you experience hard stools? 20. Do you have a feeling of incomplete bowel movement? 21. Do you have bleeding per rectum because of straining? a. How many days last week did you experience bleeding because of straining? 22. Do you have a sensation of fullness in the rectum after a bowel movement? 23. Have you had stool incontinence (i.e., have you soiled your pants?) a How many days last week did you experience faecal incontinence? 24. Have you been bothered by black, tarry stools? How often do you experience black, tarry stools?
2. GI_RELATED QUALITY OF LIFE ISSUES Daily activities refer to requirements of daily living, for example, maintenance or upkeep of your place of residence, gardening, shopping, and yard work? 25. Have you lost 20 pounds, or greater than 15% of your usual weight in the past 6 months? 26. Have you had any hospitalization related to GI problems in past 6 months? 27. a. If yes to Question-26, How many hospitalizations and what were the discharge diagnoses, if known? 28. Have you ever received, a) Total parental Nutrition (TPN), where the doctors rested your gut by feeding you by venous infusion in the past 6 months? b) Have you ever been on TPN? 29. a) Have you awakened in the middle of the night by GI pain in past 4 weeks? Last 7 days? b) How often in the last week have you awakened in the middle of the night because of GI pain? 30. Have you used non-prescription medications, herbal therapies or alternative therapies (Acupuncture, Hypnotherapy, Yoga, etc) to relieve your GI symptoms in past 6 months? If YES to Question-30 then please list the non-prescription meds and alternative therapies and their effectiveness (0–100) to relieve your symptoms.31. Do you have a history of peptic ulcer disease diagnosed by a physician? 32. Have you had to change occupations because of your GI symptoms? 33. Have you had to change any normal daily errand or task you usually perform at home or work because of GI symptoms?
If YES to questions 32 or 33, please list the changes. Please place a mark on the line to indicate your level of GI involvement in the last week to the following (VAS 0–100). 34. How much discomfort have you had because of abdominal pain? 35. How much discomfort have you had because of bloating? 36. How certain are you that you can manage your GI symptoms so that you can do the things you enjoy doing? 37. What is your level of interest in learning more about your GI problems? 38. Are you concerned with your GI problems? 39. Does it take longer for you to complete tasks with your GI problems? 40. How would you rate your ability to control your pain due to GI problems? 41. Do you feel fatigued at times? 42. How certain are you that you can manage your fatigue? 43. In the past week how much have your GI problems interfered with your daily activities? 44. How would you rate mental or physical stress that have an affect on your GI problems? 45. How effective are you coping with your GI problems? 46. Have your GI problems limited your work at your place of employment? 47. Has your GI problems limited your social life? 48. Overall, considering pain, discomfort, limitations in your daily life and other changes in your body and life; how severe would you rate your disease today? Thank you for participating in this project.
APPENDIX 2
Correlations between SF-36 Domain Scores and Items from the GI Dysmotility Questionnaire
| GIDQ items | PCS | MCS | Bodily Pain | Social Functioning | Mental Health | Vitality | Role Physical | Physical Functioning |
|---|---|---|---|---|---|---|---|---|
| Pain control # 40 | 0.73* P = 0.0008 | 0.64* P = 0.005 | 0.49 P = 0.04 | 0.48 P = 0.048 | −0.55 P = 0.02 | 0.56 P = 0.02 | 0.83* P = 0.00004 | 0.6 P = 0.01 |
| Stress effect # 44 | −0.78* P = 0.0002 | −0.67 P = 0.008 | 0.63 P = 0.006 | −0.5 P = 0.04 | −0.66 P = 0.003 | 0.64 P < 0.01 | −0.77* P = 0.0002 | |
| Coping # 45 | 0.61* P = 0.009 | 0.62 P = 0.006 | 0.4 P = 0.058 | 0.7* P = 0.002 | 0.66* P = 0.004 | 0.67* P = 0.002 | ||
| Fatigue # 41 | −0.69 P < 0.01 | −0.58 P = 0.02 | ||||||
| Certain # 36 | 0.45 P = 0.06 | 0.45 P = 0.07 | 0.51 P = 0.036 | 0.65* P = 0.004 | ||||
| Concern # 38 | −0.56 P = 0.04 | −0.58 P = 0.014 | −0.55 P = 0.02 | −0.51 P = 0.035 | ||||
| Longer time # 39 | −0.53 P = 0.03 | −0.53 P = 0.03 | −0.59 P = 0.012 | −0.62 P < 0.01 | ||||
| Work limitation # 46 | −0.56 P = 0.02 | 0.51 P = 0.04 | −0.59 P = 0.03 | |||||
| Pt dis rating # 48 | −0.52 P = 0.03 | −0.5 P = 0.04 | −0.59 P = 0.012 | |||||
| Social limitation # 47 | −0.52 P = 0.03 | 0.44 P = 0.07 |
Spearman rank order analysis performed on scores from the SSc patients’ SF-36 and GI Dysmotility Questionnaire (GIDQ). All domains of SF-36 are shown except Role-Emotional and General Health, which had no significant correlations with items of the GIDQ.
Significant after applying a Bonferroni alpha adjustment for each row of table: P < 0.05/10 = 0.005 for each GIDQ item.
References
- 1.Sjögren RW. Gastrointestinal features of scleroderma. Curr Opin Rheumatol. 1996;8:569–75. doi: 10.1097/00002281-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Shakra M, Guillemin F, Lee P. Gastrointestinal manifestations of systemic sclerosis. Semin Arthritis Rheum. 1994;24:29–39. doi: 10.1016/0049-0172(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 3.Steen V, Medsger T. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43:2437–44. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 4.Marie I, Levesque H, Ducrotte P, et al. Gastric involvement in systemic sclerosis- a prospective study. Am J Gastroenterol. 2001;96:77–83. doi: 10.1111/j.1572-0241.2001.03353.x. [DOI] [PubMed] [Google Scholar]
- 5.Sallam H, McNearney T, Chen J. Systematic review: pathophysiology and management of gastrointestinal dysmotility in systemic sclerosis (scleroderma) Aliment Pharmacol Ther. 2006;23:691–712. doi: 10.1111/j.1365-2036.2006.02804.x. [DOI] [PubMed] [Google Scholar]
- 6.Greydanus MP, Camilleri M. Abnormal postcibal antral and small bowel motility due to neuropathy or my- opathy in systemic sclerosis. Gastroenterology. 1989;96:110–5. doi: 10.1016/0016-5085(89)90770-1. [DOI] [PubMed] [Google Scholar]
- 7.Dessein PH, Joffe B, Metz RM, Millar DL, Lawson M, Stanwix AE. Autonomic dysfunction in systemic sclerosis: sympathetic overactivity and instability. Am J Med. 1992;93:143–50. doi: 10.1016/0002-9343(92)90043-b. [DOI] [PubMed] [Google Scholar]
- 8.Lock G, Straub RH, Zeuner M, et al. Association of autonomic nervous dysfunction and esophageal dysmotility in systemic sclerosis. J Rheumatol. 1998;25:1330–5. [PubMed] [Google Scholar]
- 9.Pfaffenbach B, Adamek RJ, Hagemann D, Wegener M. Gastric myoelectrical activity and gastric emptying in patients with progressive systemic sclerosis. Am J Gastroenterol. 1996;91:411–3. [PubMed] [Google Scholar]
- 10.Marycz T, Muehldorfer SM, Gruschwitz MS, et al. Gastric involvement in progressive systemic sclerosis: electrogastrographic and sonographic findings. Eur J Gastroenterol Hepatol. 1999;11:1151–6. doi: 10.1097/00042737-199910000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Young MA, Rose S, Reynolds JC. Gastrointestinal manifestations of scleroderma. Rheum Dis Clin North Am. 1996;22:797–823. doi: 10.1016/s0889-857x(05)70302-1. [DOI] [PubMed] [Google Scholar]
- 12.Czirjak L, Kumanovics G, Varju C, et al. Survival and causes of death in 366 Hungarian patients with systemic sclerosis. Ann Rheum Dis. 2008;67:59–63. doi: 10.1136/ard.2006.066340. [DOI] [PubMed] [Google Scholar]
- 13.Walker UA, Tyndall A, Czirjak L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR scleroderma trials and research group database. Ann Rheum Dis. 2007;66:754–63. doi: 10.1136/ard.2006.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franck-Larsson K, Hedenstrom H, Dahl R, Ronnblom A. Delayed gastric emptying in patients with diffuse versus limited systemic sclerosis, unrelated to gastrointestinal symptoms and myoelectric gastric activity. Scand J Rheumatol. 2003;32:348–55. doi: 10.1080/03009740410005016. [DOI] [PubMed] [Google Scholar]
- 15.Chen JDZ, Schirmer BD, McCallum RW. Serosal and cutaneous recordings of gastric myoelectrical activity in patients with gastroparesis. Am J Physiol. 1994;266:G90–8. doi: 10.1152/ajpgi.1994.266.1.G90. [DOI] [PubMed] [Google Scholar]
- 16.Simonian HP, Panganamamula K, Chen JZ, Fisher RS, Parkman HP. Multichannel electrogastrography (EGG) in symptomatic patients: a single center study. Am J Gastroenterol. 2004;99:478–85. doi: 10.1111/j.1572-0241.2004.04103.x. [DOI] [PubMed] [Google Scholar]
- 17.Hasler WL, Soudah HC, Dulai G, Owyang C. Mediation of hyperglycemia- evoked gastric slow-wave dysrhythmias by endogenous prostaglandins. Gastroenterology. 1995;108:727–36. doi: 10.1016/0016-5085(95)90445-x. [DOI] [PubMed] [Google Scholar]
- 18.Chen JDZ, Lin ZY, McCallum RW. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci. 1996;41:1538–45. doi: 10.1007/BF02087897. [DOI] [PubMed] [Google Scholar]
- 19.McNearney T, Lin X, Shrestha J, Lisse J, Chen JD. Characterization of gastric myoelectrical rhythms in patients with systemic sclerosis using multichannel surface electrogastrography. Dig Dis Sci. 2002;47:690–8. doi: 10.1023/a:1014759109982. [DOI] [PubMed] [Google Scholar]
- 20.Wollaston DE, Xiaohong X, Tokumara O, Chen JDZ, McNearney TA. Systemic Sclerosis (SSc) patients have unique and persistent alteration in gastric myoelectrical activity (GMA) with acupressure to Neiguan point PC6. J Rheumatol. 2005;32:494–501. [PubMed] [Google Scholar]
- 21.Masi AT, Rodnan GP, Medsger TA, Jr, et al. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 22.Kahaleh M, Sultany G, Smith E, Huffstutter J, Loadholt C, LeRoy E. A modified scleroderma skin scoring method. Clin Exp Rheumatol. 1986;4:367–9. [PubMed] [Google Scholar]
- 23.Pope JE, Bellamy N. Outcome measurement in scleroderma clinical trials. Semin Arthritis Rheum. 1993;23:22–33. doi: 10.1016/s0049-0172(05)80024-1. [DOI] [PubMed] [Google Scholar]
- 24.Medsger TA, Jr, Bombardieri S, Czirjak L, Scorza R, Della Rossa A, Bencivelli W. Assessment of disease severity and prognosis. Clin Exp Rheumatol. 2003;21:S42–6. [PubMed] [Google Scholar]
- 25.Chen JD, McCallum RW. Electrogastrographic parameters and their clinical significance. In: Chen JD, editor. Electrogastrography: Principles and Applications. NY: Raven; 1994. pp. 45–74. [Google Scholar]
- 26.Lembo T, Naliboff B, Munakata J, et al. Symptoms and visceral perception in patients with pain predominant irritable bowel syndrome. Am J Gastroenterol. 1999;94:1320–6. doi: 10.1111/j.1572-0241.1999.01009.x. [DOI] [PubMed] [Google Scholar]
- 27.Groll D, Vanner S, Depew W, et al. The IBS-36: a new quality of life measure for irritable bowel syndrome. Am J Gastroenterol. 2002;97:962–71. doi: 10.1111/j.1572-0241.2002.05616.x. [DOI] [PubMed] [Google Scholar]
- 28.Del Rosso A, Boldrini M, Agostino D, et al. Health-related quality of life in systemic sclerosis as measured by the Short Form 36: relationship with clinical and biologic markers. Arthritis Rheum. 2004;51:475–481. doi: 10.1002/art.20389. [DOI] [PubMed] [Google Scholar]
- 29.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 30.Steen VD, Medsger TA., Jr The value of the Health Assessment Questionnaire and special patient-generated scales to demonstrate change in systemic sclerosis patient over time. Arthritis Rheum. 1997;40:1984–91. doi: 10.1002/art.1780401110. [DOI] [PubMed] [Google Scholar]
- 31.Stewart AL, Hays RD, Ware JE., Jr The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26:724–35. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Danieli E, Paolo A, Lorenzo B, et al. Health-related quality of life measured by the Short Form 36 (S-36) in systemic sclerosis: correlations with indexes of disease activity and severity, disability, and depressive symptoms. Clin Rheumatol. 2005;24:48–54. doi: 10.1007/s10067-004-0970-z. [DOI] [PubMed] [Google Scholar]
- 33.Cossutta R, Zeni S, Soldi A, Colombelli P, Belloti Masserini A, Fantini F. Evaluation of quality of life in patients with systemic sclerosis by administering the SF-36 questionnaire. Reumatismo. 2002;54:122–7. doi: 10.4081/reumatismo.2002.122. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–48. [PubMed] [Google Scholar]
- 35.Ware JE. Measuring patients’ views: the optimum outcome measure. BMJ. 1993;306:1429–30. doi: 10.1136/bmj.306.6890.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sjögren RW. Gastrointestinal motility disorders in scleroderma. Arthritis Rheum. 1994;37:1265–82. doi: 10.1002/art.1780370902. [DOI] [PubMed] [Google Scholar]
- 37.Khanna D, Hays RD, Park GS, et al. Development of a preliminary scleroderma gastrointestinal tract 1.0 quality of life instrument. Arthritis Rheum. 2007;57:1280–6. doi: 10.1002/art.22987. [DOI] [PubMed] [Google Scholar]
- 38.Matucci-Cerinic M, Giacomelli R, Pignone A, et al. Nerve growth factor and neuropeptides circulating levels in systemic sclerosis. Ann Rheum Dis. 2001;60:487–94. doi: 10.1136/ard.60.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owyang C. Octreotide in gastrointestinal motility disorders. Gut. 1994;35(Suppl 3):S11–4. doi: 10.1136/gut.35.3_suppl.s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sjolund K, Bartosik I, Lindberg G, Scheja A, Wildt M, Akesson A. Small intestinal manometry in patients with systemic sclerosis. Eur J Gastroenterol Hepatol. 2005;17:1205–12. doi: 10.1097/00042737-200511000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Akesson A, Ekman R. Gastrointestinal regulatory peptides in systemic sclerosis. Arthritis Rheum. 1993;36:698–703. doi: 10.1002/art.1780360519. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Gong Z, Wu K, Wang B, Yuang Y. Gastrointestinal dysmotility in patients with acute pancreatitis. J Gastroenterol Hepatol. 2003;18:57–62. doi: 10.1046/j.1440-1746.2003.02898.x. [DOI] [PubMed] [Google Scholar]
- 43.Bercik P, De Giorgio R, Blennerhassett P, Verdu EF, Barbara G, Collins SM. Immune-mediated neural dysfunction in a murine model of chronic Helicobacter pylori infection. Gastroenterology. 2002;123:1205–15. doi: 10.1053/gast.2002.36024. [DOI] [PubMed] [Google Scholar]
- 44.Kawamura O, Sekiguchi T, Kusano M, Nishioka T, Itoh Z. Effect of erythromycin on interdigestive gastrointestinal contractile activity and plasma motilin concentration in humans. Dig Dis Sci. 1993;38:870–6. doi: 10.1007/BF01295913. [DOI] [PubMed] [Google Scholar]
- 45.Cohen S. The gastrointestinal manifestations of scleroderma: pathogenesis and management. Gastroenterology. 1980;79:155–66. [PubMed] [Google Scholar]
- 46.Sallam H, McNearney TA, Doshi D, Chen JD. Transcutaneous electrical nerve stimulation (TENS) improves upper GI symptoms and balances the sympathovagal activity in scleroderma patients. DigDis Sci. 2007;52:1329–37. doi: 10.1007/s10620-006-9257-3. [DOI] [PubMed] [Google Scholar]
- 47.Ciftci O, Onat AM, Yavuz B, et al. Cardiac repolarization abnormalities and increased sympathetic activity in scleroderma. J Natl Med Assoc. 2007;99:232–7. [PMC free article] [PubMed] [Google Scholar]
- 48.Herrick AL. Pathogenesis of Raynaud’s phenomenon. Rheumatology. 2005;44:587–96. doi: 10.1093/rheumatology/keh552. [DOI] [PubMed] [Google Scholar]
- 49.Hesselstrand R, Scheja A, Shen GQ, Wiik A, Akesson A. The association of antinuclear antibodies with organ involvement and survival in systemic sclerosis. Rheumatology. 2003;42:534–40. doi: 10.1093/rheumatology/keg170. [DOI] [PubMed] [Google Scholar]
- 50.Henault J, Robitaille G, Senecal JL, Raymond Y. DNA topoisomerase I binding to fibroblasts induces monocyte adhesion and activation in the presence of anti-topoisomerase I autoantibodies from systemic sclerosis patients. Arthritis Rheum. 2006;54:963–73. doi: 10.1002/art.21646. [DOI] [PubMed] [Google Scholar]