Maternal glucose during pregnancy is significantly associated with lower insulin sensitivity and greater static β-cell response in children 5–10 years of age.
Abstract
Context:
Intrauterine exposure to elevated glucose concentrations may be a mediating factor in prenatal programming of offspring diabetes risk. However, studies examining the effects of maternal glucose concentration on measures of insulin sensitivity and β-cell response in prepubertal children are limited.
Objective:
We tested the hypothesis that maternal gestational glucose concentration would be inversely associated with children's insulin sensitivity independent of adiposity and positively associated with children's β-cell response independent of adiposity and insulin sensitivity.
Design, Setting, and Participants:
This was a cross-sectional study of 21 children aged 5–10 yr in a clinical research setting.
Outcomes Measured:
Children's insulin sensitivity index and basal, static, dynamic, and total β-cell response to glucose were determined by mathematical modeling using insulin, glucose, and C-peptide values after a liquid meal tolerance test. Children's percent body fat was determined by dual-energy x-ray absorptiometry. Maternal gestational glucose concentration for the target pregnancy was determined after a 50-g, 1-h oral glucose challenge test at 24–28 wk gestation.
Results:
Maternal glucose concentration was significantly, inversely associated with children's insulin sensitivity, independent of percent fat and ethnicity (P <0.05). A significant, positive association was observed for maternal glucose concentration with static β-cell response, independent of percent fat and insulin sensitivity (P < 0.05).
Conclusions:
Maternal gestational glucose concentration was significantly associated with offspring insulin sensitivity and β-cell response independent of adiposity. These results suggest that maternal glucose may program the fetus both at the pancreas and at the level of insulin target tissues such as skeletal muscle and liver.
Evidence suggests that prenatal exposure to a diabetic intrauterine environment is associated with increased risk for impaired glucose tolerance (1, 2) and type 2 diabetes among offspring (3). Whether the prediabetic maternal environment affects offspring metabolism is less clear. Reductions in insulin sensitivity and insulin secretion precede impaired glucose tolerance and type 2 diabetes (4). Therefore, it is conceivable that intrauterine exposure to elevated maternal glucose concentrations, such as observed with pregestational and/or gestational diabetes, may chronically alter offspring insulin sensitivity and β-cell function in a manner that contributes to the development of impaired glucose tolerance and type 2 diabetes. Although not all investigations have found significant differences in insulin sensitivity among offspring with prenatal exposure to diabetes (5–8), many of these studies determined insulin sensitivity using proxy measures (5, 6). Similarly, although previous studies have suggested altered insulin secretion among offspring of diabetic pregnancies (6, 8–11), only two of these actually assessed insulin secretion by measuring C-peptide response to a glucose dose (8, 11), and none have examined β-cell responsivity to glucose. Thus, few studies have investigated associations of maternal gestational glucose with offspring insulin sensitivity and β-cell function using robust methodology.
Although the mechanism linking maternal metabolic condition to offspring glucose metabolism is not known, at least a portion of the effect is believed to be attributable to intrauterine exposure to elevated maternal glucose. Glucose freely crosses the placenta and stimulates the fetal pancreas to produce insulin (12), potentially leading to permanent alterations of the fetal pancreas and insulin-sensitive tissues. In vitro studies of the fetal pancreas, as well as animal studies of induced hyperglycemia during gestation, have shown that prenatal exposure to hyperglycemia may result in abnormal fetal β-cell development (13), altered adrenergic regulation of insulin secretion (14), reduced skeletal muscle glucose uptake (15), and reduced hepatic insulin sensitivity (16). In humans, prenatal exposure to pregestational diabetes, gestational diabetes, and elevated maternal glucose among nondiabetic women has been shown to be associated with increased fetal/neonatal insulin secretion determined by C-peptide, insulin, and glucose concentrations from amniotic fluid and umbilical cord blood (9–11). However, in later childhood and adulthood, decreased insulin secretion has been observed among offspring from diabetic pregnancies (6, 8). Although these observations suggest that prenatal exposure to a diabetic intrauterine environment and thus, presumably, elevated maternal glucose concentrations may impact offspring insulin resistance (3, 17) and insulin secretion (6, 8, 18), the association between maternal gestational glucose, specifically, and measures of insulin sensitivity and β-cell function in children has not been investigated.
Therefore, the purpose of this study was to examine associations of maternal gestational glucose with insulin sensitivity and β-cell response in children 5–10 yr of age. We hypothesized that greater maternal gestational glucose would be significantly associated with lower insulin sensitivity in children, independent of their adiposity, and an altered β-cell response, independent of their adiposity and insulin sensitivity.
Subjects and Methods
Forty prepubertal children and their biological mothers were enrolled in the study. Children were 5–10 yr of age, healthy, from a singleton pregnancy, and weighed at least 11 kg (24.2 lbs) to ensure adequate blood sampling. Per study design, children from both normal glucose-tolerant and gestational diabetic pregnancies were included in the study. Maternal glycemic status (gestational diabetes/normal) and blood glucose concentrations after a 1-h, 50-g oral glucose screening test were obtained retrospectively from medical records. It was assumed that maternal glucose concentrations after the oral glucose screen were indicative of maternal glycemic status throughout pregnancy unless subsequent blood glucose values from maternal records suggested otherwise. In three such cases, the data were eliminated in final analyses to avoid potential confounding of results.
Participants were excluded from the study if the mother did not receive prenatal care during the first trimester of pregnancy, had a body mass index (BMI) under 18 kg/m2 at time of pregnancy, or was diagnosed with type 1 or type 2 diabetes before pregnancy or if the child was born premature (<37 wk gestation), had a birth weight below 2500 g indicating intrauterine growth restriction, was born with congenital abnormalities, or was currently taking medications known to affect body composition and glucose metabolism.
Complete data were available on 21 mother-child pairs. Reasons for missing/excluded data included unavailable maternal gestational glucose concentration (10 participants), unavailable child blood samples (four participants), child use of medication believed to affect metabolism (one participant), and maternal glucose 3 sd above the mean (one participant). All study procedures were approved by the University of Alabama (UAB) Institutional Review Board, and all participants provided written informed consent and assent, where age appropriate, before enrollment in the study.
Protocol
Participants reported to the UAB's Participant and Clinical Interactions Resource at approximately 0630 h after a 12-h overnight fast for metabolic testing. After completion of metabolic testing, participants were taken to UAB's Webb Nutrition Sciences Building for body composition analysis.
Procedures
Liquid meal tolerance test
Insulin sensitivity and β-cell response were determined by mathematical modeling after a liquid meal tolerance test. For the liquid meal tolerance test, a flexible iv catheter was placed in the antecubital space of the left arm for frequent blood sampling. The meal was designed to provide approximately 1.75 g of carbohydrate per kilogram of lean body mass and consisted of Carnation Instant Breakfast (Nestlé USA, Inc., Glendale, CA) mixed with whole milk. Before consumption of the liquid meal, two baseline blood samples were drawn over a 15-min period, and values for insulin, glucose, and C-peptide were averaged to determine fasting concentrations. The meal commenced at time zero and was consumed within a 5-min period. Blood samples (n = 23) were collected at 5, 10, 15, 20, 25, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 210, and 240 min relative to time zero. Sera were separated and stored at −85 C until analyzed for glucose, insulin, and C-peptide. An index of insulin sensitivity based on insulin and glucose data and indices of β-cell response based on glucose and C-peptide data were determined using the oral glucose minimal model (19). β-Cell response indices included total β-cell response, basal β-cell response, dynamic β-cell response, and static β-cell response. Total β-cell responsivity index is considered a global estimate of β-cell response to glucose. Basal β-cell responsivity index measures β-cell sensitivity to glucose under basal condition. Dynamic β-cell responsivity index measures β-cell response to the rate of change or increase in glucose. Static β-cell responsivity index assesses β-cell response to average above basal glucose concentrations across the course of the test.
Glucose, insulin, and C-peptide assays
Glucose, insulin, and C-peptide were analyzed by the Core Laboratory of the UAB Nutrition Obesity Research Center, Diabetes Research Training Center, and Center for Clinical and Translational Science. Fasting glucose was measured with the SIRRUS analyzer (Stanbio Laboratory, Boerne, TX) using the glucose oxidase method [intraassay coefficient of variation (CV) of 1.21% and an interassay CV of 3.065%]. Fasting insulin and C-peptide were assayed using the TOSOH AIA-600 II automated analyzer (TOSOH Bioscience, Inc., San Francisco, CA), which employs an immunoenzymatic technique that uses fluorescence. Intraassay and interassay CV for insulin were 4.42 and1.49%, respectively, and the intraassay and interassay CV for C-peptide were 1.67 and 2.59%, respectively.
Body composition
Dual-energy x-ray absorptiometry (Lunar iDXA; GE Healthcare, General Electric Co., Madison, WI) was used for the determination of children's total percent body fat. Participants were scanned in the supine position with arms at their side. All participants were scanned in light clothing. Analyses of all scans were performed by the same trained individual (N.C.B.) using the enCORE adult software package version 1.33 (GE Healthcare).
Statistical analysis
Descriptive statistics were performed on all variables of interest. Pearson correlation analyses were used to examine associations among maternal gestational glucose, offspring insulin sensitivity, and offspring β-cell response indices. Multiple linear regression analyses were used to determine the independent associations of maternal gestational glucose concentrations with offspring insulin sensitivity and β-cell response adjusting for children's percent fat. In agreement with other studies (20), our preliminary analyses indicated that insulin sensitivity differed by ethnicity. Therefore, ethnicity was included as a covariate in the model for the dependent variable insulin sensitivity. Because no ethnic differences were observed for the β-cell response indices, it was not included as a covariate in the β-cell response models.
To determine whether the associations for maternal gestational glucose concentrations with children's β-cell response indices were independent of insulin sensitivity, multiple linear regression analyses were performed, adjusting for both insulin sensitivity and percent fat. This was performed only in cases where significant associations were observed for maternal gestational glucose and children's β-cell response indices.
Analyses of Studentized residuals were performed for each model to determine potential outliers (±3 sd). One outlier was identified and removed for the basal β-cell response model. All continuous variables were logarithmically (log10) transformed to approximate a normal distribution. All statistical analyses were two tailed and assumed a 5% significance level. Analyses were performed using SAS software package (version 9.1; SAS Institute, Cary, NC).
Power calculations to assess multivariate relationships among outcome variables based on a test of the squared multiple correlation (R2) for a multiple linear regression model were performed on a sample size of 21 participants. Based on a three-parameter model, a two-sided statistical test, and assuming a 5% significance level, this study had 80% power to detect an R2 of 0.40 and 90% power to detect an R2 of 0.46. Power calculations were performed using nQuery Advisor, version 7 (Janet D. Elashoff, Cork, Ireland).
Results
Maternal and child demographic and descriptive information as well as child metabolic characteristics are presented in Table 1. Maternal gestational glucose concentrations ranged from 75–229 mg/dl, and early maternal BMI during pregnancy ranged from 18.3–43.4 kg/m2. Five (24%) of the women were diagnosed with gestational diabetes during pregnancy. Children's BMI percentile ranged from approximately 17th–99th percentile. Children's fasting insulin ranged from 1–13 μIU/ml, and fasting glucose ranged from 78–108 mg/dl. All children were at the first stage of pubertal development (i.e. Tanner 1) (21).
Table 1.
Maternal and child demographic, descriptive, and metabolic characteristics
| Variable | Mean ± sd or % |
|---|---|
| Maternal characteristics | |
| Maternal gestational glucose (mg/dl) | 133 ± 35 |
| Age at delivery (yr) | 25.5 ± 6.9 |
| Maternal early pregnancy BMI (kg/m2) | 30.4 ± 7.8 |
| Gestational weight gain (kg) | 9.0 ± 4.9 |
| Child Characteristics | |
| Ethnicity | 19% EA; 81% AA |
| Gender | 43% male; 57% female |
| Age (yr) | 7.2 ± 1.3 |
| BMI percentile | 76.4 ± 24.7 |
| Percent fat | 28.1 ± 9.1 |
| Fasting insulin (μIU/ml) | 5 ± 3 |
| Fasting glucose (mg/dl) | 94 ± 6 |
| Fasting C-peptide (ng/ml) | 1.0 ± 0.5 |
| Insulin sensitivity [×10−4 dl/kg · min/(μIU/ml)] | 16.3 ± 11.0 |
| Total β-cell response (10−9 min−1) | 36.5 ± 23.8 |
| Basal β-cell response (10−9 min−1) | 4.4 ± 1.9 |
| Dynamic β-cell response (10−9) | 584.1 ± 212.7 |
| Static β-cell response (10−9 min−1) | 49.2 ± 22.1 |
AA, African-American; EA, European-American.
Pearson correlation coefficients for associations of maternal gestational glucose with children's metabolic outcomes are shown in Table 2. Maternal gestational glucose was significantly and inversely associated with children's insulin sensitivity. Significant, positive associations were observed for maternal gestational glucose with children's fasting C-peptide, total β-cell response, basal β-cell response, and static β-cell response. There was a positive trend for the relationship between maternal gestational glucose and fasting insulin.
Table 2.
Unadjusted Pearson correlation coefficients for maternal gestational glucose with children's metabolic outcomes
| Maternal gestational glucose | |
|---|---|
| Fasting insulin | 0.44a |
| Fasting glucose | 0.06 |
| Fasting C-peptide | 0.50b |
| Insulin sensitivity index | −0.50b |
| Total β-cell response | 0.49b |
| Basal β-cell response | 0.51b |
| Static β-cell response | 0.63c |
| Dynamic β-cell response | 0.27 |
| Disposition index | 0.07 |
0.05 < P < 0.1.
P < 0.05.
P < 0.01.
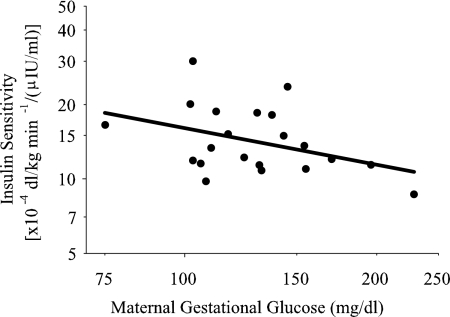
In multiple linear regression modeling, maternal gestational glucose was significantly and inversely associated with children's insulin sensitivity independent of ethnicity and percent fat (Table 3 and Fig. 1). Both ethnicity and percent fat were significantly inversely associated with children's insulin sensitivity (Table 3). Additional adjustments for age and gender did not affect the significant associations observed for maternal gestational glucose and children's insulin sensitivity.
Table 3.
Multiple linear regression analyses for associations of maternal gestational glucose with children's insulin sensitivity and β-cell response indices
| Parameter estimate ± se | Std β | Model R2 | P | |
|---|---|---|---|---|
| Insulin sensitivity index | 0.70 | <0.001 | ||
| Maternal gestational glucose | −0.66 ± 0.31 | −0.33 | 0.047 | |
| Ethnicity | −0.17 ± 0.04 | −0.62 | <0.001 | |
| Percent fat | −0.52 ± 0.24 | −0.34 | 0.041 | |
| Static β-cell response | 0.40 | 0.031 | ||
| Maternal gestational glucose | 1.12 ± 0.40 | 0.64 | 0.013 | |
| Percent fat | 0.08 ± 0.30 | 0.06 | 0.795 | |
| Insulin sensitivity index | 0.08 ± 0.20 | 0.10 | 0.674 | |
| Basal β-cell response | 0.54 | 0.005 | ||
| Maternal gestational glucose | 0.46 ± 0.32 | 0.30 | 0.171 | |
| Percent fat | 0.32 ± 0.25 | 0.27 | 0.213 | |
| Insulin sensitivity index | −0.25 ± 0.15 | −0.33 | 0.124 | |
| Total β-cell response | 0.38 | 0.014 | ||
| Maternal gestational glucose | 0.66 ± 0.50 | 0.28 | 0.204 | |
| Percent fat | 0.76 ± 0.38 | 0.43 | 0.063 |
Fig. 1.
Association of maternal gestational glucose with children's insulin sensitivity. Data were adjusted for children's percent fat and ethnicity (Std β = −0.32; P = 0.047).
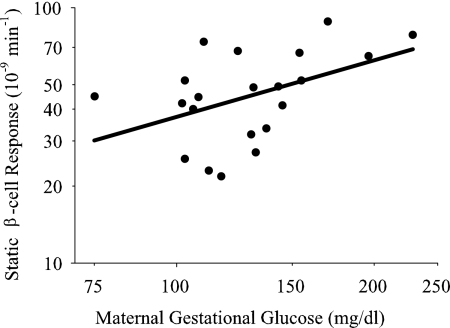
A significant, positive association was observed for maternal gestational glucose with children's static β-cell response independent of percent fat [standardized (Std) β = 0.61; P = 0.010], which remained after additional adjustment for children's insulin sensitivity (Table 3 and Fig. 2). Neither percent fat nor insulin sensitivity was significantly associated with children's static β-cell response. Although there was a trend for a significant association of maternal gestational glucose with children's basal β-cell response independent of percent fat (Std β = 0.42; P = 0.060), additional adjustment for children's insulin sensitivity attenuated these results (Table 3). There was no significant association of maternal gestational glucose with children's total β-cell response after adjusting for percent fat (Table 3). Although we did evaluate the associations of maternal gestational glucose with children's disposition index and dynamic β-cell response, these models were not significant (F ratio = 0.26; P = 0.777 and 0.82; P = 0.456, respectively).
Fig. 2.
Association of maternal gestational glucose with children's static β-cell response. Data were adjusted for children's percent fat and insulin sensitivity (Std β = 0.64; P = 0.013).
Discussion
This study was designed to assess the independent associations of maternal gestational glucose concentration with robust measures of insulin sensitivity and β-cell response in 5- to 10-yr-old children. We found that greater maternal gestational glucose concentration was associated with lower insulin sensitivity and greater static β-cell response in children independent of their body composition. Furthermore, the relationship between maternal gestational glucose and static β-cell response was independent of insulin sensitivity, indicating that greater static β-cell response was not solely a compensatory response to lower insulin sensitivity. These findings suggest that maternal gestational glucose may impact fetal programming of both the pancreas and insulin target tissues.
A novel finding of this study was the significant inverse association between maternal gestational glucose and children's insulin sensitivity independent of their adiposity. Lower insulin sensitivity in children born to mothers with elevated gestational glucose may explain previous observations of increased risk of impaired glucose tolerance and type 2 diabetes in offspring exposed to a pregestational diabetic and/or gestational diabetic intrauterine environment (1–3, 5, 17). Present results extend and provide a potential mechanism for earlier observations that third-trimester maternal postchallenge glucose was associated with offspring risk for type 2 diabetes even among individuals from mothers with normal glucose tolerance during pregnancy (17). It is important to note that not all studies have indicated associations of maternal diabetic state with offspring insulin sensitivity, acute insulin response to glucose, or proxy measures of insulin resistance (5–7). Reasons for discrepant results among studies could be due to differences in the subject population or methodology employed for assessing insulin sensitivity and secretion. Although further research in larger populations is needed to verify these findings, in our population of 5- to 10-yr-old children, insulin sensitivity was associated with maternal gestational glucose concentrations independent of children's body composition and ethnicity.
Our study is also novel in being the first to show an association between maternal gestational glucose and specific indices of β-cell function in children. Our findings indicated that static β-cell response was significantly increased in children with prenatal exposure to elevated maternal gestational glucose. This relationship was independent of children's adiposity and insulin sensitivity, indicating that it was not simply a compensatory response to lower insulin sensitivity. Maternal gestational glucose also tended to be positively associated with basal β-cell response (P = 0.06). This relationship may have been significant with a larger sample size. Although these indices of β-cell response have yet to be validated against cellular events, it is believed that static β-cell response represents, at least in part, the transport of new insulin granules from the intracellular pool to the cell surface, and basal β-cell response represents β-cell sensitivity to ambient glucose concentrations (22).
These findings support and extend those from a number of studies suggesting enhanced β-cell function among offspring of diabetic and/or hyperglycemic intrauterine environments (5, 9–11). These earlier studies used cord blood C-peptide (9–11) or 30- and 120-min insulin concentrations during an oral glucose tolerance test (OGTT) (5) as measures of insulin secretion. Our results demonstrate for the first time, using robust methodology, that maternal gestational glucose, not just maternal diabetes status, is associated with specific β-cell response indices in children.
Some studies have suggested lower insulin secretion among offspring of diabetic pregnancies (6, 8, 18). Differences among studies may relate to the age of the offspring. Our participants were 5–10 yr old, whereas a number of other studies examined older children and adults. It is possible that increased insulin secretion precedes the decline in insulin secretion. Pima Indians, a population known for their exceedingly high prevalence of type 2 diabetes mellitus, were found to have greater acute insulin response to glucose as well as greater early insulin response after an OGTT and a meal test compared with Caucasians, independent of insulin resistance (23). In addition, animal studies have demonstrated an increase in insulin secretion during early life, but a decrease in insulin secretion and β-cell response to glucose later in life among offspring of diabetic pregnancies (15, 24). Exaggerated β-cell response early in life may mediate later β-cell decline through several potential mechanisms including autocrine effects of insulin on β-cell secretion (25) and endoplasmic reticulum stress (26). Other potential sources of between-study differences include the intrauterine environment and ethnicity. Although our participants were children from mothers with and without gestational diabetes, those from other studies were offspring of mothers with gestational diabetes, type 2 diabetes, and/or type 1 diabetes (6, 8, 18). Our children were primarily African-American, in contrast to those from other studies, which included Pima Indians (6, 18) and individuals of European ancestry (8). Finally, one major difference between studies is our use of maternal gestational glucose as a continuous variable regardless of diabetes status. All but one (10) previous study simply categorized mothers according to diabetes status.
Mechanisms underlying the associations of maternal hyperglycemia with offspring insulin sensitivity and β-cell response are not fully known but may be related to molecular aspects of insulin signaling and glucose transport. It has been shown in rodent models that offspring with prenatal exposure to diabetes have decreased skeletal muscle glucose uptake due to lower glucose transporter-1 and -4 protein levels in newborns and adults, respectively (15), and increased hepatic insulin resistance related, in part, to reduced serine-AKT phosphorylation affecting insulin signaling and suppression of hepatic glucose production (16). It is also possible that prenatal exposure to high glucose affects the development of the pancreatic β-cells. Studies in both humans and animal models have indicated that intrauterine exposure to diabetes alters β-cell mass, pancreatic insulin content, and insulin secretion (13, 15, 24). In general, existing literature suggests that fetal and infant offspring of diabetic mothers likely have greater insulin secretion due to greater β-cell mass (13, 15) but that over time, insulin secretion may decline due to either reduced β-cell mass (15) or reduced β-cell response to glucose in the presence of normal islet mass (24). Our findings in 5- 10-yr-old children suggest that prenatal exposure to elevated maternal gestational glucose concentrations may result in exaggerated β-cell sensitivity to glucose during the prepubertal period. However, future studies examining long-term changes in children's β-cell response are needed to determine whether exaggerated insulin secretion precedes reduced β-cell responsivity among children exposed to high maternal glucose in utero.
Strengths of this study included the use of robust measures of insulin sensitivity, β-cell function, and body composition and the use of maternal gestational glucose as a continuous variable. This study is limited by the small sample size, which may have affected statistical power and limited our ability to adjust for all possible confounders. An additional limitation is that we had only a single measure of maternal gestational glucose from the 50-g OGTT, which may not adequately reflect maternal glucose tolerance throughout the pregnancy. In future studies, a more thorough assessment of maternal gestational glucose concentrations throughout the pregnancy would be preferable. Lastly, even though our findings suggest maternal gestational glucose affects offspring insulin sensitivity and β-cell function, we cannot rule out the possibility that a shared genotype between mother and child may have influenced these outcomes. It is conceivable that women with gestational hyperglycemia have genes placing them at increased risk for type 2 diabetes, which are transmitted to their offspring. Future research in this area should consider evaluating both the genetic as well as the prenatal environment.
In conclusion, this study offers novel findings regarding the relationship of maternal gestational glucose with offspring insulin sensitivity and β-cell function. Specifically, maternal gestational glucose concentration was inversely associated with children's insulin sensitivity and positively associated with β-cell responsivity independent of children's adiposity. Furthermore, the association of maternal gestational glucose with static β-cell response was independent of insulin sensitivity, suggesting that increased static β-cell response among children exposed to elevated maternal gestational glucose was not solely a result of lower insulin sensitivity. Although these preliminary findings offer further insight regarding the influence of maternal gestational glucose on prenatal programming of insulin sensitivity and β-cell function, they should be confirmed in a larger sample and with use of more extensive information regarding maternal gestational glucose.
Acknowledgments
We acknowledge the following individuals from UAB's Center for Women's Reproductive Health for their support during the study: Rachel Copper (administration and recruitment), Mickey Parks (study nurse practitioner), and Melissa Mancuso, M.D. (study physician). We also acknowledge Maryellen Williams and Cindy Zeng who conducted all laboratory analyses. Lastly, we thank the children and mothers who agreed to participate in this study.
This work was supported by NR-0025 (Thrasher Research Fund, to P.C.C.-L.), F32 DK 082028 (to P.C.C.-L.), P30-DK56336, P60-DK079626, UL1RR025777, and TL1RR025775 (TL1 training grant, to N.C.B.).
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- BMI
- Body mass index
- CV
- coefficient of variation
- OGTT
- oral glucose tolerance test
- Std
- standardized.
References
- 1. Plagemann A, Harder T, Kohlhoff R, Rohde W, Dörner G. 1997. Glucose tolerance and insulin secretion in children of mothers with pregestational IDDM or gestational diabetes. Diabetologia 40:1094–1100 [DOI] [PubMed] [Google Scholar]
- 2. Silverman BL, Metzger BE, Cho NH, Loeb CA. 1995. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care 18:611–617 [DOI] [PubMed] [Google Scholar]
- 3. Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. 2000. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 49:2208–2211 [DOI] [PubMed] [Google Scholar]
- 4. Bergman RN, Finegood DT, Kahn SE. 2002. The evolution of beta-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest 32(Suppl 3):35–45 [DOI] [PubMed] [Google Scholar]
- 5. Krishnaveni GV, Hill JC, Leary SD, Veena SR, Saperia J, Saroja A, Karat SC, Fall CH. 2005. Anthropometry, glucose tolerance, and insulin concentrations in Indian children: relationships to maternal glucose and insulin concentrations during pregnancy. Diabetes Care 28:2919–2925 [DOI] [PubMed] [Google Scholar]
- 6. Salbe AD, Lindsay RS, Collins CB, Tataranni PA, Krakoff J, Bunt JC. 2007. Comparison of plasma insulin levels after a mixed-meal challenge in children with and without intrauterine exposure to diabetes. J Clin Endocrinol Metab 92:624–628 [DOI] [PubMed] [Google Scholar]
- 7. Hunter WA, Cundy T, Rabone D, Hofman PL, Harris M, Regan F, Robinson E, Cutfield WS. 2004. Insulin sensitivity in the offspring of women with type 1 and type 2 diabetes. Diabetes Care 27:1148–1152 [DOI] [PubMed] [Google Scholar]
- 8. Sobngwi E, Boudou P, Mauvais-Jarvis F, Leblanc H, Velho G, Vexiau P, Porcher R, Hadjadj S, Pratley R, Tataranni PA, Calvo F, Gautier JF. 2003. Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet 361:1861–1865 [DOI] [PubMed] [Google Scholar]
- 9. Freinkel N, Metzger BE, Phelps RL, Dooley SL, Ogata ES, Radvany RM, Belton A. 1985. Gestational diabetes mellitus. Heterogeneity of maternal age, weight, insulin secretion, HLA antigens, and islet cell antibodies and the impact of maternal metabolism on pancreatic B-cell and somatic development in the offspring. Diabetes 34(Suppl 2):1–7 [DOI] [PubMed] [Google Scholar]
- 10. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. 2008. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 11. Phelps RL, Freinkel N, Rubenstein AH, Kuzuya H, Metzger BE, Boehm JJ, Mølsted-Pedersen L. 1978. Carbohydrate metabolism in pregnancy. XV. Plasma C-peptide during intravenous glucose tolerance in neonates from normal and insulin-treated diabetic mothers. J Clin Endocrinol Metab 46:61–68 [DOI] [PubMed] [Google Scholar]
- 12. Freinkel N. 1980. Banting Lecture 1980. Of pregnancy and progeny. Diabetes 29:1023–1035 [DOI] [PubMed] [Google Scholar]
- 13. Reiher H, Fuhrmann K, Noack S, Woltanski KP, Jutzi E, Hahn von Dorsche H, Hahn HJ. 1983. Age-dependent insulin secretion of the endocrine pancreas in vitro from fetuses of diabetic and nondiabetic patients. Diabetes Care 6:446–451 [DOI] [PubMed] [Google Scholar]
- 14. Gauguier D, Bihoreau MT, Picon L, Ktorza A. 1991. Insulin secretion in adult rats after intrauterine exposure to mild hyperglycemia during late gestation. Diabetes 40(Suppl 2):109–114 [DOI] [PubMed] [Google Scholar]
- 15. Boloker J, Gertz SJ, Simmons RA. 2002. Gestational diabetes leads to the development of diabetes in adulthood in the rat. Diabetes 51:1499–1506 [DOI] [PubMed] [Google Scholar]
- 16. Yamashita H, Shao J, Qiao L, Pagliassotti M, Friedman JE. 2003. Effect of spontaneous gestational diabetes on fetal and postnatal hepatic insulin resistance in Leprdb/+ mice. Pediatr Res 53:411–418 [DOI] [PubMed] [Google Scholar]
- 17. Franks PW, Looker HC, Kobes S, Touger L, Tataranni PA, Hanson RL, Knowler WC. 2006. Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes 55:460–465 [DOI] [PubMed] [Google Scholar]
- 18. Gautier JF, Wilson C, Weyer C, Mott D, Knowler WC, Cavaghan M, Polonsky KS, Bogardus C, Pratley RE. 2001. Low acute insulin secretory responses in adult offspring of people with early onset type 2 diabetes. Diabetes 50:1828–1833 [DOI] [PubMed] [Google Scholar]
- 19. Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. 2001. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 50:150–158 [DOI] [PubMed] [Google Scholar]
- 20. Gower BA, Nagy TR, Goran MI. 1999. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes 48:1515–1521 [DOI] [PubMed] [Google Scholar]
- 21. Marshall WA, Tanner JM. 1969. Variations in pattern of pubertal changes in girls. Arch Dis Child 44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cobelli C, Toffolo GM, Dalla Man C, Campioni M, Denti P, Caumo A, Butler P, Rizza R. 2007. Assessment of β-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab 293:E1–E15 [DOI] [PubMed] [Google Scholar]
- 23. Lillioja S, Nyomba BL, Saad MF, Ferraro R, Castillo C, Bennett PH, Bogardus C. 1991. Exaggerated early insulin release and insulin resistance in a diabetes-prone population: a metabolic comparison of Pima Indians and Caucasians. J Clin Endocrinol Metab 73:866–876 [DOI] [PubMed] [Google Scholar]
- 24. Holemans K, Aerts L, Van Assche FA. 2003. Lifetime consequences of abnormal fetal pancreatic development. J Physiol 547:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bouche C, Lopez X, Fleischman A, Cypess AM, O'Shea S, Stefanovski D, Bergman RN, Rogatsky E, Stein DT, Kahn CR, Kulkarni RN, Goldfine AB. 2010. Insulin enhances glucose-stimulated insulin secretion in healthy humans. Proc Natl Acad Sci USA 107:4770–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fonseca SG, Urano F, Burcin M, Gromada J. 2010. Stress hypERactivation in the β-cell. Islets 2:1–9 [DOI] [PubMed] [Google Scholar]