Abstract
To determine the ability of cultured bone marrow-derived mesenchymal stem cells (BMSCs) to differentiate into functional urothelium. BMSCs were isolated from the long bones of aborted fetal limbs by Percoll density gradient centrifugation and characterized by flow cytometry. Human fetal urinary bladders were cut into small pieces and cultured for 3–5 days until the growth of urothelial cells was established. BMSCs were then cocultured with neonatal urothelial cells and subsequently evaluated for antigen expression and ultramicrostructure, by immunocytochemistry and electron microscopy, respectively. A subset of BMSCs expressed the differentiation marker CD71. The BMSC markers CD34, CD45, and HLA-DR were barely detectable, confirming that these cells were not derived from hematopoietic stem cells or differentiated cells. In contrast, the stem cell markers CD29, CD44, CD105, and CD90 were highly expressed. BMSCs possessed the ability to differentiate into a variety of cellular subtypes, including osteocytes, adipocytes, and chondrocytes. The shapes of BMSCs changed, and the size of the cells increased, following in vitro coculture with urothelial cells. After 2 weeks of coculture, immunostaining of the newly differentiated BMSCs positively displayed the urothelial-specific keratin marker. Electron microscopy revealed that the cocultured BMSCs had microstructural features characteristic of epithelial cells. Pluripotent BMSCs can transdifferentiate into urothelial cells in response to an environment conditioned by neonatal urothelial cells, providing a means for the time-, labor- and cost-effective reconstruction of urinary bladder mucosa.
Keywords: Urothelial cells, Stem cells, Differentiation, Coculture
Introduction
Bladder carcinoma remains one of the most common malignancies in the world, and 70–80% of bladder cancer patients have superficial transitional cell carcinoma (TCC) (Grossman 1996). Superficial TCC frequently recurs because the remaining urothelial cells of bladder cancer patients often contain genetic defects that cause tumor regrowth (Wolff et al. 2005). It is currently unknown whether it may be possible to prevent bladder carcinoma recurrence by transplanting in vitro-differentiated urothelial cells, free from genetic defects, to replace resected bladder tissue.
Previous research has demonstrated that stem cell transplantation may provide significant therapeutic benefits for various cardiovascular, neurodegenerative and endocrine disorders, as well as other diseases (Yamada et al. 2008; Tondreau et al. 2008; Ianus et al. 2003; Fröhlich et al. 2008). To date, however, hematopoietic stem cells (HSCs) derived from adult peripheral blood, adult bone marrow, or umbilical cord blood have been used in human clinical transplantation (Chinen and Buckley 2010).
Stem cells are functionally defined by their capacity to self-renew and differentiate into one or more terminal cell types. Proper regulation of these properties is critical for animal development, growth control, and reproduction. Under normal conditions, the replacement of damaged cells by stem cells is an active process that occurs commonly in adult tissues, especially in rapidly dividing cells such as blood cells (Patel 2006). The cellular environment significantly influences the balance between stem cell self-renewal and terminal differentiation (Hoffmann et al. 2008). Several types of stem cells have been shown to terminally differentiate into a variety of different cell types (Bruce et al. 2007; Pepper et al. 2007), and bone marrow-derived mesenchymal stem cells (BMSCs) have been shown to be transducible in a previous study (Coleman et al. 2007). Under standard in vitro differentiation conditions, these cells have tri-lineage differentiation potential with the capacity to differentiate to osteoblasts, adipocytes, and chondroblasts. Because stem cell behavior is not the result of a cell-autonomous program but is instead the consequence of complex cell–cell and cell-environment interactions, a suitable growth environment is essential for achieving the desired differentiated phenotype. For the current investigation, we created an in vitro microenvironment that fosters the epithelial differentiation of BMSCs into urothelial cells.
Methods
Isolation and culture of MSCs from human fetal bone marrow
Permission for this study was granted by the Ethics Committee of the Second Affiliated Hospital, Tianjin Medical University. Human fetal BMSCs and bladder tissues were obtained from aborted fetuses at 3–5 months gestation in accordance with Chinese guidelines. All of the families provided informed written consent. All women donating fetal tissue had been serologically screened for syphilis, toxoplasmosis, rubella, human immunodeficiency virus 1, cytomegalovirus, hepatitis B and C, and herpes simplex types 1 and 2. Bone marrow-derived MSCs were isolated from fetal tissues according to a previously described protocol (Morganstein et al. 2010). Briefly, MSCs were flushed from the cavitas medullaris using a 2.5-mL syringe containing Dulbecco’s modified Eagle’s medium–low glucose (DMEM-LG, Gibco) culture medium. Then bone marrow cells were centrifuged in a 1.073 g/mL Percoll density gradient and the cells from the interphase were collected and cultured in DMEM-LG medium, supplemented with 10% fetal bovine serum (FBS) and antibiotics (100 units penicillin/mL and 100 μg streptomycin/mL). After 72 h in culture, non-adherent hematopoietic cells were removed and the medium was replaced. The adherent spindle-shaped MSC population was cultured for 5 passages and used in this study.
Isolation and culture of urothelial cells from human fetal urinary bladders
The fetal bladders were rinsed 3 times with physiological saline and twice with a prepared mix of DMEM and Ham’s F-12 nutrient mixture (DMEM/F12, Gibco) supplemented with 10% FBS and antibiotics (100 units penicillin/mL and 100 μg streptomycin/mL). Thereafter, the bladder was cut into small pieces in a culture dish, and incubated in DMEM/F12 supplemented with 10% FBS, epidermal growth factor (EGF, 5 ng/mL), bovine pituitary extract (BPE, 50 μg/mL), cholera toxin (30 ng/mL), and antibiotics (100 units penicillin/mL and 100 μg streptomycin/mL). Tissues were cultured for 3–5 days at 37 °C in a fully humidified atmosphere of 5% CO2 in air until urothelial cells started to grow. The tissues were then transferred to 6-well plates to isolate the growing urothelial cells.
Flow cytometric analysis of MSCs
MSCs after 4 weeks in culture were detached from the flasks by gentle trypsinization and washed twice in PBS. Cell suspension was fixed in ice-cold 2% paraformaldehyde, then incubated with fluorescein isothiocyanate (FITC)-conjugated anti-CD29, CD34, CD44, CD45, CD71, and HLA-DR antibodies, or phycoerythrin (PE)-conjugated anti-CD90 and anti-CD105 antibodies (BD Pharmingen, San Diego, CA) on ice for 30 min. Flow cytometry was performed using the FACSCalibur system (Becton–Dickinson, San Jose, CA), and data were acquired and analyzed with Cellquest graphic software (Becton–Dickinson, San Jose, CA).
Osteoblast, adipocyte and chondrocyte differentiation of MSCs
MSCs are multi-potential and must be able to differentiate to osteoblasts, adipocytes, and chondroblasts. In order to test whether the cells had the basic properties, the cells were cultured using standard in vitro tissue culture-differentiating conditions. In brief, 2 × 104 MSCs were cultured in 6-well plates with osteogenic or adipogenic medium, respectively. After 3 weeks of culture in osteogenic medium, cells were fixed with methanol (−20 °C, 2 min). Alkaline phosphatase activity in osteoblasts was determined using methyl green for 3 min at room temperature. Calcium deposition in cells was tested by performing von Kossa staining. Cells were fixed with methanol for one minute, then stained with toluylene red for one minute at room temperature. Similarly, the formation of adipocytes was evaluated after 7 days of culture in adipogenic medium by staining methanol-fixed cells with Oil Red O dye for 20 min at room temperature. For chondrogenic differentiation, 4 × 105 MSCs were cultured in chondrogenic induction medium supplemented with 10 ng/mL of transforming growth factor β (Sigma-Aldrich), 0.1 μM hexadecadrol, and 50 μg/mL ascorbic acid for 3 weeks. Cells were fixed with 4% phosphoformic acid. Chondrocyte nodules were paraffin embedded and stained for glycosaminoglycans with 1% toluidine blue for 10 min. MSCs that were not induced were processed in parallel as a negative control for three experiments.
Identification of urothelial cells
Cultured urothelial cells were fixed in 4% paraformaldehyde for 60 min and washed 3 times with PBS at room temperature. Endogenous peroxidase was inactivated by incubating slides with 3% hydrogen peroxide for 10 min. After washing the cells three times with PBS, a primary anti-human cytokeratin CKAE1/AE3 monoclonal antibody (CKAE1/AE3; ZSGB-BIO, Beijing) was added, and slides were incubated in a humidified chamber overnight at 4 °C. After rinsing three times (3 min each) with PBS, samples were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG for 30 min at 37 °C. The signals were visualized for the urothelial-specific marker by incubating with 3,3′-diaminobenzidine for 30 min and counterstained with hematoxylin. Slides were then dehydrated in a graded ethanol series and treated with xylene before mounting with neutral gummi. Normal human bladder tissue was processed in parallel as a positive control. BMSCs were cultured in DMEM/F12 supplemented with the same co-factors as described above as a negative control.
Coculture of BMSCs with urothelial cells
Fourth-passage BMSCs (cultured for approximately 4 weeks after isolation) were plated onto sterile cover slips in 6-well plates. When the BMSC density approached 40–50% confluence, cover slips containing BMSCs were inverted onto sterilized rubber rings in 6-well plates containing urothelial cells in DMEM/F12 supplemented with 10% FBS, EGF (5 ng/mL), BPE (50 μg/mL), cholera toxin (30 ng/mL), and antibiotics (100 units penicillin/mL and100 μg streptomycin/mL). The rubber rings created a space between the two cell types, preventing them from coming in direct contact with each other. Urothelial cells were replaced every week during coculturing in order to prevent the decline of induced growth by the stem cells. At different times (0, 7, 14, and 21 days), cultured cells were fixed in 4% paraformaldehyde or 3% glutaraldehyde for analysis of morphology and expression of molecular markers.
Immunocytochemical identification of differentiated cells in coculture
To examine whether the newly differentiated cells had the basic properties of urothelial cells, the expression of cytokeratin CKAE1/AE3 protein was examined by immunocytochemistry, as described above. Urothelial cells and BMSCs cultured separately were used as positive and negative controls, respectively.
Transmission electron microscopy of newly differentiated cells
Morphological changes in newly differentiated cells after coculture were detected by Transmission electron microscopy (TEM). Cells were washed with PBS and fixed in 3% glutaraldehyde for 48 h, then post-fixed in 1% osmium tetroxide. Subsequently, cells were dehydrated in graded alcohols and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate, and viewed under a JEM-1400A transmission electron microscope (JEOL, Japan).
Results
MSC culture from fetal bone marrow cells
Observations of BMSCs 3 days after the initial seeding revealed numerous colonies of small, undifferentiated cells with spindle morphology. The cells grew and divided more rapidly from day 4 after the initial seeding and formed colonies of confluent spindle cells after ~2 weeks of culture, which suggests the cells required passage. Spindle cells showed large nuclei, little endochylema, clear nucleoli and were arranged as parallel concentric circles or spirals. Cells grew more rapidly after passage. Trypsin treatment was needed for cell passage, and subsequently required trypsinization and reseeding in two or three 25-cm2 flasks approximately weekly.
Biomarker expression of MSCs in culture
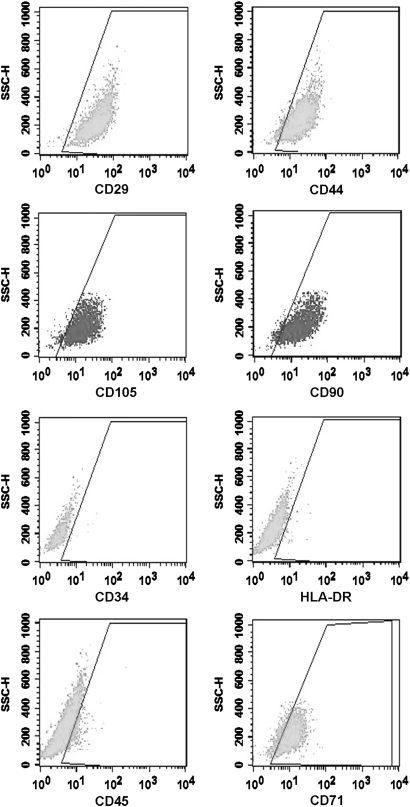
Fluorescence-activated cell sorting (FACS) analysis showed that cultured cells clearly represented a BMSC population. More than 95% of the cells expressed the stem cell markers CD29, CD44, CD105 and CD90, whereas less than 2% of the cells expressed CD34, CD45 or HLA-DR. Thus it was determined that these cells were not derived from hematopoietic stem cells or differentiated cells. A subset of BMSCs expressed the differentiation marker CD71 (Fig. 1).
Fig. 1.
Phenotypes of BMSCs as indicated by flow cytometry. BMSC markers CD29, CD44, CD105, and CD90. Hematopoietic progenitor markers CD34 and HLA-DR. Pan-leukocyte marker CD45. Differentiated marker CD71
Multi-potential differentiation of MSCs
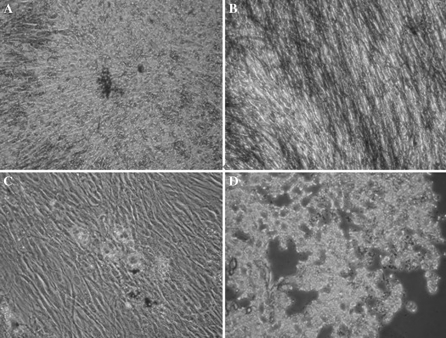
For determining the multi-potential of MSCs, cells were induced to differentiate into cells of osteocytes, adipocytes, and chondrocytes, using standard in vitro tissue culture-differentiating conditions. Cells of osteoblastic differentiation showed calcium deposition-positive particles (Fig. 2a). Similarly, the osteoblastic differentiation assay showed that culture of MSCs in osteoblastic medium resulted in the appearance of more than 90% alkaline phosphatase-positive cells (Fig. 2b). Culture of MSCs in adipocyte differentiation medium resulted in the appearance of Oil red O-incorporating adipocytes (Fig. 2c). To analyze the chondrocyte differentiation potential, MSCs were cultured in appropriate medium and stained with toluidine blue. The result was an obvious difference in the staining of differentiated cells and blue extracellular matrix. This hinted that there were lots of glycosaminoglycans in the excreted ground substance (Fig. 2d).
Fig. 2.
a Calcium deposits, characteristic of osteogenic cells, were confirmed by von Kossa staining (×200). b Alkaline phosphatase (×200). c Oil Red O staining confirmed the presence of lipid droplets indicating differentiation into an adipocyte lineage (×200). d Toluidine blue staining confirmed the presence of glycosaminoglycans in the excreted ground substance, indicating differentiation into chondrocytes (×200)
Culture and identification of urothelial cells
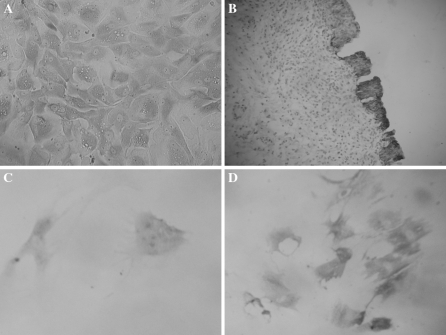
Urothelial cells derived from the fetal urinary bladder (specifically, the urachus) were separated by mechanical dissection instead of trypsinization, to minimize contamination with smooth muscle cells and fibroblasts. After 3 days, small quantities of epithelial cells, smooth muscle cells, and fibroblasts had grown. The influence of contaminating smooth muscle cells and fibroblasts was minimized by selecting tissue-derived epithelial cells and replating them into 6-well plates. Immunocytochemical analysis of the cells using the primary antibody CKAE1/AE3 confirmed that the selected cells were urothelial cells (Fig. 3a, b).
Fig. 3.
a Normal urothelial cells express the CKAE1/AE3 protein (×200). b Positive control. Normal urinary bladder mucous membrane urothelial cells (×200). c BMSCs cocultured with urothelial cells express the CKAE1/AE3 protein. There was weakly positive CKAE1/AE3 protein expression in cells on day 7 (×200). d There was strongly positive CKAE1/AE3 protein expression after 14 days of culture (×200)
Characteristics of newly differentiated urothelial-like cells in the coculture system
Fourth-passage MSCs were placed on cover slips for the coculture experiment. Chages in morphology were monitored under the microscope every day and cells began to become triangular and polygonal from the third day coculture. Most MSCs had become polygonal at about day 14 of coculture. Staining of MSCs cocultured with urothelial cells for CKAE1/CKAE3 revealed specific expression of urothelial markers in induced cells (Fig. 3c, d). Markers were completely absent in the negative control MSCs which had not been cocultured with the urothelial cells (data not show).
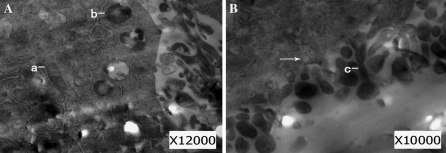
To further identify the morphological changes of newly differentiated urothelial-like cells, the cellular and subcellular characteristics were defined using TEM after coculture with urothelial cells for 2 weeks. The cells exhibited microvilli on cell surfaces and showed conspicuous plastosomes, lysosomes and fusiform vesicles in the cytoplasm (Fig. 4a, b), which suggests that the newly differentiated cells displayed features of epithelial cells.
Fig. 4.
a–b The presence of plastosomes (a), lysosomes (b), and fusiform vesicles (arrow) in the cytoplasm and microvilli (c) in the cell membrane are characteristic of the ultramicrostructure of urothelial cells
Discussion
Bladder carcinoma is one of the most common malignant urinogenital tumors worldwide. The primary treatment for superficial bladder TCC is transurethral resection of the tumor plus intravesically-instilled treatments (Bacillus Calmette–Guérin or chemotherapy). Irrigation of the bladder with chemotherapeutic agents, immunomodulators or both together is an important part of bladder-sparing therapy, and is currently the most useful and effective method for preventing post-operative recurrence and progression of superficial TCC. During the administration of chemotherapy, immunomodulators, or a combination of both, mucous membranes and urothelial cells are sloughed off, exposing lower layer tissues that support the growth of epithelial cells. The exfoliated membrane mucosa can begin to regenerate within approximately 1 month after surgery and adjuvant therapy, and is often completely restored upon cystoscopic examination 6 months after treatment (Qian et al. 2006).
However, the compensatory capacity of the bladder is limited. Large anatomical defects are often present shortly after treatment, and there is incomplete regeneration of the tissue over the long-term, despite regeneration of the membrane mucosa. Perhaps of greater concern is the fact that regeneration by existing cells after treatment may result in tumor recurrence (Kim et al. 2010). In an effort to resolve both the anatomical defects and to decrease the recurrence of bladder tumors, we created an in vitro microenvironment which fostered epithelial differentiation of BMSCs in order to obtain large amounts of normal urothelial cells that can be used to replace the resected and damaged cells.
MSCs are multipotent, self-renewing cells found in various adult and fetal tissues, including bone marrow and peripheral blood. MSCs can be easily isolated from marrow aspirates based on their ability to adhere to and grow on plastic tissue culture dishes, and cells can sometimes reach up to 50 population doublings in culture (Bianco et al. 2001). MSCs possess the ability to differentiate into a variety of cellular subtypes, including osteocytes, adipocytes, and chondrocytes.
In the present study, we used BMSCs from fetal bone marrow and induced them to differentiate into urological cells. Expanded cells in culture expressed the typical MSC phenotype. More than 95% of the cells expressed CD29, CD44, CD105 and CD90, whereas less than 2% of the cells expressed CD34, CD45 or HLA-DR. This implied that these cells were not derived from hematopoietic stem cells or their differentiated cells. A subset of BMSCs expressed the differentiation marker CD71, which suggested that some cells were undergoing differentiation. Furthermore, multi-potential was determined by inducing cells to differentiate into osteogenic, adipogenic and chondrogenic cells using standard in vitro tissue culture-differentiating conditions, which strongly suggested that these cells have a multi-potential differentiation capability and can be used in further experiments.
In vitro and in vivo studies have provided evidence that MSCs can overcome germ lineage restrictions and express molecular characteristics of cells from different germinal layers. For example, exposure of hMSCs to a sequence of growth factors that mimic the order of secretion during liver embryogenesis (FGF-4, followed by HGF, followed by HGF + ITS + dexamethasone) has been shown to promote differentiation of MSCs into hepatocyte-like cells that exhibit glycogen-storage and express CK-18. Additional exposure to trichostatin A considerably improves their endodermal differentiation, leading to acquisition of an epithelial morphology and appropriate temporal expression of hepatic proteins (Snykers et al. 2007). After being cocultured with myocytes, BMSCs differentiate into cardiomyocyte-like cells, developing a more elongated, stick-like morphology and exhibiting a contractile phenotype, first as individual cells and then as clusters of synchronously contracting cells (Li et al. 2007). BMSCs are also capable of differentiating into neuron-like cells when cultured in a specific induction medium (NPBM + cAMP + IBMX + NGF + Insulin), exhibiting an expression profile similar to that of genuine neurons within 10 days. Bone marrow has also been shown to harbor cells that have the capacity to differentiate into functionally competent, insulin-secreting pancreatic endocrine β-cells. Thus, owing to their multi-lineage differentiation potential, MSCs represent an attractive cell type for replacement therapy to treat a variety of conditions, including liver damage, cardiomyopathy, neurodegenerative diseases (Tondreau et al. 2008), and diabetes mellitus (Ianus et al. 2003).
The cellular environment significantly influences the balance between stem cell self-renewal and terminal differentiation (Hoffmann et al. 2008). When cultured under specific microenvironmental factors, stem cells have the potential to transdifferentiate into corresponding mature cells (Blazejewska et al. 2009; Altschuler et al. 2008). This approach has also been applied to BMSCs (Coleman et al. 2007). Because stem cell behavior is not the result of a cell-autonomous program, but is instead the consequence of complex cell–cell and cell-environmental interactions, a suitable growth environment is essential for achieving the desired differentiated phenotype (Roeder et al. 2007). Previous reports have revealed that BMSCs can undergo differentiation into urothelium-like cells. After being cocultured with urothelium, or cultured using urothelium-derived conditioned medium, human BMSCs express urothelium-specific genes and proteins (Tian et al. 2009). The results obtained by Anumanthan et al. (2008) support the concept that the embryonic bladder mesenchyma is a potent inductor that can regulate the differentiation of bone marrow MSCs into bladder urothelial cells (morphogenesis and cytodifferentiation).
In the present study, we showed that human fetal BMSCs cultured in vitro in an environment conditioned by human fetal urothelial cells developed into a population of cells with characteristic urothelial cell features. Viewed ultrastructurally, these cells exhibited cytoplasmic plastosomes, lysosomes and fusiform vesicles, and membrane microvilli typical of epithelial cells. They also expressed keratin, providing biochemical evidence of urothelial differentiation. Compared with the approaches designed by Tian et al. (2009) and Anumanthan et al. (2008), our model offers a simple and clear method for the culture and observation of double layer cells. The influence of contaminating smooth muscle cells and fibroblasts was minimized by removing tissue-derived epithelial cells and replating them into 6-well plates. Urothelial cells were replaced every week during coculture in order to prevent induced stem cells from declining. In addition, the ease and low cost of this culture technique can make it more readily useable in the clinic.
Most importantly, these new cells could potentially take the place of the genetically defective urothelial cells typically present in early stage or superficial bladder carcinoma, decreasing the risk of cancer recurrence and progression. In addition, together with other bladder-sparing measures, transplantation of healthy urothelial cells could facilitate bladder preservation. Clearly, preserving one’s own bladder offers quality-of-life advantages over the use of an allochthonous bladder. Bladder preservation also solves difficulties due to innervation and blood vessel distribution in transplanted tissue. Establishing the means to grow sufficient quantities of urothelial cells in vitro is only the beginning—the more difficult step of successfully transplanting induced cells lies ahead.
Conclusions
Pluripotent BMSCs can transdifferentiate into urothelial cells in response to an environment conditioned by neonatal transitional epithelium. Stem cells can thus potentially provide a means for reconstructing the urinary bladder mucosa after bladder cancer resection and intravesical adjuvant therapy.
References
- Altschuler RA, O’Shea KS, Miller JM. Stem cell transplantation for auditory nerve replacement. Hear Res. 2008;242:110–116. doi: 10.1016/j.heares.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumanthan G, Makari JH, Honea L, Thomas JC, Wills ML, Bhowmick NA, Adams MC, Hayward SW, Matusik RJ, Brock JW 3rd, Pope JC 4th (2008) Directed differentiation of bone marrow derived mesenchymal stem cells into bladder urothelium. J Urol 108:1778–1783 [DOI] [PMC free article] [PubMed]
- Bianco P, Riminucci M, Gronthos S, Robey PG (2001) Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19:180–192 [DOI] [PubMed]
- Blazejewska EA, Schlötzer-Schrehardt U, Zenkel M, Bachmann B, Chankiewitz E, Jacobi C, Kruse FE (2009) Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells 27:642–652 [DOI] [PMC free article] [PubMed]
- Bruce SJ, Gardiner BB, Burke LJ, Gongora MM, Grimmond SM, Perkins AC (2007) Dynamic transcription programs during ES cell differentiation towards mesoderm in serum versus serum-freeBMP4 culture. BMC Genomic 8:365 [DOI] [PMC free article] [PubMed]
- Chinen J, Buckley R. Transplantation immunology: solid organ and bone marrow. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S324–S335. doi: 10.1016/j.jaci.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman B, Fallon JB, Pettingill LN, de Silva MG, Shepherd RK (2007) Auditory hair cell explant cocultures promote the differentiation of stem cells into bipolar neurons. Exp Cell Res 313:232–243 [DOI] [PMC free article] [PubMed]
- Fröhlich M, Grayson WL, Wan LQ, Marolt D, Drobnic M, Vunjak-Novakovic G (2008) Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance. Curr Stem Cell Res Ther 3:254–264 [DOI] [PMC free article] [PubMed]
- Grossman HB. Superficial bladder cancer: decreasing the risk of recurrence. Oncology (Williston Park) 1996;10:1617–1624. [PubMed] [Google Scholar]
- Hoffmann M, Chang HH, Huang S, Ingber DE, Loeffler M, Galle J (2008) Noise-driven stem cell and progenitor population dynamics. PLoS One 3:e2922 [DOI] [PMC free article] [PubMed]
- Ianus A, Holz GG, Theise ND, Hussain MA (2003) In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest 111:843–850 [DOI] [PMC free article] [PubMed]
- Kim WJ, Kim EJ, Kim SK, Kim YJ, Ha YS, Jeong P, Kim MJ, Yun SJ, Lee KM, Moon SK, Lee SC, Cha EJ, Bae SC (2010) Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol Cancer 9:3 [DOI] [PMC free article] [PubMed]
- Li X, Yu X, Lin Q, Deng C, Shan Z, Yang M, Lin S (2007) Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenvironment. J Mol Cell Cardiol 42:295–303 [DOI] [PubMed]
- Morganstein DL, Wu P, Mane MR, Fisk NM, White R, Parker MG (2010) Human fetal mesenchymal stem cells differentiate into brown and white adipocytes, and reveal a role for ERRα in human UCP1 expression. Cell Res 20:434–444 [DOI] [PMC free article] [PubMed]
- Patel P. A natural stem cell therapy? How novel findings and biotechnology clarify the ethics of stem cell research. J Med Ethics. 2006;32:235–239. doi: 10.1136/jme.2005.012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper J, Sprouffske K, Maley C. Animal cell differentiation patterns suppress somatic evolution. PLoS Comput Biol. 2007;3:e250. doi: 10.1371/journal.pcbi.0030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Zhang ZG, Wen F, Fang Y (2006) Treatment of multiple superficial bladder tumors with bladder mucosal stripping in aged patients. J Med Theor Pract 19:1262–1263
- Roeder I, Braesel K, Lorenz R, Loeffler M (2007) Stem cell fate analysis revisited: interpretation of individual clone dynamics in the light of a new paradigm of stem cell organization. J Biomed Biotechnol 2007:84656 [DOI] [PMC free article] [PubMed]
- Snykers S, Vanhaecke T, De Becker A, Papeleu P, Vinken M, Van Riet I, Rogiers V (2007) Chromatin remodeling agent trichostatin A: a key-factor in the hepatic differentiation of human mesenchymal stem cells derived of adult bone marrow. BMC Dev Biol 7:24 [DOI] [PMC free article] [PubMed]
- Tian H, Bharadwaj S, Liu Y, Ma PX, Atala A, Zhang Y (2009) Differentiation of human bone marrow mesenchymal stem cells into bladder cells: potential for urologic tissue engineering. Tissue Eng Part A16:1769–1779 [DOI] [PMC free article] [PubMed]
- Tondreau T, Dejeneffe M, Meuleman N, Stamatopoulos B, Delforge A, Martiat P, Bron D, Lagneaux L (2008) Gene expression pattern of functional neuronal cells derived from human bone marrow mesenchymal stromal cells. BMC Genomic 9:166 [DOI] [PMC free article] [PubMed]
- Wolff EM, Liang G, Jones PA. Mechanisms of disease: genetic and epigenetic alterations that drive bladder cancer. Nat Clin Pract Urol. 2005;2:502–510. doi: 10.1038/ncpuro0318. [DOI] [PubMed] [Google Scholar]
- Yamada S, Nelson TJ, Crespo-Diaz RJ, Perez-Terzic C, Liu XK, Miki T, Seino S, Behfar A, Terzic A (2008) Embryonic stem cell therapy of heart failure in genetic cardiomyopathy. Stem Cells 26:2644–2653 [DOI] [PMC free article] [PubMed]