Abstract
Background
Human papillomavirus (HPV) persistence is the pivotal event in cervical carcinogenesis. We followed a large-scale community-based cohort for 16 years to investigate the role of genotype-specific HPV persistence in predicting cervical cancer including invasive and in situ carcinoma.
Methods
At the baseline examination in 1991–1992, 11 923 participants (aged 30–65 years) consented to HPV testing and cytology; 6923 participants were reexamined in 1993–1995. For HPV testing, we used a polymerase chain reaction–based assay that detected 39 HPV types. Women who developed cervical cancer were identified from cancer and death registries. Cumulative risks for developing cervical cancer among infected and persistently infected women were calculated by the Kaplan–Meier method.
Results
Of 10 123 women who were initially cytologically normal, 68 developed cervical cancer. The 16-year cumulative risks of subsequent cervical cancer for women with HPV16, HPV58 (without HPV16), or other carcinogenic HPV types (without HPV16 or HPV58) were 13.5%, 10.3%, and 4.0%, respectively, compared with 0.26% for HPV-negative women. Women with type-specific persistence of any carcinogenic HPV had greatly increased risk compared with women who were HPV-negative at both visits (hazard ratio = 75.4, 95% confidence interval = 31.8 to 178.9). The cumulative cervical cancer risks following persistent carcinogenic HPV infections increased with age: The risks were 5.5%, 14.4%, and 18.1% for women aged 30–44 years, 45–54 years, and 55 years and older, respectively. However, newly acquired infections were associated with a low risk of cervical cancer regardless of age.
Conclusions
HPV negativity was associated with a very low long-term risk of cervical cancer. Persistent detection of HPV among cytologically normal women greatly increased risk. Thus, it is useful to perform repeated HPV testing following an initial positive test.
CONTEXT AND CAVEATS
Prior knowledge
Persistent infections with carcinogenic types of human papillomaviruses (HPVs), as opposed to cleared infections, are strongly linked to the incidence of cervical cancer. However, there have been few long-term studies to follow persistent HPV infections and quantify risk.
Study design
A community-based cohort of 11 923 women from Taiwan was followed for 16 years. Participants were given baseline exams that included HPV DNA testing and cytological tests in 1991–1992; these tests were repeated for most participants during 2-year follow-up exams. Incidence of cervical cancer was determined from cancer and death registries and HPV type–specific risks were assessed using Kaplan–Meier methods.
Contribution
The 16-year risk of cervical cancer was 6.2% for women infected with any carcinogenic HPV(s), and 13.5%, 10.3%, or 4.0% for women infected with HPV16, HPV58, or other carcinogenic HPVs, respectively, vs 0.26% for HPV-negative women. Among women who were persistently infected with the same carcinogenic HPVs over the 2-year testing interval, risk of cervical cancer was 12.4% vs 0.14% for women who repeatedly tested HPV negative.
Implication
It is useful to repeat HPV testing several years after a positive test to better judge whether a woman is at risk for cervical cancer.
Limitations
HPV typing was not performed at the time of cervical cancer diagnoses and cervical cancers were identified through registries rather than from active long-term follow-up.
From the Editors
Human papillomavirus (HPV) infection is the predominant cause of cervical cancer (1–3). Most HPV-infected women do not have cervical pathology; infections found at young ages (<25 years) are typically acute, sexually transmitted, and destined to resolve (4). Thus, despite the fact that HPV testing has excellent sensitivity and etiologic relevance for cervical cancer detection, it has low positive predictive value, which makes it difficult to use for primary screening, especially among young women who have recently started sexual activity. Although HPV-negative test results are very reassuring, it would be wasteful to refer all HPV-positive women for colposcopic examinations because most of these women are not at risk for cervical cancer (5). To improve the cost-effectiveness of incorporating HPV tests in cervical cancer screening, we need to understand the natural history of HPV infection and cytological abnormalities in the women for whom screening is most commonly being recommended (ie, those aged ≥30 years) and to translate these insights into better screening procedures (6–9).
One important issue is to determine which HPV types we should screen for. Testing only those HPV types which cause most of the cancers might increase specificity while preserving sensitivity. Epidemiological, phylogenetic, and laboratory studies (10–12) have shown that 12 types of the virus (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) are definitely carcinogenic, but with major distinctions between them. For example, HPV16 causes half of the world’s cervical cancers, whereas HPV51 causes only 1%. Another type, HPV68, is probably carcinogenic, and many HPV types that are genetically related to the carcinogenic ones are categorized as “possibly carcinogenic” based partly on occasional detection as single infections in invasive cancers. On the contrary, HPV6 and HPV11 mainly cause genital warts; there is no evidence to support a role for them in cervical carcinogenesis.
Another important topic in HPV screening is how to respond to a positive test. When women are infected at any age, most HPV infections clear within months to a few years. Only persistently detectable infections seem to be associated with risk of cervical neoplasia (13–20). However, very few long-term studies have examined cervical cancer risk following HPV persistence among young women (20).
To determine the long-term risk following HPV infection and persistence on genotype-specific base among women older than 30 years, we examined follow-up data from the Community-based Cancer Screening Program (CBCSP) cohort in Taiwan, which included more than 10 000 women aged 30–65 years. The baseline prevalence of HPV among these women (in 1991–1992) has been reported elsewhere (3). The most common carcinogenic types were HPV52 (2.5%), HPV16 (2.0%), HPV56 (1.8%), HPV18 (1.6%), HPV33 (1.2%), HPV58 (1.3%), and HPV39 (1.0%). In the current study, we were able to advance the knowledge of detection and persistence of specific HPV types in relation to subsequent 16-year risk of cervical cancer.
Methods
Recruitment and Study Design of the Taiwan CBCSP Cohort
The study cohort included 11 923 women aged 30–65 years who were enrolled in January 1, 1991, to December 31, 1992; all of the participants were invited from the list of 41 380 residents from local household registration offices in seven urban and rural townships in Taiwan. They were given a baseline examination, and 6923 participants were reexamined 2 years later in 1993–1995. At recruitment, well-trained public health nurses gave detailed information about the study to each participant. Written informed consent was obtained from each participant who volunteered to participate. The sociodemographic characteristic, cigarette smoking, Pap smear history, reproductive and sexual history, and personal and family history of cancers were obtained by a structured questionnaire. The criteria for ineligibility were lack of a specimen (n = 1170) and reported hysterectomy according to the questionnaire (n = 138). After exclusion of 13 women with cervical cancer diagnosed before enrollment, 10 602 women were eligible as HPV cohort members (3). This study was approved by the Institutional Review Board of the National Taiwan University College of Public Health.
Inclusion Criteria of Participants in Long-term Follow-up
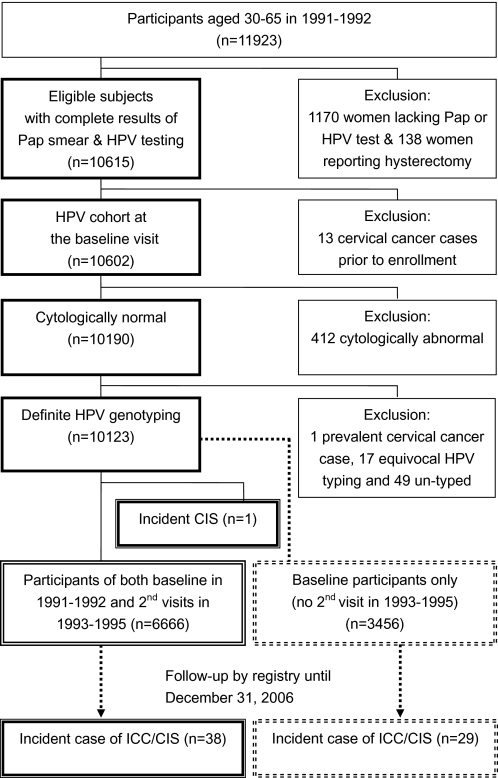
Women (n = 412) with an abnormal Pap smear were excluded; further exclusions were made for prevalent cervical cancer (n = 1), equivocal HPV typing (n = 17), and untyped specimens (n = 49). In total, 10 123 women with normal conventional cytology (Pap smears) at baseline and definite HPV testing were included for analysis (Figure 1). After exclusion of one incident carcinoma in situ (CIS), 6666 women participated in both baseline and second visits, whereas the other 3456 participants were only examined once at the baseline visit.
Figure 1.
CONSORT diagram of study participants in long-term follow-up study on cervical neoplasia, including carcinoma in situ (CIS) and invasive cervical cancer (ICC). At the baseline examination in 1991–1992, 11 923 participants (aged 30–65 years) consented to human papillomavirus (HPV) testing and cytology (Pap); following the exclusions noted, 6923 participants were reexamined in 1993–1995. Women who developed cervical cancer were identified from cancer and death registries, permitting calculation of cumulative risk related to baseline status and also related to the combination of results from baseline and second examinations. Only women who were initially normal were included for the analyses for baseline HPV infection (n = 10123) and repeated HPV testing (n = 6666).
Specimen Collection at the Baseline and the Second Visits
A Cervex Brush collection device (Rovers, Oss, the Netherlands) was used to obtain exfoliated cervical cells. The cervical smears were stained using Papanicolaou stain and read at the Taipei Institute of Pathology. Pap smears were graded according to the 1991 version of the Bethesda system (21), which categorized cytological results as normal, atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), and cancer. Abnormal cytology results regarding glandular cells, such as adenocarcinoma, were exceedingly rare and grouped with the squamous lesions of the equivalent severity.
ViraPap (Digene Diagnostic, now Qiagen, Gaithersburg, MD) kits were used (subsequently to the Cervex Brush) to collect and preserve cervical cells for HPV DNA testing. The cells were stored at −80°C at National Taiwan University until HPV DNA testing, which was performed in 2004–2005 at Yuan-Shan Research Institute.
Laboratory Methods for HPV DNA and Genotyping
Genomic and HPV DNA was prepared from a 100-μL aliquot of each thawed cervical cell sample using Qiamp 96 DNA blood kits (Qiagen Inc, Venlo, the Netherlands). For each sample, polymerase chain reaction (PCR) assays were performed once using a MY11/biotinylated GP6+ (192 bp) primer that targeted DNA amplification to the L1 region of the HPV genome and a 2-μL aliquot of purified mixed genomic and HPV DNA in a total volume of 25-μL. All specimens were tested by the EasyChip HPV genotyping array (King-Car, I-Lan, Taiwan) and gel electrophoresis. The EasyChip HPV blot simultaneously detects 39 types of HPV (types 6, 11, 16, 18, 26, 31, 32, 33, 35, 37, 39, 42, 43, 44, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 66, 67, 68, 69, 70, 71 [CP8061], 72, 74, 81 [CP8304], 82 [82 and MM4], 83 [MM7], 84 [MM8], and 85 [L1AE5]) on the basis of reverse blot hybridization. On a 9.6 × 1.44 mm nylon membrane, duplicate dots of HPV type–specific probes were deposited symmetrically, and then bound to the amplified HPV DNA from cervical samples. Every positive result was confirmed by repeating amplification and genotyping in an independent assay. After PCR, each specimen was tested by EasyChip. PCR products were subsequently subjected to agarose gel electrophoresis and stained with ethidium bromide to test for a visible band at the expected position. To minimize any chance of false-positive results, a specimen was only considered positive by EasyChip if there was also a positive result by gel electrophoresis. Each experimental batch included 89 test samples and seven control samples for quality monitoring. Two aliquots from a stock of cervical cells infected with a known HPV type were used as control samples to monitor reproducibility. Three cell line controls, HeLa (HPV18-integrated), CaSki (HPV16-integrated), and Jurkat cells (American Type Culture Collection, Manassas, VA), were also used to determine the accuracy and effectiveness of the test. As a PCR positive control, a 136-bp fragment of GAPDH (human glyceraldehyde-3-phosphate dehydrogenase) gene from a male blood sample was processed and confirmed. A sterile water control was used to monitor contamination in both the HPV and GAPDH reactions. Quality assurance of the experiments was evaluated with a triple-blind procedure that concealed sample, experimenter, and result reader identities, with 97% typing reproducibility (22). Notably, the World Health Organization HPV Laboratory Network (LabNet) examined the EasyChip assay in its 2010 HPV DNA Proficiency Study and validated its 100% proficiency and 0% false-positive rate for detection of HPV types 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68b (23).
Ascertainment of Cervical Cancer Including Carcinoma In Situ During Follow-up
Through data linkage with computerized registries of the Taiwan National Cancer Registry and National Death Certification Registry, we identified cohort members with newly diagnosed cervical cancers until December 31, 2006, using the ICD-9 code 180. All diagnoses of cancer were based on histology and were further stratified into CIS and invasive cervical cancer (ICC). Morphologically, verification was available for 98.2% of invasive cervical cancers (24).
Statistical Analysis
We used the most recent evaluation (11,12) by the International Agency for Research on Cancer (IARC) to group the HPV types detected by the EasyChip assay: Carcinogenic HPV types included 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59; probably or possibly carcinogenic types included 26, 53, 66, 67, 68,70, 73, and 82; and other HPV types were not thought to be carcinogenic or were unspecified by IARC.
We categorized HPV status on a type-specific basis according to our findings at the baseline and second examinations (2 years later). Women were deemed to have persistent HPV infections if the same HPV type was present at both examinations. They were determined to have cleared the infection if a specific HPV type was found at baseline but not at the second examination. They were said to have acquired a new infection if an HPV type was present at the second examination that was not present at the baseline examination. “Pure acquisition” was defined as complete HPV negativity at the baseline examination with the acquisition of new HPV infection(s) at the second examination.
Incidence of ICC and CIS following baseline infection was defined as the number of women with these diagnoses divided by person-time, which was measured from study entry to time of cancer diagnosis for women who developed ICC or CIS and from study entry to December 31, 2006, or date of death for women who did not develop ICC or CIS. When calculating the incidence of ICC or CIS in women who had persistent, cleared, or acquired infections, person-time was calculated from the second examination. The Kaplan–Meier method was used to estimate cumulative risk of ICC or CIS. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were estimated using Cox proportional hazard models; the proportionality of hazards was verified by modeling that showed no statistically significant change in hazard ratios with increasing follow-up time. All statistical tests were two-sided. All computations were performed using SAS 9.1.3 (SAS Institute, Cary, NC).
Results
We enrolled 10 123 cytologically normal women with a mean age of 46.3 years; the mean duration of follow-up was 14.5 years. There were 6666 participants who were included in analyses of persistent HPV infections; one woman was diagnosed with cervical cancer between the baseline and second examination and was excluded from this analysis (Figure 1). A total of 68 women with incident cervical cancers, including 34 with CIS and 34 with ICC, were diagnosed before December 31, 2006.
Incidence of Cervical Cancer by HPV Infection
Within this cohort, the incidence of cervical cancers, including CIS and ICC, was 46.4 cancers per 100 000 person-years of follow-up (Table 1). There were 252.0 cancers per 100 000 person-years among women who were HPV positive at baseline and only 15.7 cancers per 100 000 person-years for women who were HPV negative at baseline; thus, women who were HPV positive had a much higher risk of cervical cancer compared with those who were HPV negative (HR = 16.2, 95% CI = 9.6 to 27.3). When HPV infections were divided into categories of carcinogenic potential, the risk of cervical cancer was greatest among women infected with the carcinogenic types (HR = 23.8, 95% CI = 14.0 to 40.7), less for those with the probably or possibly carcinogenic HPV types when no definitely carcinogenic types were present (HR = 8.9, 95% CI = 2.6 to 29.9), and much less for those infected with other types alone (HR = 4.4, 95% CI = 1.5 to 12.9) for other types found alone compared with HPV-negative women. There were only three women who developed CIS but no one who developed ICC after infection with probably or possibly carcinogenic types (without carcinogenic types). No women who were infected with only other HPV types developed ICC.
Table 1.
Incidence (per 100 000 person-years) and risk of histologically confirmed cervical carcinoma in situ (CIS) and/or invasive cancer (ICC) in relation to human papillomavirus (HPV) status at baseline and second examinations among 10 123 women who were cytologically normal at study entry
| HPV infection | No. of women (%) | No. of cancers (ICC/CIS) | Incidence (10−5)* | HR (95% CI) | P† |
| Baseline examination | 10123 (100.0) | 68 (34/34) | 46.4 | ||
| HPV negative | 8780 (86.7) | 20 (9/11) | 15.7 | 1.0 (referent) | |
| HPV positive (any type) | 1343 (13.3) | 48 (25/23) | 252.0 | 16.2 (9.6 to 27.3) | <.001 |
| Carcinogenic types‡ | 784 (7.7) | 41 (24/17) | 370.8 | 23.8 (14.0 to 40.7) | <.001 |
| Probably or possibly carcinogenic types§ | 161 (1.6) | 3 (0/3) | 136.2 | 8.9 (2.6 to 29.9) | <.001 |
| Other HPV types alone‖ | 398 (3.9) | 4 (1/3) | 69.1 | 4.4 (1.5 to 12.9) | .007 |
| Second examination¶ (subgroup) | 6666 (100.0) | 37 (18/19) | 42.5 | ||
| HPV negative | 5769 (86.5) | 9 (3/6) | 13.2 | 1.0 (referent) | |
| HPV positive (any type) | 897 (13.5) | 28 (15/13) | 243.4 | 20.6 (9.7 to 43.6) | <.001 |
| Carcinogenic types‡ | 479 (7.2) | 24 (13/11) | 395.7 | 33.5 (15.6 to 72.0) | <.001 |
| Probably or possibly carcinogenic types§ | 115 (1.7) | 1 (0/1) | 67.2 | 5.7 (0.7 to 43.1) | .098 |
| Other HPV types alone‖ | 303 (4.5) | 3 (2/1) | 75.9 | 6.4 (1.7 to 23.7) | .005 |
Incidence rate: per 100 000 person-years.
Hazard ratios (HRs) and their 95% confidence intervals (CIs) were estimated using Cox proportional hazard models. All statistical tests were two-sided.
Carcinogenic types include HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59.
Probably or possibly carcinogenic types include: HPV68 and HPV26, 53, 66, 67, 70, 73, and 82 (without any carcinogenic type[s], as listed previously).
Other HPV types, including HPV6, 11, 32, 37, 42, 43, 44, 54, 55, 61, 62, 69, 71, 72, 74, 81, 83, 84, and 85 (without any carcinogenic or any probably or possibly carcinogenic type[s], as listed previously).
HPV infection status at the second examination 2 years later (regardless of HPV status at baseline).
When we performed a similar analysis of a subgroup of 6666 women based on single time-point results at the second examination, women with carcinogenic HPV infections were again at much higher risk for incident ICC or CIS than women with HPV negativity (HR = 33.5, 95% CI = 15.7 to 72.0).
Prediction of Cervical Cancer by HPV Genotype
HPV16 was the most commonly detected HPV type at baseline before a cervical cancer diagnosis (Table 2): HPV16 positivity predicted 13 cancers (nine ICC and four CIS), followed by HPV52 (four ICC and six CIS), HPV58 (seven ICC and two CIS), HPV18 (five ICC and two CIS), and HPV31 (four ICC and two CIS). Multiple infections with these HPV types were rare, ruling out meaningful confounding between them. Because ICC or CIS rarely developed among HPV-negative women, infection with any of these carcinogenic types was associated with high hazard ratios (for HPV16, HR = 43.6 [95% CI =21.7 to 87.6]; for HPV52, HR = 24.3 [95% CI = 11.4 to 51.8]; for HPV58, HR = 49.4 [95% CI = 22.5 to 108.5]; for HPV18, HR = 21.7 [95% CI = 9.2 to 51.3]; and for HPV31, HR =56.9 [95% CI = 22.9 to 141.8]; Table 2).
Table 2.
Incidence (per 100 000 person-years) of cervical carcinoma in situ (CIS) and invasive cancer (ICC) among 10 123 cytologically normal women by type-specific human papillomavirus (HPV) infections at baseline and second examinations*
| Cohort with baseline examination (n = 10 123) |
Cohort with both baseline and second examinations (n = 6666) |
||||||||||||||
| Baseline HPV infection |
Genotype-specific persistence |
Acquisition† |
Clearance |
||||||||||||
| HPV type | No. of women | No. of cancers (ICC/CIS) | Incidence (10−5)§ | HR (95% CI) | P‖ | No. of women | No. of cancers (ICC/CIS) | Incidence (10−5)§ | HR (95% CI) | P‖ | No. of women | No. of cancers (ICC/CIS) | No. of women | No. of cancers (ICC/CIS) | Ratio of persistence vs baseline infection ‡ |
| HPV negative | 8780 | 20 (9/11) | 15.7 | 1.0 (referent) | 5396 | 7 | 9.9 | 1.0 (referent) | |||||||
| Carcinogenic types¶ | |||||||||||||||
| Any carcinogenic type | 784 | 41 (24/17) | 370.8 | 23.8 (14.0 to 40.7) | <.001 | 205 | 21 | 838.3 | 85.1 (36.2 to 200.3) | <.001 | 300 | 5 | 345 | 5 | 3.4 |
| 16 | 139 | 13 (9/4) | 675.5 | 43.6 (21.7 to 87.6) | <.001 | 21 | 5 | 2204.6 | 216.7 (68.7 to 683.3) | <.001 | 48 | 0 | 64 | 1 | 5.0 |
| 52 | 189 | 10 (4/6) | 376.9 | 24.3 (11.4 to 51.8) | <.001 | 74 | 6 | 676.9 | 69.5 (23.3 to 206.79) | <.001 | 54 | 2 | 56 | 0 | 2.9 |
| 58 | 85 | 9 (7/2) | 766.5 | 49.4 (22.5 to 108.5) | <.001 | 32 | 4 | 1045.8 | 105.1 (30.7 to 358.9) | <.001 | 30 | 0 | 22 | 0 | 2.1 |
| 18 | 145 | 7 (5/2) | 338.1 | 21.7 (9.2 to 51.3) | <.001 | 23 | 2 | 688.5 | 68.3 (14.2 to 328.4) | <.001 | 43 | 1 | 66 | 1 | 3.1 |
| 31 | 48 | 6 (4/2) | 888.2 | 56.9 (22.9 to 141.8) | <.001 | 16 | 3 | 1545.6 | 156.3 (40.4 to 604.9) | <.001 | 14 | 0 | 14 | 0 | 2.7 |
| 33 | 94 | 4 (2/2) | 292.0 | 18.6 (6.3 to 54.3) | <.001 | 14 | 4 | 2189.4 | 221.3 (64.8 to 756.1) | <.001 | 21 | 1 | 54 | 0 | 11.9 |
| 56 | 86 | 4 (2/2) | 327.1 | 21.1 (7.2 to 61.7) | <.001 | 9 | 3 | 2601.9 | 269.6 (69.7 to 1043.6) | <.001 | 38 | 0 | 44 | 1 | 12.8 |
| 35 | 36 | 3 (2/1) | 598.9 | 38.9 (11.6 to 130.9) | <.001 | 5 | 1 | 1529.1 | 151.1 (18.6 to 1228.1) | <.001 | 11 | 0 | 18 | 2 | 3.9 |
| 39 | 84 | 2 (2/0) | 166.6 | 10.7 (2.5 to 45.9) | .001 | 21 | 2 | 725.7 | 73.9 (15.4 to 356.1) | <.001 | 29 | 0 | 32 | 0 | 6.9 |
| 45 | 48 | 2 (2/0) | 293.6 | 18.9 (4.4 to 81.2) | <.001 | 6 | 1 | 1322.8 | 134.5 (16.5 to 1094.0) | <.001 | 23 | 1 | 27 | 0 | 7.1 |
| 51 | 64 | 2 (1/1) | 215.0 | 13.7(3.2 to 58.5) | <.001 | 12 | 2 | 1282.1 | 132.8 (25.6 to 639.4) | <.001 | 25 | 0 | 31 | 0 | 9.7 |
| 59 | 69 | 0 | 0.0 | 0.0# | 4 | 0 | 0.0 | 0.0# | 8 | 0 | 11 | 0 | — | ||
| Probably or possibly carcinogenic types** | |||||||||||||||
| Any probably or possible carcinogenic type | 281 | 8 (4/4) | 204.7 | 13.3 (5.8 to 30.1) | <.001 | 70 | 5 | 560.5 | 57.3 (18.2 to 180.5) | <.001 | 118 | 2 | 105 | 0 | 4.3 |
| 68 | 55 | 2 (1/1) | 264.1 | 17.2 (4.0 to 73.6) | <.001 | 13 | 1 | 577.0 | 57.4 (7.1 to 466.7) | <.001 | 23 | 0 | 22 | 0 | 3.3 |
| 53 | 97 | 4 (2/2) | 293.35 | 19.0 (6.5 to 55.6) | <.001 | 22 | 2 | 748.8 | 75.7 (15.7 to 364.5) | <.001 | 39 | 1 | 29 | 0 | 4.0 |
| 70 | 84 | 3 (2/1) | 259.8 | 17.0 (5.1 to 57.3) | <.001 | 26 | 3 | 900.1 | 91.3 (23.6 to 353.2) | <.001 | 25 | 1 | 28 | 0 | 5.4 |
| 67 | 27 | 1 (1/0) | 258.2 | 16.3 (2.2 to 121.6) | .006 | 3 | 1 | 2512.6 | 252.8 (31.1 to 2056.4) | <.001 | 6 | 0 | 12 | 0 | 15.5 |
| 82 | 19 | 1 (1/0) | 374.3 | 24.4 (3.3 to 182.0) | .002 | 7 | 1 | 1132.5 | 116.4 (14.3 to 947.6) | <.001 | 13 | 0 | 9 | 0 | 4.8 |
| 26 | 11 | 0 | 0.0 | 0.0# | 3 | 0 | 0.0 | 0.0# | 8 | 0 | 6 | 0 | — | ||
Some women were infected with more than one HPV type. There were 6667 initially normal women who participated in the second visit; among them, 38 cervical cancers occurred after the second visit. One cancer that occurred between the two visits was excluded from the analysis of 2-year persistence. Only 3456 initially normal women participated in the baseline visit and 29 of them developed cervical cancer (Figure 1).
Acquisition of given type indicated that the woman was negative for the given type at baseline.
The ratio is the quotient of hazard ratio of persistent infection divided by hazard ratio of baseline infection from the given type.
Incidence rate: per 100 000 person-years.
Hazard ratios (HRs) and their 95% confidence intervals (CIs) were estimated using Cox proportional hazard models. All statistical tests were two-sided.
Carcinogenic types include HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59.
There was no incident cancer for calculation of 95% confidence intervals.
Probably or possibly carcinogenic types include HPV68 and HPV26, 53, 66, 67, 70, 73, and 82 (without any carcinogenic type[s], as listed above).
Prediction of Cervical Cancer by Persistence, Acquisition, and Clearance
In type-by-type analyses, persistence of genotype-specific HPVs at the second examination predicted extremely high incidences and hazard ratios of ICC or CIS for virtually all carcinogenic types and some possibly carcinogenic types, compared with persistent HPV negativity (Table 2, right columns). The hazard ratios ranged from 2.1 (HPV58) to 12.8 (HPV56) times greater for persistent infections than for baseline (single time-point) infections. Only one woman with ICC had a single persistent infection with an HPV type thought not to be carcinogenic (HPV81), suggesting that HPV81 might be carcinogenic or, more likely, that a carcinogenic infection was not detected at enrollment.
Acquisition of HPV at the second visit rarely led to ICC or CIS over the period studied; during 14 years of follow-up, only eight cancers were linked to incident infections, and seven of them had concurrent persistence of other carcinogenic HPV types; the remaining one was associated with acquisition of a putative noncarcinogenic HPV type, HPV62, between the two visits. Clearance was also rarely followed by a diagnosis of ICC or CIS.
Cumulative Risk of Cervical Cancer by HPV Infection and Persistence
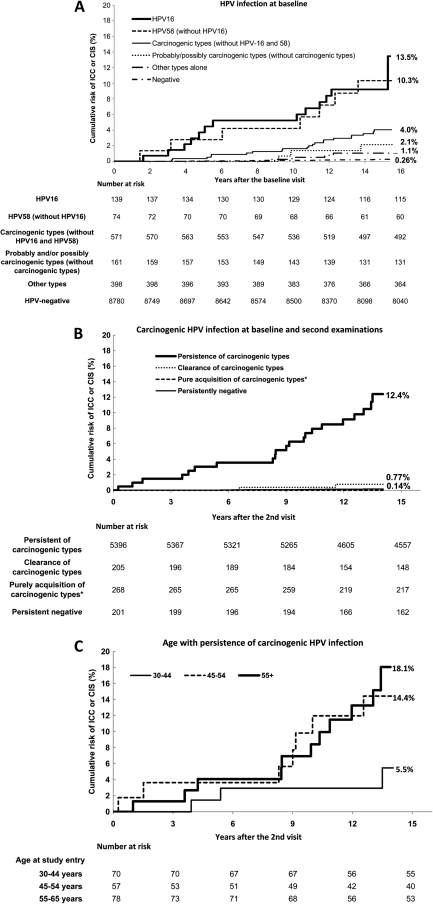
From our data, we graphed (Figure 2, A) the cumulative risks of an ICC or a CIS diagnosis with up to 16 years of follow-up after baseline infection by HPV16 (13.5%), by HPV58 without HPV16 (10.3%), by any carcinogenic type without HPV16 or HPV58 (4.0%), by any probably or possibly carcinogenic type without a definitely carcinogenic one (2.1%), and by other HPV types (1.1%). HPV-negative women had a very low risk of ICC or CIS (0.26%). The corresponding risk was 6.2% among women infected with any carcinogenic HPV. We also graphed the risk of ICC or CIS among women whose HPV status was determined at baseline and second (2-year) examinations (Figure 2, B). We found high risk following persistence of carcinogenic HPV types (12.4%) compared with the low risk following pure acquisition of carcinogenic types (0%).
Figure 2.
Kaplan–Meier estimates of cumulative risk (%) of histologically confirmed cervical carcinoma in situ (CIS) and invasive cancer (ICC) during 16-year follow-up by type-specific human papillomavirus (HPV) infection. The cumulative risk by various HPV infection status and age at study entry were derived by using Kaplan–Meier method. A) Cumulative risk by type-specific HPV infection at baseline examination: 13.5% (95% confidence interval [CI] = 6.5% to 28.0%) for HPV16 infection, 10.3% (95% CI = 4.9% to 21.7%) for HPV58 infection, 4.0% (95% CI = 2.6% to 6.2%) for carcinogenic types (without HPV16 and HPV58) infection, 2.1% (95% CI = 0.7% to 6.6%) for probably and/or possibly carcinogenic types (without carcinogenic types) infection, 1.1% (95% CI = 0.4% to 2.8%) for other types infection, and 0.26% (95% CI = 0.17% to 0.41%) for HPV-negative women. B) Cumulative by HPV infection of carcinogenic types at baseline and second examinations: 12.4% (95% CI = 8.0% to 19.2%) for persistent of carcinogenic types, 0.77% (95% CI = 0.19% to 3.1%) for clearance of carcinogenic types, 0.0% for purely acquisition of carcinogenic types, and 0.14% (95% CI = 0.07% to 0.3%) for persistent negative. Asterisk indicates there were no cases of CIS or cancer among women with pure acquisition of carcinogenic HPV types in the absence of persistence or clearance of other types. C) Cumulative risk by age at study entry for persistent carcinogenic type (on a type-specific basis): 5.5% (95% CI = 1.7% to 17.7%) for women aged 30–44 years, 14.4% (95% CI = 6.9% to 30.4%) for women aged 45–54 years, 18.1% (95% CI = 9.8% to 33.3%) for women aged 55–65 years.
Age Effect
The risk of ICC or CIS following persistence of baseline infections with carcinogenic HPVs increased with age (Figure 2, C). With up to 14 years follow-up since the second examination, cumulative risks for women aged 30–44, 45–54, and 55–65 years at study entry were 5.5%, 14.4%, and 18.1%, respectively (Figure 2, C). There were very low risks of ICC or CIS among women who were HPV negative at baseline and across age groups (0.2%–0.3%, data not shown). At the extreme, women aged 55 years and older who were persistently HPV negative as of 1993–1995 had an extremely low risk (0.08%) of subsequently developing cervical cancer; only one woman was diagnosed with cervical intraepithelial neoplasia 3 (CIN3) after 9.6 years. However, we found age differences in risk of ICC or CIS only among the women who had prevalent HPV infections at baseline; in our study, age did not modify the consistently low risk of new infections acquired between the two examinations.
To further explore the effects of age, we compared hazard ratios for incident CIS or ICC by presence of HPV infection at baseline and second examination for women who were aged 30–44 years, 45–54 years, or 55 years and older (Table 3). Women with persistence of any carcinogenic HPV type had much more risk of incident cervical carcinoma compared with women who were HPV negative at both visits (HR = 75.4, 95% CI = 31.8 to 178.9). The hazard ratios for CIS or ICC among women who were infected by carcinogenic HPV types at the baseline examination, compared with HPV-negative women, were 11.0, 35.2, and 48.5, respectively, for women who were aged 30–44 years, 45–54 years, and 55 years or older. Notably, hazard ratios among women who were persistently infected by carcinogenic HPV types increased to 37.8 (95% CI = 7.6 to 187.6) for women who were 30–44 years old, to 71.2 (95% CI = 8.4 to 275.4) for women at 45–54 years, and to 190.9 (95% CI = 24.6 to 1477.5) for women aged 55 years and older.
Table 3.
Hazard ratios (HRs) with 95% confidence intervals (CIs) of incident cervical carcinoma in situ (CIS) and invasive cancer (ICC) by age at study entry and human papillomavirus (HPV) infections at baseline and second examinations
| HR (95% CI) per age at study entry | ||||||||
| HPV infection | Entire cohort* | P† | 30–44 years | P† | 45–54 years | P† | ≥ 55 years | P† |
| Baseline examination | ||||||||
| HPV negative | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | ||||
| Carcinogenic HPV type(s)‡ | 23.0 (13.5 to 39.5) | <.001 | 11.0 (4.7 to 25.4) | <.001 | 35.2 (12.7 to 97.7) | <.001 | 48.5 (14.2 to 165.5) | <.001 |
| Second examination | ||||||||
| Persistently HPV negative | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | ||||
| Pure acquisition of any carcinogenic type(s)§ | 0.0‖ | 0.0 | 0.0 | 0.0 | ||||
| Persistence of any carcinogenic type(s)¶ | 75.4 (31.0 to 178.9) | <.001 | 37.9 (7.6 to 187.6) | <.001 | 71.2 (18.4 to 275.4) | <.001 | 190.7 (24.6 to 1477.5) | <.001 |
TheHRs for the entire cohort were adjusted with age at study entry.
Hazard ratios and their 95%CIs were estimated using Cox proportional hazard models. All statistical tests were two-sided.
Carcinogenic types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59).
They were HPV negative at baseline.
There was no incident case for calculation of 95% confidence intervals.
Persistence was defined on a type-specific basis.
Discussion
In this long-term (16-year) prospective study, we confirmed the critical role of persistent carcinogenic HPV infections in predicting risk of subsequent cervical neoplasia in women aged 30 years and older.
The incidence of ICC or CIS following baseline infection by the 12 carcinogenic HPV types was 0.37% per person-year, which was more than 20 times the risk in HPV-negative women. We observed a sustained low risk of ICC or CIS in absence of any carcinogenic HPV type at baseline. In fact, no ICC and only one CIS developed among HPV-negative women who were aged 55 years and older. The extraordinary risk stratification of cervical cancer provided by HPV testing in this cohort supports the usefulness of HPV testing, at long intervals, for cervical cancer screening.
Persistence of carcinogenic HPV infections was critical to the magnitude of ICC or CIS risk. Type-specific HPV persistence for a duration of 2 years elevated cervical cancer risk substantially for all individual carcinogenic HPV types: Risks were elevated two- to tenfold compared with single time-point detection. HPV infections that are detected only at a single time-point tend to clear rapidly; clearance gradually reaches a plateau around 3–5 years of follow-up (25). Two-year persistence (40.9%) in our study served as a biomarker of chronic or long-term infection, which was linked to increased risk of subsequent cancer development. As the duration of infection increased, risk increased; thus, the risk of ICC or CIS following persistence increased with age, in line with the known natural history of HPV infections that are usually acquired sexually at young ages. Our findings suggest that, if upon testing an HPV infection is found, retesting 2 years later would provide useful guidance as to the duration of infection and its risk. Although the theoretical importance of persistence cannot be overemphasized, many women and their clinicians are reluctant to wait for clearance because the first positive result (especially at older ages) does indicate increased risk compared with a negative result. Clinical guidelines will need to achieve a balance of clinical attention and restraint.
When baseline HPV infections cleared rather than persisted, subsequent cervical cancer risk was extremely low. This finding demonstrated that overt persistence rather than one-time infection is associated with a high risk of cancer. In fact, we observed low cervical cancer risk following HPV acquisition at any age, confirming that age does not modify the cancer risk following incident infections (17). Because it is the duration of overtly detectable infection that most strongly predicts cancer risk, it would not be useful to repeat HPV testing on a frequent basis. Any new infections that might be found would be low-risk, and could not be interpreted like prevalent (possibly persistent) infections found on the first screen. The proper interval for repetition of HPV screening is not yet known, but it may exceed 5 years (26).
Our findings extend the conclusions of recent randomized clinical trials that showed a high sensitivity of HPV testing to predict the risk of CIS over shorter intervals of a few years (6–9). However, in our study, HPV testing at a single time point did not perfectly predict outcome during the entire follow-up period. Only 13% of participants were HPV positive at baseline, and they comprised 70% of all of the women who developed ICC or CIS. In other words, 30% of the cancers were diagnosed in the remaining 87% of participants who were HPV negative initially. These cancers were presumably caused by new infections that were acquired after entry into the study. New infections acquired after 30 years of age confer low risk (ie, in terms of the fraction of cancers, including CIS, that they cause compared with infections acquired earlier in life) but they are not without risk. It is quite possible that some cases of CIS and even ICC were caused by carcinogenic HPV infections that were acquired after the baseline and/or after the second examination 2 years later. However, the fraction of ICC diagnoses without HPV detection did not increase with time since the last screening (data not shown). In other words, a fraction of the women with ICC who were diagnosed throughout follow-up were HPV negative when we initially measured them, for unknown reasons. As the only tentative clue, women with cancers who were not preceded by HPV detection tended to be younger than those in whom we did find HPV infections. It is important to point out that during 16 years follow-up, only nine out of 8780 women who were baseline HPV negative developed ICC, and only three out of 5396 women who tested negative at both the baseline and second examinations developed ICC. These estimates might be useful to guide publicly funded prevention efforts, which will need to consider how often to rescreen women who initially tested HPV negative.
The importance of HPV16, HPV58, HPV52, HPV18, HPV31, and HPV33 in this study was consistent with our previous cross-sectional study reporting the association between prevalent cervical cancer and HPV infection at baseline (3), and the distribution of HPV types in cervical cancers diagnosed in Asia (27–29). Moreover, our findings complement a recent long-term follow-up among young women aged 20–29 years in Denmark that observed similar risk stratification related to HPV persistence vs HPV negativity (20) and are consistent with other cohort data (30).
Compared with detection of all carcinogenic HPV types as a pool, genotyping may provide further information to improve the specificity of HPV testing. Even among the carcinogenic types, HPV16 and HPV58 were found to cause a higher risk of ICC or CIS than other carcinogenic HPV types. Full typing of HPVs may be useful to optimize the sensitivity and specificity of HPV test for detecting cervical cancers; alternatively, partial typing might provide as much risk stratification as clinicians need.
The major limitation of our study was the lack of HPV typing at the time of cervical cancer diagnoses. We might have misclassified the causal HPV types in some cases due to the transient nature of HPV infections. Moreover, our use of registry linkage rather than active follow-up probably led to under-ascertainment of cervical cancers, particularly for CIS. In this context, we did note a higher proportion of ICC among all of the cervical cancers in this prospective series (34/68 = 50.0%) than in the data from baseline (20/56 = 35.7%). Along the same lines, we noticed an apparent difference between the cumulative risk of ICC or CIS in our study and cumulative risk of CIN3, which includes CIS, in Western countries (20). In Western countries, the cumulative incidence of CIN3 is relatively high, and it is much more frequent than ICC. In comparison, in Taiwan, we saw less CIS, even at baseline. The difference in CIN3 diagnosis might be due in part to CIS being a slightly more stringent and serious diagnosis, or to less-intensive screening in Taiwan. Also, many women with the precursors to CIN3 may have experienced regression of their disease over time before they entered the age range of the cohort.
In conclusion, this cohort study extends to 16 years the usefulness of HPV testing for cervical cancer screening. In women older than 30 years who test HPV positive, it is helpful to perform a repeated HPV test 2 years later to improve the predictive value and specificity of cervical cancer screening. The accumulated evidence suggests that it is time to include HPV testing in cancer screening programs for the general population. HPV negative women will obtain superior reassurance of reduced risk. The challenge that remains is to devise optimal management guidelines for HPV-positive women, which may include a careful wait-and-see approach to monitor viral persistence vs clearance.
Funding
This work was supported by the Bureau of Health Promotion, Department of Health (93-028) and the National Science Council (NSC 94-2314-B-001-011 and 95-2314-B-001-007; 96-2314-B-001-004 and 97-2314-B-001-001-MY3) in Taiwan. There were no financial contributions from any other organization or industry, such as Merck and Company or King-Car Company. Dr Schiffman and Dr Hsing were supported by the Intramural Research Program of the National Institutes of Health, USA.
Footnotes
The study sponsors played no role in the design of the study; the collection, analysis, or interpretation of the data; neither the writing of the article, and the decision to submit the article for publication. Kai-Li Liaw holds stock in Merck and Company, Inc, the maker of Gardasil, and is currently an employee of Merck and Company. The other authors claim no conflicts of interest with this work.
The authors are grateful to research assistants in the genomics research center of Academia Sinica and to colleagues at the Yuan-Shan Institute of King-Car Co. We appreciate the help of Mr. Ruey-Wen Lin and Ms. Chiou-Mein You for setting up the standard operation protocols of HPV genotyping, and Dr Mahboobeh Safaeian (NCI and NIH) for sharing important comments. The enrollment of participants and collection of specimens were co-funded by the Department of Health (Taiwan) and the National Institutes of Health (USA).
References
- 1.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87(11):796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 2.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Chen HC, You SL, Hsieh CY, et al. Prevalence of genotype-specific human papillomavirus infection and cervical neoplasia in Taiwan: a community-based survey of 10602 women. Int J Cancer. 2011;128(5):1192–1203. doi: 10.1002/ijc.25685. [DOI] [PubMed] [Google Scholar]
- 4.Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132(2):277–284. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman M, Khan MJ, Solomon D, et al. A study of the impact of adding HPV types to cervical cancer screening and triage tests. J Natl Cancer Inst. 2005;97(2):147–150. doi: 10.1093/jnci/dji014. [DOI] [PubMed] [Google Scholar]
- 6.Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 7.Naucler P, Ryd W, Tornberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357(16):1589–1597. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 8.Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370(9601):1764–1772. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- 9.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11(3):249–257. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 10.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 11.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009;10(4):321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 12.Schiffman M, Clifford G, Buonaguro F. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer. 2009;4(1):8. doi: 10.1186/1750-9378-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ylitalo N, Sorensen P, Josefsson AM, et al. Consistent high viral load of human papillomavirus 16 and risk of cervical carcinoma in situ: a nested case-control study. Lancet. 2000;355(9222):2194–2198. doi: 10.1016/S0140-6736(00)02402-8. [DOI] [PubMed] [Google Scholar]
- 14.Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA. 2001;286(24):3106–3114. doi: 10.1001/jama.286.24.3106. [DOI] [PubMed] [Google Scholar]
- 15.Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ. 2002;325(7364):572. doi: 10.1136/bmj.325.7364.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kjaer S, Hogdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66(21):10630–10636. doi: 10.1158/0008-5472.CAN-06-1057. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102(5):315–324. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munoz N, Hernandez-Suarez G, Mendez F, et al. Persistence of HPV infection and risk of high-grade cervical intraepithelial neoplasia in a cohort of Colombian women. Br J Cancer. 2009;100(7):1184–1190. doi: 10.1038/sj.bjc.6604972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trottier H, Mahmud SM, Lindsay L, et al. Persistence of an incident human papillomavirus infection and timing of cervical lesions in previously unexposed young women. Cancer Epidemiol Biomarkers Prev. 2009;18(3):854–862. doi: 10.1158/1055-9965.EPI-08-1012. [DOI] [PubMed] [Google Scholar]
- 20.Kjaer SK, Frederiksen K, Munk C, et al. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010;102(19):1478–1488. doi: 10.1093/jnci/djq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liaw KL, Hsing AW, Chen CJ, et al. Human papillomavirus and cervical neoplasia: a case-control study in Taiwan. Int J Cancer. 1995;62(5):565–571. doi: 10.1002/ijc.2910620513. [DOI] [PubMed] [Google Scholar]
- 22.Lin CY, Chen HC, Lin RW, et al. Quality assurance of genotyping array for detection and typing of human papillomavirus. J Virol Methods. 2007;140(1–2):1–9. doi: 10.1016/j.jviromet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Eklund C, Wallin KL, Forslund O, et al. Report on HPV DNA Proficiency Panel 2010, WHO Global Reference Laboratory for HPV Diagnosis and Control. Malmo, Sweden: WHO HPV Laboratory Network (LabNet); 2011. [Google Scholar]
- 24.Taiwan Cancer Registry Task Force Cancer Registry Annual Report 1991–2006. Taiwan: Department of Health; Executives Yuan; http://crs.cph.ntu.edu.tw/ [Google Scholar]
- 25.Rodriguez AC, Schiffman M, Herrero R, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100(7):513–517. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337(7676):a1754. doi: 10.1136/bmj.a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121(3):621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 28.Li N, Franceschi S, Howell-Jones R, et al. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2010;128(4):927–935. doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- 29.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 30.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97(14):1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]