Abstract
4-Thujanol, a bicyclic monoterpene alcohol, is present in the essential oils of many medicinal and aromatic plants. It is commonly used as a fragrance and flavouring ingredient in a lot of different products. The potential genotoxic effects of 4-thujanol on human peripheral blood lymphocytes (PBLs) were investigated in vitro by the chromosome aberrations (CAs), sister chromatid exchanges (SCEs), and micronucleus (MN) tests. The cells were treated with 13, 26 and 52 μg/mL 4-thujanol in the presence and absence of a metabolic activator (S9 mix). 4-Thujanol induced CA (P < 0.001) and MN formation (P < 0.05) at all concentrations (13, 26 and 52 μg/mL) in the presence and absence of the S9 mix without a concentration-dependent manner. However, the treatment of peripheral lymphocytes with 4-thujanol did not produce a statistical difference in the frequency of SCEs when compared with control group. Furthermore, this monoterpene did not significantly decrease the mitotic index (MI), proliferation index (PI), and nuclear division index (NDI). In conclusion, 4-thujanol had a significant clastogenic effect at the tested concentrations (13, 26 and 52 μg/mL) for human PBLs. In addition, no cytotoxic and/or cytostatic effects were observed regardless of the concentrations used. This work presents the first report on genotoxic properties of 4-thujanol.
Keywords: 4-Thujanol, Human peripheral blood lymphocytes, Genotoxicity, Chromosome aberrations, Sister chromatid exchanges, Micronucleus
Introduction
4-Thujanol (sabinene hydrate), a natural bicyclic monoterpene alcohol, is present in the essential oils of many medicinal and aromatic plants such as Laurus nobilis L. (Lauraceae) (Sangun et al. 2007; Al-Kalaldeh et al. 2010); Sideritis erythrantha Boiss. and Heldr. (Lamiaceae) (Köse et al. 2010); Achillea millefolium L. (Asteraceace) (De Sant’anna et al. 2009). 4-Thujanol is a fragrance ingredient found in many different products including fine fragrances, shampoos, soaps, household cleaners and detergents (Bhatia et al. 2008). Due to its woody, minty and spicy odour (Mosciano 1997), 4-thujanol has also been commonly used as a flavouring agent in a variety of food products and beverages (VCF 2010). Therefore, human exposure to 4-thujanol through the diet or environment is widespread. Since the exposure to 4-thujanol is very common it is necessary to identify its possible effects on humans.
Essential oils extracted from plants, consisting of a variety of monoterpenes, are endowed with many biological activities, including antioxidant, antimicrobial, anti-inflammatory, and antitumor properties (Pattnaik et al. 1997; Moteki et al. 2002; Candan et al. 2003; Tepe et al. 2004; Kordalı et al. 2005). Although monoterpenes are commonly regarded as safe substances, some extracts from plants containing monoterpenoid compounds and also some isolated plant monoterpenes have been found genotoxic, mutagenic and cytotoxic in various test systems (Nishida et al. 2005; Azirak and Rencuzogullari 2008; Buyukleyla and Rencuzogullari 2009; De Sant’anna et al. 2009). Thus, an assessment of the genotoxic potential of 4-thujanol is necessary to ensure its safe use as a fragrance and flavouring ingredient.
However, there is no study available on the toxicologic effects of 4-thujanol (Bhatia et al. 2008). Furthermore, to the best of our knowledge, no studies have been carried out that focus on the genotoxic effects of this substance. Chromosome aberrations (CAs), sister chromatid exchanges (SCEs) and micronucleus (MN) formation in human peripheral blood lymphocytes (PBLs) are among the most widely used cytogenetic markers for the detection of early biological effects induced by DNA damaging agents (Carrano and Natarajan 1988). CAs are the result of DNA-level damage (Bonassi et al. 2007). SCEs involve the exchange of DNA segments between two sister chromatids in a single chromosome during cell proliferation and are regarded as a manifestation of damage to the genome (Tucker et al. 1993; Helleday 2003).
MN are formed by the effect of compounds that induce chromosomal breaks or agents that damage the spindle apparatus. MN can be formed from acentric chromosomal fragments or whole chromosomes fail to be segregated to the daughter nuclei during mitotic cellular division (Fenech and Bonassi 2011). Thus, these genotoxicity tests are well-established markers for the determination of the genotoxic effects of compounds.
Genotoxic potential of crude plant extracts and of isolated compounds can be assessed with the CA, SCE and MN tests that are highly sensitive (Rencuzogullari et al. 2006; Ananthi et al. 2010; Di Sotto et al. 2010; Kayraldiz et al. 2010).
For this reason, the purpose of this study was to determine the genotoxic effect of 4-thujanol using CA, SCE and MN tests in cultured human PBLs in the presence and absence of an exogenous metabolic activation systems (S9 mix).
Materials and methods
Test samples and chemicals
The study was carried out using human peripheral blood samples from two healty, non-smoking volunteer donors aged 21 years old. Both donors had no exposure to known genotoxicants.
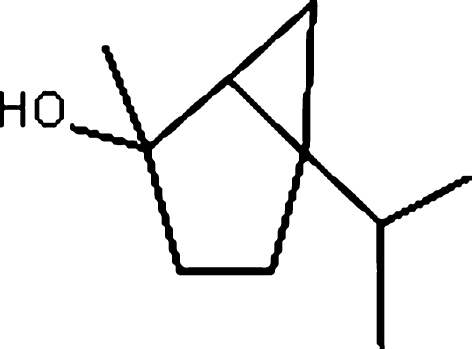
4-Thujanol (Fluka) was used as the test substance for the in vitro tests. Test substance was purchased from Sigma–Aldrich. The chemical structure of 4-thujanol is shown in Fig. 1. The test substance was dissolved in 50% ethanol supplied by Merck (Darmstadt, Germany), which was also used as solvent control. 5-bromodeoxyuridine (B-5002), colchicine (C-9754) and cytochalasin B (C-6762) were purchased from Sigma (St. Louis, MO). In the cultures without metabolic activation, the positive control was mitomycin-C (MMC, Sigma M-05030) at 0.25 μg/mL for treatments. For cultures with metabolic activation, cyclophosphamide monohydrate (CP, 28 μg/mL, Sigma C-0768) was used as a positive control at 28 μg/mL for in vitro tests. Giemsa and all other chemicals were purchased from Merck (Darmstadt, Germany). All test solutions were freshly prepared prior to each experiment.
Fig. 1.
The chemical structure of 4-thujanol
In vitro assay (without S9 mix)
The CA and SCE tests were performed using the methods developed by Evans (1984) and Perry and Thompson (1984), with minor modification. This study was conducted according to IPCS guidelines (Albertini et al. 2000). Lymphocyte cultures were set up by adding 0.2 mL of whole blood from two healty donors to 2.5 mL of chromosome medium PB Max (Gibco, cat. no. 12552-013) supplemented with 10 μg/mL of bromodeoxyuridine. Cultures were incubated at 37 °C for 72 h. The test concentrations were chosen as 13, 26 and 52 μg/mL based on the top concentration that resulted in approximately 50% (LD50) reduction in mitotic index (MI) (52 μg/mL). The cells were treated with 13, 26 and 52 μg/mL concentrations of 4-thujanol that dissolved in 50% ethanol, for 24 h (4-thujanol was added 48 h after initiating the culture) and 48 h (4-thujanol was added 24 h after initiating the culture). A control (i.e., untreated control), a solvent control (50% ethanol, 4 μL/mL) and a positive control (MMC, 0.25 μg/mL) were also used. To arrest the cells in metaphase, the cells were exposed to 0.06 μl/mL colchicine 2 h before harvesting. The cells were treated with a hypotonic solution (0.4% KCl) for 15 min at 37 °C and then fixed three times in a cold solution consisting of methanol:glacial acetic acid (3:1 v/v) at room temperature (22 °C ± 1 °C). Finally, the centrifuged cells were dropped onto clean slides. The staining of the air-dried slides was performed following the standard methods using 5% Giemsa stain for CA analysis (i.e., with 5% Giemsa in Sorensen Buffer, pH 6.8, 15 min), and the modified Fluorescence Plus Giemsa method (FPG) for SCE analysis (Speit and Haupter 1985). For FPG staining, 1-day-old slides were covered with Sorensen Buffer (pH 6.8). Then, slides were irradiated with 30 W, 254 nm UV lamp at 15 cm distance for 30 min. After irradiation, the slides were incubated in 1 X SSC (standard saline citrate) at 58–60 °C for 60 min and then stained with 5% Giemsa prepared with Sorensen buffer for 20 min.
For the MN test, 0.2 mL of fresh blood was used to establish the cultures and the cultures were incubated for 68 h. The cells were treated with 13, 26 and 52 μg/mL concentrations of 4-thujanol for 24 and 48 h (4-thujanol was added 44 and 20 h after initiating the culture, respectively) treatment periods. Cytochalasin B (final concentration of 6 μg/mL) was added after 44 h of incubation in order to block cytokinesis and obtain binucleated cells. After an additional 24 h incubation at 37 °C, cells were harvested by centrifugation and slides were prepared for MN test (Fenech 2000; Kirsch-Volders et al. 2003).
In vitro assay (with S9 mix)
In general, the same procedure for the CA, SCE and MN assays described earlier were used to assess the indirect genotoxic effect of 4-thujanol with minor modifications depending on the methodology of the metabolic activation. The lymphocytes were cotreated with 13, 26 and 52 μg/mL concentrations of 4-thujanol and 0.5 mL S9 mix for 3 h. 4-Thujanol and S9 mix were added 48 h after initiating the culture. For every culture of lymphocytes with the tested compound, a control, a solvent control (50% ethanol, 4 μL/mL) and a positive control (cyclophosphamide monohydrate, 28 μg/mL) were also performed. The test chemical and S9 mix were removed from the culture by centrifugation 4 min at 2,500 rpm. The pellet of lymphocytes was washed twice with 2.5 mL RPMI 1,640 medium (Biochrom AG, F 1215) and resuspended in fresh complete medium (chromosome medium PB Max). The cultures were incubated for a total of 72 h at 37 °C for the CA and SCE assays and 68 h at 37 °C for the MN assay.
Preparation of S9
The albino male rats (Rattus norvegicus var. albinos) weighing 200 g were pretreated with 80 mg/kg concentration of 3-methylcholanthrene (dissolved in sunflower oil) for 5 days. For the preparation of S9 fraction and the S9 mix, the method described by Maron and Ames (1983) was employed.
Microscopic evaluation
For each donor, a total of 100 well-spread metaphases were investigated (a total of 200 metaphase spreads for two donors) in order to score the CAs at each concentration and treatment period that showed structural and/or numerical chromosome aberrations. However, only the structural CAs were taken into consideration to determine genotoxicity. The CA was classified according to ISCN (International System for Human Cytogenetic Nomenclature) (Paz-y-Miño et al. 2002) and evaluated as chromatid-type (breaks, sister unions, and exchanges) and chromosome-type (breaks, dicentrics, rings, and fragments) aberrations. Gaps were not considered as CA according to Mace et al. (1978). Percentage of cells with structural chromosome aberrations (SCAs) have been calculated following the scoring of CAs.
The scoring of SCE was performed according to the IPCS guidelines (Albertini et al. 2000). To score SCE, 25-well-differentiated second-division metaphase cells were analyzed per donor (a total of 50 s-division metaphase for each concentration) and the frequency of SCE per cell was recorded.
The scoring criteria used for binuclear cell and MN evaluation were adopted from the Human MicroNucleus Project (Bonassi et al. 2001). To determine MN formation, 2,000 binucleated cells were analyzed for each donor (4,000 binucleated cells were scored per concentration). We evaluated the micronucleated binuclear lymphocytes containing one or more MN per 2,000 binucleated cells.
Mitotic index (MI), proliferation index (PI) and nuclear division index (NDI)
The mitotic index was calculated from the number of metaphases in 3,000 cells analyzed per culture for each donor (6,000 cells per concentration) in the CA assay.
In the SCE assay, a total of 200 cells (100 cells from each donor) were scored for the proliferation index. PI was calculated according to formula as follows: PI = [(1 × M1) + (2 × M2) + (3 × M3)]/N, where M1, M2 and M3 represent those metaphases corresponding to first, second and third divisions, and N is the total number of metaphases scored (Lamberti et al. 1983).
Moreover, in the micronucleus assay, cytostaticity was calculated by using the nuclear division index (NDI). To this aim, 1,000 lymphocytes per donor were scored. The number of viable cells with one to four nuclei was determined in 1,000 cells. NDI was calculated using the following formula: NDI = [(1 × M1) + (2 × M2) + (3 × M3) + (4 × M4)]/N; where M1 through M4 represent the number of cells with one to four nuclei and N is the total number of viable cells scored (Eastmond and Tucker 1989; Fenech 2000).
Statistical analysis
One-Way ANOVA was used for the statistical significance of all parameters. The comparisons between groups were made using a post-hoc analysis, LSD test. Concentration–response relationships were determined from the correlation and regression coefficients for the percentage of cells with CA, the mean SCE, the percentage of MN and BNMN, as well as for the MI, PI and NDI.
Results
In these experiments, three tests for evaluation of genotoxic activity of 4-thujanol were used in the presence and absence of metabolic activation.
The effects of 4-thujanol on CAs, in the presence and absence of S9 mix are summarized in Table 1. 4-Thujanol statistically significantly increased (P < 0.001) the formation of structural CA when compared with the control and the solvent control at all concentrations (13, 26 and 52 μg/mL) in 24 and 48 h treatment periods in the absence of S9 mix; however, the aberrations were significantly lower in comparison with the respective positive control. In parallel, human lymphocyte cultures treated with all concentrations of 4-thujanol showed a statistically significant increase (P < 0.001) in the percentage of cells with structural CA when compared with both the control and solvent controls in the presence of S9 mix. As shown in Table 1, the chromatid-type aberrations were more common than the chromosome-type aberrations.
Table 1.
The structural chromosome aberrations (SCAs) in cultured human lymphocytes treated with 4-thujanol in the presence and absence of S9 mix
| Test substance | Treatment | SCAs | Percentage of cells with SCAs ± SE | |||
|---|---|---|---|---|---|---|
| Time (h) | S9 mix | Conc. (μg/mL) | Chromatid type | Chromosome type | ||
| Control | − | − | − | 9 | − | 4.50 ± 0.50 |
| Ethanol (50%) | 24 | − | 4 μL | 9 | 2 | 5.50 ± 0.50 |
| PC (MMC) | 24 | − | 0.25 | 46 | 33 | 32.50 ± 1.50 a3b3 |
| 4-thujanol | 24 | − | 13 | 28 | 3 | 13.00 ± 1.00 a3b2c3 |
| 26 | 28 | 2 | 13.50 ± 1.50 a3b3c3 | |||
| 52 | 32 | 1 | 14.50 ± 0.50* a3b3c3 | |||
| Ethanol (50%) | 48 | − | 4 μL | 8 | 2 | 5.00 ± 1.00 |
| PC (MMC) | 48 | − | 0.25 | 140 | 54 | 58.50 ± 1.50* a3b3 |
| 4-thujanol | 48 | − | 13 | 27 | 4 | 14.00 ± 1.00 a3b3c3 |
| 26 | 35 | 5 | 16.50 ± 0.50 a3b3c3 | |||
| 52 | 35 | 4 | 17.00 ± 1.00 a3b3c3 | |||
| Control | − | + | − | 7 | 2 | 4.50 ± 0.50* |
| Ethanol (50%) | 3 | + | 4 μL | 9 | 1 | 5.00 ± 0.00 |
| PC (CP) | 3 | + | 28 | 24 | 8 | 14.50 ± 0.50* a3b3 |
| 4-thujanol | 3 | + | 13 | 17 | 4 | 10.50 ± 0.50 a3b2c2 |
| 26 | 21 | 5 | 11.50 ± 1.50 a3b3c1 | |||
| 52 | 23 | 4 | 11.50 ± 0.50 a3b3c1 | |||
PC positive control, MMC mitomycin C, CP cyclophosphamide
*A polyploid cell was scored but its number not included in the ratio of structural CAs
a, significant from control; b, significant from solvent control; c, significant from positive control
a1b1c1: P < 0.05; a2b2c2: P < 0.01, a3b3c3: P < 0.001
The observed frequencies of SCE in the human PBLs are given in Table 2. As can be seen in Table 2, despite the slight increase in the mean frequency of SCE at the highest concentration of 4-thujanol when compared with the solvent control, the difference was not found statistically significant in the absence and presence of the S9 mix (P > 0.05). Furthermore, there was no concentration–response relationship in the frequency of SCE under conditions with and without S9 mix.
Table 2.
The mean sister chromatid exchange (SCE) values in cultured human lymphocytes treated with 4-thujanol in the presence and absence of S9 mix
| Test substance | Treatment | Min-Max SCE | SCE/Cell ± SE | ||
|---|---|---|---|---|---|
| Time (h) | S9 mix | Conc. (μg/mL) | |||
| Control | − | − | − | 1–14 | 6.30 ± 0.42 |
| Ethanol (50%) | 24 | − | 4 μL | 1–14 | 6.36 ± 0.36 |
| PC (MMC) | 24 | − | 0.25 | 10–33 | 10.20 ± 0.16 a3b3 |
| 4-thujanol | 24 | − | 13 | 2–14 | 6.84 ± 0.44 c3 |
| 26 | 2–14 | 6.28 ± 0.28c3 | |||
| 52 | 3–15 | 6.66 ± 0.34 c3 | |||
| Ethanol (50%) | 48 | − | 4 μL | 2–12 | 6.44 ± 0.08 |
| PC (MMC) | 48 | − | 0.25 | 28–68 | 46.16 ± 1.96 a3b3 |
| 4-thujanol | 48 | − | 13 | 1–11 | 6.02 ± 0.66 c3 |
| 26 | 3–12 | 7.32 ± 0.14 c3 | |||
| 52 | 2–15 | 6.30 ± 0.58 c3 | |||
| Control | − | + | − | 0–13 | 7.02 ± 0.34 |
| Ethanol (50%) | 3 | + | 4 μL | 2–16 | 7.16 ± 0.36 |
| PC (CP) | 3 | + | 28 | 7–34 | 18.26 ± 0.94 a3b3 |
| 4-thujanol | 3 | + | 13 | 2–15 | 7.00 ± 0.12 c3 |
| 26 | 3–12 | 6.86 ± 0.22 c3 | |||
| 52 | 3–14 | 7.74 ± 0.10 c3 | |||
PC Positive control, MMC Mitomycin C, CP Cyclophosphamide
a, significant from control; b, significant from solvent control; c, significant from positive control
a1b1c1: P < 0.05; a2b2c2: P < 0.01, a3b3c3: P < 0.001
For the evaluation of clastogenic and/or aneugenic effects of 4-thujanol, we used the cytokinesis-block micronucleus assay on human PBLs (Table 3). In the absence of the S9 mix, 4-thujanol increased the percentage of the micronucleated binuclear cell (MNBN %) at two higher concentrations (26 and 52 μg/mL) for 24 h and at all concentrations (13, 26 and 52 μg/mL) for 48 h treatment periods when compared with the control (P < 0.05) but not with the solvent control. Increasing 4-thujanol concentrations caused a statistically significant increase in the percentage of MN (MN %) only for 48 h treatment period in the absence of S9 mix (P < 0.01). Additionally, 4-thujanol induced a statistically significant increase in MNBN and MN % when compared with the control at the 13 and 26 μg/mL concentrations (P < 0.05) and also when compared with both the control and solvent control at the 52 μg/mL concentration (P < 0.001) in the presence of the S9 mix (Table 3).
Table 3.
The percentage of micronucleus (MN) and the percentage of micronucleated binuclear cells (MNBN) in cultured human lymphocytes treated with 4-thujanol in the presence and absence of S9 mix
| Test substance | Treatment | The percentage of micronucleated binuclear cells ± SE (%) | The percentage of micronucleus ± SE (%) | ||
|---|---|---|---|---|---|
| Time (h) | S9 mix | Conc. (μg/mL) |
|||
| Control | − | − | − | 0.20 ± 0.05 | 0.25 ± 0.00 |
| Ethanol (50%) | 24 | − | 4 μL | 0.37 ± 0.02 | 0.47 ± 0.07 |
| PC (MMC) | 24 | − | 0.25 | 0.57 ± 0.02 a3b1 | 0.95 ± 0.05 a3b3 |
| 4-thujanol | 24 | − | 13 | 0.15 ± 0.05 c3 | 0.19 ± 0.09 c3 |
| 26 | 0.35 ± 0.05 a1c2 | 0.35 ± 0.05 c3 | |||
| 52 | 0.37 ± 0.02 a1c1 | 0.37 ± 0.02 c3 | |||
| Ethanol (50%) | 48 | − | 4 μL | 0.47 ± 0.02 | 0.53 ± 0.02 |
| PC (MMC) | 48 | − | 0.25 | 1.60 ± 0.10 a3b3 | 2.34 ± 0.04 a3b3 |
| 4-thujanol | 48 | − | 13 | 0.62 ± 0.07 a2c3 | 0.65 ± 0.05 a2c3 |
| 26 | 0.52 ± 0.07 a1c3 | 0.60 ± 0.05 a2c3 | |||
| 52 | 0.52 ± 0.02 a1c3 | 0.60 ± 0.10 a2c3 | |||
| Control | − | + | − | 0.12 ± 0.07 | 0.12 ± 0.07 |
| Ethanol (50%) | 3 | + | 4 μL | 0.28 ± 0.01 | 0.28 ± 0.01 |
| PC (CP) | 3 | + | 28 | 0.46 ± 0.01 a2b1 | 0.51 ± 0.01 a2b1 |
| 4-thujanol | 3 | + | 13 | 0.31 ± 0.08 a1 | 0.32 ± 0.09 a1c1 |
| 26 | 0.40 ± 0.02 a1 | 0.40 ± 0.02 a2 | |||
| 52 | 0.67 ± 0.02 a3b3c1 | 0.68 ± 0.02 a3b3 | |||
PC Positive control, MMC Mitomycin C, CP Cyclophosphamide
a, significant from control; b, significant from solvent control; c, significant from positive control
a1b1c1: P < 0.05; a2b2c2: P < 0.01, a3b3c3: P < 0.001
With respect to the cytotoxic and cytostatic effects of the test compound, the values of the MI, PI and NDI parameters for all treatments of 4-thujanol in the presence and absence of S9 mix are presented in Table 4. The MI (percentage of cells in mitosis) is a parameter used to determine possible cytotoxic or mitogenic effects. In the lymphocyte cultures, no decrease in the percentage of mitosis (MI) was detected for all treatments with 4-thujanol under experimental conditions (P > 0.05). Antiproliferative and cytostatic effects of 4-thujanol were measured by PI and NDI, respectively. In the presence and absence of the S9 mix, the PI did not significantly decrease in comparison with the control and solvent control. Similarly, except for the highest concentration (52 μg/mL) for 48 h treatment, 4-thujanol did not cause a significant reduction in the NDI when compared with the control groups (Table 4).
Table 4.
Mitotic index (MI), proliferation index (PI) and nuclear division index (NDI) values in cultured human lymphocytes treated with 4-thujanol in the presence and absence of S9 mix
| Test substance | Treatment | MI ± SE | PI ± SE | NDI ± SE | ||
|---|---|---|---|---|---|---|
| Time (h) | S9 mix | Conc. (μg/mL) | ||||
| Control | − | − | − | 3.63 ± 0.53 | 2.14 ± 0.01 | 1.63 ± 0.04 |
| Ethanol (50%) | 24 | − | 4 μL | 3.24 ± 0.21 | 1.87 ± 0.11 | 1.38 ± 0.05 |
| PC (MMC) | 24 | − | 0.25 | 2.95 ± 0.42 | 1.59 ± 0.02 a1 | 1.36 ± 0.01 a1 |
| 4-thujanol | 24 | − | 13 | 3.86 ± 0.06 | 2.09 ± 0.06 c1 | 1.44 ± 0.01 |
| 26 | 3.13 ± 0.56 | 1.80 ± 0.12 | 1.47 ± 0.06 | |||
| 52 | 2.89 ± 0.83 | 1.78 ± 0.21 | 1.44 ± 0.24 | |||
| Ethanol (50%) | 48 | − | 4 μL | 4.20 ± 0.40 | 2.24 ± 0.15 | 1.60 ± 0.01 |
| PC (MMC) | 48 | − | 0.25 | 3.15 ± 0.22 | 1.60 ± 0.01 a2b3 | 1.39 ± 0.01 a2b2 |
| 4-thujanol | 48 | − | 13 | 4.01 ± 0.18 | 2.34 ± 0.02 c3 | 1.54 ± 0.04 c1 |
| 26 | 3.71 ± 0.21 | 2.35 ± 0.09 c3 | 1.53 ± 0.03 c1 | |||
| 52 | 3.53 ± 0.83 | 2.30 ± 0.08 c3 | 1.45 ± 0.02 a2c1 | |||
| Control | − | + | − | 3.92 ± 0.45 | 2.19 ± 0.11 | 1.19 ± 0.02 |
| Ethanol (50%) | 3 | + | 4 μL | 3.80 ± 0.47 | 2.19 ± 0.00 | 1.18 ± 0.11 |
| PC (CP) | 3 | + | 28 | 3.45 ± 0.38 | 2.21 ± 0.02 | 1.17 ± 0.02 |
| 4-thujanol | 3 | + | 13 | 3.68 ± 0.78 | 2.20 ± 0.02 | 1.18 ± 0.05 |
| 26 | 3.63 ± 0.63 | 2.29 ± 0.03 | 1.23 ± 0.03 | |||
| 52 | 3.33 ± 0.17 | 2.09 ± 0.10 | 1.17 ± 0.03 | |||
PC Positive control, MMC Mitomycin C, CP Cyclophosphamide
a, significant from control; b, significant from solvent control; c, significant from positive control
a1b1c1: P < 0.05; a2b2c2: P < 0.01, a3b3c3: P < 0.001
Discussion
The results of the present study revealed that in general, 4-thujanol significantly increased the percentage of cells with structural CAs and MN formation at all concentrations (13, 26 and 52 μg/mL) when compared with the controls in the presence and absence of the S9 mix. However, the treatment of peripheral lymphocytes with 4-thujanol in the presence and absence of the S9 mix did not produce a statistical difference in the frequency of SCEs when compared with control group (P > 0.05). In addition, with regard to CA, SCE and MN data, there were no concentration-dependent effects of 4-thujanol on any of these parameters.
The induction of structural CAs, and micronucleated lymphocytes by all concentrations of 4-thujanol (13, 26 and 52 μg/mL) suggests the clastogenic potential of the test compound at these concentrations. It is generally acknowledged that the CA and MN assays are the mutagenicity tests for the detection of chromosome mutations, whereas the SCE assay is an indicator test for genotoxic exposure. Hence, the results of the CA and MN assays should be considered of higher significance than the results of an SCE test (Eke and Çelik 2008).
Micronuclei are formed from acentric chromosomal fragments which arise as a result of chromosome breaks after clastogenic effect or whole chromosomes that do not migrate during anaphase as a result of aneugenic affects (Heddle et al. 1991). The results of the CA test showed that 4-thujanol induced more chromatid breaks than other structural or numerical CAs. Therefore, it can be said that MN can be formed from acentric chromosomal fragments in this study.
There are no studies in relation to 4-thujanol genotoxicity or mutagenicity and toxicity. This study is the first report on detecting the genotoxic and/or cytotoxic effects of 4-thujanol using CA, SCE and MN assay in PBLs in vitro. However, few studies have been carried out on the potential genotoxic effects of some monoterpenoid compounds.
Menthol (a monocyclic monoterpene alcohol) and linalool (an acyclic monoterpene alcohol) did not increase chromosomal aberrations at concentrations of up to 200 μg/mL and 250 μg/mL, respectively, when incubated with Chinese hamster fibroblast cells (Ishidate et al. 1984). However, in our study, the lower concentrations of 4-thujanol induced chromosomal abnormalities. The lack of agreement of our results could be due to the differences in chemical structure of the tested monoterpenes and of different cell lines used.
Hilliard et al. (1998) found that menthol racemic induced a weak but statistically significant increase in chromosome aberrations at concentrations of 1.6–1.9 mM in Chinese hamster ovary cells without metabolic activation. Additionally, it induced a significant increase in chromosome aberrations at 1.2 mM in human TK6 cells. Aydın et al. (2005) reported that concentrations above 0.1 mM thymol (approximately 15 μg/mL) (a phenolic monoterpene) and gamma-terpinene (approximately 14 μg/mL) (a monocyclic monoterpene hydrocarbon) and 0.05 mM carvacrol (approximately 7.5 μg/mL) (a phenolic monoterpene) significantly induced DNA damage in human lymphocytes in the comet assay. Azirak and Rencuzogullari (2008) found that both carvacrol (10, 30, 50 and 70 mg/kg b.w., intraperitoneally) and thymol (40, 60, 80 and 100 mg/kg b.w., intraperitoneally) significantly induced structural CA in rat bone marrow cells for all treatment periods (6, 12 and 24 h). These results are consistent with the results of our study, although different cell types and/or test systems were used.
Finally, similar to the results of our study, Buyukleyla and Rencuzogullari (2009) reported that thymol significantly increased the structural CA and MN formation at 25, 50, 75 and 100 μg/mL concentrations for 24 and 48 h treatment period in human PBLs without S9 mix.
Results show that 4-thujanol most probably, has a genotoxic risk at the tested concentrations (13, 26 and 52 μg/mL) in human PBLs in the presence and absence of the S9 mix. In addition, 4-thujanol caused particularly structural CAs instead of numerical CA, meaning that 4-thujanol as a clastogen can lead to formation of CA by breaking the phosphodiester back bone of DNA. 4-Thujanol also may act on proteins and DNA with production of reactive oxygen species (ROS) that may cause DNA strand breaks.
4-Thujanol did not significantly decrease the MI, PI and NDI in the presence and absence of the S9 mix (P > 0.05). Furthermore, there was no concentration–response relationship for MI, PI and NDI. Therefore, it could be indicated that 4-thujanol is neither a cytotoxic nor cytostatic agent in human peripheral blood lymphocytes. These data also suggest that the metabolites of 4-thujanol did not have significant cytotoxic and cytostatic effects on cell proliferation in vitro. Similar to our results, Horváthová et al. (2007) found that 1,8-cineole, the monoterpene cyclic ether known as eucalyptol, did not show cytotoxic effects on cultured human leukemic K562 cells. Buyukleyla and Rencuzogullari (2009) reported that thymol had a cytotoxic effect via decreasing the MI, RI (replication index) and NDI at the two highest concentrations (75 and 100 μg/mL) in human lymphocytes. In our study, 4-thujanol did not show cytotoxic/cytostatic effects in the same cell culture system. But, the highest concentration used in the present study was 52 μg/mL which is lower than 75 and 100 μg/mL. Based on this aspect, our results are not contradictory to the results obtained from this study.
Horváthová et al. (2009) reported that high concentrations of borneol (34.28 mg/kg/day) showed a cytotoxic effect in primary rat hepatocytes due to the increased levels of DNA damage and that this effect resulted probably from the induction of apoptotic and necrotic DNA fragmentation. Furthermore, Bakkali et al. (2008) reported that the main mechanisms of cytotoxic effects of essential oils in mammalian cells were apoptosis and necrosis. Whereas in our study, all concentrations of 4-thujanol (13, 26 and 52 μg/mL) caused an increase of chromatid breaks, while at the same concentrations, we did not observe a significant increase of cytotoxicity in human lymphocytes. Therefore, it can be thought that the chromosomal aberrations caused by 4-thujanol were not potent enough to induce apoptosis.
Taken as a whole, these findings suggest that 4-thujanol had a significantly clastogenic effect at the tested concentrations (13, 26 and 52 μg/mL) for human lymphocytes. And, no cytotoxic and/or cytostatic effects were observed with or without metabolic activation regardless of the concentrations used. Therefore, we propose that it is necessary to be careful when using 4-thujanol as a fragrance and flavouring ingredient. However, the lack of other data in relation to the genotoxic effect of 4-thujanol, it should be further examined in different test systems to better understand its potential genotoxicity.
Acknowledgements
This investigation was supported by a grant from The Scientific and Technical Research Council of Turkey, Ankara (TUBITAK, Project No. 109T546).
Abbreviations
- CA
Chromosome aberration
- SCE
Sister chromatid exchange
- MN
Micronucleus
- PBLs
Peripheral blood lymphocytes
- MNBN
Micronucleated binuclear cell
- MI
Mitotic index
- PI
Proliferation index
- NDI
Nuclear division index
- MMC
Mitomycin-C
- CP
Cyclophosphamide
- Cyt-B
Cytochalasin B
References
- Albertini RJ, Anderson D, Douglas GR, Hagmar L, Hemminki K, Merlo F, Natarajan AT, Norppa H, Shuker DEG, Tice R, Waters MD, Aitio A. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat Res. 2000;463:111–172. doi: 10.1016/S1383-5742(00)00049-1. [DOI] [PubMed] [Google Scholar]
- Al-Kalaldeh JZ, Abu-Dahab R, Afifi FU. Volatile oil composition and antiproliferative activity of Laurus nobilis, Origanum syriacum, Origanum vulgare, and Salvia triloba against human breast adenocarcinoma cells. Nutr Res. 2010;30:271–278. doi: 10.1016/j.nutres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Ananthi R, Chandra N, Santhiya ST, Ramesh A. Genotoxic and antigenotoxic effects of Hemidesmus indicus R. Br. root extract in cultured lymphocytes. J Ethnopharmacol. 2010;127:558–560. doi: 10.1016/j.jep.2009.10.034. [DOI] [PubMed] [Google Scholar]
- Aydın S, Başaran AA, Başaran N. Modulating effects of thyme and its major ingredients on oxidative DNA damage in human lymphocytes. J Agric Food Chem. 2005;53:1299–1305. doi: 10.1021/jf0402375. [DOI] [PubMed] [Google Scholar]
- Azirak S, Rencuzogullari E. The in vivo genotoxic effects of carvacrol and thymol in rat bone marrow cells. Environ Toxicol. 2008;23:728–735. doi: 10.1002/tox.20380. [DOI] [PubMed] [Google Scholar]
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils-a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Bhatia SP, Letizia CS, Api AM. Fragrance material review on 4-thujanol. Food Chem Toxicol. 2008;46:295–296. doi: 10.1016/j.fct.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Bonassi S, Fenech M, Lando C, et al. Human micronucleus project: international database comparison for results with the cytokinesis block micronucleus assay in human lymphocytes: effect of laboratory protocol, scoring criteria, and host factors on the frequency of micronuclei. Environ Mol Mutagen. 2001;37:31–45. doi: 10.1002/1098-2280(2001)37:1<31::AID-EM1004>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Bonassi S, Znaor A, Ceppi M, Lando C, Chang WP, Holland N, Kirsch-Volders M, Zeiger E, Ban S, Barale R, Bigatti MP, Bolognesi C, Cebulska-Wasilewska A, Fabianova E, Fucic A, Hagmar L, Joksic G, Martelli A, Migliore L, Mirkova E, Scarfi MR, Zijno A, Norppa H, Fenech M. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis. 2007;28:625–631. doi: 10.1093/carcin/bgl177. [DOI] [PubMed] [Google Scholar]
- Buyukleyla M, Rencuzogullari E. The effects of thymol on sister chromatid exchange, chromosome aberration and micronucleus in human lymphocytes. Ecotoxicol Environ Saf. 2009;72:943–947. doi: 10.1016/j.ecoenv.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Candan F, Unlü M, Tepe B, Daferera AD, Polissiou M, Sökmen A, Akpulat HA. Antioxidant and antimicrobial activity of essential oil and methanol extracts of Achillea millefolium subsp. Millefolium Afan. (Asteraceae) J Ethnopharmacol. 2003;87:215–220. doi: 10.1016/S0378-8741(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Carrano AV, Natarajan AT. Consideration for population monitoring using cytogenetic techniques. Mutat Res. 1988;204:379–406. doi: 10.1016/0165-1218(88)90036-5. [DOI] [PubMed] [Google Scholar]
- Sant’anna JR, Da Silva Franco CC, Miyamoto CT, Cunico MM, Miguel OG, Cocco LC, Yamamoto CI, Junior CC, Castro-Prado AA. Genotoxicity of Achillea millefolium essential oil in diploid cells of Aspergillus nidulans. Phytother Res. 2009;23:231–235. doi: 10.1002/ptr.2596. [DOI] [PubMed] [Google Scholar]
- Di Sotto A, Mazzanti G, Carbone F, Hrelia P, Maffei F. Inhibition by β-caryophyllene of ethyl methanesulfonate-induced clatogenicity in cultured human lymphocytes. Mutat Res. 2010;699:23–28. doi: 10.1016/j.mrgentox.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Eastmond DA, Tucker JD. Identification of aneuploidy-inducing agents using cytokinesis-blocked human lymphocytes and an anti-kinetochore antibody. Environ Mol Mutagen. 1989;13:34–43. doi: 10.1002/em.2850130104. [DOI] [PubMed] [Google Scholar]
- Eke D, Çelik A. Genotoxicity of thimerosal in cultured human lymphocytes with and without metabolic activation sister chromatid exchange analysis proliferation index and mitotic index. Toxicol In Vitro. 2008;22:927–934. doi: 10.1016/j.tiv.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Evans HJ. Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. In: Kilbey BJ, Legator M, Nichols W, Ramel C, editors. Handbook of mutagenicity test procedures. 2. Amsterdam: Elsevier Science Publishers BV; 1984. pp. 405–427. [Google Scholar]
- Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/S0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Fenech M, Bonassi S. The effect of age, gender, diet and lifestyle on DNA damage measured using micronucleus frequency in human peripheral blood lymphocytes. Mutagenesis. 2011;26:43–49. doi: 10.1093/mutage/geq050. [DOI] [PubMed] [Google Scholar]
- Heddle JA, Cimino MC, Hayashi M, Romagna F, Shelby MD, Tucker JD, Vanparys Ph, Mac Gregor JT. Micronuclei as an index of cytogenetic damage: past, present, and future. Environ Mol Mutagen. 1991;18:277–291. doi: 10.1002/em.2850180414. [DOI] [PubMed] [Google Scholar]
- Helleday T. Pathways for mitotic homologous recombination in mammalian cells. Mutat Res. 2003;532:103–115. doi: 10.1016/j.mrfmmm.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Hilliard CA, Armstrong MJ, Bradt CI, Hill RB, Greenwood SK, Galloway SM. Chromosome aberrations in vitro related to cytotoxicity of nonmutagenic chemicals and metabolic poisons. Environ Mol Mutagen. 1998;31:316–326. doi: 10.1002/(SICI)1098-2280(1998)31:4<316::AID-EM3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Horváthová E, Turcaňiová V, Slameňova D. Comparative study of DNA-damaging and DNA-protective effects of selected components of essential plant oils in human leukemic cells K562. Neoplasma. 2007;54:478–483. [PubMed] [Google Scholar]
- Horváthová E, Slameňova D, Maršálková L, Šramková M, Wsólová L. Effects of borneol on the level of DNA damage induced in primary rat hepatocytes and testicular cells by hydrogen peroxide. Food Chem Toxicol. 2009;47:1318–1323. doi: 10.1016/j.fct.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Ishidate Jr, Sofuni T, YoshikawaA K, Hayashi M, Nohmi T, Sawada M, Matsuoka A. Primary mutagenicity screening of food additives currently used in Japan. Food Chem Toxicol. 1984;22:623–636. doi: 10.1016/0278-6915(84)90271-0. [DOI] [PubMed] [Google Scholar]
- Kayraldiz A, Kocaman AY, Rencüzoğulları E, İstifli ES, İla HB, Topaktaş M, Dağlıoğlu YK. The genotoxic and antigenotoxic effects of Aloe vera leaf extract in vivo and in vitro . Turk J Biol. 2010;34:235–246. [Google Scholar]
- Kirsch-Volders M, Sofuni T, Aardema M, Albertini S, Eastmond D, Fenech M, Ishidate M, Jr, Kirchner S, Lorge E, Morita T, Norppa H, Surrallés J, Vanhauwaert A, Wakata A. Report from the in vitro micronucleus assay working group. Mutat Res. 2003;540:153–163. doi: 10.1016/j.mrgentox.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Kordalı S, Kotan R, Mavi A, Çakır A, Ala A, Yıldırım A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, Artemisia dracunculus, Artemisia santonicum and Artemisia spicigera essential oils. J Agric Food Chem. 2005;53:9452–9458. doi: 10.1021/jf0516538. [DOI] [PubMed] [Google Scholar]
- Köse EO, Deniz İG, Sarıkürkçü C, Aktaş Ö, Yavuz M. Chemical composition, antimicrobial and antioxidant activities of the essential oils of Sideritis erythrantha Boiss. and Heldr. (var. erythrantha and var. cedretorum P.H. Davis) endemic in Turkey. Food Chem Toxicol. 2010;48:2960–2965. doi: 10.1016/j.fct.2010.07.033. [DOI] [PubMed] [Google Scholar]
- Lamberti L, Ponzetto BP, Ardito G. Cell kinetics and sister chromatid exchange frequency in human lymphocytes. Mutat Res. 1983;319:193–199. doi: 10.1016/0165-7992(83)90163-X. [DOI] [PubMed] [Google Scholar]
- Mace MLJ, Daskal Y, Wray W. Scanning electron microscopy of chromosome aberrations. Mutat Res. 1978;52:199–206. doi: 10.1016/0027-5107(78)90141-0. [DOI] [PubMed] [Google Scholar]
- Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Mosciano G (1997) P&F 22 No. 3, 47
- Moteki H, Hibasami H, Yamada Y, Katsuzaki H, Imai K, Komiya T. Specific induction of apoptosis by 1, 8 cineole in two human leukemia cell lines, but not in human stomach cancer line. Oncol Rep. 2002;9:757–760. [PubMed] [Google Scholar]
- Nishida N, Tamotsu S, Nagata M, Saito C, Sakai A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J Chem Ecol. 2005;31:187–1203. doi: 10.1007/s10886-005-4256-y. [DOI] [PubMed] [Google Scholar]
- Pattnaik S, Subramanyam VR, Bapaji M, Köle CR. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios. 1997;89:39–46. [PubMed] [Google Scholar]
- Paz-y-Miño C, Bustamante G, Sánchez ME, Leone PE. Cytogenetic monitoring in a population occupationally exposed to pesticides in Ecuador. Environ Health Perspect. 2002;110:1077–1080. doi: 10.1289/ehp.021101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry PE, Thompson EJ. The methodology of sister chromatid exchanges. In: Kilbey BJ, Legator M, Nichols W, Ramel C, editors. Handbook of mutagenicity test procedures. 2. Amsterdam: Elsevier Science Publishers; 1984. pp. 495–529. [Google Scholar]
- Rencuzogullari E, Ila HB, Kayraldiz A, Diler SB, Yavuz A, Arslan M, Kaya FF, Topaktas M. The mutagenic and antimutagenic effects of Ecballium elaterium fruit juice in human peripheral lymphocytes. Russ J Genet. 2006;42:623–627. doi: 10.1134/S1022795406060068. [DOI] [PubMed] [Google Scholar]
- Sangun MK, Aydin E, Timur M, Karadeniz H, Caliskan M, Ozkan A. Comparison of chemical composition of the essential oil of Laurus nobilis L. leaves and fruits from different regions of Hatay, Turkey. J Environ Biol. 2007;28:731–733. [PubMed] [Google Scholar]
- Speit G, Haupter S. On the mechanism of differential giemsa staining of bromodeoxyuridine-substituted chromosomes. II. differences between the demonstration of sister chromatid differentiation and replication patterns. Hum Genet. 1985;70:126–129. doi: 10.1007/BF00273070. [DOI] [PubMed] [Google Scholar]
- Tepe B, Donmez E, Unlu M, Candan F, Daferera D, Vardar-Unlu G, Polissiou M, Sokmen A. Antimicrobial and antioxidative activities of the essential oils and methanol extracts of Salvia cryptantha (Montbret et Aucher ex Benth.) and Salvia multicaulis (Vahl) Food Chem. 2004;84:519–525. doi: 10.1016/S0308-8146(03)00267-X. [DOI] [Google Scholar]
- Tucker JD, Auletta A, Cimino MC, Dearfield KL, Jacobson-Kram D, Tice RR, Carrano AV. Sister-chromatid exchange: second report of the gene-tox program. Mutat Res. 1993;297:101–180. doi: 10.1016/0165-1110(93)90001-4. [DOI] [PubMed] [Google Scholar]
- VCF (2010) Volatile compounds in food:database/Nijssen, In: Ingen-Visscher LM, Van CA, Donders, JJH (eds)–Version 12.2—Zeist (The Netherlands):TNO quality of life, pp 1963–2010