Abstract
Human adipose derived mesenchymal stem cells (ADMSCs) are multipotential stem cells, originated from the vascular stromal compartment of fat tissues which can be used as an alternative cell source for many different cell therapies. However, their response to chemotherapeutic agants remains unknown. Here we assessed the acute direct effects of individual chemotherapeutic drug on ADMSCs. Using an in vitro culture system, the response of ADMSCs to the three chemotherapeutic agents cisplatin, comptothecin and vincristine was determined in comparison with that of testicular germ cell tumour (TGCT) cell line. The recovery of cell numbers following exposure to chemotherapeutic agents were also evaluated. Our results showed that human ADMSCs were resistant to chemo-therapeutic agents which are commonly used in clinic, the full recovery was seen respectively in ADMSCs after the drug treatment. Moreover, ADMSCs maintained their stem cell characteristics in vitro after the exposure to all chemotherapeutic agents.
Keywords: Adipose tissue, MSCs, Chemoresistance, Cisplatin, Vincristine, Camptothecin
Introduction
In the past several years, great progresses have burgeoned worldwide in the adult stem cells field. Among them mesenchymal stem cells (MSCs) have received much attention for their prospective clinical and research use. They can differentiate into various cell lineages including osteoblasts, chondrocytes, adipocytes and other cell types (Kopen et al. 1999; Liechty et al. 2000; Muraglia et al. 2000; Pereira et al. 1998; Pittenger et al. 1999; Toma et al. 2002; Wakitani et al. 1995; Woodbury et al. 2000). Numerous studies with a variety of animal models have shown that MSCs may be useful in the process of repairation or regeneration of damaged bones, cartilages, or myocardial tissues, thus representing a new source to treat congenital or degenerative disorders (Muguruma et al. 2003; Pak et al. 2003; Parsons et al. 2004).
Table 1.
The IC50 (in μM) value of ADMSCs and 2102EP to the three assessed agents
| Drug | ADMSCs | 2102EP | Fold rate |
|---|---|---|---|
| Cisplatin | 22.3 ± 3.8 | 0.72 ± 0.075 | 30.97 |
| Vincristine | 0.0047 ± 0.0004 | <0.0005 | NA |
| Camptothecin | 0.076 ± 0.003 | 0.0042 ± 0.0005 | 18.1 |
The results represent the mean ± SEM of triplicate cultures of one representative experiment
Fold rate, the mean IC50 value for ADMSCs divided by the mean IC50 value for 2101EP
NA, not available
Although the human bone marrow is the most often used resource for obtaining MSCs, other tissues have been found to contain MSCs, among which human adipose tissues represent the most promising site for aquiring MSCs population because of easy isolation process (Zuk et al. 2002). Being found by Zuk et al. (2005) firstly, adipose tissues MSCs share almost every similar phenotype, multilineage differentiation potentials with those of bone marrow MSCs thus suggesting an substitute to pluripotent ES cells in both the lab and the clinic (Zuk et al. 2002).
Accordingly to previous reports, bone marrow derived MSCs are resistant to chemotherapeutic agents and irradiation (Chen et al. 2006; Li et al. 2004; Mueller et al. 2006). In contrast, there are no studies in the literature regarding for the chemosensitivity of ADMSCs, this prompted us to analyze the acute and direct reaction of cultured ADMSCs exposed to single chemotherapeutic agent in vitro compared with that of TGCT cell line 2102EP which has been known of high sensitivity. Moreover, we also evaluated the recovery of cell numbers following exposure to chemotherapeutic agents.
Materials and methods
Isolation and culture of human ADMSCs
Human subcutaneous raw lipoaspirates were collected after obtaining necessary informed consent from patients undergoing selective suction-assisted lipectomy. All the procedures were approved by the Ethics Committee at Anhui Medical Univeristy. The procedure was described by Cao et al. (2005) with some modifications. Briefly, the lipoaspirates were extensively washed with D-Hanks’s solution to remove contaminating blood cells and local anesthetics. Then the extracellular matrix was digested with 0.2% collagenase II (Sigma) at 37 °C for 30 min to release the cellular fractions. The cells were collected and resuspended in 57% Dulbecco’s modified Eagle medium (low glucose DMEM/F12), supplemented with 40% MCDB-201 (Sigma, USA), 2% fetal bovine serum (FBS; Gibco Life Technologies, Paisley, United Kingdom), 1-insulin transferring selenium (Gibco Life Technologies), 10−9 M dexamethasone (Sigma), 10−4 M ascorbic acid 2-phosphate (Sigma), 10 ng/ml epidermal growth factor (Sigma), 10 ng/ml platelet-derived growth factor BB (Sigma), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco) and then plated in culture flask (1 × 106 cells/ml) in a humidified environment containing 5% CO2 at 37 °C. Once adherent cells reached 70–80% confluence, they were detached with 0.125% trypsin and 0.01% EDTA and replated at a 1:3 dilution under the same culture conditions. All experiments were done in the 5th passage.
Sensitive cell lines
The human testicular germ cell tumor (TGCT) cell line 2012EP was cultured in RPMI1640 medium supplemented with 10% fetal bovine serum and maintained at 37 °C in a humidified atmosphere with 5% CO2. Medium was changed every 2 days and cells were passaged every 4–5 days.
Immunophenotype
Cells were detached and washed with phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA; Sigma), and incubated with following primary antibodies CD29, CD31, CD34, CD44, CD45, CD105 and CD11a (BD Biosciences) at the concentration in 10–20 ng/ml for 30 min at 4 °C. To detect intracellular antigens, cells were fixed in 2% paraformaldehyde at 4 °C for 15 min and then permeabilized with 0.1% saponin (Sigma) at room temperature for 1 h. Same species and isotype irrelevant antibodies were used as negative control. After washing with PBS containing 0.5% bovine serum albumin, the cells were incubated with fluorescein iso-thiocyanate (FITC) or phycoerythrin (PE)-conjugated secondary antibodies at 4 °C for 30 min. Then cells were resuspended in PBS and analyzed by a FACS Calibur flow cytometer and the results were analyzed by CellQuest Pro software (BD Biosciences).
Differentiation induction in ADMSCs
Osteogenic differentiation: Cells were seeded at a density of 2 × 104/cm2 and were then cultures in the following osteogenic differentiation medium for 2–3 weeks: Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FCS, 10 mmol/L β-glycerophosphate, 10−7 mol/L dexamethasone, and 0.2 mmol/L ascorbic acid (all from Sigma). Then the cells were stained with von Kossa staining (from Sigma) to show osteogenic differentiation.
Adipogenic differentiation: Cells at 2 × 104/cm2 were induced for 3 weeks in DMEM supplemented with 10% FCS, 0.5 μmol/L hydrocortisone, 0.5 mmol/L isobutylmethylxanthine, and 50 μg/ml indomethacin (all from Sigma). At the end of the induction, the cells were fixed in 10% formaldehyde for 10 min and stained with fresh Oil red-O solution (Sigma) to show lipid droplets.
XTT assay
Cells were seeded into flat-bottom 96-well plates at a density of 3 × 103 cells per well in 100 μl of culture medium. After 24 h, cells were treated with chemotherapeutic drugs, the culture was continued for another 2–3 days and cell survival was measured by XTT (2,3-bis(2-methoxy-4-nitro-5-sulphophenyl)-5-((phenylamino)carbonyl)2H-tetrazolium hydroxide) (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturers’ instruction. Data shown are representives of three independent experiments. The concentrations that inhibited cell growth by 50% (IC50) were determined for each treatment schedule from semilogarithmic dose–response plots.
Results
Morphology, phenotype and differentiation potential of cultured ADMSCs
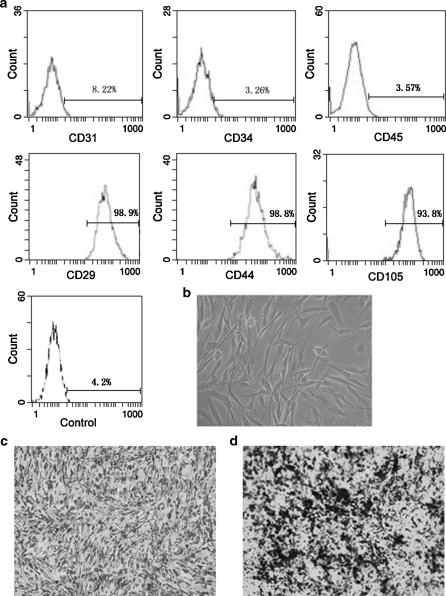
ADMSCs displayed a fibroblast-like morphology when cultured in specific medium (Fig. 1b): the morphology was maintained through repeated passages under non-stimulating conditions. Flow cytometry showed that they were positive for CD29, CD44, CD105 but did not express CD31, CD34, CD45 (Fig. 1a). When subjected to the osteogenic and adipogenic differentiation inducing medium, ADMSCs showed a typical morphological change of differentiation respectively with extracellular calcium deposition by Von Kossa staining and intracellular lipid droplets stained by Oil-red (Fig. 1c, d).
Fig. 1.
In vitro phenotypic characteristics of ADMSCs. a ADMSCs were harvested at passage 1 or 2 and a set of cell surface antigen was analyzed by flow cytometry. Data are shown as histograms of mean channel fluorescence. Data are representatives of several independent experiments. b Cultured ADMSCs show a fibroblast-like morphology (magnification, × 200). c, d ADMSCs were incubated in adipogenic or osteogenic differentiation inducing medium for a couples of days and then samples were stained with Oil-Red (c) or von Kossa (d) to show lipid droplets or calcium deposits, respectively (magnification, ×100)
ADMSCs are resistant to chemotherapeutic substances versus 2102EP
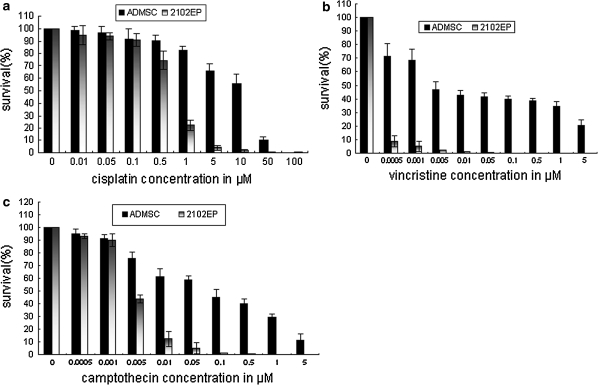
We analyzed the in vitro response of ADMSCs to three chemotherapeutic agents commonly used in clinic in comparison with TGCT cell line 2102EP which has been known of high sensitivity by XTT assays. Here, results from one representative experiment are presented. ADMSCs showed a reduced sensitivity to increasing concentrations of cisplatin, vincristine and camptothecin in a dose-dependent manner compared with that of 2102EP cells (Fig. 2). Moreover we also compared the IC50 value of ADMSCs and 2102EP to three agents and the results showed that each value of ADMSCs was significantly higher than that of 2102EP cell line which was 30.97 fold for cisplatin and 18.1 fold for camptothecin (data not available for vincristine). Although the relative statistical comparison of IC values from a single cell line with data from primary cells of multiple sources was limited, these data suggested that ADMSCs are more resistant to chemotherapy-induced damage.
Fig. 2.
ADMSCs are resistant to several chemotherapeutic substances. ADMSCs and TGCT cells 2102EP were treated with different concentration of cisplatin (10−2–102 μM) (a) vincristine (0.0005–5 μM) (b) and camptothecin (0.0005–5 μM) (c) for 72 h and cell survival was analyzed in XTT assay. Results of a–c are represented as mean ± SD from at least three independent experiments. IC50 values of ADMSCs and 2102EP cells are shown in the Table 1
ADMSCs keep their stem cell characteristic after genotoxic treatment in vitro
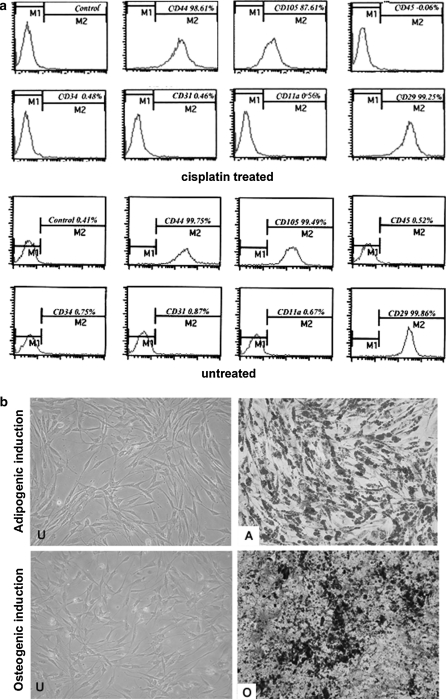
According to the data from growth kinetics, we speculated that cultured ADMSCs may retain their stem cell characteristics after chemotherapeutic treatment in vitro. In order to prove this hypothesis, we analyzed the stem cell phenotype of ADMSCs after the treatment with 3 μM cisplatin or 0.005 μM camptothecin for 2 h, repeated four times every 24 h, which indicate the dosage of clinically relevant serum concentrations. Flow cytometry demonstrated an identical phenotype with CD31−, CD34−, CD105+, CD44+ and CD11a−, CD45−, CD29+ before and after the cisplatin treatment (Fig. 3a). Finally, upon induction of osteogenic and adipogenic differentiation, cisplatin and camptothecin treated ADMSCs showed signs of specific differentiation which altogether indicated that ADMSCs may keep their stem cell characteristics after genotoxic damage (Fig. 3b).
Fig. 3.
ADMSCs retain their stem cell characteristics after in vitro treatment with DNA damaging substances. a Flow cytometry showed almost the same phenotype for selected markers in cisplatin treated and untreated ADMSCs. b Treated or untreated ADMSCs were incubated in differentiation-inducing medium or control growth medium, resulting in typical signs of differentiation, that is, lipid droplets stained by oil red or calcium deposits stained by von Kossa respectively. (U): Uninduced. (A) Adipogenic induced. (O) Osteogenic induced (magnification, ×100)
Recovery of ADMSCs following treatment of individual chemotherapeutic agents
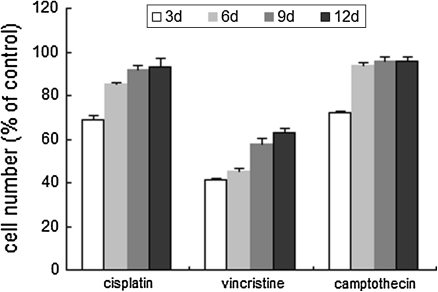
After the exposure to cisplatin, vincristine and comptothecin at the dose of 3, 0.1 and 0.005 μM for 3 days, respectively, which represented doses corresponding to clinically relevant plasma serum concentrations measured in patients receiving intensive chemotherapy, significant cell loss was found in every group especially in that of vincristine. For cells treated with cisplatin and comptothecin the cell count was reduced to 71 and 74%. However, after the withdrawal of the drugs, each group showed a significant recovery of proliferation which returned to the control level on about the 12th day except for the case of vincristine, for which the full recovery was achieved on about the 15th day (data not shown) (Fig. 4). Altogether, these results demonstrated further that in vitro cultured ADMSCs are resistant to the genotoxic damage.
Fig. 4.
Recovery of ADMSCs following exposure to individual chemotherapeutic agents. Cells were incubated with a chemotherapeutic agent (cisplatin 3 μmol/L, vincristine 0.1 μmol/L, camptothecin 0.005 μmol/L) for 3 days and then cultured for another 9 days. The y-axis shows the cell number, expressed as a percentage of control. The results represent the mean ± SEM of triplicate cultures of one representative experiment
Discussion
MSCs represent a promising tool for the regeneration of damaged tissues in clinical applications (Garcia-Gomez et al. 2004). Although bone marrow was the first source reported to contain MSCs, however, for clinical use, the isolation and the harvest of bone marrow MSCs is a highly invasive procedure and the number, differentiation potential, and maximal life span of bone marrow MSCs decline with increasing age and could be clinically inefficient when derived from elderly patients (Kern et al. 2006). Adipose tissues are another alternative source for MSCs which can be obtained in large numbers by a less invasive method: cosmetic liposuctions. These cells can grow easily under specific tissue culture conditions and their multilineage differentiation capacity has been long confirmed (Kern et al. 2006).
Recently, several studies have shown that bone marrow MSCs in patients under whole body irradiation and high-dose chemotherapy seem mainly to originate from the host after the process of allogeneic hematopoietic stem cell transplantation (Devine and Hoffman 2000; Koc et al. 2002); this phenomenon leads to the studies which investigate the reaction of bone marrow MSCs under the treatment of chemotherapeutic substances and irradiation. These results demonstrate that the cultured human bone marrow MSCs are generally resistant to the chemotherapeutic and irradiation damage (Chen et al. 2006; Li et al. 2004). ADMSCs share almost every similar characteristic with bone marrow MSCs with respect to morphology, colony frequency, expansion potential and mutiple differentiation capacity, although a little difference in phenotype exists (Grisendi et al. 2003). Presently: there are no systematic reports in the literature concerning the chemosensitivity of human ADMSCs. Thus in this paper, we isolated MSCs consistently from human liposuction donors and examined the effect of three commonly used chemotherapeutic agents on ADMSCs in vitro, we found that ADMSCs are resistant to cisplatin, comptothecin and vincristine and can recover proliferation capacity after withdrawal of the drugs. Furthermore, it may be questioned that the surviving cells may no longer be stem cells after using chemotherapeutic drugs on ADMSCs population, so the cell surface marker and the effects of these chemotherapeutic agents on the osteogenic and adipogenic capacity were also evaluated. Our experiments showed that ADMSCs were able to maintain their phenotype and osteogenic, adipogenic differentiation potential in vitro after the treatment with various chemotherapeutic agents. This is the first direct demonstration showing that MSCs from human adipose have enhanced chemosensitivity.
Here we speculate that the enhanced resistance to DNA damage may be a general characteristic of adult stem cells. In stem cells, the degree of resistance to genotoxic damage correlates with the relevance of the particular cell type for the generation of progeny (Heyer et al. 2000). Embryonic stem cells within the developing embryo which can generate germ cells become hypersensitive to DNA damage during pre-implantation embryonic development, and after exposure to a DNA damaging agent, undergo p53-dependent apoptosis without cell cycle arrest, presumably to avoid a high rate of embryonic malformations (Heyer et al. 2000). However, in contrast, adult stem cells like MSC which do not participate directly in the generation of germ cells are always under the influence of harmful environmental factors during the entire process of human life as the seed cells for reparation and substitution of tissues and organs, thus it is crucial for them to keep the genetic stability. The differential response to the genotoxic damage could secure genomic integrity in the progeny but also maintain the function of adult stem cells in the organism.
In our study, investigations were only focused on three clinically used chemotherapeutic drugs. Moreover, MSCs are a quiescent cell population in vivo, the response of rapidly dividing MSCs in culture system may not represent their actual behaviour in vivo. Some studies suggested that the stromal response in vivo to acute injury, including chemotherapy, is complex and may involve induction of differentiation, cell migration and proliferation (Almohamad et al. 2003; Gimble et al. 1996). So the results inferred form our research were limited and comparative experiments are needed to assess the responsiveness of ADMSCs toward more chemotherapeutive substances.
In conclusion, our studies showed that human ADMSCs were relatively resistant to chemotherapeutic agents commonly used in the clinic, full recovery was seen in ADMSCs treated with high doses of cisplatin, comptothecin and vincristine, respectively. ADMSCs maintained their stem cell characteristics in vitro after exposure to all chemotherapeutic agents. Further investigation of the underlying mechanisms which is responsible for the resistance of ADMSCs to DNA damage agents is crucial for the application of ADMSCs in the clinic.
Acknowledgments
This work was supported by grants from the “863 Projects” of Minsitry of Science and Technology of PR China (No 2002 AA 205061); and from Beijing Minsitry of Science and Technology (No. 2002-489).
Conflict of interest The authors declare no conflict of interest.
Abbreviations
- ADMSC
Adipose derived mesenchymal stem cell
- MSCs
Mesenchymal stem cells
- XTT
2,3-Bis(2-methoxy-4-nitro-5-sulphophenyl)-5-((phenylamino)carbonyl)2H-tetrazolium hydroxide
- FITC
Fluoresein-5-isothiocyanate
- FACS
Fluorescence-activated cell sorting
References
- Almohamad K, Thiry A, Hubin F, Belaid Z, Humblet C, Boniver J, Defresne MP. Marrow stromal cell recovery after radiation-induced aplasia in mice. Int J Rad Biol. 2003;79:259–267. doi: 10.1080/0955300031000085740. [DOI] [PubMed] [Google Scholar]
- Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- Chen MF, Lin CT, Chen WC, Yang CT, Chen CC, Liao SK, Liu JM, Lu CH, Lee KD. The sensitivity of human mesenchymal stem cells to ionizing radiation. Int J Rad Oncol Biol Phys. 2006;66:244–253. doi: 10.1016/j.ijrobp.2006.03.062. [DOI] [PubMed] [Google Scholar]
- Devine SM, Hoffman R. Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr Opin Hematol. 2000;7:358–363. doi: 10.1097/00062752-200011000-00007. [DOI] [PubMed] [Google Scholar]
- Garcia-Gomez I, Elvira G, Zapata AG, Lamana ML, Ramirez M, Castro JG, Arranz MG, Vicente A, Bueren J, Garcia-Olmo D. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2004;10:1453–1468. doi: 10.1517/14712598.2010.519333. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Robinson CE, Wu X, Kelly KA, Rodriguez BR, Kliewer SA, Lehmann JM, Morris DC. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol. 1996;50:1087–1094. [PubMed] [Google Scholar]
- Grisendi G, Bussolari R, Cafarelli L, Petak I, Rasini V, Veronesi E, Santis G, Spano C, Tagliazzucchi M, Barti-Juhasz H, Scarabelli L, Bambi F, Frassoldati A, Rossi G, Casali C, Morandi U, Horwitz EM, Paolucci P, Conte P, Dominici M. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res. 2003;70:3718–3729. doi: 10.1158/0008-5472.CAN-09-1865. [DOI] [PubMed] [Google Scholar]
- Heyer BS, MacAuley A, Behrendtsen O, Werb Z. Hypersensitivity to DNA damage leads to increased apoptosis during early mouse development. Gen Dev. 2000;14:2072–2084. [PMC free article] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells (Dayton, Ohio) 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marr Transplant. 2002;30:215–222. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Law HK, Lau YL, Chan GC. Differential damage and recovery of human mesenchymal stem cells after exposure to chemotherapeutic agents. Br J Haematol. 2004;127:326–334. doi: 10.1111/j.1365-2141.2004.05200.x. [DOI] [PubMed] [Google Scholar]
- Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- Mueller LP, Luetzkendorf J, Mueller T, Reichelt K, Simon H, Schmoll HJ. Presence of mesenchymal stem cells in human bone marrow after exposure to chemotherapy: evidence of resistance to apoptosis induction. Stem Cells (Dayton, Ohio) 2006;24:2753–2765. doi: 10.1634/stemcells.2006-0108. [DOI] [PubMed] [Google Scholar]
- Muguruma Y, Reyes M, Nakamura Y, Sato T, Matsuzawa H, Miyatake H, Akatsuka A, Itoh J, Yahata T, Ando K, Kato S, Hotta T. In vivo and in vitro differentiation of myocytes from human bone marrow-derived multipotent progenitor cells. Exp Hematol. 2003;31:1323–1330. doi: 10.1016/j.exphem.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113(Pt 7):1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- Pak HN, Qayyum M, Kim DT, Hamabe A, Miyauchi Y, Lill MC, Frantzen M, Takizawa K, Chen LS, Fishbein MC, Sharifi BG, Chen PS, Makkar R. Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a Swine model of myocardial infarction. J Cardiovasc Electrophysiol. 2003;14:841–848. doi: 10.1046/j.1540-8167.2003.03124.x. [DOI] [PubMed] [Google Scholar]
- Parsons CH, Szomju B, Kedes DH. Susceptibility of human fetal mesenchymal stem cells to Kaposi sarcoma-associated herpesvirus. Blood. 2004;104:2736–2738. doi: 10.1182/blood-2004-02-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira RF, O’Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Zuk PA. The adipose-derived stem cell: looking back and looking ahead. Mol Biol Cell. 2005;21:1783–1787. doi: 10.1091/mbc.E09-07-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]