Abstract
Despite promising results in clinical trials conducted to date, little is known about how cardiac contractile modulation (CCM) mediated inotropic enhancement occurs and how CCM affects the electrophysiological characteristics of the heart. The aims of the present study were to 1) investigate how the stimulation parameters of the CCM signal and the location of stimulus delivery influence the contractile response, 2) characterise the effect of CCM on ventricular electrophysiology, and 3) investigate the potential physiological mechanisms underlying these acute inotropic and electrophysiological effects. Experiments were conducted in isolated rabbit hearts with simultaneous measurement of ventricular contractility and monophasic action potential duration (MAPD). Biphasic square wave pulses were applied to the left ventricle, timed to coincide with the absolute refractory period. CCM mediated responses were assessed over a range of signal amplitudes (2–30 mA), durations (2–15 ms) and delays from the activation of the locally recorded monophasic action potential (0–30 ms). Responses were assessed during perfusion with the β1-adrenoceptor antagonist metoprolol (1.8 μM) and HMR 1556 (500 nM), an inhibitor of the slow delayed rectifying potassium current. Norepinephrine content was collected and assessed by ELISA from samples of coronary effluent collected during CCM. CCM induced a significant increase in left ventricular pressure (LVP) in a manner dependent upon the amplitude and duration of the CCM signal but independent of the delay of the stimulus within the action potential plateau and was associated with an increase in norepinephrine in coronary effluent (Mean: 46 ± 9 pg/ml). CCM promoted a shortening of MAPD-90% close to the site of stimulation (− 19 ± 3%) but had no effect on those recorded at distant sites (0 ± 1%). The increase in LVP (4.7 ± 1.8 vs. 0.7 ± 0.9%, P < 0.01) and shortening of local MAPD-90% (− 15 ± 3 vs. 1 ± 1%, P < 0.01) was abolished with metoprolol. Perfusion with HMR 1556 caused a significant inhibition of local MAPD shortening (− 27 ± 2 vs. − 21 ± 3 ms, P < 0.05). CCM is associated with a shortening of ventricular MAPD in a manner dependent upon β-adrenoceptor stimulation resulting from catecholamine release, a finding which may be of clinical significance in regard to the development of malignant ventricular arrhythmias. This article is part of a Special Issue entitled Possible Editorial.
Keywords: Cardiac contractility modulation, Non-excitatory stimulation, Rabbit, Sympathetic nerves, Langendorff
Research highlights
► CCM induced responses are related to the amplitude and duration of the stimulus. ► CCM causes a shortening of ventricular monophasic action potential duration. ► Inotropic and electrophysiological responses to CCM are β1-adrenoceptor dependent. ► CCM signal application stimulates catecholamine release. ► Action potential shortening with CCM is partially mediated via IKs.
1. Introduction
Application of electrical currents to the myocardium during the absolute refractory period of the ventricular action potential enhances ventricular inotropy in vivo and in a wide range of isolated cardiac tissue preparations [1–4]. This effect, termed cardiac contractility modulation (CCM) is a novel therapeutic treatment for use in patients with heart failure who are unresponsive to conventional drug therapies and/or ineligible for/unresponsive to cardiac resynchronisation therapy. Despite several studies on CCM, little is known about the mechanisms by which this form of non-excitatory stimulation enhances ventricular contractility.
It is possible that electrical stimulation of the myocardium could stimulate autonomic nerves within the myocardium and thereby promote the release of adrenergic neurotransmitters from sympathetic nerve terminals. However, experimental evidence to date has been conflicting. Early reports in isolated rabbit papillary muscles demonstrated that inotropic response to CCM was unchanged during pharmacological β-adrenoceptor blockade [4]. Similarly the inotropic effects of CCM were not blocked in isolated ferret hearts perfused with the β adrenoceptor antagonist propranolol [3], although there was a trend for towards a reduction in left ventricular inotropy and systolic level of the Ca2+ transient. More recently, Meyer et al. reported that application of electrical signals within the coronary sinus during the absolute refractory period (similar to CCM) in sheep promoted an increase in ventricular pressure development that was abolished when these animals were pre-treated with propranolol [5], providing indirect evidence that application of electrical signals to the heart during the refractory period could stimulate the release of sympathetic neurotransmitters.
In addition to an increase in contractility, early studies in isolated papillary muscles [4] suggested that CCM prolongs action potential duration and indicated a potential direct effect of CCM on sarcolemmal ion channels (e.g. the L-type Ca2+ channel). To our knowledge these findings have yet to be confirmed in the whole heart. As the activation and recovery of specific depolarising and repolarising currents change over the cardiac cycle, it is important to assess whether differences in the parameters of the CCM signal would influence the inotropic and electrophysiological response to CCM. This is supported by the lack of such data within the literature. In addition, knowledge of the effect of CCM signal parameters on the response may be of clinical importance to establish clinical efficacy. The role of adrenergic neurotransmitter release and concurrent β-adrenoceptor activation in the acute mechanisms of CCM induced inotropy require further investigation as do the electrophysiological effects of CCM in the whole heart.
The aims of the present study are to1) investigate how the stimulation parameters of the CCM signal and the location of stimulus delivery influence the contractile response in, 2) characterise the effect of CCM on ventricular electrophysiology, and 3) investigate the potential physiological mechanisms underlying these acute inotropic and electrophysiological effects in isolated rabbit hearts.
2. Materials and methods
2.1. Animal welfare
All procedures were undertaken in accordance with ethical guidelines set out by the UK Animals (Scientific Procedures) Act 1986 and conformed to the Guide for the Care and Use of Laboratory Animals Published by the US National Institutes of Health (NIH Publication No.85-23, revised 1985).
2.2. Langendorff tissue isolation and perfusion
Adult male New Zealand White rabbits (2.0–2.5 kg, n = 21) were pre-medicated with ketamine (Ketaset, 10 mg/kg, Fort Dodge, Southampton, UK), medetomidine hydrochloride (Sedator, 0.2 mg/kg, Dechra, Shrewsbury, UK) and butorphanol (Torbugesic, 0.05 mg/kg, Fort Dodge Southhampton, UK) (i.m.). 15–20 min later, animals were sacrificed with an overdose of pentobarbitone sodium (Sagatal, Rhone Merieux, Harlow, UK; 0.8 mg/kg. body weight, i.v.) containing Heparin (1000 IU, Multiparin, Wrexham, United Kingdom) via the marginal ear vein. The hearts were rapidly excised and retrogradely perfused through the ascending aorta (40 ml/min) using a Gilson Minipulse 3 peristaltic pump (Anachem, Luton, UK). The heart was perfused with Tyrode solution of the following composition (mM): Na+ 138.0, K+ 4.0, Ca2+ 1.8, Mg+ 1.0, HCO3− 24.0, H2PO4− 0.4, Cl− 121.0, glucose 11.0 and acetate 20.0. The solution was continuously bubbled with 95% O2–5% CO2 to maintain a constant pH of 7.4. Temperature was maintained at 37 °C. A 3F polypropylene catheter (Portex, Kent, United Kingdom) was inserted at the apex of the left ventricle (LV) for drainage of Thebesian venous effluent. Intra-ventricular left ventricular pressure (LVP) was monitored with a fluid-filled latex balloon connected to a pressure transducer (MTL0380, ADInstruments Ltd, Chalgrove, UK) and inserted into the LV via the left atrium. Left ventricular end diastolic pressure was adjusted to zero. Aortic perfusion pressure (AP) was monitored with a second pressure transducer connected in a series with the aortic cannula.
2.3. Cardiac electrical recording and pacing
A pair of platinum electrodes (Grass Instruments, Astro-Medical Inc., Slough, UK) were inserted into the right atrial appendage for recording of atrial electrograms. Contact electrodes were used to record monophasic action potentials (MAPs) from the epicardial surface of the LV using a DC-coupled high input impedance differential amplifier (Joint Biomedical Workshop, University of Leicester, UK). MAPs were recorded from the left ventricular free wall close to (within 5 mm) and distant from the site of stimuli delivery at either apex or base depending on the experimental protocol. All hearts were paced at 200 bpm via another contact electrode inserted into the right ventricular apex.
2.4. CCM signal generation and delivery
Square wave electrical pulses were generated using a Neurolog modular system (Digitimer, Welwyn Garden City, UK) and signal strength was amplified through a constant current stimulator (Model A385, World Precision Instruments, Stevenage, UK). The CCM signal was applied directly to the epicardial surface of the left ventricular myocardium through a pair of platinum hook electrodes (Grass Instruments, Astro-Medical Inc., Slough, UK) as biphasic pulses with equal positive and negative phase amplitudes. Stimulus amplitude, duration and delay from activation of MAP (Fig. 1) were varied according to experimental protocols (see below). CCM signal was delivered over a period of 2–3 min, allowing for at least a 30-s period of steady state response, with a 10-min rest period between sequential stimulations.
Fig. 1.
CCM signal. A square wave bipolar signal is applied directly to the surface of the LV during the absolute refractory period. Signal delay is timed from the point of activation of a locally recorded mono-phasic action potential.
2.5. Protocols
2.5.1. Stimulus location
The effect of different stimulus locations was studied to obtain the optimal location for CCM signal delivery, denoted by the maximal inotropic response to a stimulation of predefined parameters. Signal parameters were chosen based on preliminary experiments—signal amplitude: 20 mA, signal duration: 20 ms (10 ms per phase), delay: + 10 ms following the activation of locally recorded MAP. Stimuli were separately applied to the apex, midlevel and base regions of the LV (Apex–Base = 25–30 mm). MAPs were measured at the apex and base of the LV simultaneously.
2.5.2. Stimulus parameters
In this protocol CCM signals were delivered solely to basal regions (electrode spacing: 5 mm). All 3 following parameters were examined in the same hearts (n = 6).
2.5.2.1. Stimulus amplitude
Signal duration = 10 ms per phase, Signal delay = + 10 ms, Signal amplitude was varied between 4 and 30 mA during sequential stimulations.
2.5.2.2. Stimulus duration
Signal amplitude = 20 mA, Signal delay = + 10 ms. Signal duration was varied between 4 and 30 ms (2 and 15 ms per phase respectively).
2.5.2.3. Stimulus delay
Signal amplitude = 20 mA, signal duration = 10 ms per phase. Signal delay was timed to coincide with the activation of locally recorded MAPs (delay of 0 ms = time of activation). Signal delay was increased in 10 ms steps, in sequential stimulations, from 0 to 40 ms.
2.5.3. Mechanisms of CCM
Signal amplitude = 20 mA, signal duration = 10 ms per phase, Signal delay = + 10 ms. The CCM response was optimised from data obtained from the experiments described above.
2.5.3.1. Role of β-adrenoceptor activation
Responses were compared before and during perfusion with the β1-adrenoceptor antagonist metoprolol (1.8 μM, Sigma-Aldrich, Gillingham, UK).
2.5.3.2. Release of noradrenaline
Samples of coronary effluent (1 ml) were collected before, during and after CCM at both low (5 mA) and high (20 mA) amplitudes of CCM stimulation. Noradrenaline content in these samples was measured directly using a competitive enzyme linked immunosorbance assay (ELISA) (BA E-5200, Griffin Life Sciences, Abingdon, UK).
2.5.3.3. Mechanism of CCM activated MAPD shortening
The slow delayed rectifier potassium current is known to be involved in β-adrenoceptor induced shortening of action potential duration [6–10]. In the current study, alterations in monophasic action potential duration (MAPD) were examined during perfusion with a specific inhibitor of IKs, [(3R,4S)-(+)-N-[3-hydroxy-2,2-dimethyl-6-(4,4,4-trifluorobutoxy)chroman-4-yl]-N-methylmethanesulfonamide (HMR1556 500nM, Sanofi-Aventis, Frankfurt, Germany). The efficacy of HMR 1556 was confirmed during perfusion with the non-selective β-adrenoceptor agonist isoprenaline (ISO), at a dose (5 nM) that produces a similar degree of inotropy and MAPD shortening to that noted during CCM. Responses to ISO and CCM (signal amplitude = 20 mA, signal duration = 10 ms per phase, Signal delay = + 10 ms) were compared before and after perfusion of HMR 1556. All hearts were paced at a rate of 250 bpm to circumvent the chronotropic effects of ISO on the sino-atrial node.
2.6. Signal measurements and analysis
All signals were recorded with a PowerLab 8s system and digitised at 2 kHz using Chart and Scope software (ADInstruments Ltd). Left ventricular contractile performance was assessed from an average of 20 beats during baseline and during the steady response plateau. MAPD was measured at 90% repolarisation (MAPD90) averaged over 20 beats recorded during steady state before and immediately after the cessation of CCM stimulation. This was necessary due to signal interference from the CCM signal preventing measurement of MAPs during CCM signal application. MAPD90 calculations were performed using custom written analysis software (NewMap, Dr. F. Burton, University of Glasgow, UK).
2.7. Statistics
Statistical comparisons were made using Student paired t tests, one or two way ANOVA where appropriate with Bonferroni post hoc test. All data are presented as mean values ± SEM. P < 0.05 was considered significant.
3. Results
3.1. Inotropic and electrophysiological response to CCM
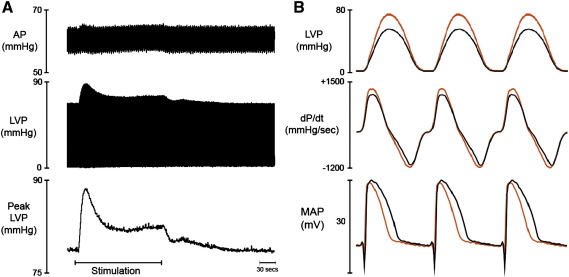
Fig. 2 illustrates the effect of CCM on perfusion pressure, left ventricular pressure, rate of pressure production and relaxation, and left ventricular epicardial monophasic action potentials in a typical experiment. Localised application of electrical signals to the epicardium of the LV promoted an increase in global pressure development (peak LVP) and rates of pressure development (dP/dtmax). The increase in peak LVP occurred within 2 to 5 s after application of the CCM signal and increased to an initial peak before decreasing to a new steady state that was higher than baseline. Following cessation of CCM, peak LVP gradually returned to baseline values within 2 to 3 min. There was no significant effect from CCM on the rate of ventricular relaxation (dP/dtmin, baseline = − 976 ± 95 mm Hg/s) or on perfusion pressure (AP, baseline = 63 ± 5 mm Hg). Left ventricular end diastolic pressure was similarly unaffected during CCM (baseline = 5±1 mm Hg). CCM resulted in a shortening of monophasic action potential, compared immediately before and immediately after CCM, which recovered to baseline values 2–3 min following the cessation of stimulation.
Fig. 2.
Effect of CCM signal delivery on left ventricular contractility and electrophysiology. Left panel. Raw data illustrating the effect of CCM on perfusion pressure (AP), left ventricular pressure (LVP) and peak LVP. Right panel. Averaged LVP profiles taken from Left panel, illustrating the rate of pressure development and relaxation together with the corresponding monophasic action potentials at baseline (black) and during steady state CCM (red). Stimulation parameters: Duration = 10 ms, Delay = 10 ms, Amplitude = 20 mA. (n = 1).
3.2. Effect of stimulus location on the response to CCM
Mean data illustrating the percentage change in peak LVP, dP/dtmax and MAPD90, recorded from multiple sites simultaneously, are shown in Fig. 3. CCM signals applied to basal (75 ± 6 vs. 78 ± 6 mm Hg, P < 0.01) and midlevel (74 ± 5 vs. 77 ± 5 mm Hg, P < 0.01) regions promoted significant increases in peak LVP. dP/dtmax was similarly significantly enhanced during CCM stimulation at basal (1205 ± 129 vs. 1355 ± 148 mm Hg/s, P < 0.01) and midlevel (1200 ± 104 vs. 1351 ± 126 mm Hg/s, P < 0.01) regions. CCM in apical regions was not associated with any significant change in ventricular inotropy. MAPD90 shortening was evident at regions close to the site of stimulation. During application of CCM at basal regions there was significant − 21 ± 4 ms (108 ± 6 vs. 88 ± 7 ms, P < 0.01) decrease in locally recorded MAPD90 and no change demonstrated in distant MAPD90 recorded from the left ventricular apex (106 ± 6 vs. 106 ± 6 ms). Stimulation in apical regions resulted in a significant − 16 ± 5 ms (110 ± 4 vs. 91 ± 4 ms, P < 0.05) reduction in locally recorded MAPD90 and no significant change in basal MAPD90 (110 ± 4 vs. 111 ± 3 ms). Midlevel stimulation promoted a significant reduction in basal (− 12 ± 5 ms) but not apical MAPD90. There was no difference in the degree of shortening of locally recorded MAPD90 between apical and basal stimulations (− 21 ± 4 vs. − 16 ± 5 ms, P = NS).
Fig. 3.
Heterogeneity of CCM response across the heart. Data illustrating the percentage change in peak left ventricular pressure (LVP, Panel A), maximal rate of change of pressure increase (dP/dtmax, Panel B) and apical and basal monophasic action potential duration (MAPD90, Panel C) during CCM at different epicardial sites. Sites of stimulation; A = Apex, M = Midlevel, B = Base. (n = 6) Heterogeneity of response to regional stimulation: *P < 0.05, **P < 0.01, ***P < 0.001. Proximal vs. distal MAP (see text for discussion): #P < 0.05, ##P < 0.01.
3.3. Effect of varying the stimulus parameters on the response to CCM
3.3.1. Amplitude
The effect of CCM signal amplitude on peak LVP, dP/dtmax and MAPD90 from 6 hearts is shown in Fig. 4. Significant increases in peak LVP were noted at stimulus amplitudes of 15 (3.4 ± 0.9 mm Hg, P < 0.01), 20 (3.9 ± 1.2 mm Hg, P < 0.001) and 30 mA (3.7 ± 0.9 mm Hg, P < 0.001). Similarly dP/dtmax was enhanced at stimulus amplitudes of 15 mA or greater. The percentage change in peak LVP and dP/dtmax was related to the stimulus amplitude in a monotonic manner, increasing to a plateau at stimulus amplitudes > 15 mA.
Fig. 4.
Effect of varying signal amplitude on the ventricular responses to CCM. Data illustrating the effect of varying the duration of the CCM signal on peak left ventricular pressure (LVP, Panels A & B), rate of pressure development (dP/dtmax, Panels C & D) and mono-phasic action potential duration (MAPD90, Panel E & F) (n = 6). CCM vs. Baseline; *P < 0.05,**P < 0.01,***P < 0.001.
A significant shortening of locally recorded MAPD90 was noted at stimulus amplitudes of 15 (− 28 ± 7 ms, P < 0.01), 20 (− 35 ± 5 ms, P < 0.001) and 30 mA (− 37 ± 7 ms, P < 0.001). A monotonic relationship was observed between the percentage change in MAPD90 and the stimulus amplitude, demonstrating a plateau at stimulus amplitudes above 20 mA.
3.3.2. Duration
The effect of CCM signal duration on peak LVP, dP/dtmax and MAPD90 from 6 hearts is shown in Fig. 5. A significant increase in peak LVP was noted at stimulus durations of 2 (2.0 ± 0.4 mm Hg, P < 0.01), 5 (2.5 ± 0.4 mm Hg, P < 0.001), 10 (3.5 ± 0.6 mm Hg, P < 0.001) and 15 ms (3.3 ± 0.5 mm Hg, P < 0.001). Similarly dP/dtmax was enhanced at all stimulus durations. The percentage change in peak LVP and dP/dtmax was related to the stimulus duration in a monotonic manner, increasing to a plateau at stimulus durations > 10 ms.
Fig. 5.
Effect of varying signal duration on the ventricular responses to CCM. Data illustrating the effect of varying the duration of the CCM signal on peak left ventricular pressure (LVP, Panels A and B), rate of pressure development (dP/dtmax, Panels C & D) and mono-phasic action potential duration (MAPD90, Panels E and F) (n = 6). CCM vs. Baseline; *P < 0.05, **P < 0.01, ***P < 0.001.
A significant shortening of locally recorded MAPD90 was noted at stimulus durations of 2 (− 18 ± 4 ms, P < 0.01), 5 (− 22 ± 4 ms, P < 0.001), 10 (− 29 ± 6 ms, P < 0.001) and 15 ms (− 26 ± 6 ms, P < 0.001). A monotonic relationship was observed between the percentage change in MAPD90 and stimulus amplitude, demonstrating a plateau at stimulus amplitudes > 10 ms.
3.3.3. Delay
The effect of time delay from MAP activation to CCM signal on peak LVP, dP/dtmax and MAPD90 from 6 hearts is shown in Table 1. A significant increase in peak LVP and dP/dtmax was noted at each delay investigated (0–30 ms). The increase in peak LVP (3.5 ± 0.5 mm Hg, P < 0.05) and dP/dtmax (126 ± 14 mm Hg/s, P < 0.05) during CCM was not significantly different at any delay studied. The shortening of MAPD90 was similarly unrelated to the timing of CCM signals during the absolute refractory period (− 23 ± 3 ms, P < 0.01).
Table 1.
Effect of varying CCM signal delay on the ventricular responses to CCM.
| Peak LVP (mm Hg) |
dP/dtmax (mm Hg/s) |
MAPD90 (ms) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Signal delay (ms) | Baseline | Steady state | % Change | Baseline | Steady state | % Change | Baseline | Steady state | % Change |
| 0 | 74 ± 6 | 77 ± 6* | 5 ± 2 | 1156 ± 125 | 1291 ± 151* | 12 ± 1 | 112 ± 6 | 91 ± 2* | − 18 ± 5 |
| 10 | 73 ± 5 | 76 ± 5* | 5 ± 1 | 1153 ± 116 | 1263 ± 140* | 9 ± 2 | 113 ± 6 | 87 ± 2** | − 21 ± 5 |
| 20 | 75 ± 7 | 79 ± 7* | 6 ± 3 | 1163 ± 148 | 1297 ± 177* | 11 ± 1 | 115 ± 3 | 87 ± 5** | − 23 ± 5 |
| 30 | 75 ± 5 | 79 ± 6* | 5 ± 1 | 1212 ± 139 | 1341 ± 165* | 10 ± 2 | 114 ± 5 | 91 ± 2** | − 19 ± 4 |
Data illustrating the effect of varying the delay of the CCM signal on peak left ventricular pressure (LVP), rate of pressure development (dP/dtmax) and mono-phasic action potential duration (MAPD90) (n = 6). CCM vs. Baseline:*P < 0.05, **P < 0.01.
3.4. Mechanisms of CCM induced inotropy and electrophysiological changes
3.4.1. Role of β-adrenoceptors
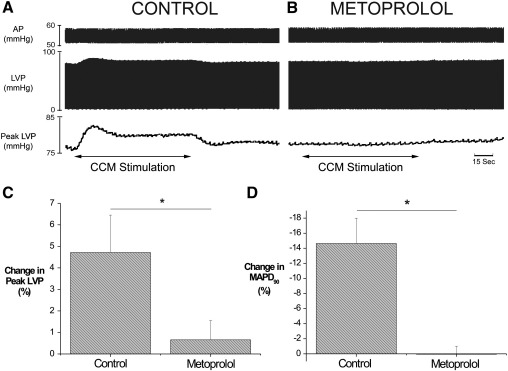
To investigate the role of β-adrenoceptor stimulation, we examined the effects of CCM in the absence and presence of the β1-adrenoceptor antagonist metoprolol. Raw and mean data illustrating the effect of CCM on peak LVP and locally recorded MAPD90 are presented in Fig. 6. Perfusion with metoprolol abolished the increase in peak LVP (3.3 ± 1 vs. 0.3 ± 0.4 mm Hg, P < 0.05), dP/dtmax (166 ± 30 vs. 44 ± 4 mm Hg/s, P < 0.01) and the shortening of locally recorded MAPD90 induced by CCM (− 17 ± 4 vs. 0.2 ± 1.24 ms, P < 0.05).
Fig. 6.
Role of β1-adrenoceptor stimulation in the ventricular response to CCM. Raw data illustrating the effect of CCM during control (Panel A) and during β1-adrenoceptor blockade using metoprolol (1.8 μM, Panel B) on left ventricular pressure (LVP), peak LVP and aortic perfusion pressure (AP). Mean data representing the effect of CCM on peak LVP and MAPD90 (Panels C and D) (n = 6). The effects of metoprolol: *P < 0.05.
3.4.2. Role of catecholamine release
To establish whether CCM stimulated noradrenaline release was an underlying mechanism of action for the effects observed, we measured noradrenaline content in samples of coronary effluent collected before, during and after CCM. Mean data from 4 hearts illustrating noradrenaline release and the change in peak LVP from baseline values at two stimulation amplitudes are illustrated in Fig. 7. CCM at both low (5 mA) and high (20 mA) amplitudes resulted in the detection of noradrenaline increase in the coronary effluent. Noradrenaline content returned to baseline values following the cessation of stimulation. Significantly more noradrenaline was measured during stimulation at high stimulus amplitude when compared to low stimulus amplitude (46 ± 9 vs.17 ± 5 pg/ml, P < 0.05). The mean steady state increase in peak LVP was greater during high than low stimulus amplitudes (7.2 ± 1.7 vs. 2.9 ± 0.7 mm Hg, P < 0.05).
Fig. 7.
Role of catecholamine release in the ventricular response to CCM. Mean data showing the effect of low (5 mA) and high (20 mA) amplitude CCM signals on the noradrenaline content in samples of coronary effluent (Panel A) and the change in peak left ventricular pressure (LVP) from baseline values (Panel B). The time course of CCM is demonstrated by the grey box (0–180 s). The sample at 180 s was collected prior to the cessation of CCM (n = 4).
3.4.3. Mechanism of MAPD shortening
To investigate if MAPD shortening was mediated via the slow activating delayed rectifier channel IKs, the effects of CCM were studied during perfusion with HMR 1556 (500 nM), an inhibitor of IKs and compared with the effects of perfusion with isoprenaline (ISO). Mean data from 6 hearts illustrating the effects of HMR 1556 on the shortening of MAPD induced by CCM and ISO are shown in Fig. 8. ISO caused a significant 5.1 ± 1.6 mm Hg increase in peak LVP (P < 0.05) and a − 27 ± 3 ms reduction in basal MAPD90 at baseline (P < 0.001). In the presence of HMR 1556, the effects of ISO on MAPD were reduced by 58 ± 4% (− 27 ± 3 vs. − 11 ± 1 ms, P < 0.05). The change in peak LVP associated with ISO was unaffected by HMR 1556. The shortening of MAPD90 associated with CCM was reduced by 26 ± 9% (− 33 ± 2 vs. − 25 ± 4 ms, P < 0.05) in the presence of HMR 1556. HMR 1556 had no effect on the increase in peak LVP associated with CCM (3.4 ± 0.9 vs. 2.9 ± 0.9 mm Hg, P = NS).
Fig. 8.
Role of the slow delayed rectifying potassium current in CCM induced shortening of ventricular mono-phasic action potential duration. Raw data illustrating the effect of CCM (Panel A) and isoprenaline (ISO, 5 nM) (Panel B) on monophasic action potential duration (MAPD) recorded from basal regions (BL = baseline). Mean data showing the effect of HMR 1556 (500 nM) on the shortening of MAPD induced by CCM (Panel C) and ISO (Panel D). The mean effects of HMR 1556 on the change in peak LVP induced by CCM (Panel E) and ISO (Panel F) (n = 6 CCM, 3 ISO).
4. Discussion
To be best of our knowledge, this is the first demonstration that the acute changes in inotropy induced by CCM are associated with a shortening of ventricular MAPD. In addition, we have shown that the inotropic and electrophysiological responses to CCM are dependent upon the location, amplitude and duration of stimulus delivery and independent of the timing of the stimulus within the refractory period (delay). Perfusion with metoprolol abolished the effects of CCM demonstrating a clear dependence upon the activation of myocardial β1-adrenoceptors. The increase in noradrenaline in the coronary effluent during CCM indicates that activation of β-adrenoceptors occurs as a result of the release of sympathetic neurotransmitters. Finally, we have demonstrated that the CCM signal alters proximal and not distal action potential duration an effect that is, in part, dependent on activation of IKs.
4.1. Stimulus parameter dependence of CCM
In the current study, the response to CCM was investigated over a range of signal parameters and demonstrated that the degree of contractile force enhancement is dependent upon the anatomical location of signal delivery, the stimulus amplitude and the duration of the stimulus but unrelated to the timing of the signal within the action potential (i.e. the delay). The finding that CCM efficacy is dependent on stimulus amplitude and signal duration is supported by studies in isolated rabbit papillary muscles [4] and during stimulation of the coronary sinus in sheep (amplitude only) [5]. It is conceivable that an increase in signal amplitude increases the area of the myocardium which is affected by the signal (i.e. greater current spread) and thus promotes a greater increase in global contractile force (a spatial effect). Increases in signal duration result in a greater period of stimulation and enhanced ventricular inotropy up to a plateau (a temporal effect). The plateau of the duration–ventricular inotropy relationship demonstrates the existence of a maximal response within a given spatial region. The role of stimulated noradrenaline release in these relationships is considered later in this discussion.
In our study, the inotropic response to CCM was not dependent on the timing of the stimulus within the action potential plateau. This suggests that, while the timing of the CCM signal must occur within the absolute refractory period to avoid triggering an additional ventricular contraction, there is a stable window of opportunity in which CCM signals can be applied. The previously reported linear dependence between contractile enhancement and signal delay in isolated rabbit papillary muscles [4] is at odds with the current study. This may reflect differences in the experimental preparation utilised (i.e. isolated muscle strip vs. whole organ responses) and/or highlight the multi-factorial mechanisms of CCM induced inotropic enhancement.
4.2. Intracardiac noradrenaline release and CCM
We have shown that the acute effects of CCM are abolished in the presence of the β1-adrenoceptor antagonist metoprolol and that CCM is associated with an increase in noradrenaline in samples of coronary effluent in a manner correlated with the magnitude of inotropic enhancement. It is therefore clear that noradrenaline release and concurrent activation of β1-adrenoceptors underlies the increase in LV inotropy and alterations in electrophysiology acutely associated with CCM in the rabbit heart. Our data are supported by the findings of Meyer et al. who found that the inotropic enhancement associated with refractory stimulation of the coronary sinus in sheep was completely abolished during β-adrenoceptor blockade [5]. Mohri et al. also reported a modest, but incomplete, inhibition of the inotropic response to CCM during perfusion with propranolol [3]. In contrast, the acute inotropic effects of CCM were preserved in the presence of propanolol in experiments performed in left and right isolated ventricular rabbit papillary muscles [4]. Previous investigators have reported that CCM is associated with an increase in the amplitude of the Ca2+ transient [3], phosphorylation of phospholamban [11] and that the inotropic response is dependent upon the entry of Ca2+ through the L-type Ca2+ channels [4]. These findings correlate with the well-described intracellular mechanisms whereby β-adrenoceptor stimulation results in myocardium inotropic enhancement [13]. Whilst our study does not provide any specific details of intracellular calcium homeostasis during CCM inotropy, it is likely that adrenergic activation will stimulate the L-type calcium channel, promote calcium release from the sarcoplasmic reticulum and stimulate an increase in calcium reuptake into the sarcoplasmic reticulum via SERCA (secondary to phospholamban phosphorylation), although the lack of enhancement of the rate of pressure relaxation in the current study may refute this point.
The ventricular myocardium is richly innervated with efferent sympathetic nerve fibres and it is likely that these fibres are stimulated to release noradrenaline by the delivery of electrical signals to the epicardial surface. Importantly, several studies have previously shown that electrical signals, not specifically timed to the absolute refractory period, can be used to stimulate catecholamine release from the perfused whole hearts and from isolated samples of cardiac tissue [14,15]. The distribution of sympathetic nerve fibres throughout the ventricle has been shown to be heterogenous with a gradient of fibre density from basal (highest) to apical (lowest) regions [16,17]. We have previously shown that the expression of tyrosine hydroxylase protein, an enzyme expressed in sympathetic nerve terminals, is greater in homogenates of rabbit ventricular tissue taken from basal than in those taken from apical regions [18]. Other studies have demonstrated a graded distribution of ventricular catecholamine content from the base to the apex in the canine heart [19] and reported greater proportion of adrenergic fibres innervating basal regions of the human and canine ventricular tissue [16,17]. These anatomical/histological findings provide a possible explanation for the differential inotropic effects noted during basal, midlevel and apical stimulation. In addition these data provide a greater understanding of the previously discussed relationships between signal amplitude (spatial), signal duration (temporal) and ventricular inotropy. The degree of inotropy likely reflects the proportion of sympathetic fibres stimulated within a given region or over a given period of time. Increases in signal amplitude will result in a greater spread of the electrical field and an increase in the current density (i.e. field intensity). As the spread and intensity of the electrical field is increased then a greater proportion of nerve fibres are likely to be stimulated resulting in a greater release of noradrenaline. This is reflected in our finding of a greater concentration of noradrenaline in samples of coronary effluent collected during CCM at high amplitudes. A further point for consideration is that stimulation within a given region may propagate the stimulation of nerves and release of noradrenaline throughout the ventricle. It is known that the myocardium exhibits a rich intrinsic innervation and that sympathetic efferent fibres run from basal to apical regions and innervate both epicardial and endocardial portions of the myocardium [20]. It seems unlikely that alterations in the signal delay should influence the contractile responses to CCM if such changes are mediated by catecholamine release from the sympathetic innervations of the LV, as our data indicate. Instead, varying CCM signal delivery is likely to be of critical clinical importance to avoid arrhythmias induced by inappropriately timed stimuli.
In the present study, CCM induced an increase in ventricular pressure development and contractility without significantly altering ventricular relaxation. These data are supported by the findings of Mohri et al. in the dog who reported no enhancement of either global or regional ventricular relaxation during CCM delivery to the ventricular epicardial surface [2]. In contrast, Meyer et al. found enhanced rates of ventricular relaxation during electrical stimulation in sheep [5]. It seems probable that this reflects the proportion of the myocardium, or more probably the proportion of sympathetic fibres, stimulated during CCM. Previous histological studies have identified a significant proportion of sympathetic fibres which cross the coronary sinus [20]. Therefore electrical stimulation in this region may result in a large and more homogenously distributed release of noradrenaline within the ventricle. The proportion of sympathetic fibres stimulated in the present study, and in that of Mohri et al.[2], may be insufficient to significantly alter global ventricular relaxation. In a recently published study Zhang et al. reported on the effects of CCM on cardiac function in rabbits with coronary ligation induced heart failure [21]. Acute CCM enhanced ventricular inotropy during stimulation at the anterior LV free wall and to a lesser degree during stimulation at the right ventricular septum. Ventricular lusitropy was only enhanced during LV free wall stimulation. While no specific information is presented on the site and size of region stimulated in this study these data do provide evidence that the location of signal delivery is an important determinant of the effects of CCM on ventricular function. The findings of the present study do not rule out alterations in regional relaxation which are not reflected in global measures.
4.3. CCM and ventricular electrophysiology
We have shown that the duration of MAPs recorded proximal to the site of stimulation are significantly shortened in a manner dependent upon the amplitude, duration and location of the stimulus. To the best of our knowledge this is the first report of the electrophysiological effects directly recorded from sites close to the CCM signal in the whole heart and represents a finding of potential clinical importance (see later). In keeping with our theory of the adrenergic dependence of CCM, we found that the shortening of MAPD was abolished during perfusion with metoprolol. Conventionally accepted evidence in isolated myocytes [6] and the whole heart [22] indicate that activation of the β1-adrenoceptors promotes shortening of ventricular action potential duration. From a mechanistic standpoint we have demonstrated that the shortening of locally recorded MAPD is partially dependent on the activation of IKs. IKs forms the slow component of the delayed rectifier outward potassium current is responsible for the increased rate of repolarisation associated with adrenergic activation and is present in a number of animal species including the rabbit [6–10]. While IKs is reported to have little effect in un-stimulated conditions, it is activated by β-adrenoceptor stimulation and promotes a shortening of ventricular action potential duration (a result of protein kinase A dependent phosphorylation) [6]. The incomplete inhibition of CCM induced MAPD shortening by HMR 1556 suggests that other repolarising currents may be activated during CCM. β-adrenoceptor stimulation is known to increase the flow of ionic currents through the L-type Ca2+ channels (ICaL), Na+/Ca2+ exchanger (INCX), IKS and chloride channels (ICl,Ca and ICl-cAMP). Activation of ICl will increase the outward current of negatively charged chloride ions and could contribute to the shortening of ventricular APD during CCM. In addition the activation of Ca2+ uptake by the sarcoplasmic reticulum ATPase (SERCA) and the enhancement of Ca2+ release by the ryanodine receptors should produce a Ca2+ transient of greater amplitude but of reduced duration, though our functional evidence and the previous reports, using aequorin Ca2+ fluorescence in the ferret heart, suggest that the Ca2+ duration is unaffected by CCM. We also cannot rule out the actions of other co-released neurotransmitters from adrenergic stimulation other than noradrenaline.
Previous investigators have suggested that CCM can induce enhanced calcium entry into the myocytes through direct modification of the L-type calcium channels. These early studies in isolated rabbit papillary muscles suggest that an enhancement of calcium influx prolongs APD [4]. In contrast, we have found that CCM induces shortening of ventricular MAPD in a manner dependent upon β1-adrenocptor stimulation, likely occurring secondary to catecholamine release from sympathetic nerve fibres. While it cannot be ruled out that these differences reflect the species of animal, the type of preparation and/or the methods utilised in each study, our data provide the first evidence of the electrophysiological effects of CCM in the whole heart. The inotropic and electrophysiological effects of β-adrenoceptor stimulation (see above) are well described and can account for a number of the molecular changes noted in previous studies with CCM (i.e. increased L-type calcium current and phosphorylation of phospholamban).
4.4. Clinical implications
Our study indicates that β1-adrenoceptor activation is primarily responsible for the acute inotropic enhancement and electrophysiological changes associated with CCM. This has obvious clinical implications since current treatment guidelines indicate the use of β-adrenoceptor antagonists in the treatment of patients with systolic heart failure. Interestingly, experimental studies in dogs with microembolism induced heart failure have shown additive beneficial effects when CCM therapy was combined with β-adrenoceptor antagonism [11]. Moreover, the results of clinical studies conducted to date show that CCM therapy used in conjunction with traditional pharmacological therapies increases patient exercise capacity improves quality of life measures and is able to reverse the detrimental molecular and structural remodelling associated with heart failure [23–25]. It is unlikely that complete inhibition of the β-adrenoceptors is achieved in the clinical situation, owing to the adverse side-effects of the high doses of β-adrenoceptor blocking drugs that would be required, and so CCM may still be effective in these patients. It is known that acute inotropic effects of CCM were still observed in early feasibility studies conducted in patients with systolic heart failure who were receiving optimised medical therapy (including β-adrenoceptor antagonists) [26]. The intermittent nature of CCM therapy, which utilises periods with and without CCM stimulation, may be akin to exercise therapy which has been shown to have beneficial effects in patients with varying degrees of heart failure and who are already receiving β-blockade therapy [12]. It also cannot be ruled out that the long-term benefitic of CCM therapy are mediated by other mechanisms than those which mediate the acute inotropic effects, this could include the release of other autonomic factors.
Previous attempts to utilise inotropic pharmacological agents in the treatment of heart failure have been unsuccessful; resulting in a greater number of patient deaths in the treatment arm of these studies [27]. However results from large scale clinical trials with CCM therapy (i.e. FIX-HF4 [23] and the preliminary findings of FIX-HF5 [24] suggest that CCM does not alter patient mortality rates at 3–6 months follow up. It is tempting to hypothesise that the localised stimulation of catecholamine release avoids the systemic complications and chronic effects of pharmacological agents, though greater patient numbers and longer follow-up data are required.
The local specific electrophysiological effects of CCM raises the question of whether CCM is pro-arrhythmic as regional variations in repolarisation could promote the development of re-entrant arrhythmias. In addition, we have previously shown that sympathetic activation increases the susceptibility to ventricular fibrillation in experimental conditions [28] and there is clear link between sympathetic drive and an increase in arrhythmic risk in clinical studies [29,30]. Therefore it seems plausible that CCM has a potential to increase the risk of ventricular arrhythmias, although preliminary anecdotal evidence does not support this suggestion. Clinical studies conducted to date suggest that CCM is not associated with any increase in hospitalisations for arrhythmic events [23,24], though more robust and rigorous scientific evidence is needed to support these short term epidemiological findings.
5. Conclusions
We have demonstrated that the acute inotropic and electrophysiological effects of CCM therapy are associated with the release of noradrenaline and concurrent stimulation of β1-adrenoceptors. In contrast to early reports we found that CCM was associated with a shortening of ventricular MAPD in a manner partially dependent upon the activation of IKs, a finding which may be of clinical importance.
6. Disclosures
The funding bodies played no role in the study design; collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit this work for publication.
Acknowledgements
This work is supported by funds from a British Heart Foundation Project Grant. The study is part of the research portfolio supported by the Leicester NIHR Biomedical Research Unit in Cardiovascular Disease. HMR 1556 was the kind gift of Dr. Jürgen Pünter (Aventis Pharma Deutchland Gmbh).
Glossary
- CCM
Cardiac contractility modulation
- LVP
Left ventricular pressure
- MAP
Monophasic action potential
- MAPD
Monophasic action potential duration
- dP/dtmin
Minimum rate of change of pressure
- dP/dtmax
Maximum rate of change of pressure
References
- 1.Pappone C., Rosanio S., Burkhoff D., Mika Y., Vicedomini G., Augello G. Cardiac contractility modulation by electric currents applied during the refractory period in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2002;90(12):1307–1313. doi: 10.1016/s0002-9149(02)02868-0. [DOI] [PubMed] [Google Scholar]
- 2.Mohri S., He K.-L., Dickstein M., Mika Y., Shimizu J., Shemer I. Cardiac contractility modulation by electric currents applied during the refractory period. Am J Physiol Heart Circ Physiol. 2002;282(5):H1642–H1647. doi: 10.1152/ajpheart.00959.2001. [DOI] [PubMed] [Google Scholar]
- 3.Mohri S., Shimizu J., Mika Y., Shemer I., Wang J., Ben-Haim S. Electric currents applied during refractory period enhance contractility and systolic calcium in the ferret heart. Am J Physiol Heart Circ Physiol. 2003;284(4):H1119–H1123. doi: 10.1152/ajpheart.00378.2002. [DOI] [PubMed] [Google Scholar]
- 4.Brunckhorst C.B., Shemer I., Mika Y., Ben-Haim S.A., Burkhoff D. Cardiac contractility modulation by non-excitatory currents: studies in isolated cardiac muscle. Eur J Heart Fail. 2006;8(1):7–15. doi: 10.1016/j.ejheart.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Meyer C., Rana O.R., Saygili E., Gemein C., Becker M., Nolte K.W. Augmentation of left ventricular contractility by cardiac sympathetic neural stimulation. Circulation. 2010;121(11):1286–1294. doi: 10.1161/CIRCULATIONAHA.109.874263. [DOI] [PubMed] [Google Scholar]
- 6.Volders P.G.A., Stengl M., van Opstal J.M., Gerlach U., Spatjens R.L.H.M.G., Beekman J.D.M. Probing the contribution of iks to canine ventricular repolarization: key role for {beta}-adrenergic receptor stimulation. Circulation. 2003;107(21):2753–2760. doi: 10.1161/01.CIR.0000068344.54010.B3. [DOI] [PubMed] [Google Scholar]
- 7.Thomas G.P., Gerlach U., Antzelevitch C. HMR 1556, a potent and selective blocker of slowly activating delayed rectifier potassium current. J Cardiovasc Pharmacol. 2003;41(1):140–147. doi: 10.1097/00005344-200301000-00018. [DOI] [PubMed] [Google Scholar]
- 8.So P.P., Hu X.D., Backx P.H., Puglisi J.L., Dorian P. Blockade of IKs by HMR 1556 increases the reverse rate-dependence of refractoriness prolongation by dofetilide in isolated rabbit ventricles. Br J Pharmacol. 2006;148(3):255–263. doi: 10.1038/sj.bjp.0706721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lengyel C., Iost N., Virag L., Varro A., Lathrop D.A., Papp J.G. Pharmacological block of the slow component of the outward delayed rectifier current (I(Ks)) fails to lengthen rabbit ventricular muscle QT(c) and action potential duration. Br J Pharmacol. 2001;132(1):101–110. doi: 10.1038/sj.bjp.0703777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gogelein H., Bruggemann A., Gerlach U., Brendel J., Busch A.E. Inhibition of IKs channels by HMR 1556. Naunyn Schmiedebergs Arch Pharmacol. 2000 Dec;362(6):480–488. doi: 10.1007/s002100000284. [DOI] [PubMed] [Google Scholar]
- 11.Imai M., Rastogi S., Gupta R.C., Mishra S., Sharov V.G., Stanley W.C. Therapy with cardiac contractility modulation electrical signals improves left ventricular function and remodeling in dogs with chronic heart failure. J Am Coll Cardiol. 2007;49(21):2120–2128. doi: 10.1016/j.jacc.2006.10.082. [DOI] [PubMed] [Google Scholar]
- 12.Flynn K.E., Pina I.L., Whellan D.J., Lin L., Blumenthal J.A., Ellis S.J. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bers D.M. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 14.Kurz T., Richardt G., Hagl S., Seyfarth M., Schömig A. Two different mechanisms of noradrenaline release during normoxia and simulated ischemia in human cardiac tissue. J Mol Cell Cardiol. 1995;27(5):1161–1172. doi: 10.1016/0022-2828(95)90052-7. [DOI] [PubMed] [Google Scholar]
- 15.Seyfarth M., Feng Y., Hagl S., Sebening F., Richardt G., Schomig A. Effect of myocardial ischemia on stimulation-evoked noradrenaline release. Modulated neurotransmission in rat, guinea pig, and human cardiac tissue. Circ Res. 1993;73(3):496–502. doi: 10.1161/01.res.73.3.496. [DOI] [PubMed] [Google Scholar]
- 16.Dahlstrom A., Fuxe K., Mya-Tu M., Zetterstrom B.E.M. Observations on adrenergic innervation of dog heart. Am J Physiol. 1965;209(4):689–692. doi: 10.1152/ajplegacy.1965.209.4.689. [DOI] [PubMed] [Google Scholar]
- 17.Kawano H., Okada R., Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18(1):32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- 18.Mantravadi R., Gabris B., Liu T., Choi B.-R., de Groat W.C., Ng G.A. Autonomic nerve stimulation reverses ventricular repolarization sequence in rabbit hearts. Circ Res. 2007;100(7):e72–e80. doi: 10.1161/01.RES.0000264101.06417.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angelakos E.T. Regional distribution of catecholamines in the dog heart. Circ Res. 1965;16:39–44. doi: 10.1161/01.res.16.1.39. [DOI] [PubMed] [Google Scholar]
- 20.Brandys J.C., Randall W.C., Armour J.A. Functional anatomy of the canine mediastinal cardiac nerves located at the base of the heart. Can J Physiol Pharmacol. 1986;64(2):152–162. doi: 10.1139/y86-023. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Cui C, Hu D. Effects of electric stimulations applied during absolute refractory period on cardiac function of rabbits with heart failure. J Huazhong Univ Sci Technol. 30(2): 155–8. [DOI] [PubMed]
- 22.Vanoli E., Priori S.G., Nakagawa H., Hirao K., Napolitano C., Diehl L. Sympathetic activation, ventricular repolarization and Ikr blockade: implications for the antifibrillatory efficacy of potassium channel blocking agents. J Am Coll Cardiol. 1995;25(7):1609–1614. doi: 10.1016/0735-1097(95)00046-7. [DOI] [PubMed] [Google Scholar]
- 23.Borggrefe M.M., Lawo T., Butter C., Schmidinger H., Lunati M., Pieske B. Randomized, double blind study of non-excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur Heart J. 2008;29(8):1019–1028. doi: 10.1093/eurheartj/ehn020. [DOI] [PubMed] [Google Scholar]
- 24.Cleland J.G., Coletta A.P., Clark A.L., Cullington D. Clinical trials update from the American College of Cardiology 2009: ADMIRE-HF, PRIMA, STICH, REVERSE, IRIS, partial ventricular support, FIX-HF-5, vagal stimulation, REVIVAL-3, pre-RELAX-AHF, ACTIVE-A, HF-ACTION, JUPITER, AURORA, and OMEGA. Eur J Heart Fail. 2009;11(6):622–630. doi: 10.1093/eurjhf/hfp071. [DOI] [PubMed] [Google Scholar]
- 25.Yu C.-M., Chan J.Y.-S., Zhang Q., Yip G.W.K., Lam Y.-Y., Chan A. Impact of cardiac contractility modulation on left ventricular global and regional function and remodeling. JACC Cardiovasc Imaging. 2009;2(12):1341–1349. doi: 10.1016/j.jcmg.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Stix G., Borggrefe M., Wolpert C., Hindricks G., Kottkamp H., BŐcker D. Chronic electrical stimulation during the absolute refractory period of the myocardium improves severe heart failure. Eur Heart J. 2004;25(8):650–655. doi: 10.1016/j.ehj.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 27.Packer M. The development of positive inotropic agents for chronic heart failure: how have we gone astray? J Am Coll Cardiol. 1993;22(4, Supplement 1):A119–A126. doi: 10.1016/0735-1097(93)90474-f. [DOI] [PubMed] [Google Scholar]
- 28.Ng G.A., Brack K.E., Patel V.H., Coote J.H. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res. 2007;73(4):750–760. doi: 10.1016/j.cardiores.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz P.J. The autonomic nervous system and sudden death. Eur Heart J. 1998;19(Suppl F):F72–F80. [PubMed] [Google Scholar]
- 30.Verrier R.L., Hagestad E.L. Role of the autonomic nervous system in sudden death. Cardiovasc Clin. 1985;15(3):41–63. [PubMed] [Google Scholar]