Abstract
Very little is currently known about the cerebral characteristics that underlie the complex processes of meditation as only a limited number of studies have addressed this topic. Research exploring structural connectivity in meditation practitioners is particularly rare. We thus acquired diffusion tensor imaging (DTI) data of high angular and spatial resolution and used atlas-based tract mapping methods to investigate white matter fiber characteristics in a well-matched sample of long-term meditators and controls (n=54). A broad field mapping approach estimated the fractional anisotropy (FA) for twenty different fiber tracts (i.e., nine tracts in each hemisphere and two inter-hemispheric tracts) that were subsequently used as dependent measures. Results showed pronounced structural connectivity in meditators compared to controls throughout the entire brain within major projection pathways, commissural pathways, and association pathways. The largest group differences were observed within the corticospinal tract, the temporal component of the superior longitudinal fasciculus, and the uncinate fasciculus. While cross-sectional studies represent a good starting point for elucidating possible links between meditation and white matter fiber characteristics, longitudinal studies will be necessary to determine the relative contribution of nature and nurture to enhanced structural connectivity in long-term meditators.
Keywords: Age, DTI, Fractional Anisotropy, Meditators, MRI, Tractography, White Matter
Introduction
Meditation is a cognitive practice directed at stilling the fluctuations of the mind (Baerentsen et al., 2009). Meditators, especially long-term practitioners, provide an ideal human model for investigating brain plasticity given their ongoing, frequent, and regular cognitive efforts. The effect of meditation on brain function has been addressed in a large number of functional studies (Cahn and Polich, 2006). However, research exploring possible links between meditation and brain structure is still surprisingly sparse. With only a small number of structural MRI studies published, existing findings point to larger brain regions (thicker cortices, more brain tissue, and a diminished age-related atrophy, respectively) in meditators compared to control subjects (Grant et al., 2010; Luders et al., 2009b; Holzel et al., 2008; Vestergaard-Poulsen et al., 2008; Pagnoni and Cekic, 2007; Lazar et al., 2005). Recent longitudinal MRI studies complement these cross-sectional outcomes by revealing actual meditation-induced increases in gray matter (GM) density as a consequence of mindfulness-based stress reduction interventions over eight weeks (Holzel et al., 2010). Interestingly, existing findings appear to support the notion that significant links between meditation and brain anatomy are wide-spread throughout the entire brain involving both cortical and subcortical regions (e.g., superior, middle and inferior frontal gyrus, orbito-frontal cortex, paracentral regions [including somatosensory cortex], inferior temporal, superior temporal, fusiform, and cingulate gyrus, insula, thalamus, putamen, and hippocampus) as well as the brain stem and the cerebellum. Consequently, one might also expect enhanced brain connectivity in meditators, particularly with respect to fiber tracts connecting those aforementioned brain regions shown to be linked to meditation.
Diffusion tensor imaging (DTI) is an exciting, relatively new imaging modality providing valuable insights into the structural connectivity of the brain by quantifying the overall orientation of white matter bundles. Nevertheless, DTI studies in meditators are surprisingly rare. In fact, to our knowledge, only one study incorporated DTI-based data and assessed the effect of an Integrative Body–Mind Training (IBMT) in a subsample of 22 subjects (Tang et al., 2010). Indeed, this longitudinal study demonstrated that as few as 11 hours of IBMT (spread over one month) is sufficient to raise the fractional anisotropy (FA) – an indicator of white matter integrity – of several fiber tracts, including the superior and anterior corona radiata, the genu and body of the corpus callosum, and the superior longitudinal fasciculus. However, given the lack of any additional reports regarding DTI-based findings, further studies are clearly necessary to advance this field of research. Thus, we set out to compare fiber characteristics utilizing DTI and atlas-based tract mapping methods in a relatively large sample of long-term meditators and well-matched controls. To expand the extremely sparse literature with respect to DTI-based findings and to provide a foundation against which future outcomes can be compared, we applied a broad field mapping approach and investigated 20 different fiber tracts (i.e., 9 tracts in each hemisphere and 2 inter-hemispheric tracts).
Materials and Methods
Subjects
Our sample consisted of 27 active meditation practitioners (mean age ± SD: 51.6 ± 12.3 years) and 27 sex- and age-matched controls (mean age ± SD: 51.4 ± 12.4 years). Both the meditation and the control group contained 11 men and 16 women. The maximum allowed age difference within a sex-matched pair across groups was two years. Altogether, age ranged between 25 and 71 years. Both groups were comparable with respect to their educational background with 89% of all mediators and 93% of all controls having, at least, some college experience (7 mediators as well as 7 controls had a Master's degree or higher; 3 mediators and 2 controls had a high school degree or lower). While the scans for the controls were obtained from the International-Consortium-for-Brain-Mapping (ICBM) database of normal adults (http://www.loni.ucla.edu/ICBM/Databases/), meditators were newly recruited from various meditation venues. Years of meditation practice ranged between 5 and 46 years (mean ± SD: 23.3 ± 12.2 years), where self-reported meditation styles included Shamatha, Vipassana, and Zazen (which were practiced by about 55% of the meditators, either exclusively or in combination with other styles). A detailed overview about all subject-specific meditation styles is provided in Supplemental Table 1. The current sample (n=54) was partly overlapping with a sample (n=44) which has been described previously (Luders et al., 2009b). More specifically, 67% of the current subjects (81% of the meditators; 52% of the controls) had been included in our prior study investigating gray matter. All left-handers, however, have now been excluded and the sample only contained right-handers based on self-reports of hand preference for selected activities. All subjects were required to be free of any neurological and psychiatric disorders and gave informed consent according to institutional guidelines (Institutional Review Board of the University of Los Angeles, California).
Image Acquisition
DTI data was acquired on a 1.5T Siemens Sonata scanner (Erlangen, Germany) using an 8-channel head coil. The DTI acquisition protocol included a whole-brain sequence with 5 non-diffusion-weighted images (b=0 s/mm2) and 30 directionally sensitized diffusion-weighted images (b=1000 s/mm2), with 55 brain slices oriented obliquely to the AC–PC line (TR=6400 ms, TE=83 ms, FOV: 240×240 mm, matrix: 96×96, voxel dimensions: 2.5 mm × 2.5mm × 2.5 mm). The DTI sequence was designed to have minimal eddy current induced distortions (Reese et al., 2003), whereas parallel imaging was employed to substantially reduce EPI distortions (Heidemann et al., 2003). High-resolution T1-weighted MPRAGE sequences were collected in addition (TR=1900 ms; TE=4.38 ms; flip angle: 15°, FOV: 256×256; voxel size: 1 mm3; NEX=4; TI=1100). All image data were visually inspected for apparent artifacts due to subject motion and instrumental malfunction.
Data Processing
Images were corrected for motion artifacts using a 3D rigid body registration (Woods et al., 1998a) and for eddy current induced distortions using a 2D nonlinear registration algorithm (Woods et al., 1998b), where all diffusion-weighted images were registered to the first non-diffusion-weighted image in the series. Using the CLAPACK library (Anderson et al., 1999) and in-house software written in C, the diffusion tensor was computed at each voxel using a linear least-squares method to fit the log-transformed data of the signal intensities (Basser et al., 1994). Finally, the resultant eigenvalues were used to compute the FA.
Tract-based Atlasing
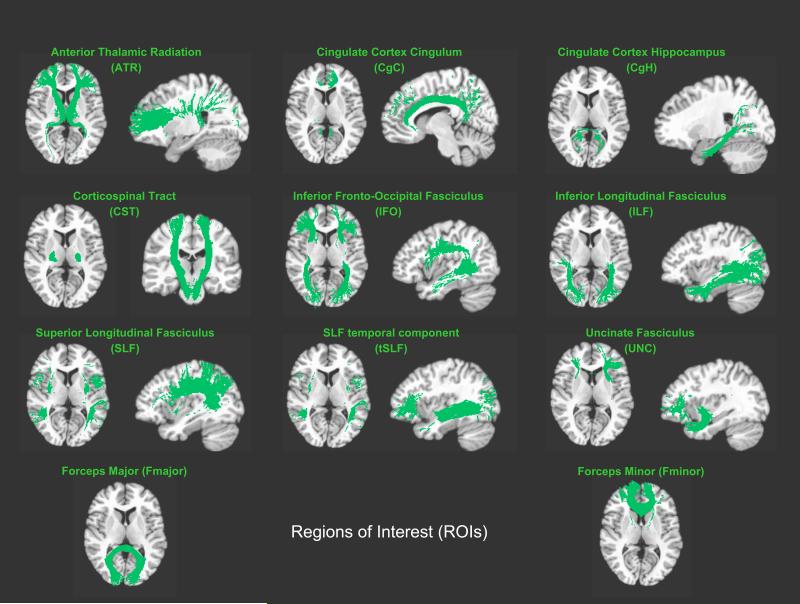
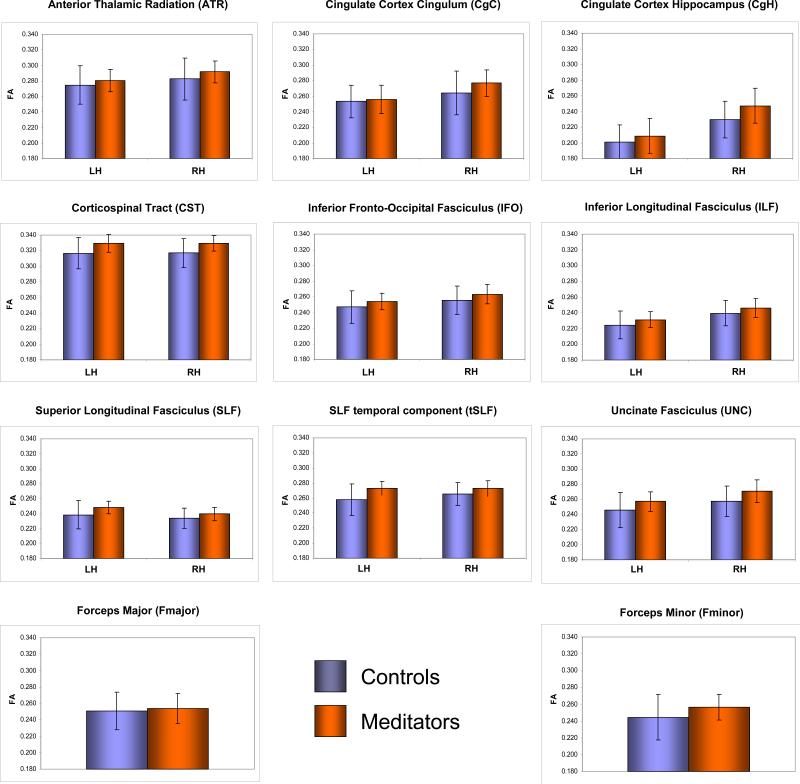
Analyses were focused on the mean FA within 20 regions of interest (ROIs) defined in the Johns Hopkins University (JHU) white matter tractography atlas (Hua et al., 2008; Wakana et al., 2007): anterior thalamic radiation (ATR), cingulum – cingulate gyrus (CgC), cingulum – hippocampus (CgH), corticospinal tract (CST), inferior fronto-occipital fasciculus (IFO), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), SLF temporal component (tSLF), and uncinate fasciculus (UNC) within each hemisphere, and the forceps major (Fmajor) and minor (Fminor) across hemispheres (see Figure 1). ROI-specific mean FA was computed within each subject's native space. For this purpose, we spatially aligned the T1-weighted reference brain from the JHU tractography atlas to each subject's T1-weighted image using a non-linear registration algorithm (Woods et al., 1998b). This resulted in one transformation file for each subject. A second transformation file was generated by computing a rigid-body registration between each subject's T1-weighted image and each subject's first non-diffusion-weighted image1 (Woods et al., 1998a). Then, the two transformation files were combined and applied to each ROI in the JHU atlas essentially resulting in ROIs within each subject's native space. ROIs were overlaid onto each subject's MR image in native space and visually inspected to ensure that automatically generated labels corresponded to individual brain anatomy. Subsequently, for each subject, mean FA was computed within each of the 20 ROIs (for ROI-specific mean FA ± SD within meditators and controls see Figure 2).
Figure 1. Regions of interest (ROIs).
Illustrated are the 11 white matter tracts (9 bilateral, 2 midline) overlaid onto the T1-weighted Colin brain (Collins et al., 1998).
Figure 2. ROI-specific FA within controls (blue) and meditators (orange).
Shown are group-specific mean FA values (coded on the Y-axes) and standard deviations (error bars), for the left hemisphere (LH), the right hemisphere (RH), or across hemispheres, respectively (i.e., for Fmajor and Fminor).
FA Differences between Long-term Meditators and Controls
All statistical analyses were conducted in PASW Statistics 18 (http://www.spss.com/). We used two separate statistical models; one to accommodate tracts with both left- and right-hemispheric measurements and the other for midline tracts only. Specifically, for the 9 bilateral tracts, left- and right-hemispheric FA values were included as repeated measures in a multivariate analysis of covariance (MANCOVA) with Group (meditators / controls) as a between-subjects factor, Tract and Hemisphere as within-subjects factors and Age as covariate. For the 2 callosal ROIs (that were measured at midline only), we did not run an omnibus test; instead, we applied post hoc comparisons with appropriate Bonferroni corrections.
For the 9 bilateral tracts, follow-up analyses were only performed in the presence of significant omnibus effects from the multivariate analyses where p<0.05 (two-tailed) was determined as the threshold for statistical significance. That is, significant Group-by-Tract Interaction and significant Group-by-Tract-by-Hemisphere interactions were followed by determining effects for each ROI separately (hemisphere was again treated as a repeated measure for the bilateral tracts). The ROI-specific outcomes are shown in Table 1 (Group / Group-by-Hemisphere Interaction). Finally, significant ROI-specific Group-by-Hemisphere interactions were followed by comparing meditators and controls with respect to the left- and right-hemispheric ROIs, separately. The hemisphere-specific outcomes are also shown in Table 1 (Left Hemisphere / Right Hemisphere).
Table 1.
FA differences between meditators and controls
| ROI | Statistics | |||
|---|---|---|---|---|
| Group | Group-by-Hemisphere Interaction | Left Hemisphere | Right Hemisphere | |
| ATR | F(1, 51) = 4.566* | F(1, 51) = 2.677 | # | # |
| CgC | F(1, 51) = 2.528 | F(1, 51) = 5.960* | F(1, 51) = 0.400 | F(1, 51) = 4.874* |
| CgH | F(1, 51) = 5.484* | F(1, 51) = 5.581* | F(1, 51) = 1.826 | F(1, 51) = 8.892** |
| CST | F(1, 51) = 11.734*** | F(1, 51) = 0.007 | # | # |
| IFO | F(1, 51) = 6.020* | F(1, 51) = 0.066 | # | # |
| ILF | F(1, 51) = 4.986* | F(1, 51) = 0.051 | # | # |
| SLF | F(1, 51) = 7.083** | F(1, 51) = 3.744T | F(1, 51) = 8.257** | F(1, 51) = 4.210* |
| tSLF | F(1, 51) = 12.008*** | F(1, 51) = 9.476** | F(1, 51) = 15.820*** | F(1, 51) = 5.580* |
| UNC | F(1, 51) = 12.545*** | F(1, 51) = 0.493 | # | # |
| Fmajor | F(1, 51) = 0.411 | n/a | n/a | n/a |
| Fminor | F(1, 51) = 10.521** | n/a | n/a | n/a |
Post-hoc analyses were only performed in the presence of significant (or trend level) interactions.
Significant at the 0.001 level.
Significant at the 0.01 level.
Significant at the 0.05 level.
Significant at a trend level (p=0.059).
n/a = not applicable
Correlation between FA and the Amount of Meditation Experience
In addition, we explored the link between the amount of individual meditation experience and white matter fiber connectivity. For this purpose, Pearson's correlations (after removing the partial effects of age) were used to examine the relationships between ROI-specific FA and three different indicators of meditation experience: (1) the number of meditation years; (2) the current frequency of the meditation practice (i.e., minutes per week); and (3) the accumulated life-time minutes (i.e., the current weekly minutes extrapolated over the number of meditation years). As a safeguard against type I error, Bonferroni corrections for multiple comparisons were applied.
Supplemental Analyses
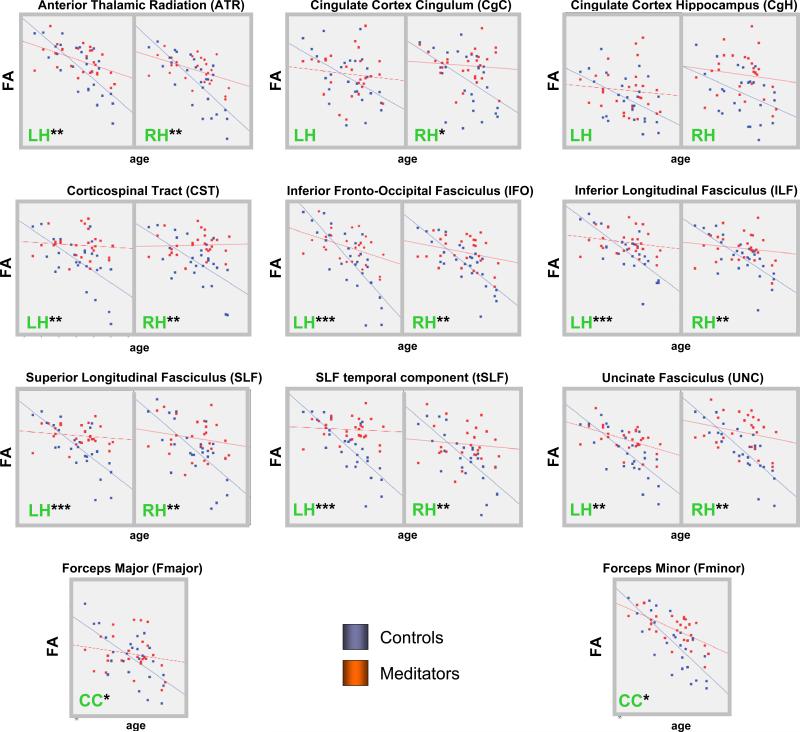
In order to further explore the possible underlying mechanisms for the observed group differences with respect to FA, we conducted two supplementary analyses: First, we obtained related diffusivity measures, such as axial diffusivity (λll) and radial diffusivity (λ⊥) as detailed elsewhere (Song et al., 2002). We then compared these ROI-specific measures between meditators and controls using the same statistical models as described above for FA. Second, we investigated whether ROI-specific FA is correlated with age, and whether these correlations are significantly different between meditators and controls. For this purpose, we used linear regressions to model the association between FA and age for each group (meditators and controls), and we compared the betas associated with the dependent variable (i.e. age) between the two groups using the homoscedasticity assumption for the errors in the two populations (Cohen, 1983). In addition, we generated group-specific scatter plots and regression slopes for each ROI (see Figure 3).
Figure 3. Correlations between FA and Age.
Shown are ROI-specific correlations for the left hemisphere (LH), the right hemisphere (RH), or across hemispheres (CC). Scatterplots and regression lines were generated separately for controls (blue) and meditators (orange). The X-axes display age; the Y-axes display FA. The asterisks indicate the significance with respect to the group differences of the correlations (***p≤0.001; **p≤0.01; *p≤0.05).
Results
FA Differences between Long-term Meditators and Controls
The repeated-measures omnibus model including the 9 bilateral tracts yielded a significant Group-by-Tract Interaction (F[8,44]=3.332; p=0.005) and a significant Group-by-Tract-by-Hemisphere interaction (F[8,44]=2.452; p=0.027).
As summarized in Table 1 (Group), we detected significantly larger FA in meditators than in controls within the following fiber tracts: anterior thalamic radiation (ATR), cingulum - hippocampus (CgH), corticospinal tract (CST), inferior fronto-occipital fasciculus (IFO), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), SLF temporal component (tSLF), uncinate fasciculus (UNC), and forceps minor (Fminor). Moreover, as shown in Table 1 (Group-by-Hemisphere Interaction), we detected significant interactions for CgC, CgH and tSLF as well as a trend for a significant interaction for SLF.
As further summarized in Table 1 (Left Hemisphere / Right Hemisphere), post hoc comparisons revealed significantly larger FA in meditators within the right CgC and right CgH but not within the left CgC and left CgH. In contrast, larger FA in meditators within tSLF and SLF were significant in both hemispheres, albeit effects were more pronounced in the left hemisphere than in the right hemisphere. Importantly, there were no fiber tracts that showed significantly larger FA measures in controls compared to meditators.2 The overall trend for smaller FA values in controls is also reflected in the ROI-specific mean FA ± SD, as illustrated in Figure 2.
Correlation between FA and the Amount of Meditation Experience
Significant correlations between ROI-specific FA and the number of meditation years were absent. Similarly, we detected no significant correlations between ROI-specific FA and the current frequency of the meditation practice (i.e., minutes per week). Although we detected significant positive correlations between the accumulated life-time minutes and the FA of the left CgH (r=.404; p=0.041) as well as the right CgC, on a trend level (r=.364; p=0.068) these findings did not survive when applying Bonferroni corrections for multiple comparisons.
Supplemental Analysis I: Axial and Radial Diffusivity
Meditators and controls did not differ significantly with respect to either axial diffusivity or radial diffusivity for any of the ROIs (the only exception was the UNC, where meditators had a slightly larger axial diffusivity). More specifically, with respect to axial diffusivity, the repeated-measures omnibus model including the 9 bilateral tracts yielded a significant Group-by-Tract Interaction (F[8,44]=3.003; p=0.009) and a significant Group-by-Tract-by-Hemisphere interaction (F[8,44]=2.242; p=0.042). As summarized in Supplemental Tables 3, follow-up analyses revealed a significantly larger axial diffusivity in meditators for the uncinate fasciculus (UNC). With respect to radial diffusivity, the repeated-measures omnibus model including the 9 bilateral tracts yielded a significant Group-by-Tract Interaction (F[8,44]=2.866; p=0.012) and a significant Group-by-Tract-by-Hemisphere interaction (F[8,44]=2.552; p=0.022). However, as summarized in Supplemental Tables 4, follow-up analyses did not reveal significant group differences for any of the tracts.
Supplemental Analysis II: Correlation between FA and Age
As shown in Table 2 (Controls / Meditators), FA and age were highly correlated with smaller FA measures in older subjects. These negative correlations were evident both in meditators and in controls (with the exception of the right CST in meditators which showed a positive correlation). However, as also reflected in the slopes of the regression lines (Figure 3), the age-related decline in meditators was much less prominent than in controls. This group difference with respect to the correlations between FA and age reached statistical significance for all of the ROIs, except the left and right CgH and the left CgC (Table 2; Controls versus Meditators).
Table 2.
Correlations between FA and age
| ROI |
Correlation |
Group Difference |
||
|---|---|---|---|---|
| |
Controls |
Meditators |
Controls versus Meditators |
|
| ATR | LH | r(25) = -0.753*** | r(25) = -0.495** | F(1, 50) = 7.44** |
| |
RH |
r(25) = -0.756***
|
r(25) = -0.465*
|
F(1, 50) = 9.82**
|
| CgC | LH | r(25) = -0.483* | r(25) = -0.117 | F(1, 50) = 2.48 |
| |
RH |
r(25) = -0.497**
|
r(25) = -0.096 |
F(1, 50) = 4.32*
|
| CgH | LH | r(25) = -0.482* | r(25) = -0.094 | F(1, 50) = 2.15 |
| |
RH |
r(25) = -0.401*
|
r(25) = -0.140 |
F(1, 50) = 1.08 |
| CST | LH | r(25) = -0.601*** | r(25) = -0.100 | F(1, 50) = 7.80** |
| |
RH |
r(25) = -0.600***
|
r(25) = 0.043 |
F(1, 50) = 10.67**
|
| IFO | LH | r(25) = -0.783*** | r(25) = -0.442* | F(1, 50) = 13.07*** |
| |
RH |
r(25) = -0.786***
|
r(25) = -0.278 |
F(1, 50) = 10.76**
|
| ILF | LH | r(25) = -0.761*** | r(25) = -0.212 | F(1, 50) = 13.66*** |
| |
RH |
r(25) = -0.720***
|
r(25) = -0.155 |
F(1, 50) = 8.97**
|
| SLF | LH | r(25) = -0.726*** | r(25) = -0.162 | F(1, 50) = 16.13*** |
| |
RH |
r(25) = -0.683***
|
r(25) = -0.186 |
F(1, 50) = 7.78**
|
| tSLF | LH | r(25) = -0.760*** | r(25) = -0.087 | F(1, 50) = 20.86*** |
| |
RH |
r(25) = -0.716***
|
r(25) = -0.107 |
F(1, 50) = 10.36**
|
| UNC | LH | r(25) = -0.729*** | r(25) = -0.497** | F(1, 50) = 7.37** |
| |
RH |
r(25) = -0.812***
|
r(25) = -0.244 |
F(1, 50) = 10.88**
|
| Fmajor |
r(25) = -0.645***
|
r(25) = -0.178 |
F(1, 50) = 5.24*
|
|
| Fminor | r(25) = -0.762*** | r(25) = -0.628*** | F(1, 50) = 6.38* | |
LH = Left Hemisphere; RH = Right Hemisphere
Significant at the 0.001 level.
Significant at the 0.01 level.
Significant at the 0.05 level.
Discussion
We detected larger FA throughout the entire brain when comparing long-term meditators against a well-matched sample of healthy controls. FA is a measure of how anisotropic (cigar-shaped) the fitted tensor is within a given voxel. That is, FA indicates the degree of directional sensitivity of water diffusion within the voxel (Basser and Pierpaoli, 1996). As summarized recently (Thomason and Thompson, 2010), higher FA measures reflect fibers that are more numerous, more dense, more myelinated, or more coherent in orientation which, in turn, influences the ability to rapidly relay electrical signals. Although, paradoxically, a loss of crossing white-matter fibers also leads to increased FA (because that implies a higher proportion of fibers running in the principal diffusion direction), larger FA values are generally interpreted as consistent with an enhanced connectivity; either achieved through the more numerous transmitting units and/or their superior efficiency as a result of axonal morphology and myelination.
Correspondence with Previous Findings
Larger FA values in meditators were particularly evident (i.e., p≤0.001) within the temporal component of the superior longitudinal fasciculus (tSLF) in the left hemisphere, as well as within the uncinate fasciculus (UNC) and the corticospinal tract (CST) in both hemispheres. To our knowledge, only one previous study assessed the link between FA and meditative practices (Tang et al., 2010). However, since that particular study was based on longitudinal analyses in meditation novices (rather than cross-sectional analyses in long-term practitioners) the relevance of these findings to ours is less direct. Given the lack of comparable DTI-based findings, the subsequent paragraphs attempt to relate our current observations to other MRI-based findings from meditation studies. Although the inter-relations between different anatomical substrates require further investigation, this discussion may provide a useful context for considering how meditation practices may influence cerebral macro- and microstructure at the regional level. Eventually, a more direct comparison between measures across different imaging modalities might lead to the identification of brain regions, networks, and system involved in the process of meditation.
The SLF traverses through the superior temporal gyrus. Thus, the observed larger FA of the tSLF may be related to thicker cortices in meditators in the caudal area of the temporal lobe (Lazar et al., 2005). Moreover, it might also correspond to previous findings within the inferior temporal gyrus indicating larger GM volumes / concentration in meditators compared to controls as well as a positive correlations between cumulated meditation hours and GM concentration (Luders et al., 2009b; Holzel et al., 2008). Although the inferior temporal gyrus (Luders et al., 2009b; Holzel et al., 2008) contains less direct tSLF projections than the superior temporal gyrus (Lazar et al., 2005), all three studies reported alterations within the left hemisphere. Intriguingly, these hemisphere-specific effects agree well with our observations of pronounced group differences with the left tSLF (and also left SLF). Recent DTI-based outcomes complement these findings by demonstrating meditation-induced FA changes within the left SLF (Tang et al., 2010). Whether hemispheric shifts pertaining to group differences within tSLF/SLF are related to hemisphere-specific functional associations, perhaps within the language domain, remains to be established in future studies.
The UNC has a ventral part that connects the orbital cortex with the amygdala and the hippocampal gyrus (Kier et al., 2004). Thus, the larger FA of this fiber tract may relate to the larger GM volumes within the orbito-frontal cortex and larger hippocampal volumes as were previously reported in an overlapping sample of meditators (Luders et al., 2009b). Moreover, the larger FA of the UNC may be linked to the higher GM density observed in meditators within the inferior frontal lobe (Vestergaard-Poulsen et al., 2008) and likewise, contribute to the positive correlations between cumulated meditation hours and GM concentration within the orbito-frontal cortex (Holzel et al., 2008). Since both orbito-frontal and hippocampal regions have been implicated in emotional regulation and emotional response control (Quirk and Beer, 2006; Davidson et al., 2000), we had suggested previously that “larger volumes in these regions might account for meditators’ singular abilities and habits to cultivate positive emotions, retain emotional stability, and engage in mindful behavior” (Luders et al., 2009b). The currently observed pronounced connectivity of the UNC in long-term meditators adds further support to this hypothesis.
The larger FA of the CST in meditators may be more difficult to interpret as this fiber tract “originates from an extensive cortical territory including somatosensory and parietal cortex, but most fibres stem from primary motor and premotor areas” (Westerhausen et al., 2007). Clearly, further research will be necessary to elucidate the functional relevance of links between meditation practices and structural alterations in motor areas of the brain. However, the current outcomes might be related to observations of enhanced GM in meditators (correlations between cortical thickness and hours of meditation experience, respectively) within paracentral and somatosensory areas (Grant et al., 2010; Luders et al., 2009b; Lazar et al., 2005), particularly when thresholds were lowered to detect significance trends. Moreover, given that the CST passes through the medulla oblongata, its currently observed larger FA might also correspond to findings of larger brain stem GM density in experienced meditators (Vestergaard-Poulsen et al., 2008). The nuclei of the medulla oblongata are involved in respiratory and cardiac control, as discussed by Vestergaard-Poulsen (2008). Thus, the pronounced connectivity of the CST in meditators might be related to alterations in breathing and heart rate during (or as consequence of) meditation (An et al., 2010; Zeidan et al., 2010; Peressutti et al., 2010; Tang et al., 2009).
Nature versus Nurture
Our current study revealed significantly larger FA in long-term meditators compared to well-matched controls. Given the accumulating evidence for neuroplasticity on a macro-anatomical level (Driemeyer et al., 2008; Boyke et al., 2008; May et al., 2007; Draganski et al., 2006; Draganski et al., 2004) it is tempting to assume that the observed group differences constitute actual meditation-induced effects. However, due to the current cross-sectional design we cannot exclude the possibility that meditators might have brains that are fundamentally different to begin with. For example, a particular brain anatomy may have drawn an individual to meditation and/or helped maintain an ongoing practice. In order to reveal possible indicators (albeit not evidences) for meditation-induced effects, we examined the relationships between ROI-specific FA and amount of meditation experience. As previously argued, “a positive correlation would corroborate the causal role of meditation practice” (Holzel et al., 2008). Our study did not reveal such significant positive correlations contrasting with previous findings with respect to GM attributes (Grant et al., 2010; Holzel et al., 2008; Lazar et al., 2005). Indeed, this may imply that the observed larger FA in meditators constitutes a predisposition (rather than being the consequence of the practice).
Notwithstanding, a possible explanation for the lack of significant correlations might be the confounding effects of age. That is, older subjects have the longest meditation history in general but are also more prone to age-related declines in white matter FA, as demonstrated in our current study but also by others (Sullivan et al., 2010; Madden et al., 2009; Sullivan and Pfefferbaum, 2006). Although the variance associated with age was controlled for in our statistical model, the trajectory of age effects might be regionally variable which may have masked any significant associations between meditation experience and FA. Moreover, it is possible that the chosen indicators of meditation experience may be less accurate for determining the actual extent / intensity of the individual training. That is, although we have subject-specific estimates with respect to frequency and length of their current meditation sessions, extrapolations over lengthy periods (up to 46 years) are subjective rather than precise. The problem is further complicated by the fact that meditation styles vary across meditators; however, even if all meditators practiced one particular style (and consistently over time), they are likely to be engaged differently in their mental exercises. Altogether, this may explain the lack of any significant correlations between FA and the amount of meditation experience. Longitudinal DTI studies are clearly necessary to determine whether larger FA within long-term meditation practitioners was actually induced by meditation, whether it was an innate prerequisite for the start and continuation of meditation, or whether it was a combination of nature and nurture (Wan and Schlaug, 2010).
Importantly, there is already some DTI-based evidence for actual meditation-induced changes within the left SLF after only one month of an Integrative Body–Mind Training (Tang et al., 2010). Moreover, a longitudinal MRI study revealed tissue enlargement of the left hippocampus and at the left temporo-parietal junction as a consequence of an 8-week mindfulness-based intervention (Holzel et al., 2010). Thus, the currently observed effects within tSLF (i.e., a fiber tract constituting the temporal component of the SLF originating/terminating in the vicinity of the temporo-parieto junction) and within UNC (i.e., a fiber tract linked to the hippocampus) may indeed represent actual effects of meditation, especially since the mean duration of meditation practice was more than 23 years. Similarly, the outcomes of the two existing longitudinal studies (Tang et al., 2010; Holzel et al., 2010) suggest that larger FA within SLF (i.e., the entire fiber tract connecting frontal and temporo-parietal regions) and CgH (i.e., another fiber bundle linked to the hippocampus) were induced by active meditation practices. Since the CgH also connects with the cingulate cortex, supplementary support for its susceptibility to meditation effects is provided by longitudinal effects with respect to cingulate fiber tracts (i.e., corona radiata) and cingulate GM tissue, as outlined below.
The two aforementioned longitudinal studies (Tang et al., 2010; Holzel et al., 2010) revealed additional effects of meditation (i.e., larger FA or GM density) within the superior and anterior corona radiata, the callosal genu and body, as well as within the posterior cingulate and the cerebellum. Thus, the currently observed larger FA within forceps minor [Fminor] (i.e., the frontal projection of the corpus callosum) and within the right cingulate bundle [CgC] (i.e., the fiber tract corresponding to the corona radiata) might indeed constitute meditation-induced features in long-term practitioners. Future research will be necessary to determine whether larger FA within the remaining regions (i.e., anterior thalamic radiation [ATR], inferior fronto-occipital fasciculus [IFO], and inferior longitudinal fasciculus [ILF]) was the cause or consequence of active meditation practices. Existing cross-sectional MRI studies, reporting larger GM volumes within frontal, temporal, and thalamic regions (Luders et al., 2009b; Holzel et al., 2008; Vestergaard-Poulsen et al., 2008) seem in agreement with respect to the spatial location of current DTI findings.
Possible Underlying Mechanisms and Relevance
If we assume that (at least some of) the observed larger FA in meditators is caused by actively meditating (rather than an innate prerequisite for the start and/or continuation of meditation) the question arises for the underlying mechanism. FA may reflect differences in the degree of angular distribution, differences in the degree of myelination, as well as differences in the number and/or density of fibers (Alexander et al., 2010; Madler et al., 2008; Song et al., 2002). Others have suggested that characteristics of axonal membranes play the dominant role in determining the amount of anisotropy (Imfeld et al., 2009; Beaulieu, 2002). On the one hand, it is possible that actively meditating (especially regularly meditating over a long period of time) can induce plastic changes on a micro-anatomical level, such as myelinogenesis (Demerens et al., 1996). As a consequence, FA in meditators may increase and possibly lead to macroscopic effects observable via DTI. On the other hand, if practiced regularly and over years, meditation might slow down aging-relating brain atrophy, perhaps due to altering (i.e., positively affecting) autonomic regulation and immune activity (Cysarz and Bussing, 2005; Davidson et al., 2003; Kubota et al., 2001). This assumption is consistent with our observation of significantly reduced rates of FA decline in long-term meditators compared to age-matched controls. Similar observations indicating typical age-related decreases in controls but not in meditation practitioners have been previously reported with respect to gray matter and cortical thickness (Pagnoni and Cekic, 2007; Lazar et al., 2005). It is certainly reasonable to assume that some brain regions or networks in long-term meditators are changed through training whereas others are only better maintained. However, it is equally plausible, that it is a combination of both effects.
Regardless the exact underlying mechanism, it is worth noting that the observed group differences are not only confined to a particular core region but rather involve large-scale networks which include the frontal, temporal, parietal, occipital lobes, the anterior corpus callosum, as well as limbic structures and the brain stem. Thus, meditation appears to be a powerful mental exercise with the potential to change the physical structure of the brain at large. Collecting evidence that active, frequent, and regular meditation practices cause alterations of white matter fiber tracts that are profound and sustainable (i.e., outlasting the actual duration of the meditation session), may become relevant for patient populations suffering from axonal demyelination and white matter atrophy. However, given the extremely sparse data more research (especially long-term analyses) in normative samples (rather than patient populations) is required before taking meditation into clinical trial studies.
Implications for Future Research
Future studies may expand this line of research by complementing anatomical measures with neuropsychological measures (Pagnoni and Cekic, 2007). For example, the SLF has been suggested to be involved in regulating spatial attention (Makris et al., 2005). Thus, the observed larger FA of the tSLF and SLF in mediators may be directly related to the importance assigned to attentional self-regulation, a core characteristic of meditation practices. Subsequent studies could therefore address if meditators show increased capacities for sustained attention that link more directly with increased FA within the SLF (tSLF). Moreover, since numerous studies revealed associations between brain anatomy and intelligence (Luders et al., 2009a), matching meditators and controls for IQ (and/or for various aspects of life style), will ensure that these variables do not contribute to the observed results. In addition, while the current work may capture the underlying anatomical substrates for the common ‘nucleus’ that characterizes meditation in general (Baerentsen et al., 2009), future studies, if sufficiently powered, may want to consider exploring possible differential effects of various meditation styles.
Supplementary Material
Research Highlights.
Little is known about the cerebral characteristics that underlie meditation.
Our DTI study revealed enhanced structural connectivity in meditators.
FA was larger within projection, commissural, and association pathways.
Largest effects were observed bilaterally within CST and UNC, and within left tSLF.
Meditation might be a powerful tool to change the physical structure of the brain.
Acknowledgements
We warmly thank all participants for their dedication and partaking in our study. We are also grateful to Trent Thixton who assisted with the acquisition of the image data. For generous support the authors thank the Brain Mapping Medical Research Organization, the Robson Family and Northstar Fund, and the following Foundations: Brain Mapping Support, Pierson-Lovelace, Ahmanson, Tamkin, William M. & Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community, Jennifer Jones-Simon, and Capital Group Companies. This study was also supported by the National Institutes of Health (U54 RR021813, P41 RR013642, and M01 RR000865), including the National Center for Research Resources (RR12169, RR13642, and RR00865). Further support was provided by grants from the Human Brain Project (P20-MHDA52176 and 5P01-EB001955) and the following additional National Institutes: Biomedical Imaging & Bioengineering, Mental Health, Drug Abuse, Cancer, Neurologic Disease & Stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The reason for registering the T1-weighted image to the non-diffusion-weighted image (rather than to the diffusion-weighted image) is the higher signal-to-noise ratio (i.e., the non-diffusion-weighted image has a contrast of a T2-weighted image). Note, due to the preceding motion correction, the non-diffusion-weighted image is spatially aligned to all of the diffusion-weighted images within a subject.
When conducting these analyses without including age as covariate, findings were very similar but slightly less significant. Supplemental Table 2 illustrates the ROI-specific outcomes without co-varying for age. Note, while the direction of the effect (i.e., meditators > controls) did not change for any of the comparisons, the group effect for ATR was no longer significant. Similarly, we lost the right-hemispheric effect for SLF. Nevertheless, the group effect, the group-by-hemisphere interaction, and the left-hemispheric effect were still significant for SLF.
Reference List
- Alexander DC, Hubbard PL, Hall MG, Moore EA, Ptito M, Parker GJ, Dyrby TB. Orientationally invariant indices of axon diameter and density from diffusion MRI. Neuroimage. 2010;52:1374–1389. doi: 10.1016/j.neuroimage.2010.05.043. [DOI] [PubMed] [Google Scholar]
- An H, Kulkarni R, Nagarathna R, Nagendra H. Measures of heart rate variability in women following a meditation technique. Int.J Yoga. 2010;3:6–9. doi: 10.4103/0973-6131.66772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EE, Bai Z, Bischof C, Blackford S, Demmel J, Dongarra J, Du Croz J, Greenbaum A, Hammarling S, McKenney A, Sorensen D. LAPACK Users’ Guide. Third edn Society for Industrial and Applied Mathematics; Philadelphia, PA: 1999. [Google Scholar]
- Baerentsen KB, Stodkilde-Jorgensen H, Sommerlund B, Hartmann T, msgaard-Madsen J, Fosnaes M, Green AC. An investigation of brain processes supporting meditation. Cogn Process. 2009 doi: 10.1007/s10339-009-0342-3. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J.Magn Reson.B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson.B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol.Bull. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Cohen A. Comparing regression coefficients across subsamples: a study of the statistical test. Sociological Methods & Research. 1983;12:77–94. [Google Scholar]
- Collins DL, Zijdenbos AP, Kollokian V, Sled JG, Kabani NJ, Holmes CJ, Evans AC. Design and construction of a realistic digital brain phantom. IEEE Trans.Med.Imaging. 1998;17:463–468. doi: 10.1109/42.712135. [DOI] [PubMed] [Google Scholar]
- Cysarz D, Bussing A. Cardiorespiratory synchronization during Zen meditation. Eur.J Appl.Physiol. 2005;95:88–95. doi: 10.1007/s00421-005-1379-3. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol.Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, Urbanowski F, Harrington A, Bonus K, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosom.Med. 2003;65:564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc.Natl.Acad.Sci.U.S.A. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Buchel C, May A. Changes in gray matter induced by learning--revisited. PLoS.ONE. 2008;3:e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JA, Courtemanche J, Duerden EG, Duncan GH, Rainville P. Cortical thickness and pain sensitivity in zen meditators. Emotion. 2010;10:43–53. doi: 10.1037/a0018334. [DOI] [PubMed] [Google Scholar]
- Heidemann RM, Ozsarlak O, Parizel PM, Michiels J, Kiefer B, Jellus V, Muller M, Breuer F, Blaimer M, Griswold MA, Jakob PM. A brief review of parallel magnetic resonance imaging. Eur.Radiol. 2003;13:2323–2337. doi: 10.1007/s00330-003-1992-7. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, Lazar SW. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Research: Neuroimaging. 2010 doi: 10.1016/j.pscychresns.2010.08.006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D. Investigation of mindfulness meditation practitioners with voxel-based morphometry. SCAN. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jancke L. White matter plasticity in the corticospinal tract of musicians: a diffusion tensor imaging study. Neuroimage. 2009;46:600–607. doi: 10.1016/j.neuroimage.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. AJNR Am.J Neuroradiol. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Sato W, Toichi M, Murai T, Okada T, Hayashi A, Sengoku A. Frontal midline theta rhythm is correlated with cardiac autonomic activities during the performance of an attention demanding meditation procedure. Brain Res.Cogn Brain Res. 2001;11:281–287. doi: 10.1016/s0926-6410(00)00086-0. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, McGarvey M, Quinn BT, Dusek JA, Benson H, Rauch SL, Moore CI, Fischl B. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Toga AW. Neuroanatomical Correlates of Intelligence. Intelligence. 2009a;37:156–163. doi: 10.1016/j.intell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Toga AW, Lepore N, Gaser C. The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter. Neuroimage. 2009b;45:672–678. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol.Rev. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madler B, Drabycz SA, Kolind SH, Whittall KP, Mackay AL. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson.Imaging. 2008;26:874–888. doi: 10.1016/j.mri.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr., Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb.Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- May A, Hajak G, Ganssbauer S, Steffens T, Langguth B, Kleinjung T, Eichhammer P. Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb.Cortex. 2007;17:205–210. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- Pagnoni G, Cekic M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiol.Aging. 2007;28:1623–1627. doi: 10.1016/j.neurobiolaging.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Peressutti C, Martin-Gonzalez JM, Garcia-Manso M, Mesa D. Heart rate dynamics in different levels of Zen meditation. Int.J Cardiol. 2010;145:142–146. doi: 10.1016/j.ijcard.2009.06.058. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr.Opin.Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson.Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav.Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol.Aging. 2010;31:464–481. doi: 10.1016/j.neurobiolaging.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Geng X, Stein EA, Yang Y, Posner MI. Short-term meditation induces white matter changes in the anterior cingulate. Proc.Natl.Acad.Sci.U.S.A. 2010;107:15649–15652. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Fan Y, Feng H, Wang J, Feng S, Lu Q, Hu B, Lin Y, Li J, Zhang Y, Wang Y, Zhou L, Fan M. Central and autonomic nervous system interaction is altered by short-term meditation. Proc.Natl.Acad.Sci.U.S.A. 2009;106:8865–8870. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Thompson PM. Diffusion Imaging, White Matter, and Psychopathology. Annu.Rev.Clin.Psychol. 2010 doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- Vestergaard-Poulsen P, van BM, Skewes J, Bjarkam CR, Stubberup M, Bertelsen J, Roepstorff A. Long-term meditation is associated with increased gray matter density in the brain stem. Neuroreport. 2008 doi: 10.1097/WNR.0b013e328320012a. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van ZP, Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Schlaug G. Music making as a tool for promoting brain plasticity across the life span. Neuroscientist. 2010;16:566–577. doi: 10.1177/1073858410377805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhausen R, Huster RJ, Kreuder F, Wittling W, Schweiger E. Corticospinal tract asymmetries at the level of the internal capsule: is there an association with handedness? Neuroimage. 2007;37:379–386. doi: 10.1016/j.neuroimage.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J.Comput.Assist.Tomogr. 1998a;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J.Comput.Assist.Tomogr. 1998b;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Gordon NS, Merchant J, Goolkasian P. The effects of brief mindfulness meditation training on experimentally induced pain. J Pain. 2010;11:199–209. doi: 10.1016/j.jpain.2009.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.