Abstract
Nanotechnology has emerged to be one of the most powerful engineering approaches in the past half a century. Nanotechnology brought nanomaterials for biomedical use with diverse applications. In the present manuscript we summarize the recent progress in adopting nanobiomaterials for bone healing and repair approaches. We first discuss the use of nanophase surface modification in manipulating metals and ceramics for bone implantation, and then the use of polymers as nanofiber scaffolds in bone repair. Finally we briefly present the potential use of the nanoparticle delivery system as adjunct system in promoting bone regeneration following fracture.
Keywords: Bone repair, Nanotechnology, Scaffolds, Nanoparticle, Gene delivery, Ceramics, Self-assembling peptides
Introduction
Nanotechnology has emerged to be one of the most powerful engineering approaches in the past half a century, since Feynman’s famous talk in 1959. Nanotechnology represents the manner to manipulation atoms and molecules over the scale of nanometer, and generates the materials with at least one dimension in nanoscale. The nanomaterials posses many specific characteristics in comparison to the bulk material due to the “quantum mechanical effect” (Sato and Webster 2004; Wang 2005; Powell and Kanarek 2006; Slocik and Naik 2010). Nanomaterials also have a much larger surface to volume ratio when compared to bulk materials. Nowadays, most nanomaterials can be applied to many aspects in biomedical research. One intriguing application of nanomaterials is to mimic the natural tissues and provide the proper extracellular environment for cells to grow and survive inside of the material (Venugopal et al. 2008; Scheller et al. 2009; Khang et al. 2010); moreover, these bio-compatible biomaterials, pre-implanted with cells or not, implied prospective approaches for tissue repair and regeneration in injured or disease conditions.
Orthopaedic surgeons have recognized the needs for proper materials to repair large defects in bone fracture (Hing 2004; Laurencin et al. 2009; Pellegrini et al. 2009). Many kinds of tissue grafts including allo-grafts and auto-grafts were used in past days; however problems including the immune rejection, infection, pain and inflammation, limited availability and ethic questions exist. Moreover, the surface of these materials might not be cell-coated and tissue compatibility is very low. Biomaterials were therefore synthesized as alternative sources for transplantation. Their characteristics vary according to the size of pores, water content, surface interaction, physical properties and the ability to host cells inside or on the surface. Nanomaterials being developed for repair and regeneration of bones included nanoparticle-containing materials with enhanced mechanical properties, nanofibrous scaffolds that can host cells, and nano-delivery systems for delivering drugs into the injured area that promote bone healing (Khan et al. 2008; Laurencin et al. 2009).
Bone healing and repair with grafts
In small bone fraction cases, initial inflammation is followed by soft callus formation, hard callus formation, and, ultimately, bone remodelling. Such automatic recovery does not happen for large bone fractures, which suggests a need for bone repair with grafts to fill the gap. The easiest way is to fix the two ends of broken bones with different metal plates or rods, which was called “internal fixation” (Venable and Stuck 1948; Burch 1958; Deyerle and Bowers 1962; Schatzker et al. 1975). In many years of study, people have optimized stainless steel, cobalt chrome alloys, titanium, and titanium alloy materials with surface modifications and proper screws for internal fixation over other materials (Schatzker et al. 1975; Uhthoff et al. 1981; Head et al. 1995; Disegi and Eschbach 2000). However all these metal-based materials were not bioresorbable, and were susceptible for long-term fatigue or even fracture (Khan et al. 2008); sometimes they also caused immune reactions in the surrounding tissues (Torgersen et al. 1995; Voggenreiter et al. 2003).
The second way is to employ bones from humans (including both autograft and allograft) and animals in repair. Autografts often contain osteogenic cells, bone marrow cells and the existing collagen matrix can promote the healing processes; this method was also considered as the “gold standard” (Fleming et al. 2000). However the harvest of autograft leads to donor site deficiency, and surgical pain. At the same time, allografts from other individuals or animals could bring diseases to the recipient host and immunological rejection with a long-term failure in follow up studies (CDC 2001; Wheeler and Enneking 2005). In recent decades, the use of synthetic materials for bone repair has achieved significant progress with the progresses in nanotechnology, and brought new approaches in clinical bone repair.
Nanotechnology and nanomaterials for bone repair
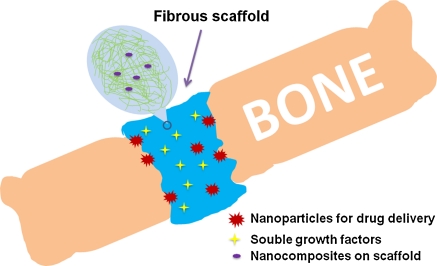
Feynman suggested: “there’s plenty of room at the bottom”. Nanotechnology has emerged to be one of the most powerful technologies in applied biomedical sciences. Nanomaterials include all types of materials with at least one dimension in less than 100 nm. The scale differences and surface modifications let nanomaterials vary in physical and chemical properties. Currently there are several major applications of nanotechnology for bone repair materials. One important property of bone repair materials is the mechanical property, and many nanomaterials have superior mechanical characteristics (Balasundaram and Webster 2006; Webster and Ahn 2007). The other way is to perform surface modification at nano-level, which provide better matrix for osteoblasts to grow and to function. For instance, osteoblasts on nanosized Ti, Ti6Al4V, and CoCrMo powder-modified metal surfaces as well as ceramics have improved adhesion and functions when compared to macrophase ones (Webster et al. 2000a; Webster and Ejiofor 2004; Webster and Smith 2005). The third way is to generate degradable polymers or use nanotechnology to modify some natural polymers such as collagen. The nano-scaffolds are much more porous and could better mimic the real extracellular matrix (ECM) in terms of number, sizes of pores and physical properties when compared to micro-scaffolds. It has been found that these nano-rough materials could also improve osteoblast functions when compared to macro-rough scaffolds (Balasundaram and Webster 2006; Marquis et al. 2009; Scheller et al. 2009; Tran and Webster 2009). Finally, nanoparticles enabling delivery of drugs and growth factors were used to promote healing and functional recovery (Fig. 1; Table 1).
Fig. 1.
Nanotechnology enables repair of bone fracture in bone fracture cases, nanomaterials were implanted into the wound area. The figure represents the use of nanocomposites of polymer scaffolds and ceramics as well as nanoparticles. Many drugs and growth factors can be included in the scaffold for controllable release over the time period to promote bone healing
Table 1.
Available nanomaterials being employed for bone repair
| Type | Major application | Bio-compatibility | Stability |
|---|---|---|---|
| Metal | Internal fixation; bone replacement; physical supports | Could be improved with surface modification | Long-term efficiency |
| Ceramics | |||
| Polymers | Cell seeding; soft tissue replacement; surface application; weak physical support | Depending on materials | Depending on biodegradation |
| Scaffolds, hydrogels | High | ||
| Nanoparticles | Drug delivery; gene delivery; protein delivery; controlled and targeted release | Could be improved with surface modification | Could be controlled |
Nanotechnology based metal and ceramic materials
It is easier to perform surface modifications on currently used metal plates and screws in comparison to generate a whole plate of nanomaterials for internal fixation. The bio-compatibility of these materials was largely determined by surface properties, such as immunogenicity, hydrophilicity, the ability to host cells and processes, and adhesion of osteoblasts. These modifications were reported to elongate the integration of implanted plates and bone tissues over extended time periods (Buser et al. 1999; Ferris et al. 1999; Webster and Ejiofor 2004; Oyane et al. 2005; Liu et al. 2006; Fan et al. 2007; Yokoyama et al. 2007; Harvey et al. 2010). As mentioned above, with nanosized Ti, Ti6Al4V, and CoCrMo powder-modified (pressed onto the surface) metal surfaces, seeded osteoblasts showed improved functions including adhesion, proliferation, and deposition of calcium-containing minerals (Webster et al. 2000a; Webster and Ejiofor 2004; Webster and Smith 2005). Another way to change the surface property of metals is the anodization that can bring nano-sized pores (Yao et al. 2007; Yao et al. 2008), which could be further used for installation of other nano-structures such as carbon nanotubes as biosensors for bone regrowth (Sirivisoot et al. 2007). The fact that nanometer roughness is most suitable for biological tissues to grow one can emphasize the importance of nanotechnology in bone repair in the coming decades.
Besides metals, people have found nanostructured ceramics promoted bone functions when compared to micro-structured ones (Webster et al. 1999; Webster et al. 2000a; Webster et al. 2000b; Li et al. 2009). These include: (1) metallic oxides such as aluminium, zirconium, and titanium; (2) calcium phosphates such as hydroxyapatite (HA), tricalcium phosphate (TCP) and calcium tetraphosphate; (3) glassceramics such as Bioglass and Ceravital (Tran and Webster 2009). These materials showed similar material properties as surface modification of metals, such as increased osteoblasts adhesion, proliferation, alkaline phosphatase activity, and calcium deposition (Webster et al. 1999; Webster et al. 2000a, b; Kay et al. 2002). More updates of ceramics such as protein based surface modifications are now being developed and explored (Webster et al. 2000b; Balasundaram and Webster 2006; Colilla et al. 2008; Tran and Webster 2009).
Nanomaterials based polymers and scaffolds
Synthetic materials also include polymers that could form scaffold like structures, and when seeded with osteoblasts, provide ideal environment for cell proliferation and growth matrix. During the process of bone healing, these materials could be degraded and even used (as in the case of self-assembling peptides), without causing any immunological response. Because they are synthetic, there is no risk of viral infection or bringing diseases to the recipient hosts. The degradation time depends on the property of the scaffold itself, the density of scaffold, and the available enzymes in the bone tissues (Marquis et al. 2009; Harvey et al. 2010; Khang et al. 2010; Kubinova and Sykova 2010; Vallet-Regi 2010). Therefore this is a system with controllable degradation, which could be combined with drug or growth factor release during bone healing.
Collagen was one of the first nanofiber scaffolds that was generated due to its common presence in natural tissues (Laurencin et al. 2009; Prabhakaran et al. 2009). The high biocompatibility suggested collagen to be the ideal choice for soft tissue repair or transplantation when seeded together with types of cells of interest. However, there is lack of good mechanical properties and the use for bone repair is therefore weakened. Some other candidates with better mechanical characteristics include poly-lactic acid (PLA), poly-glycolic acid (PGA), and poly-lactic-co-glycolic acid (PLGA). They have been approved by the US Food and Drug Administration (FDA) for clinical uses. When these polymers form nanofiber scaffolds, these materials were found to be able to enhance the protein adsorption functions of osteoblasts (Wei and Ma 2004; Xiao et al. 2008). Additionally, the polymer casts of nanophase carbon fibers rather than conventional fibers showed improved properties in supporting the functions of osteoblasts (Price et al. 2003, 2004). In other studies, Ceramic/Polymer nanocomposites were designed and developed to create better materials as bone implant scaffolds with improved mechanical strength. It was found that the mixed material showed better support for osteoblasts than each individual component (Marra et al. 1999; Ma et al. 2001; Blaker et al. 2003; Jung et al. 2005). More and more nanocomposites of different compositions and thus different mechanical properties as well as diverse biocompatibilities are yet to be developed.
Among different types of nanofiber scaffolds, one family was found to be interesting and prospective in regenerative medicine. That is the self-assembling peptides (SAP). The concept of self-assembling peptides, which is very common in biological activities such as protein aggregation, suggested that biomaterials could be designed to support cell functions in a controllable manner (Semino 2008). In past decades, many different types of SAP were designed and reported, which would start gelation in polarized solvents, such as physiological solutions. These include EAK16, RAD16-I, RAD16-II, DN1, KLN12, etc. For instance, RAD16-I would form nanofiber scaffold in ionic solutions, which has been shown to be able to support growth and proliferation of many types of cells, and when transplanted in vivo, to repair injured tissue with functional recovery (Bokhari et al. 2005; Genove et al. 2005; Garreta et al. 2007; Dubois et al. 2008; Dégano et al. 2009; Tang and Zhao 2010). RAD16-I was found to be able to promote bone regeneration and to lead to new bridge formation, as well as to inhibit demineralization (Misawa et al. 2006; Garreta et al. 2007; Kirkham et al. 2007). It was also suggested that by modification of anionic groups of the side-chains, the SAP could have better properties in attracting calcium and inducing salt precipitation (Kirkham et al. 2007), which is critical for new bone formation. The best news is that people could design any type of SAP they wanted as long as the basic physical laws are respected, which provides almost endless possibilities in new materials development and discoveries.
Last but not least, carbon nanotubes could form scaffolds for bone repair. Both single-wall and multi-wall carbon nanotubes were found to be able to interact with the biological tissues and to be useful for bone repair (Tutak et al. 2009; Zhang et al. 2009; Bhattacharya et al. 2011; Joshi et al. 2010; Mendes et al. 2010; Niu et al. 2010; Sahithi et al. 2010; Tutak et al. 2010). This has been well presented in the published literature and will not be further discussed here (see above references).
Nanoparticle delivery system for bone healing
Besides the contribution as bone implant, nanotechnology provides excellent drug and molecule delivery systems with high targeting efficiency. Currently available nanoparticles for drug delivery mainly include polymeric nanoparticle, PEG-ylation modified particles, micelle, liposome, dendrimer, and nanosized inorganic materials (Kim and Fisher 2007). These systems vary in terms of their efficiency in different biological systems, toxicity, the sizes of genetic sequences being carried, penetration depth in tissue, and targeting efficiency (Goldberg et al. 2007; Zhang and Uludag 2009). For bone healing, both genes and proteins could be delivered to promote proliferation of osteoblasts, the formation of new blood vessels, and the secretion of calcium salts. For instance, the system of VEGF-DNA loaded PLGA nanoparticles was tested in vitro and was shown to penetrate the cytoplasm and to attain the nucleus (Yi et al. 2006). Also, cationic liposomes with BMP-2 cDNA could enhance bone regeneration in a rabbit model of cranial bone defects (Ono et al. 2004). Similar studies with BMP-2 gene delivery showed effects on cultured cells and rat models (Matsuo et al. 2003; Park et al. 2003). Given the fact that many signalling molecules for bone healing and regeneration have been identified in the past years, it is believed that with nanoparticle delivery and controlled release bone repair could be largely facilitated in the future.
Summary
In summary, in the recent years nanotechnology had greatly promoted the development of new methods and approaches for bone repair. One major aspect is the emergence of diverse nanomaterials with abundant properties for different types of applications, and experts can still further engineer these materials for individual medicinal use. This is prospective novelty. The other side of nanotechnology is nanodelivery, which is more efficient and precise than conventional approaches. Currently there is lack of sufficient pre-clinical and clinical studies with nanomaterials for bone repair: while there is evidence that human stem cells could be seeded onto nanomaterials for growth and amplification (Soumetz et al. 2008; Sundelacruz and Kaplan 2009; Dupont et al. 2010). The authors believe that the application of nanotechnology in modern bone repair will finally bring further benefits for patients in the future.
Acknowledgments
The authors declare no conflicts of interest, and were supported by National Scientific Funding (No: 30800572).
References
- Balasundaram G, Webster TJ. Nanotechnology and biomaterials for orthopaedic medical applications. Nanomedicine (Lond) 2006;1:169–176. doi: 10.2217/17435889.1.2.169. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M, Wutticharoenmongkol-Thitiwongsawet P, Hamamoto DT, Lee D, Cui T, Prasad HS, Ahmad M. Bone formation on carbon nanotube composite. J Biomed Mater Res A. 2011;96:75–82. doi: 10.1002/jbm.a.32958. [DOI] [PubMed] [Google Scholar]
- Blaker JJ, Gough JE, Maquet V, Notingher I, Boccaccini AR. In vitro evaluation of novel bioactive composites based on bioglass-filled polylactide foams for bone tissue engineering scaffolds. J Biomed Mater Res A. 2003;67:1401–1411. doi: 10.1002/jbm.a.20055. [DOI] [PubMed] [Google Scholar]
- Bokhari MA, Akay G, Zhang S, Birch MA. The enhancement of osteoblast growth and differentiation in vitro on a peptide hydrogel-polyHIPE polymer hybrid material. Biomaterials. 2005;26:5198–5208. doi: 10.1016/j.biomaterials.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Burch JE. Bone reaction to stainless steel fixation material. South Med J. 1958;51:1390–1394. doi: 10.1097/00007611-195811000-00006. [DOI] [PubMed] [Google Scholar]
- Buser D, Nydegger T, Oxland T, Cochran DL, Schenk RK, Hirt HP, Snétivy D, Nolte LP. Interface shear strength of titanium implants with a sandblasted and acid-etched surface: a biomechanical study in the maxilla of miniature pigs. J Biomed Mater Res. 1999;45:75–83. doi: 10.1002/(SICI)1097-4636(199905)45:2<75::AID-JBM1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- CfDCaP (CDC) Septic arthritis following anterior cruciate ligament reconstruction using tendon allografts—Florida and Louisiana, 2000. MMWR Morb Mortal Wkly Rep. 2001;50:1081–1083. [PubMed] [Google Scholar]
- Colilla M, Manzano M, Vallet-Regi M. Recent advances in ceramic implants as drug delivery systems for biomedical applications. Int J Nanomedicine. 2008;3:403–414. doi: 10.2147/ijn.s3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dégano IR, Quintana L, Vilalta M, Horna D, Rubio N, Borrós S, Semino C, Blanco J. The effect of self-assembling peptide nanofiber scaffolds on mouse embryonic fibroblast implantation and proliferation. Biomaterials. 2009;30:1156–1165. doi: 10.1016/j.biomaterials.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Deyerle WM, Bowers RV. Internal fixation of bone with a metal pin (1862). Report of a case with a century of follow-up study. N Engl J Med. 1962;26:820–822. doi: 10.1056/NEJM196204192661609. [DOI] [PubMed] [Google Scholar]
- Disegi JA, Eschbach L. Stainless steel in bone surgery. Injury. 2000;31(Suppl 4):2–6. doi: 10.1016/S0020-1383(00)80015-7. [DOI] [PubMed] [Google Scholar]
- Dubois G, Segers VF, Bellamy V, Sabbah L, Peyrard S, Bruneval P, Hagège AA, Lee RT, Menasché P. Self-assembling peptide nanofibers and skeletal myoblast transplantation in infarcted myocardium. J Biomed Mater Res B Appl Biomater. 2008;87:222–228. doi: 10.1002/jbm.b.31099. [DOI] [PubMed] [Google Scholar]
- Dupont KM, Sharma K, Stevens HY, Boerckel JD, Garcia AJ, Guldberg RE. Human stem cell delivery for treatment of large segmental bone defects. Proc Natl Acad Sci USA. 2010;107:3305–3310. doi: 10.1073/pnas.0905444107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Ikoma T, Tanaka J, Zhang X. Surface structural biomimetics and the osteoinduction of calcium phosphate biomaterials. J Nanosci Nanotechnol. 2007;7:808–813. doi: 10.1166/jnn.2007.501. [DOI] [PubMed] [Google Scholar]
- Ferris DM, Moodie GD, Dimond PM, Gioranni CW, Ehrlich MG, Valentini RF. RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials. 1999;20:2323–2331. doi: 10.1016/S0142-9612(99)00161-1. [DOI] [PubMed] [Google Scholar]
- Fleming JE, Jr, Cornell CN, Muschler GF. Bone cells and matrices in orthopaedic tissue engineering. Orthop Clin North Am. 2000;31:357–374. doi: 10.1016/S0030-5898(05)70156-5. [DOI] [PubMed] [Google Scholar]
- Garreta E, Gasset D, Semino C, Borros S. Fabrication of a three-dimensional nanostructured biomaterial for tissue engineering of bone. Biomol Eng. 2007;24:75–80. doi: 10.1016/j.bioeng.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Genove E, Shen C, Zhang S, Semino CE. The effect of functionalized self-assembling peptide scaffolds on human aortic endothelial cell function. Biomaterials. 2005;26:3341–3351. doi: 10.1016/j.biomaterials.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Langer R, Jia X. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym Ed. 2007;18:241–268. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EJ, Henderson JE, Vengallatore ST. Nanotechnology and bone healing. J Orthop Trauma. 2010;24(Suppl 1):S25–S30. doi: 10.1097/BOT.0b013e3181ca3b58. [DOI] [PubMed] [Google Scholar]
- Head WC, Bauk DJ, Emerson RH., Jr Titanium as the material of choice for cement less femoral components in total hip arthroplasty. Clin Orthop Relat Res. 1995;15:85–90. [PubMed] [Google Scholar]
- Hing KA. Bone repair in the twenty-first century: biology, chemistry or engineering? Philos Transact A Math Phys Eng Sci. 2004;362:2821–2850. doi: 10.1098/rsta.2004.1466. [DOI] [PubMed] [Google Scholar]
- Joshi B, Gupta S, Kalra N, Gudyka R, Santhanam KS. A new material with atomized cobalt-multiwalled carbon nanotubes: a possible substitute for human implants. J Nanosci Nanotechnol. 2010;10:3799–3804. doi: 10.1166/jnn.2010.1997. [DOI] [PubMed] [Google Scholar]
- Jung Y, Kim SS, Kim YH, Kim SH, Kim BS, Kim S, Choi CY. A poly(lactic acid)/calcium metaphosphate composite for bone tissue engineering. Biomaterials. 2005;26:6314–6322. doi: 10.1016/j.biomaterials.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Kay S, Thapa A, Haberstroh KM, Webster TJ. Nanostructured polymer/nanophase ceramic composites enhance osteoblast and chondrocyte adhesion. Tissue Eng. 2002;8:753–761. doi: 10.1089/10763270260424114. [DOI] [PubMed] [Google Scholar]
- Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. Tissue engineering of bone: material and matrix considerations. J Bone Joint Surg Am. 2008;90(Suppl 1):36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- Khang D, Carpenter J, Chun YW, Pareta R, Webster TJ. Nanotechnology for regenerative medicine. Biomed Microdevices. 2010;12:575–587. doi: 10.1007/s10544-008-9264-6. [DOI] [PubMed] [Google Scholar]
- Kim K, Fisher JP. Nanoparticle technology in bone tissue engineering. J Drug Target. 2007;15:241–252. doi: 10.1080/10611860701289818. [DOI] [PubMed] [Google Scholar]
- Kirkham J, Firth A, Vernals D, Boden N, Robinson C, Shore RC, Brookes SJ, Aggeli A. Self-assembling peptide scaffolds promote enamel remineralization. J Dent Res. 2007;86:426–430. doi: 10.1177/154405910708600507. [DOI] [PubMed] [Google Scholar]
- Kubinova S, Sykova E. Nanotechnologies in regenerative medicine. Minim Invasive Ther Allied Technol. 2010;19:144–156. doi: 10.3109/13645706.2010.481398. [DOI] [PubMed] [Google Scholar]
- Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:6–10. doi: 10.1002/wnan.25. [DOI] [PubMed] [Google Scholar]
- Li B, Chen X, Guo B, Wang X, Fan H, Zhang X. Fabrication and cellular biocompatibility of porous carbonated biphasic calcium phosphate ceramics with a nanostructure. Acta Biomater. 2009;5:134–143. doi: 10.1016/j.actbio.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Liu H, Slamovich EB, Webster TJ. Increased osteoblast functions among nanophase titania/poly(lactide-co-glycolide) composites of the highest nanometer surface roughness. J Biomed Mater Res A. 2006;78:798–807. doi: 10.1002/jbm.a.30734. [DOI] [PubMed] [Google Scholar]
- Ma PX, Zhang R, Xiao G, Franceschi R. Engineering new bone tissue in vitro on highly porous poly(alpha-hydroxyl acids)/hydroxyapatite composite scaffolds. J Biomed Mater Res. 2001;54:284–293. doi: 10.1002/1097-4636(200102)54:2<284::AID-JBM16>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Marquis ME, Lord E, Bergeron E, Drevelle O, Park H, Cabana F, Senta H, Faucheux N. Bone cells-biomaterials interactions. Front Biosci. 2009;14:1023–1067. doi: 10.2741/3293. [DOI] [PubMed] [Google Scholar]
- Marra KG, Szem JW, Kumta PN, DiMilla PA, Weiss LE. In vitro analysis of biodegradable polymer blend/hydroxyapatite composites for bone tissue engineering. J Biomed Mater Res. 1999;47:324–335. doi: 10.1002/(SICI)1097-4636(19991205)47:3<324::AID-JBM6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Sugita T, Kubo T, Yasunaga Y, Ochi M, Murakami T. Injectable magnetic liposomes as a novel carrier of recombinant human BMP-2 for bone formation in a rat bone-defect model. J Biomed Mater Res A. 2003;66:747–754. doi: 10.1002/jbm.a.10002. [DOI] [PubMed] [Google Scholar]
- Mendes RM, Silva GA, Caliari MV, Silva EE, Ladeira LO, Ferreira AJ. Effects of single wall carbon nanotubes and its functionalization with sodium hyaluronate on bone repair. Life Sci. 2010;87:215–222. doi: 10.1016/j.lfs.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Misawa H, Kobayashi N, Soto-Gutierrez A, Chen Y, Yoshida A, Rivas-Carrillo JD, Navarro-Alvarez N, Tanaka K, Miki A, Takei J, Ueda T, Tanaka M, Endo H, Tanaka N, Ozaki T. PuraMatrix facilitates bone regeneration in bone defects of calvaria in mice. Cell Transplant. 2006;15:903–910. doi: 10.3727/000000006783981369. [DOI] [PubMed] [Google Scholar]
- Niu L, Kua H, Chua DH. Bonelike apatite formation utilizing carbon nanotubes as template. Langmuir. 2010;26:4069–4073. doi: 10.1021/la9034722. [DOI] [PubMed] [Google Scholar]
- Ono I, Yamashita T, Jin HY, Ito Y, Hamada H, Akasaka Y, Nakasu M, Ogawa T, Jimbow K. Combination of porous hydroxyapatite and cationic liposomes as a vector for BMP-2 gene therapy. Biomaterials. 2004;25:4709–4718. doi: 10.1016/j.biomaterials.2003.11.038. [DOI] [PubMed] [Google Scholar]
- Oyane A, Uchida M, Yokoyama Y, Choong C, Triffitt J, Ito A. Simple surface modification of poly(epsilon-caprolactone) to induce its apatite-forming ability. J Biomed Mater Res A. 2005;75:138–145. doi: 10.1002/jbm.a.30397. [DOI] [PubMed] [Google Scholar]
- Park J, Ries J, Gelse K, Kloss F, der Mark K, Wiltfang J, Neukam FW, Schneider H. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes. Gene Ther. 2003;10:1089–1098. doi: 10.1038/sj.gt.3301960. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Seol YJ, Gruber R, Giannobile WV. Pre-clinical models for oral and periodontal reconstructive therapies. J Dent Res. 2009;88:1065–1076. doi: 10.1177/0022034509349748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell MC, Kanarek MS. Nanomaterial health effects—part 1: background and current knowledge. WMJ. 2006;105:16–20. [PubMed] [Google Scholar]
- Prabhakaran MP, Venugopal J, Ramakrishna S. Electrospun nanostructured scaffolds for bone tissue engineering. Acta Biomater. 2009;5:2884–2893. doi: 10.1016/j.actbio.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Price RL, Gutwein LG, Kaledin L, Tepper F, Webster TJ. Osteoblast function on nanophase alumina materials: influence of chemistry, phase, and topography. J Biomed Mater Res A. 2003;67:1284–1293. doi: 10.1002/jbm.a.20011. [DOI] [PubMed] [Google Scholar]
- Price RL, Ellison K, Haberstroh KM, Webster TJ. Nanometer surface roughness increases select osteoblast adhesion on carbon nanofiber compacts. J Biomed Mater Res A. 2004;70:129–138. doi: 10.1002/jbm.a.30073. [DOI] [PubMed] [Google Scholar]
- Sahithi K, Swetha M, Ramasamy K, Srinivasan N, Selvamurugan N. Polymeric composites containing carbon nanotubes for bone tissue engineering. Int J Biol Macromol. 2010;46:281–283. doi: 10.1016/j.ijbiomac.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Sato M, Webster TJ. Nanobiotechnology: implications for the future of nanotechnology in orthopedic applications. Expert Rev Med Devices. 2004;1:105–114. doi: 10.1586/17434440.1.1.105. [DOI] [PubMed] [Google Scholar]
- Schatzker J, Sanderson R, Murnaghan JP. The holding power of orthopaedic screws in vivo. Clin Orthop Relat Res. 1975;240:115–126. doi: 10.1097/00003086-197505000-00019. [DOI] [PubMed] [Google Scholar]
- Scheller EL, Krebsbach PH, Kohn DH. Tissue engineering: state of the art in oral rehabilitation. J Oral Rehabil. 2009;36:368–389. doi: 10.1111/j.1365-2842.2009.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semino CE. Self-assembling peptides: from bio-inspired materials to bone regeneration. J Dent Res. 2008;87:606–616. doi: 10.1177/154405910808700710. [DOI] [PubMed] [Google Scholar]
- Sirivisoot S, Yao C, Xiao X, Sheldon BW, Webster TJ. Greater osteoblast functions on multiwalled carbon nanotubes grown from anodized nanotubular titanium for orthopedic applications. Nanotechnology. 2007;18:365102. doi: 10.1088/0957-4484/18/36/365102. [DOI] [Google Scholar]
- Slocik JM, Naik RR. Probing peptide-nanomaterial interactions. Chem Soc Rev. 2010;39:3454–3463. doi: 10.1039/b918035b. [DOI] [PubMed] [Google Scholar]
- Soumetz FC, Pastorino L, Ruggiero C. Human osteoblast-like cells response to nanofunctionalized surfaces for tissue engineering. J Biomed Mater Res B Appl Biomater. 2008;84:249–255. doi: 10.1002/jbm.b.30867. [DOI] [PubMed] [Google Scholar]
- Sundelacruz S, Kaplan DL. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin Cell Dev Biol. 2009;20:646–655. doi: 10.1016/j.semcdb.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Zhao X. Interaction between a self-assembling peptide and hydrophobic compounds. J Biomater Sci Polym Ed. 2010;21:677–690. doi: 10.1163/156856209X434683. [DOI] [PubMed] [Google Scholar]
- Torgersen S, Gjerdet NR, Erichsen ES, Bang G. Metal particles and tissue changes adjacent to miniplates. A retrieval study. Acta Odontol Scand. 1995;53:65–71. doi: 10.3109/00016359509005948. [DOI] [PubMed] [Google Scholar]
- Tran N, Webster TJ. Nanotechnology for bone materials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:336–351. doi: 10.1002/wnan.23. [DOI] [PubMed] [Google Scholar]
- Tutak W, Park KH, Vasilov A, Starovoytov V, Fanchini G, Cai SQ, Partridge NC, Sesti F, Chhowalla M. Toxicity induced enhanced extracellular matrix production in osteoblastic cells cultured on single-walled carbon nanotube networks. Nanotechnology. 2009;20:255101. doi: 10.1088/0957-4484/20/25/255101. [DOI] [PubMed] [Google Scholar]
- Tutak W, Chhowalla M, Sesti F. The chemical and physical characteristics of single-walled carbon nanotube film impact on osteoblastic cell response. Nanotechnology. 2010;21:315102. doi: 10.1088/0957-4484/21/31/315102. [DOI] [PubMed] [Google Scholar]
- Uhthoff HK, Bardos DI, Liskova-Kiar M. The advantages of titanium alloy over stainless steel plates for the internal fixation of fractures. An experimental study in dogs. J Bone Joint Surg Br. 1981;63-B:427–484. doi: 10.1302/0301-620X.63B3.7263759. [DOI] [PubMed] [Google Scholar]
- Vallet-Regi M. Nanostructured mesoporous silica matrices in nanomedicine. J Intern Med. 2010;267:22–43. doi: 10.1111/j.1365-2796.2009.02190.x. [DOI] [PubMed] [Google Scholar]
- Venable CS, Stuck WG. Results of recent studies and experiments concerning metals used in the internal fixation of fractures. J Bone Joint Surg Am. 1948;30A:247–250. [PubMed] [Google Scholar]
- Venugopal J, Low S, Choon AT, Ramakrishna S. Interaction of cells and nanofiber scaffolds in tissue engineering. J Biomed Mater Res B Appl Biomater. 2008;84:34–48. doi: 10.1002/jbm.b.30841. [DOI] [PubMed] [Google Scholar]
- Voggenreiter G, Leiting S, Brauer H, Leiting P, Majetschak M, Bardenheuer M, Obertacke U. Immuno-inflammatory tissue reaction to stainless-steel and titanium plates used for internal fixation of long bones. Biomaterials. 2003;24:247–254. doi: 10.1016/S0142-9612(02)00312-5. [DOI] [PubMed] [Google Scholar]
- Wang J. Nanomaterial-based amplified transduction of biomolecular interactions. Small. 2005;1:1036–1043. doi: 10.1002/smll.200500214. [DOI] [PubMed] [Google Scholar]
- Webster TJ, Ahn ES. Nanostructured biomaterials for tissue engineering bone. Adv Biochem Eng Biotechnol. 2007;103:275–308. doi: 10.1007/10_021. [DOI] [PubMed] [Google Scholar]
- Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25:4731–4739. doi: 10.1016/j.biomaterials.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Webster TJ, Smith TA. Increased osteoblast function on PLGA composites containing nanophase Titania. J Biomed Mater Res A. 2005;74:677–686. doi: 10.1002/jbm.a.30358. [DOI] [PubMed] [Google Scholar]
- Webster TJ, Siegel RW, Bizios R. Osteoblast adhesion on nanophase ceramics. Biomaterials. 1999;20:1221–1227. doi: 10.1016/S0142-9612(99)00020-4. [DOI] [PubMed] [Google Scholar]
- Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21:1803–1810. doi: 10.1016/S0142-9612(00)00075-2. [DOI] [PubMed] [Google Scholar]
- Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J Biomed Mater Res. 2000;51:475–483. doi: 10.1002/1097-4636(20000905)51:3<475::AID-JBM23>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25:4749–4757. doi: 10.1016/j.biomaterials.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005;83:36–42. doi: 10.1097/01.blo.0000165850.58583.50. [DOI] [PubMed] [Google Scholar]
- Xiao X, Liu R, Huang Q. Preparation and characterization of nano-hydroxyapatite/polymer composite scaffolds. J Mater Sci Mater Med. 2008;19:3429–3435. doi: 10.1007/s10856-008-3499-x. [DOI] [PubMed] [Google Scholar]
- Yao C, Storey D, Webster TJ. Nanostructured metal coatings on polymers increase osteoblast attachment. Int J Nanomedicine. 2007;2:487–492. [PMC free article] [PubMed] [Google Scholar]
- Yao C, Slamovich EB, Webster TJ. Enhanced osteoblast functions on anodized titanium with nanotube-like structures. J Biomed Mater Res A. 2008;85:157–166. doi: 10.1002/jbm.a.31551. [DOI] [PubMed] [Google Scholar]
- Yi F, Wu H, Jia GL. Formulation and characterization of poly (d, l-lactide-co-glycolide) nanoparticle containing vascular endothelial growth factor for gene delivery. J Clin Pharm Ther. 2006;31:43–48. doi: 10.1111/j.1365-2710.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Oyane A, Ito A. Biomimetic coating of an apatite layer on poly(l-lactic acid); improvement of adhesive strength of the coating. J Mater Sci Mater Med. 2007;18:1727–1734. doi: 10.1007/s10856-007-3024-7. [DOI] [PubMed] [Google Scholar]
- Zhang S, Uludag H. Nanoparticulate systems for growth factor delivery. Pharm Res. 2009;26:1561–1580. doi: 10.1007/s11095-009-9897-z. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen L, Wells T, El-Gomati M. Bamboo and herringbone shaped carbon nanotubes and carbon nanofibres synthesized in direct current-plasma enhanced chemical vapour deposition. J Nanosci Nanotechnol. 2009;9:4502–4506. doi: 10.1166/jnn.2009.M84. [DOI] [PubMed] [Google Scholar]