Abstract
The pro-apoptotic effects of hydrogen peroxide and the purported anti-apoptotic effects of Vitamin C on chicken embryonic fibroblasts were investigated. Hydrogen peroxide induced morphological changes in a dose dependent manner, and a myriad of autophagosomes were observed using transmission electron microscopy. Doxorubicin elicited alterations were not inhibited by co-incubation with Vitamin C except that mitochondrial structure was slightly improved. TUNEL assay, cytotoxicity analysis and flow cytometry revealed that the cytotoxicity, DNA fragmentation and apoptotic rates were dose dependent upon treatment with hydrogen peroxide. Calcium homeostasis was disrupted in a dose dependent manner, and cell cycle was blocked at G2/M checkpoint at low concentration and S/G2 checkpoint at high concentration respectively upon treatment with hydrogen peroxide. The administration of Vitamin C only has a modest effect against doxorubicin induced apoptosis, calcium homeostasis disruption and cell cycle arrest. This research demonstrated that the elevation of reactive oxygen species is sufficient to induce the apoptosis of chicken embryonic fibroblasts, whereas the administration of Vitamin C does not necessarily have certain anti-apoptotic effects, especially when the stimulus is not directly linked with redox state.
Keywords: Chicken, Embryonic fibroblasts, Hydrogen peroxide, Vitamin C, Apoptosis, Redox state
Introduction
Complex IV (cytochrome oxidase) in the mitochondrial respiratory chain, as well as other redox centers in the electron transport chain, may leak electrons to oxygen (1–2%), partially reducing this molecule to superoxide anion (O2−) and resulting in the propagation of oxidative chain reactions (Turrens 2003). Reactive oxygen species (ROS) is responsible for cataract, infertility, diabetic nephropathy, bone disorder, neurological disorders, ischemic/reperfusion injury, rheumatoid arthritis, atherosclerosis and ageing by means of lipid peroxidation, DNA damage and mutation and redox-sensitive signaling pathways (Gupta et al. 2009; Hamada et al. 2009; Liu et al. 2009; Makker et al. 2009; Wagener et al. 2009). Supplementation with antioxidants was assumed to antagonize atherosclerosis, pre-eclampsia, hypertension, neurodegenerative diseases and carcinogenesis that are closely linked with oxidative stress, however, experimental results seemed controversial, and an inappropriate administration may lead to harmful effects (Pourova et al. 2010).
Vitamin C, whose chemical name is ascorbic acid, is generally considered as a potent reductant. Vitamin C in cells undergoing hypoxia-reperfusion are linked with a reduction of ROS level, prevention of cytochrome c release and a stabilized mitochondrial membrane potential and a decreased activation of caspase-3 and caspase-9 (Mandl et al. 2009). While high intake of Vitamin C (2,000 mg/d) has not been consistently reported to cause any side effects, its benefits to normal people have never been established, and what have been discovered is only that Vitamin C exerts some inhibitory effects on gastric metaplasia, chronic gastritis and lung and colorectal cancer in vulnerable population (Valko et al. 2006).
Avian species are usually more resistant to oxidative stress and accordingly have a longer life span (Finkel and Holbrook 2000). This research is to investigate the apoptotic effects of redox state on chicken embryonic fibroblasts (CEFs), an avian cell line presupposed to be more resistant to oxidative stress. Hydrogen peroxide was used as the source of ROS, and doxorubicin was administrated as a pro-apoptotic stimulus not directly relevant to ROS formation to verify the putative anti-apoptotic effects of Vitamin C. Three aspects, i.e. the apoptotic morphology, apoptotic effects and apoptotic mechanisms, were studied via confocal microscopy, electron microscopy, MTT assay and flow cytometry. The present study revealed the apoptotic effects of elevated ROS level on chicken embryonic fibroblasts, as well as whether the administration of Vitamin C is preventive to non-ROS induced apoptosis, thereby providing precious insights for theoretical and therapeutic trials.
Materials and methods
Reagents
Microplates (Cat. No.: 3516) and Petri dishes (Cat. No.: 14831) were purchased from Corning (USA). MEM medium was obtained from Gibco (Carlsbad/CA, USA, Cat. No.: 41500-034). Fetal bovine serum (FBS) was from HyClone (South Logan/UT, USA, Cat. No.: S0415. Vitamin C, doxorubicin, and hydrogen peroxide were form Sigma (St. Louis/MO, USA). Unless it was specially noted somewhere, all reagents for this research were purchased from Sigma (St. Louis/MO, USA). Vitamin C, doxorubicin, and hydrogen peroxide were solubilised in MEM medium and then filtered with 0.22-μm filter membrane to eliminate potential microbes.
Primary cell culture and serial passage
Chicken embryonic fibroblasts were prepared using nine-day old embryos isolated from Beijing Fatty chicken (Gallus gallus) eggs (Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing, China), rinsed three times with phosphate buffered saline (PBS, pH 7.4), chopped into 1.0 mm3 pieces, and then plated on the bottom of a tissue culture flask containing MEM+10% (v/v) fetal bovine serum in incubator at 37 °C with 5% CO2 as previously described (Wu et al. 2008). Upon confluence, the cells were purified via serial passages. Experimental cells were used in exponential phase and from passages 3–6.
Treatment of CEFs
The media were removed and the cells were cultured in media containing appropriate concentrations of Vitamin C, doxorubicin, and hydrogen peroxide for 24 and 48 h.
AO/EB (acridine orange/ethidium bromide) staining
AO & EB (Sigma, St. Louis/MO, USA) (both 2 mg/mL in ethanol) solution of 10 μL was added into 3 mL cell suspension harvested from a well of a 6-well microplate. Incubated for 5 min in the dark at room temperature, different samples were observed under a confocal microscope (Nikon TE-2000-E, Japan) with excitation wavelengths of 488 and 543 nm.
TEM (transmission electron microscopy) observation
Cells were harvested and fixed with 2.5% (m/v) glutaraldehyde, washed with 0.1 M phosphate buffer and subjected to serial dehydration with 30, 50, 70, 80, 90 and 100% acetone (v/v). The samples were embedded with epoxy resin (SPURR) for polymerization, and then sectioned with ultramicrotome (LEICAUC6i). After double staining with uranyl acetate and lead citrate, they were observed under transmission electron microscope (JEM-123O) and photographed.
Apoptotic detection
MTT assay was used to evaluate cytotoxicity as described by Mosmann (1983). The CEFs were plated in 96-well plates at the concentration of 5 × 104 per well. Forty-eight hours after treatment, the media were replaced with 200 μL serum-free MEM media and 20 μL MTT solution (5 mg/mL in PBS). After 4-h incubation at 37 °C the media were removed, and 200 μL DMSO was added. The OD values at 490 nm were detected under an enzymatic reader. Air dried cell samples were fixed with 4% (m/v) paraformaldehyde (in PBS, pH 7.4, freshly prepared). In Situ Cell Death Detection Kit (Roche, UK) was then used to perform TUNEL assay. Annexin V-FITC Apoptosis Detection Kit I (BD, Franklin Lakes, NJ) was used to stain cell suspension, which was then analyzed with a flow cytometer (BD FACSCalibur, USA).
Ca2+ homeostasis
Cells were stained with 200 μL of Fluo-3/AM (Invitrogen, Carlsbad/CA, USA) (15 μM in 30 mM HEPES solution) and observed using confocal microscopy (Nikon TE-2000-E, Japan) with the excitation wavelength of 488 nm.
Cell cycle analysis
Cells were harvested, suspended in precooled 70% (v/v) ethanol at 4 °C overnight, stained with PI solution (PI 0.05 mg/mL, RNase 0.02 mg/mL, NaCl 0.585 g/mL, sodium citrate 1 mg/mL, pH 7.2–7.6) at 4 °C for 30 min in the dark, and then analyzed with a flow cytometer (BD FACSCalibur, USA).
Statistical analysis
Cytotoxicity data, apoptotic rates and cell cycle data were analyzed using the GLM procedure in Statistical Analysis System (SAS Inc., Cary, NC, USA) and compared with a multiple comparison test (DUNCAN). A value of P < 0.05 was thought of as statistically significant.
Results and discussion
Morphological observation
Previous research on hydrogen peroxide-induced cell death observed nuclear shrinkage, condensation or other kinds of alterations, chromatin condensation, and swelling of organelles, while pretreatment with antioxidant appeared to be able to relieve the symptoms (Ben-juan and Zeng-tong 2007; Goto et al. 2009; Juknat et al. 2005; Lim et al. 2002).
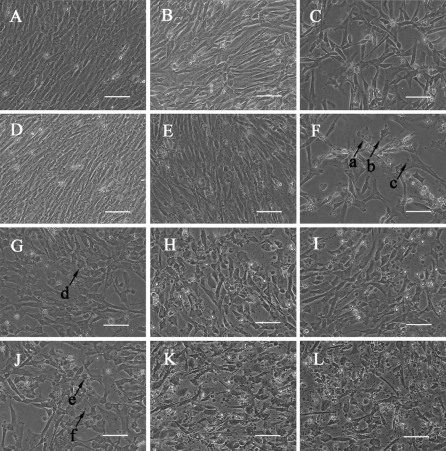
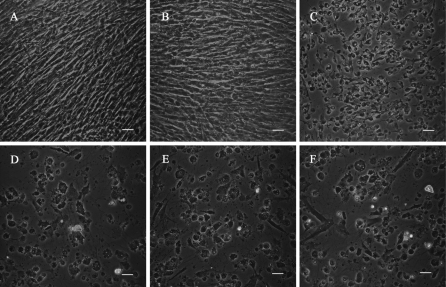
In the present study, normal fibroblasts exhibited typical fusiform and fibrous morphology with oval-shaped nuclei (Fig. 1a, d). Morphological alterations, for example, cell shrinkage (Fig. 1, arrow a), cytoplasm condensation (Fig. 1, arrow b) and emergence of well packaged apoptotic bodies (Fig. 1, arrow c), took place following incubation with H2O2 in a dose dependent manner (Fig. 1b, c, e, f). Aforementioned changes (Fig. 1, arrows d–f) also took place in doxorubicin treated cells, and Vitamin C administration made no obvious differences (Fig. 1g–l).
Fig. 1.
Morphological observation of samples treated (a–c), (g–i) for 24 h; (d–f) (j–l) for 48 h; (a, d) controls; and those incubated with (b, e) H2O2 10 μM; (c, f) H2O2 100 μM; (g, j) Vitamine C (Vc) 0 μM; (h, k) Vc 50 μM; and (i, l) Vc 500 μM. Cells for functional investigation of Vc are cultured in media supplemented with 2 μg/mL doxorubicin. Arrows a and e, cellular shrinkage; arrows b and f, cytoplasm condensation; arrows c and g, apoptotic bodies. Scale bars = 50 μm
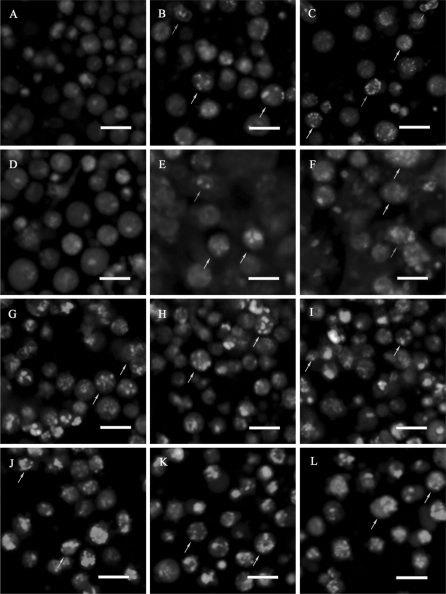
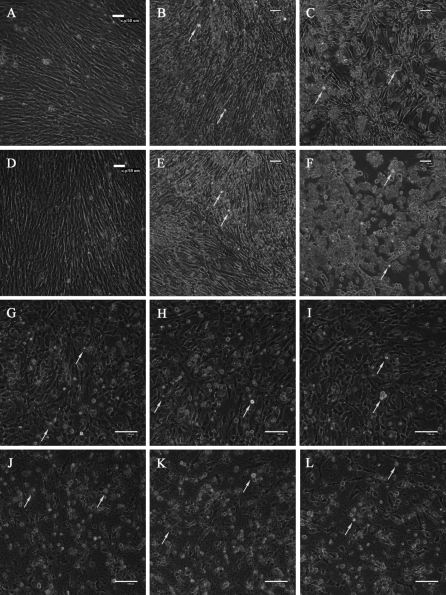
The cells were stained with AO/EB to visualize nuclear morphology and membrane permeability. It was observed that in controls (Fig. 2a, d), most cells were viable and there existed a few necrotic ones, whereas exposure to H2O2 induced nuclear condensation and increased apoptotic and necrotic rates increased in a dose and duration dependent manner (Fig. 2b, c, e, f). Doxorubicin treated cells exhibited obvious nuclear condensation and chromatin margination (Fig. 2g, j), upon which Vitamin C exerted no observable effects (Fig. 2h, i, k, l). It was also noteworthy that apoptotic rates were higher at 48 h.
Fig. 2.
Morphological observation of CEFs using AO/EB double staining. (a–c) (g–i) samples treated for 24 h; (d–f) (j–l) samples treated for 48 h; (a, d) controls; and cells treated with (b, e) H2O2 10 μM; (c, f) H2O2 100 μM; (g, j) Vc 0 μM; (H, K) Vc 50 μM; and (i, l) Vc 500 μM. Cells for functional investigation of Vc are cultured in media supplemented with 2 μg/mL doxorubicin. Normal cells displayed evenly distributed brown fluorescence; apoptotic cells possessed brown cytosol and condensed brown nuclei; necrotic cells exhibited red cytosol and condensed red nuclei; dead cells through other pathways displayed evenly distributed red fluorescence. Brown arrows point to representative apoptotic cells, and red arrows point to representative necrotic cells. Scale bars = 20 μm. (Color figure online)
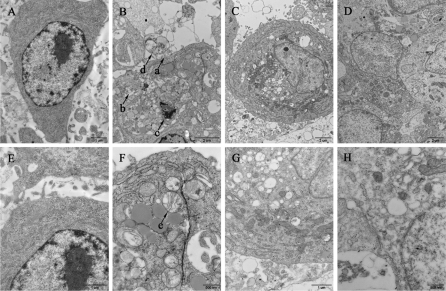
Subcellular alterations were observed by TEM. The cells in control displayed homogenous and plump cytoplasm, clear nucleoli, intact karyotheca and seldom any vacuoles (Fig. 3a, e). Mitochondria are a major resource and target of oxidative stress, and even play a central role in “the Free Radical Theory of Aging” (Alexeyev 2009; Cadenas and Davies 2000). Evidence is accumulating that links oxidative stress with mitochondrial impairment such as fragmentation, loss of transmembrane potential, swelling, crista abnormalities and respiratory chain deficiency (Baregamian et al. 2011; Romano et al. 2010; Wu et al. 2011; Chang et al. 2010). Keeping up with this notion, in H2O2 treated CEFs, morphological events, including membrane blebbing (Fig. 3b, arrow a), vacuolization (Fig. 3b, arrow b), nuclear and cytoplasmic condensation (Fig. 3b, arrow c) and partitioning into well-packaged apoptotic bodies (Fig. 3b, arrow d), took place (Fig. 3b, f). Most mitochondria are swollen and crista shrunken, their contents have flowed out (Fig. 3b, arrow e). A myriad of autophagosomes with mitochondria in the progress of being digested were observed, indicating that the signaling pathway of H2O2 elicited cell death was flexible and might have switched to other mechanisms. In comparison, doxorubicin treated cells displayed serious vacuolization, and the mitochondria were swollen but intact (Fig. 3c, g). The only difference caused by the treatment with 500 μM Vitamin C was that as opposed to the round shape of swollen mitochondria in doxorubicin only group, those in Vitamin C treated cells mostly displayed normal elliptical shapes (Fig. 3h), as a slight improvement of mitochondrial structure and morphology (Fig. 3d, h). Vitamin C in cells undergoing hypoxia-reperfusion are linked with a reduction of ROS level, prevention of cytochrome c release and a stabilized mitochondrial membrane potential and a decreased activation of caspase-3 and caspase-9 (Mandl et al. 2009). In FAS-mediated apoptosis administration of Vitamin C was associated with diminished levels of ROS, reduced activity of caspases, and partial preservation of mitochondrial membrane integrity (Perez-Cruz et al. 2003). While high intake of Vitamin C (2,000 mg/d) has not been consistently reported to cause any side effects, its benefits to normal people have never been established, and what have been discovered is only that Vitamin C exerts some inhibitory effects on gastric metaplasia, chronic gastritis and lung and colorectal cancer in vulnerable population (Valko et al. 2006). Furthermore, it can also suppress tumorigenesis by means of cell cycle arrest and apoptosis-related gene expression regulation (Zhai et al. 2010). In the present study, Vitamin C, as an antioxidant, may have some effects in scavenging ROS generated in apoptotic signaling, thus improving mitochondrial structure and function. But for an apoptotic cascade triggered by a stimulus not directly related to ROS level, doxorubicin administration in this research, it may be ineffective.
Fig. 3.
Subcellular observation using TEM at 24 h upon treatment. (a, e) controls; and cells treated with (b, f) H2O2 100 μM; (c, g) Vc 0 μM; and (d, h) Vc 500 μM. Cells for functional investigation of Vc are cultured in media supplemented with 2 μg/mL doxorubicin. Arrow a, membrane blebbing; arrow b, vacuolization; arrow c, nuclear and cytoplasmic condensation; arrow d, apoptotic bodies and arrow e, damaged mitochondria, Scales bars, 1 μm in (a–d), 1 μm in (e, g) and 500 nm in (f, h)
Apoptotic effects
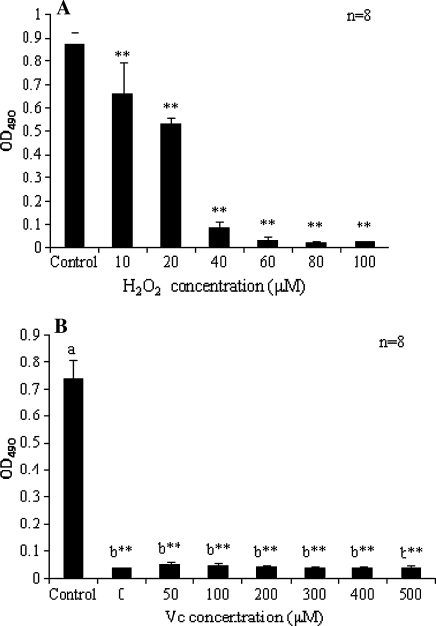
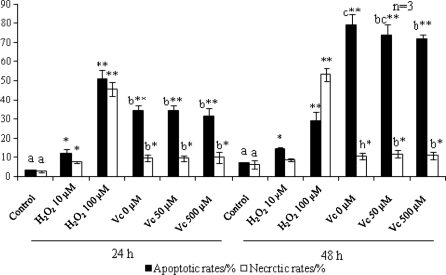
A plethora of antioxidants have been shown to have protective effects against apoptosis, however, there are a few exceptions, e.g. 2,6-di-tert-butyl-4-methylphenol (BHT) promotes a time- and concentration-dependent induction of apoptosis in human U937 cells (Hong and Liu 2004; Palomba et al. 1999). In the present study, MTT assay suggested that the cytotoxicity of H2O2 (Fig. 4a) was dose dependent. Incubation with doxorubicin lead to a significant decline (P < 0.0001) in viable cells numbers, whereas the administration of Vitamin C exerted no significant effects (P > 0.05) in the full concentration range (Fig. 4b). TUNEL assay was performed to detect DNA fragmentation. There were few positive cells in control (Fig. 5a), whereas exposure to H2O2 induced DNA fragmentation in a dose dependent manner. Doxorubicin elicited DNA fragmentation in some, but not all, of the treated cells, upon which Vitamin C exerted no observable effects (Fig. 5d–f). The results of Annexin V-FITC/PI assay (Fig. 6) suggested that both the apoptotic and necrotic rates increased in the H2O2 treated group in a dose dependent manner. Both the rates increased upon doxorubicin treatment, and intragroup comparison revealed no significant difference (P > 0.05) except that at 48 h the apoptotic rates were significantly reduced following treatment with 500 μM Vitamin C (P < 0.05), indicating that it may have, even though not considerable, some anti-apoptotic effects. The apoptotic rates of doxorubicin treated samples were time dependent.
Fig. 4.
Cytotoxicity analysis of cells treated with a H2O2 and b Vc coincubated with 2 μg/mL Doxorubicin. OD values are corresponding to viable cell population. Different letters signify statistically significance (P < 0.05). Statistical significance compared with corresponding controls is marked with **P < 0.0001)
Fig. 5.
In situ labeling of DNA fragmentation with TUNEL assay at 48 h following treatment. a control; and samples treated with b H2O2 10 μM; c H2O2 100 μM; d Vc 0 μM; e Vc 50 μM; and f Vc 500 μM. Cells for functional investigation of Vc are cultured in media supplemented with 2 μg/mL doxorubicin. Positive cells were green fluorescent under confocal microscope, indicating that DNA fragmentation had taken place. Scale bars = 50 μm
Fig. 6.
Apoptotic rates and necrotic rates upon exposure to H2O2 and Vc. Cells for functional investigation of Vc are cultured in media supplemented with 2 μg/mL doxorubicin. Apoptotic cells are Annexin V-FITC +/PI−; necrotic cells are Annexin V-FITC +/PI +. After flow cytometry the percentages are calculated to plot the bar chart. Statistical significance to corresponding controls is marked with *P < 0.05 and **P < 0.0001. Different letters signify statistical difference (P < 0.05)
Calcium homeostasis
Ample evidence suggested that the disruption of calcium homeostasis is sufficient to trigger apoptotic signaling (Jiang et al. 1994), and it was reported that H2O2-induced mitochondrial apoptosis is probably dependent on calcium signaling (Bejarano et al. 2008). In the present study, Ca2+ release in controls (Fig. 7a, d) was negligible, and upon the treatment of H2O2 and doxorubicin, many positive cells, the ones with disturbed calcium homeostasis as revealed by green fluorescence, occurred in the population. Positive cell numbers in H2O2 treated population were dose dependent, whereas no obvious differences were observed in the Vitamin C treated cells. The disruption of calcium homeostasis was in proportion to the number of morphologically irregular cells in treated samples, implying that calcium overload might be a constitutive event rather than a direct apoptotic trigger herein.
Fig. 7.
Intracellular calcium homeostasis. (a–c) (g–i) samples treated for 24 h; (d–f) (j–l) samples treated for 48 h; (a, d) controls; and cells treated with (b, e) H2O2 10 μM; (c, f) H2O2 100 μM; (g, j) Vc 0 μM; (h, k) Vc 50 μM; and (i, l) Vc 500 μM. Cells for functional investigation of Vc are cultured in media supplemented with 2 μg/mL doxorubicin. Perturbations of intracellular calcium homeostasis were characterized via the green fluorescence emitted by its specific binding with the molecular probe Fluo-3/AM. Scale bars, 50 μm in (a–f), 100 μm in (g–l). Arrows point to representative positive cells
Cell cycle analysis
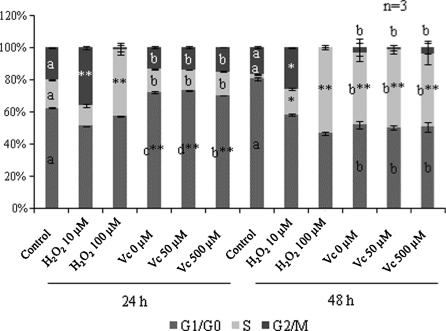
Accumulating evidence shows that upon the receipt of apoptotic-inducing stimuli, p53 can be activated to arrest cell cycle progression at G1/S or S/G2 checkpoint (Levine 1997). It was reported that in Fanconi’s anemia cell lines accumulated in G2 phase to a greater extent than normal lymphoblasts after H2O2 treatment (Zunino et al. 2001). In human leukemia HL-60 cells upon H2O2 treatment there was a block at G1 to S transition and apoptotic cells were mainly derived from S and G2 phases (Lee et al. 2000).
Significant increase in G2/M phase was detected upon exposure to 10 μM H2O2 at both 24 h (P < 0.0001) and 48 h (P < 0.05), whereas exposure to 100 μM H2O2 arrested cell cycle at S phase (Fig. 8). Doxorubicin arrested cell cycle at G1/G0 phase and S phase significantly (P < 0.0001) at 24 and 48 h respectively, and intragroup data only revealed that compared with non-Vitamin C-treated samples, proportion in G1/G0 phase increased (P < 0.05) in 50 μM Vitamin C treated cells and decreased (P < 0.05) in 500 μM Vitamin C treated ones at 24 h, implying that different mechanisms might be involved at low and high Vitamin C concentration respectively.
Fig. 8.
Cell cycle analysis of H2O2 and Vc treated CEFs. Statistically significant increase to corresponding controls is marked with *P < 0.05 and **P < 0.0001. Cells for functional investigation of Vc are cultured in media supplemented with 2 μg/mL doxorubicin. Different letters signify statistical difference (P < 0.05)
Previous research was focused on the effects of antioxidants on ROS induced apoptosis in mammalian cells rather than those caused by non-ROS stimuli in avian cells. The present study demonstrated that excessive accumulation of ROS is sufficient to induce programmed cell death in CEFs, whereas Vitamin C does not necessarily have considerable anti-apoptotic effects on the fibroblasts, especially when the apoptotic stimuli have no direct relationships with cellular redox state.
Acknowledgments
This work was supported by “863” National Major Research Program (2006AA10Z198, 2007AA10Z170), the Ministry of Agriculture of China for Transgenic Research Program (2008ZX08009-003) and National Key Technology R&D Program (2006BAD13B08, 2008BADB2B01).
Abbreviations
- AO
Acridine orange
- CEFs
Chicken embryonic fibroblasts
- EB
Ethidium bromide
- MTT
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrasolium bromide
- PI
Propidium iodide
- ROS
Reactive oxygen species
- TEM
Transmission electron microscopy
Footnotes
D.P. Jin and C.Y. Li equally contributed to this paper.
Contributor Information
W. J. Guan, Phone: +86-10-62815992, FAX: +86-10-62815992, Email: wjguan301@126.com
Y. H. Ma, Phone: +86-10-62813463, FAX: +86-10-62813463, Email: yhmaxumusuo@126.com
References
- Alexeyev MF. Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS J. 2009;276:5768–5787. doi: 10.1111/j.1742-4658.2009.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baregamian N, Song J, Papaconstantinou J, Hawkins HK, Evers BM, Chung DH (2011) Intestinal mitochondrial apoptotic signaling is activated during oxidative stress Pediatr Surg Int. doi:10.1007/s00383-011-2880-x [DOI] [PMC free article] [PubMed]
- Bejarano I, Lozano GM, Ortiz A, García JF, Paredes SD, Rodríguez AB, Pariente JA (2008) Caspase 3 activation in human spermatozoa in response to hydrogen peroxide and progesterone. Fertil Steril 90:1340–1347 [DOI] [PubMed]
- Ben-juan W, Zeng-tong Z. Hydrogen peroxide induced apoptosis in SV-40 transformed human salivary gland acinar cells. Oral Oncol. 2007;43:248–251. doi: 10.1016/j.oraloncology.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJA. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/S0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Chang JC, Kou SJ, Lin WT, Liu CS. Regulatory role of mitochondria in oxidative stress and atherosclerosis. World J Cardiol. 2010;2:150–159. doi: 10.4330/wjc.v2.i6.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Goto K, Takemura G, Maruyama R, Nakagawa M, Tsujimoto A, Kanamori H, Li L, Kawamura I, Kawaguchi T, Takeyama T, Fujiwara H, Minatoguchi S. Unique mode of cell death in freshly isolated adult rat ventricular cardiomyocytes exposed to hydrogen peroxide. Med Mol Morphol. 2009;42:92–101. doi: 10.1007/s00795-009-0439-x. [DOI] [PubMed] [Google Scholar]
- Gupta S, Malhotra N, Sharma D, Chandra A, Ashok A. Oxidative stress and its role in female infertility and assisted reproduction: clinical implications. Int J Fertil Steril. 2009;2:147–164. [Google Scholar]
- Hamada Y, Fujii H, Fukagawa M. Role of oxidative stress in diabetic bone disorder. Bone. 2009;45:S35–S38. doi: 10.1016/j.bone.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Hong H, Liu GQ. Protection against hydrogen peroxide-induced cytotoxicity in PC12 cells by scutellarin. Life Sci. 2004;74:2959–2973. doi: 10.1016/j.lfs.2003.09.074. [DOI] [PubMed] [Google Scholar]
- Jiang S, Chow SC, Nicotera P, Orrenius S (1994) Intracellular Ca2+ signals activate apoptosis in thymocytes: studies using the Ca2+-ATPase inhibitor thapsigargin. Exp Cell Res 212:84–92 [DOI] [PubMed]
- Juknat AA, Del Valle Armanino Méndez M, Quaglino A, Fameli CI, Mena M, Kotler ML. Melatonin prevents hydrogen peroxide-induced Bax expression in cultured rat astrocytes. J Pineal Res. 2005;38:84–92. doi: 10.1111/j.1600-079X.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- Lee JE, Sohn J, Lee JH, Lee KC, Son CS, Tockgo YC. Regulation of bcl-2 family in hydrogen peroxide-induced apoptosis in human leukemia HL-60 cells. Exp Mol Med. 2000;32:42–46. doi: 10.1038/emm.2000.8. [DOI] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/S0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Lim CS, Lee JC, Kim SD, Chang DJ, Kaang BK. Hydrogen peroxide-induced cell death in cultured Aplysia sensory neurons. Brain Res. 2002;941:137–145. doi: 10.1016/S0006-8993(02)02646-X. [DOI] [PubMed] [Google Scholar]
- Liu CY, Lee CF, Wei YH. Role of reactive oxygen species-elicited apoptosis in the pathophysiology of mitochondrial and neurodegenerative diseases associated with mitochondrial DNA mutations. J Formos Med Assoc. 2009;108:599–611. doi: 10.1016/S0929-6646(09)60380-6. [DOI] [PubMed] [Google Scholar]
- Makker K, Agarwal A, Sharma R. Oxidative stress and male infertility. Indian J Med Res. 2009;129:357–367. [PubMed] [Google Scholar]
- Mandl J, Szarka A, Bánhegyi G. Vitamin C: update on physiology and pharmacology. Br J Pharmacol. 2009;157:1097–1110. doi: 10.1111/j.1476-5381.2009.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Palomba L, Sestili P, Cantoni O. The antioxidant butylated hydroxytoluene induces apoptosis in human U937 cells: the role of hydrogen peroxide and altered redox state. Free Radic Res. 1999;31:93–101. doi: 10.1080/10715769900301601. [DOI] [PubMed] [Google Scholar]
- Perez-Cruz I, Carcamo JM, Golde DW. Vitamin C inhibits FAS-induced apoptosis in monocytes and U937 cells. Blood. 2003;102:336–343. doi: 10.1182/blood-2002-11-3559. [DOI] [PubMed] [Google Scholar]
- Pourova J, Kottova M, Voprsalova M, Pour M. Reactive oxygen and nitrogen species in normal physiological processes. Acta Physiol. 2010;198:15–35. doi: 10.1111/j.1748-1716.2009.02039.x. [DOI] [PubMed] [Google Scholar]
- Romano AD, Serviddio G, Matthaeis A, Bellanti F, Vendemiale G. Oxidative stress and aging. J Nephrol. 2010;23(Suppl 15):S29–S36. [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Wagener FADTG, Dekker D, Berden JH, Scharstuhl A, Vlag J. The role of reactive oxygen species in apoptosis of the diabetic kidney. Apoptosis. 2009;14:1451–1458. doi: 10.1007/s10495-009-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Guan W, Li H, Ma Y. Establishment and characteristics of white ear lobe chicken embryo fibroblast line and expression of six fluorescent proteins in the cells. Cell Biol Int. 2008;32:1478–1485. doi: 10.1016/j.cellbi.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Wu S, Zhou F, Zhang Z, Xing D. Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission-fusion proteins. FEBS J. 2011;278:941–954. doi: 10.1111/j.1742-4658.2011.08010.x. [DOI] [PubMed] [Google Scholar]
- Zhai P, Zeng J, Tan N, Wang J, Huang L, She W. Effects of Vitamin C on A549 cell proliferation, apoptosis and expressions of caspase, survivin. Chin J Lung Cancer. 2010;13:89–93. doi: 10.3779/j.issn.1009-3419.2010.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunino A, Degan P, Vigo T, Abbondandolo A. Hydrogen peroxide: effects on DNA, chromosomes, cell cycle and apoptosis induction in Fanconi’s anemia cell lines. Mutagenesis. 2001;16:283–288. doi: 10.1093/mutage/16.3.283. [DOI] [PubMed] [Google Scholar]