Abstract
Purpose
Mammalian target of rapamycin (mTOR) inhibitors mediate AKT activation through a type 1 insulin-like growth factor receptor (IGF-1R)-dependent mechanism. Combining the mTOR inhibitor temsirolimus with cixutumumab, a fully human IgG1 monoclonal antibody directed against IGF-1R, was expected to enhance mTOR-targeted anticancer activity by modulating resistance to mTOR inhibition. Objectives of this Phase I study were to evaluate the tolerability and activity of temsirolimus and cixutumumab.
Experimental Design
Patients in sequential cohorts (“3+3” design) received escalating doses of temsirolimus with cixutumumab weekly for 28 days. At MTD, 21 patients were randomized into three separate drug sequence treatment groups for serial blood draws and FDG-PET/CT scans for pharmacodynamic analyses (PD).
Results
Forty-two patients with advanced cancer (19M/23F, median age = 53, median number of prior therapies = 4) were enrolled. MTD was reached at cixutumumab, 6 mg/kg IV and temsirolimus, 25 mg IV. DLTs included Grade 3 mucositis, febrile neutropenia, and Grade 4 thrombocytopenia. Most frequent toxicities were hypercholesterolemia, hypertriglyceridemia, hyperglycemia, thrombocytopenia, and mucositis. Tumor reduction was observed in 2 of 3 patients with Ewing's sarcoma and in 4 of 10 patients with adrenocortical carcinoma. PD data suggest that cixutumumab alone or combined with temsirolimus increased plasma IGF-1 and IGFBP3. FDG-PET/CT showed the odds of achieving stable disease decreased by 58% (P =0.1213) with a one-unit increase in absolute change of SUV from baseline to Day 3.
Conclusions
Temsirolimus combined with cixutumumab was well tolerated. We are currently enrolling expansion cohorts at the MTD for Ewing's sarcoma and adrenocortical carcinoma.
Keywords: Phase I Clinical Trials, IGF-1R pathway, mTOR pathway
INTRODUCTION
The PI3K/AKT/mTOR pathway is normally regulated by upstream receptor tyrosine kinases, particularly the insulin receptor and type 1 insulin-like growth factor receptor (IGF-1R)1. Recent studies in in vitro and in vivo models as well as using tumor biopsies from patients have demonstrated that treatment with mTOR inhibitors leads to upregulation of AKT phosphorylation in tumors, which may antagonize the antiproliferative effects of mTOR inhibition2,3. Several studies have shown that mTOR inhibitors mediate AKT activation through an IGF-1R-dependent mechanism and that IGF-1R inhibitors may abrogate or reduce AKT phosphorylation induced by mTOR inhibitors2–4. AKT activation is related to the escape/resistance mechanism of mTOR inhibitors, but combination studies with rapamycin and IGF-1R inhibitors suggest additive antitumor effects compared to treatment with single agents alone.5 Thus, combining an mTOR inhibitor and IGF-1R inhibitor may be an appropriate strategy to enhance mTOR-targeted anticancer therapy. Furthermore, as mTOR is involved in signal transduction downstream of IGF-1R, the combination may also potentially enhance the activity of IGF-1R inhibitors.
We report the results of the first Phase I study that combines an mTOR inhibitor (temsirolimus; CCI-779) and an IGF-1R monoclonal antibody (cixutumumab; IMC-A12) to assess safety and tolerability as primary objectives and to evaluate biologic effects and assess tumor metabolism and clinical tumor response as secondary objectives.
PATIENTS AND METHODS
Eligibility Criteria
Eligible patients had advanced or metastatic, histologically proven malignant tumors and patients enrolled in the maximum-tolerated dose (MTD) expansion cohort must have disease that is accessible to biopsy. Further requirements were age 16 years or older, ECOG performance status of 0 or 1 and life expectancy of more than 12 weeks. Patients must have absolute neutrophil count ≥ 1500/mL; platelets ≥ 100,000/mL; creatinine ≤ 2× ULN; bilirubin ≤ 1.5 × ULN; AST(SGOT) and/or ALT(SGPT) ≤ 5× ULN. There was no limit to prior numbers of treatment, including IGF-1R inhibitors or mTOR inhibitors. Treatment with radiotherapy (except palliative radiation to control symptoms), endocrine therapy, or chemotherapy must have ceased at least 4 weeks before starting treatment. Patients with well-controlled diabetes and hyperlipidemia were allowed in the dose expansion cohort, but were excluded in the dose escalation portion. Further patient exclusions were treatment with concurrent strong CYP3A modifiers, major surgery within 4 weeks; significant comorbidity, brain metastases and pregnant or nursing females.
Although biopsies were planned, many could not be completed due to patient refusal, absence of tumor in the sample, financial limitations, and other problems. Together, these precluded drawing a meaningful statistical result from the 10 paired biopsies that were done.
Study Design
This study used a standard 3+3 design and patients were enrolled across four dose cohorts as shown in Table 1. At the MTD (cixutumumab 6 mg/kg intravenously [IV] weekly and temsirolimus 25 mg IV weekly), 21 patients were randomized to three separate treatment arms (Table 2): 7 patients received cixutumumab before the combination of both agents (Arm A), 6 patients received temsirolimus before the combination of both agents (Arm B), and 8 patients received the combination of both agents at the onset of the study (Arm C). During Cycle 1 only, FDG-PET/CT scans and tumor biopsies with corresponding blood draws for peripheral blood mononuclear cells [PBMCs] were required for all 21 patients. The rationale for establishing 3 separate treatment arms was to evaluate the biological effect of each drug individually and in combination and to allow comparison of pharmacodynamic endpoints, including FDG-PET changes, after treatment with cixutumumab or temsirolimus alone and in combination. We did not perform pharmacokinetic (PK) evaluation since the published data on monoclonal antibodies such as bevazicumab with chemotherapy or small molecules suggested that antibody (which is cleared by the reticuloendothelial system) does not affect the PK of small molecules or cytotoxic agents.6
Table 1.
Dose Scheme (n = 42 patients)
| Dose level | Dose | |
|---|---|---|
| Cixutumumab | Temsirolimus | |
| 1 | 3 mg/kg | 25 mg |
| 2 | 5 mg/kg | 25 mg |
| 3 | 6 mg/kg | 25 mg |
| 4 | 6 mg/kg | 37.5 |
Table 2.
Dosing Schema for Patients in Expansion Cohort (1 Cycle consists of 28 Days)
| 1st Biopsy & baseline PET scan (Within 8 days before Cycle 1 Day 1) | Cycle 1, Day 1 | 1st post-treatment PET (Day 3) | Cycle 1, Day 8 | 2ndpost-treatment PET + Bx (Day 11) | Cycle 1, Day 15, 22, Cycle 2 and beyond (Days 1, 8, 15, 22) | Between days 23 and day 28 of every even cycle | |
|---|---|---|---|---|---|---|---|
| Arm A (7* patients) | Yes | Cixutumumab | Yes | Cixutumumab | Yes | Temsirolimus + Cixutumumab | Restaging as well as PET |
| Arm B (6 patients) | Yes | Temsirolimus | Yes | Temsirolimus | Yes | Temsirolimus + Cixutumumab | Restaging as well as PET |
| Arm C (8 patients) | Yes | Temsirolimus + Cixutumumab | Yes | Temsirolimus + Cixutumumab | Yes | Temsirolimus + Cixutumumab | Restaging as well as PET |
Randomization was blindly assigned by computer
This study was performed according to the principles embodied in the Declaration of Helsinki and after approval by the institutional review boards of both centers that enrolled patients. Informed consent was obtained from all patients enrolled on the study.
Dose-Limiting Toxicity and Maximum-Tolerated Dose
Dose-limiting toxicity (DLT) was defined as possible/probably/definitely drug-related Grade 3 to 4 nonhematologic toxicity (excluding Grade 3 nausea or Grade 3 to 4 vomiting or diarrhea in patients who had not received optimal antiemetic and antidiarrheal treatment), Grade 3 to 4 thrombocytopenia lasting 7 days or thrombocytopenia associated with active bleeding or requiring platelet transfusion, Grade 3 anemia, Grade 4 neutropenia, and drug-related death. MTD was defined as a dose level below the dose at which more than or equal to 33% (2 out of 6 patients) of the patient population experienced a DLT.
Evaluation of Safety
Adverse events were recorded for patients who received at least one dose of cixutumumab or temsirolimus. Severity was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 3.0. Temperature, blood pressure, and pulse were measured before each infusion. Regular monitoring of hematology, blood chemistry and urinalysis, and physical examinations was also performed.
Evaluation of Efficacy
Treatment efficacy was evaluated by computed tomography (CT) or magnetic resonance imaging (MRI) per Response Evaluation Criteria in Solid Tumors (RECIST)7 prior to treatment and every 8 weeks thereafter. Briefly, complete response (CR) was disappearance of all lesions, partial response (PR) was ≥ 30% reduction in the sum of the longest diameters of the lesions, stable disease (SD) was denoted in patients whose sum of longest diameters was not decreased more than 30% and not increased more than 20%, and progressive disease (PD) was a ≥ 20% increase in the sum of the longest diameters of the lesions.
IGF-1 and IGFBP3 Analysis
Plasma samples were collected at baseline and then before infusion on Days 8, 15 and 22 from patients enrolled in the dose escalation cohorts up to dose level 3 and in the dose expansion cohort (n = 21). Total IGF-1 and IGFBP3 protein levels were measured by non-extraction ELISA kits from Diagnostics System Laboratories (Webster, TX) according to the manufacturer's protocol. Descriptive statistics were calculated to summarize the IGF-1 and IGFBP3 data. Graphical displays were used to show the patterns of mean levels over time in each of the three treatment arms of the MTD expansion cohort (n = 21). The approximate normality of IGF-1 and IGFBP3 was confirmed at each of the 4 time points (cycle 1, Days 0, 8, 15, and 22) using 4 different statistical tests for non-normality.
Incomplete mixed model repeated measures analysis of variance (ANOVA) was used to model the mean levels of IGF-1, and of IGFBP3 (in separate models) to take into account missing data at any of the 4 time points being assessed. This allowed analysis of all available data, consistent with the intention to treat principle. We assumed that the scant missing data values were missing at random (MAR). Modeling each IGF variable was conducted using the MIXED procedure in SAS Version 9.2 computer software (SAS Institute, Inc., Cary, NC). Given that there were data from only 21 patients, 3 terms were included in each mixed model: treatment group (Arm), the linear effect of time (i.e., slope), and the interaction effect of Arm with time (to test for differences in slope by treatment arm).
For each IGF variable, statistical modeling was done after preliminary analysis to find the best of 14 covariance structures for its 4 repeated measures based on the smallest (absolute) value of Akaike's Information Criterion (AIC). Comparisons and testing of differences by time points and/or by treatment arms were performed using ESTIMATE statements in the MIXED procedure in SAS. For each IGF variable, 9–10 specific contrast estimates were tested. The false discovery rate method of Benjamini and Hochberg was used to correct any resulting multiple comparisons.
Tumor Metabolism
2[18F]fluoro-2-deoxy-D-glucose positron emission tomography combined with x-ray computed tomography (FDG-PET/CT) was performed in patients enrolled in the expansion cohort. Standard uptake value (SUV) data were collected on Day 0 (baseline, SUV1), Day 3 (SUV2), Day 11 (SUV3), and Day 51 (at the end of cycle 2, SUV4). Descriptive statistics were used to summarize the patients' demographic, clinical characteristics, and clinical responses. Box plots were displayed of the repeated measures of SUV by clinical responses, PD and SD, respectively. A hierarchical logistic regression was used to model the effect of SUV change from baseline to Day 3 or to Day 11 on the probability of achieving SD, in which the baseline SUV was used as a covariate. This hierarchical logistic model accounted for random variation among SUV measurements within the same patient. The SUV change was calculated as both the absolute change and the percent relative change. For example, from Day 0 to Day 3, absolute change is defined as (SUV2 – SUV1), and the percent of relative change is defined as (SUV2 – SUV1)/SUV1 *100. A p-value less than 0.05 denoted statistical significance. These statistical analyses were carried out using SAS 9.1 (SAS Institute, Cary, NC) and S-Plus, version 8.0 (Insightful Corp., Seattle, WA).
RESULTS
Patient Characteristics
Forty-two patients were enrolled across two study centers (The University of Texas MD Anderson Cancer Center, Houston, TX, and Barbara Ann Karmanos Cancer Institute, Detroit, MI). Patient demographic and clinical characteristics at study entry are summarized in Table 3. The majority of patients were heavily pretreated, with the median number of prior therapies being 4 (range 1–12). Tumor types included breast cancer (n=9), adrenocortical carcinoma (n=10), Ewing's sarcoma (n=3), desmoplastic small-round-cell tumor (n=3), ovarian cancer (n=2), colorectal cancer (n=2), neuroendocrine cancer (n=2), and one patient each had 11 other tumor types.
Table 3.
Patient Characteristics
| Median age, yrs | 53 (range 20–79) |
| Median # prior therapies | 4 (range 1–12) |
| No. pts (n=42) | |
| Male/Female | 19/23 (45%/55%) |
| Diagnosis | |
| Adrenocortical carcinoma | 10 (24%) |
| Breast cancer | 9 (21%) |
| Ewing's sarcoma | 3 (7%) |
| Desmoplastic small-round-cell tumor | 3 (7%) |
| Ovarian cancer | 2 (5%) |
| Colorectal cancer | 2 (5%) |
| Neuroendocrine cancer | 2 (5%) |
| Other * | 11 (26%) |
n=1 each: Nasopharynx, non-small cell lung cancer, small cell lung cancer, myxoid sarcoma, rhabdomyosarcoma, uterine leiomyosarcoma, endometrial, histiocytoma, melanoma, prostate, gastroesophageal junction.
Toxicities
DLT occurred in two of six patients enrolled at dose level 4 (cixutumumab 6 mg/kg and temsirolimus 37.5 mg). One patient experienced Grade 4 thrombocytopenia and another patient experienced febrile neutropenia. Because the criteria for MTD were exceeded at dose level 4, dose level 3 (cixutumumab 6 mg/kg and temsirolimus 25 mg) was determined to be the MTD for this combination. This dose cohort was expanded to 29 patients, which was composed of eight patients at the MTD and another 21 patients added to the expansion cohort. Among the 29 patients treated at dose level 3, one patient experienced a DLT of Grade 3 mucositis.
The most frequent treatment-related toxicities were hyperglycemia (≥ Grade 3 in 4.8% of patients), hypertriglyceridemia (≥ Grade 3 in 2.4% of patients), hypercholesterolemia (≥ Grade 3 in 2.4% of patients), thrombocytopenia (≥ Grade 3 in 4.8% of patients) and mucositis (≥ Grade 3 in 2.4% of patients) (Table 4). Hyperglycemia was managed by a collaborating endocrinologist, which included the use of insulin and oral diabetic agents. Management of mucositis included the use of xyloxylin (1:1:1 ratio of diphenhydramine, Maalox, lidocaine; 10 mL swish/swallow QID as needed), Caphasol (sodium phosphate; 15 mL swish/spit every 4 hours as needed), valacyclovir (500 mg po TID for treatment), Biotene (MW every 4 hours as needed), and Carafate (1 gm/10 mL; 10 mL swish/swallow or spit QID as needed).
Table 4.
Treatment-Related Toxicities
| Dose Cixutumumab, Temsirolimus | Cohort I 3 mg/kg, 25 mg | Cohort II 5 mg/kg, 25 mg | Cohort III 6 mg/kg, 25mg | Cohort IV 6 mg/kg, 37.5mg | ||||
|---|---|---|---|---|---|---|---|---|
| N=3 | N=4 | N=29 | N=6 | |||||
| NCI CTCAE Grade | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 |
| Endocrine | ||||||||
| Hypercholesterolemia | 2 | 3 | 15 | 1 | 4 | |||
| Hypertriglyceridemia | 3 | 3 | 18 | 1 | 5 | |||
| Hyperglycemia | 3 | 4 | 17 | 2 | 4 | |||
| Hematologic | ||||||||
| Neutropenia | 1 | 1 | 3 | 2 | 1 | |||
| Febrile Neutropenia | 1 (DLT) | |||||||
| Thrombocytopenia | 1 | 1 | 12 | 1 | 4 | 1 (DLT) | ||
| Non-hematologic | ||||||||
| Fatigue | 2 | 6 | 2 | 4 | ||||
| Rash/Pruritis | 1 | 2 | 10 | 4 | ||||
| Mucositis | 3 | 9 | 2 (1 DLT) | 5 | ||||
| Anorexia/Weight loss | 1 | 4 | 7 | 1 | 1 | |||
| Nausea/Vomiting | 2 | 1 | 5 | 3 | ||||
| Elevated Creatinine/Acute Renal Failure | 6 | 1 | ||||||
| Elevated AST/ALT | 1 | 3 | 2 | 1 | ||||
| Allergic Reaction | 4 | |||||||
| Ocular flashes of light | 4 | |||||||
One patient had Grade 4 renal failure and required hemodialysis. However, examination of a kidney biopsy in conjunction with clinical presentation suggested that the patient's renal failure was not related to the study drugs and the patient died from disease progression.”
Antitumor Activity
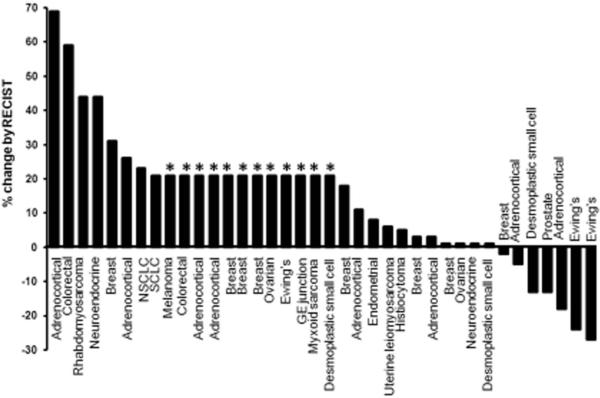
Of the 42 study patients, 36 patients reached their first restaging evaluation and two patients came off study early for progression (total = 38). Four patients did not reach restaging due to withdrawal for adverse events. Best overall responses (n=38) are shown in Figure 1. Eighteen patients (47%) had a best response of SD. Nine of these patients had SD ≥ 5 months (prostate cancer, n=1; breast cancer, n=1; desmoplastic small-round-cell tumor, n=1; adrenocortical carcinoma, n=4; Ewing's sarcoma, n=2) and one other patient with desmoplastic small-round-cell tumor is currently in his third month of treatment and has achieved SD thus far. The greatest tumor reduction was observed in 2 of 3 patients with Ewing's sarcoma (24% and 27% decrease), one of whom had SD for 8 months, and the other having SD for 14 months. Four of 10 patients with adrenocortical carcinoma achieved SD for 8+ months.
Figure 1.
Best response in 38 of 42 patients treated by RECIST criteria. Patients with early clinical progression or with new lesions are assigned on the graph as a 21% increase (*).
IGF-1 and IGFBP3 Levels
We performed pharmacodynamic evaluation of IGF-1 and IGFBP3 in the plasma of 36 patients. The rationale for exploring IGFBP3 and IGF-1 is based on the hypothesis that IGF-1R blockade by cixutumumab may lead to changes in free IGFBP3 and/or IGF-1. IGFBP3 and IGF-1 plasma levels were analyzed by ELISA on blood samples collected at baseline, and at Days 8, 15 and 22 of treatment for 36 patients. The median IGFBP3 and IGF-1 levels increased over time. The median IGF-1 levels among all 36 patients at baseline was 136.7 ng/mL (standard deviation (StDv) 15.8; range: 15.9 – 411.7), which increased to 366.5 ng/mL (StDv 161.1; range: 34.2–772.7) by Day 22. Similarly, the median IGFBP3 levels among all 36 patients at baseline was 60.1 ng/mL (StDv 24.5; range: 9.2 – 102.8), which gradually increased to 99.0 ng/mL (StDv 23.7; range: 46.0 – 144.0) by Day 22.
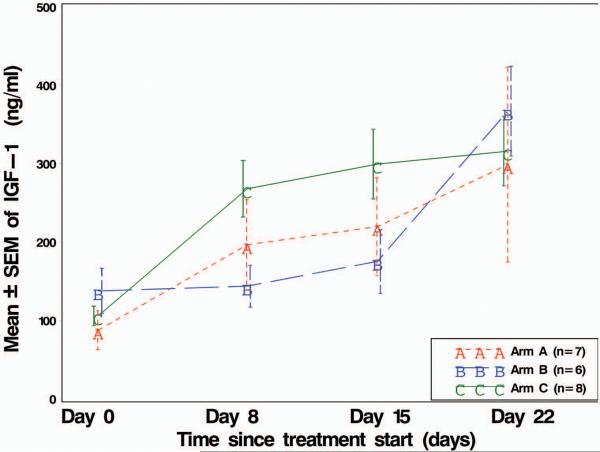
In the dose expansion cohort, in which 21 patients were randomized among three treatment arms, there was a significant overall time slope (p < 0.0001) in mean IGF-1 levels for all 21 patients combined (Figure 2A). At each of 3 time points (Days 8, 15, and 22), mean IGF-1 for all 21 patients combined was significantly higher (p < 0.05) than it was at Day 0 (even after adjusting for 9 multiple comparisons). The same was true for only the 7 patients on Arm A. In addition, for Arm B, the steep rise in mean IFG-1 from Day 15 to Day 22 was statistically significant (p < 0.05, in the multiple comparisons). Taken together, that would suggest that the rise in mean IGF-1 levels was associated with the administration of cixutumumab.
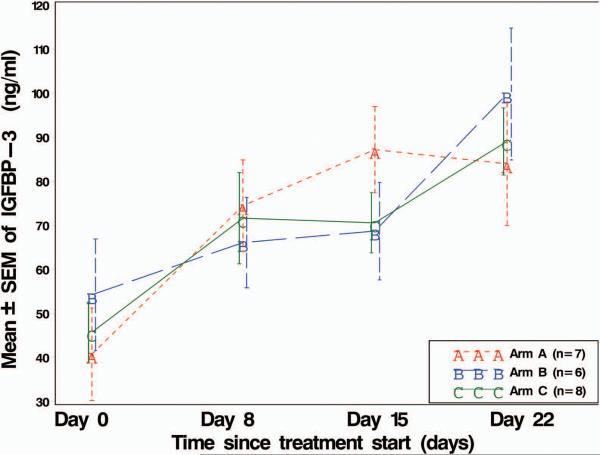
Figure 2.
Line plot of mean IGF-1 and IGFBP3 levels over time, by drug sequence treatment arm. SEM = standard error of the mean. IGF-1 and IGFBP3 plasma levels were analyzed by ELISA from the 21 patients assigned to treatment arms: Arm A, patients received cixutumumab before the combination of both agents; Arm B, patients received temsirolimus before the combination of both agents; Arm C, patients received the combination of both agents at onset of study.
The treatment by time interaction effect was significant (p = 0.0002) indicating some type of variation by treatment arm. Given the small sample sizes, only the visually apparent largest (two) contrasts shown in Figure 2A were tested, which were mean IGF-1 level in Arm C vs. Arm B at Day 8, and again at Day 15. After adjusting for a total of 9 multiple comparisons, neither of these two differences in means was statistically significant.
Temsirolimus alone given before the combination (Arm B) did not appear to impact the IGF-1 level until cixutumumab was added. The combination given at the beginning of the treatment caused higher IGF-1 plasma levels than cixutumumab alone, indicative that temsirolimus might enhance the inhibition of IGF-1R. So, time certainly affected mean IGF-1 levels, and drug sequence did also, but only at Days 8 and 15.
For IGFBP3, we also found a significant overall time slope (p < 0.0001) in mean levels for all 21 patients combined (Figure 2B). At each of Days 8, 15, and 22, the mean IGFBP3 of all 21 patients combined was significantly higher (p < 0.05) than it was at Day 0 (even after adjusting for 10 multiple comparisons). In addition, for Arm B (also for Arm C, and also for all patients combined) the steep rise in mean IGFBP3 from Day 15 to Day 22 was statistically significant (p < 0.05, in the multiple comparisons).
The treatment by time interaction effect was only modestly significant (p = 0.0488) indicating only a slight variation in mean IGFBP3 levels by treatment arm. Even the apparently largest treatment arm differences in mean IGFBP3 (at either Day 15 or at Day 22) were not statistically significant (p > 0.05, in the multiple comparisons). Hence, drug sequence did not seem to affect mean IGFBP3 levels, although the length of time did.
No association between change in levels of either IGFBP3 or IGF-1 and tumor type or response by RECIST was seen.
Tumor Metabolism
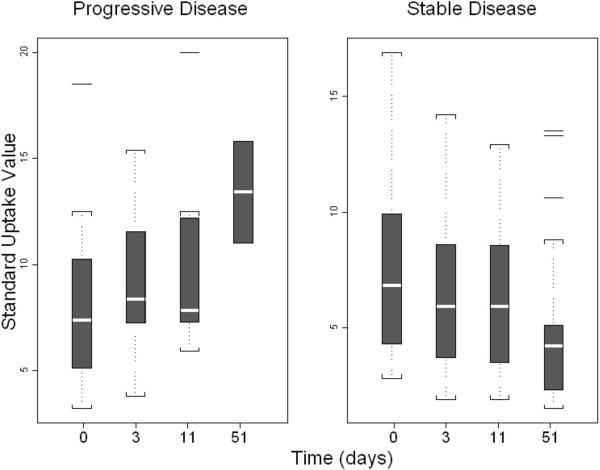
FDG-PET/CT scans were performed at baseline, Day 3, and Day 11. The absolute and relative changes were compared to Day 0 to Day 3 to Day 11 and after Day 56. FDG-PET/CT studies were reviewed, with up to three reference lesions identified per patient. For each reference lesion, a SUV was measured, representing the maximum SUV using a body-weight calculation. For patients with more than one reference lesion, an average of the individual SUV measurements was generated. In addition to assessing the reference lesions, follow-up scans were reviewed for the presence of progressive, non-reference lesions. An absolute increase of SUV from baseline to Day 3 trended toward an increased risk of disease progression (Odds ratio [OR] 2.38; 95% CI 0.82 – 6.92; P = 0.12), though this did not achieve statistical significance. Similarly, a relative increase of SUV from baseline to Day 3 was associated with an insignificant increased risk of disease progression (OR 1.08; 95% CI 0.40 – 1.20; P = 0.11) (Figure 3).
Figure 3.
Box plot of longitudinal measurements of Standard Uptake Value (SUV) by PET scan at Days 0, 3, 11, and 51 among 21 patients in the expansion cohort. Patients were stratified by whether SD was achieved rather than disease progression.
Among 17 patients with available SUV changes from Day 0 to Day 3, seven patients had a decrease in SUV in all locations, five patients had a mixture of SUV decrease/increase in different locations, and five patients had increased SUV in all disease sites. Among 14 patients with SUV changes available from Day 0 to Day 11, five patients had a decrease in all sites, five patients had a mixture of SUV decrease/increase in different sites, and four patients had increased SUV in all locations. Among nine patients with available SUV changes from Day 0 to Day 56, five patients had a decrease in all sites, one patient had a mixture of SUV decrease/increase in different locations, and three patients had an increased SUV in all sites where there was tumor.
DISCUSSION
Weekly administration of cixutumumab, a fully human IgG1 monoclonal antibody directed against the IGF-1R, combined with temsirolimus, an mTOR inhibitor, was well tolerated. MTD was reached at the dose of cixutumumab 6 mg/kg and temsirolimus 25 mg. Three of 42 patients experienced a DLT, which included Grade 3 mucositis, febrile neutropenia, and Grade 4 thrombocytopenia. One patient had Grade 4 renal failure and required hemodialysis. However, examination of a kidney biopsy in conjunction with clinical presentation suggested that the patient's renal failure was not related to the study drugs and the patient died from disease progression.
Metabolic complications (hyperglycemia and hyperlipidemia) were the most prevalent. During the dose escalation phase, diabetic and hyperlipidemic patients were excluded, however, they were included during dose expansion if they were well controlled prior to enrollment. We worked closely with an endocrinology collaborator to observe and carefully treat those patients when they developed adverse events. Only two patients (known diabetics) developed Grade 3 hyperglycemia, which was correctable with oral agents and insulin fairly rapidly. Only two patients developed Grade 3 or 4 hypercholesterolemia or hypertriglyceridemia and, again, with appropriate treatment (statins, fibrates and lifestyle changes), they were correctable. With appropriate oversight, consultations and treatment, associated metabolic toxicities were treatable and major complications were avoided.
Three patients with Ewing's sarcoma were treated, of whom two had durable SD (lasting 8 and 14+ months). Responses in Ewing's sarcoma have been reported with other IGF-1R antibodies, such as AMG479 and R15078–10, suggesting that some patients with this tumor may be particularly sensitive to IGF-1R antagonists, supporting the role of IGF-1R signaling in this malignancy. The lack of a response in all patients with Ewing's sarcoma may reflect alterations in pathways that are not abrogated by IGF-1R inhibition.9 In addition to the observed clinical activity in Ewing's sarcoma, SD was reported in other solid tumor types. In our study, four of 10 patients with adrenocortical carcinoma had durable SD (lasting 8+ months) and one of nine patients with breast cancer had SD lasting 6 months.
We performed pharmacodynamic evaluation of IGF-1 and IGFBP3 in plasma to explore whether or not IGF-1R blockade by cixutumumab might lead to changes in free IGFBP3 and/or IGF-1. IGFs and IGFBPs are secreted molecules and can be detected in the circulation. When bound to IGFs, IGFBPs function by regulating their transport between intra- and extravascular spaces as well as through interaction with their receptors, prolonging IGF-1/2 half-life and precluding their mitogenic activity. IGFBP3 is the largest and is the major carrier of IGF-1 in the blood. It is very likely that when IGF-1R is blocked by cixutumumab, changes in free IGFBP3 and/or IGF-1 will be observed. We observed that overall, IGFBP3 and IGF-1 plasma levels increased significantly over time, and that a rise in IGF-1 levels was associated with the administration of cixutumumab. A similar pattern was seen with the change over time in IGFBP3 levels in cohorts where temsirolimus or cixutumumab was given alone before the combination. Overall, the plasma IGF-1 and IGFBP3 levels suggest that cixutumumab and its combination with temsirolimus facilitates upregulation of IGF-1 and IGFBP3. Increased circulating IGF-1 levels most likely result from a disrupted negative feedback mechanism that regulates growth hormone secretion. Cixutumumab blocks IGF-1R in the pituitary, resulting in increased secretion of growth hormone, which stimulates IGF-1 production in peripheral tissues, primarily the liver. IGFBP3 is more stable when it forms a complex with IGF-1. Therefore, in the presence of elevated circulating IGF-1, IGFBP3 levels may increase as well. Due to various factors, we were not able to perform a meaningful statistical analysis for immunohistochemical and reverse phase protein array assays.
Although FDG-PET/CT is becoming well established as a means to assess metabolic response of many tumor types and was used in this study, it has limitations in some settings. Specifically, studies in the literature identified a variable correlation between FDG uptake and treatment response to mTOR inhibition.12,13 In a study with both animal and human subjects14, a poor correlation between FDG uptake and clinical response was observed. The changes in FDG uptake seen in treated subjects were in large part due to direct effects on the AKT activation pathway, rather than on the intrinsic metabolic machinery of the tumor cells. Another possibility is that FDG uptake may depend on the type of malignancy studied15.
For the assessment of metabolic response and FDG-PET/CT findings, we applied SUV as a continuous variable, rather than setting a threshold for response/non-response. In our statistical analysis, this resulted in finding that a decrease in the SUV by a unit of 1 (absolute measurement) between baseline and Day 3 or Day 11 correlated with a trend towards higher odds of achieving a negative clinical benefit. This finding is suggestive of response, but given the inherent limitations in SUV reproducibility due to uncontrollable factors, it would be difficult to apply this narrow of a threshold to routine clinical practice. Higher patient numbers will be necessary to further validate the correlation between SUV and response.
In conclusion, further investigations are warranted to establish whether dual inhibition of the IGF-1R and mTOR pathways will improve clinical outcomes in patients with cancer. We are currently enrolling expansion cohorts at the MTD for Ewing's sarcoma and adrenocortical carcinoma, tumor types in which clinical benefit was observed.
TRANSLATIONAL RELEVANCE.
This 3+3 multi-center Phase I study enrolled 42 patients with advanced cancer and a median of four prior therapies. The trial combined the mTOR inhibitor temsirolimus with cixutumumab, a fully human IgG1 monoclonal antibody directed against IGF-1R. The combination was expected to enhance mTOR-targeted anticancer activity by modulating resistance to mTOR inhibition. Objectives of this study were to evaluate the tolerability and activity of temsirolimus and cixutumumab. Clinical benefit was observed in adrenocortical carcinoma and the combination was well tolerated. Two of three patients with Ewing's sarcoma had tumor reduction >20%. Responses in Ewing's sarcoma have been reported with other IGF-1R antibodies, such as AMG479 and R1507, suggesting that some patients with this tumor may be particularly sensitive to IGF-1R antagonists, supporting the role of IGF-1R signaling in this malignancy and treatment with the combination of temsirolimus and cixutumumab.
ACKNOWLEDGMENT
The authors would like to thank Joann Aaron, MA, in the Department of Investigational Cancer Therapeutics for scientific review and editing of this manuscript.
This study was supported by R21CA13763301A1 (Aung Naing), U01CA62461 (Razelle Kurzrock), and U01CA62487 (Patricia LoRusso)
Footnotes
Presented in part at the American Association for Cancer Research-National Cancer Institute-European Organization for Research and Treatment of Cancer Molecular Targets and Cancer Therapeutics, November 15–19 2009, Boston, MA; the National Cancer Institute/Cancer Therapy Evaluation Program Early Drug Development Meeting, October, 2009, Bethesda MD; and the 46th Annual Meeting of the American Society of Clinical Oncology, June 4–8 2010, Chicago, IL
REFERENCES
- 1.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–40. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 3.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 4.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurmasheva RT, Dudkin L, Billups C, Debelenko LV, Morton CL, Houghton PJ. The insulin-like growth factor-1 targeting antibody, CP-751871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res. 2009;69:7662–71. doi: 10.1158/0008-5472.CAN-09-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denlinger CS, Blanchard R, Xu L, Bernaards C, Litwin S, Spittle C, et al. Pharmacokinetic analysis of irinotecan plus bevacizumab in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2009;65:97–105. doi: 10.1007/s00280-009-1008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, Wanders JN, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Kurzrock R, Patnaik A, Aisner J, Warren T, Leong S, Benjhamin R, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2458–65. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- 9.Garofalo C, Manara MC, Nicoletti G, Marino MT, Lollini P-L, Astolfi A, et al. Efficacy of and resistance to anti-IGF-1R therapies in Ewing's sarcoma is dependent on insulin receptor signaling. Oncogene. 2011 doi: 10.1038/onc.2010.640. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Olmos D, Postel-Vinay S, Molife LR, Okuno SH, Schuetze SM, Paccagnella ML, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. Lancet Oncol. 2009;11:129–35. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tolcher AW, Sarantopoulos J, Patnaik A, Papadopoulos K, Lin CC, Rodon J, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27:5800–7. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 12.Brepoels L, Stroobants S, Verhoef G, De Groot T, Mortelmans L, De Wolf-Peeters C, et al. (18)F-FDG and (18)F-FLT uptake early after cyclophosphamide and mTOR inhibition in an experimental lymphoma model. J Nucl Med. 2009;50:1102–9. doi: 10.2967/jnumed.109.062208. [DOI] [PubMed] [Google Scholar]
- 13.Wei LH, Su H, Hildebrandt IJ, Phelps ME, Czernin J, Weber WA. Changes in tumor metabolism as readout for mammalian target of rapamycin kinase inhibition by rapamycin in glioblastoma. Clin Cancer Res. 2008;14:3416–26. doi: 10.1158/1078-0432.CCR-07-1824. [DOI] [PubMed] [Google Scholar]
- 14.Ma WW, Jacene H, Song D, Vilardell F, Messersmith WA, Laheru D, et al. [18F]fluorodeoxyglucose positron emission tomography correlates with Akt pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy. J Clin Oncol. 2009;27:2697–704. doi: 10.1200/JCO.2008.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Giorgi U, Amadori D. [18F]Fluorodeoxyglucose positron emission tomography for outcome prediction of mammalian target of rapamycin inhibitor therapy. J Clin Oncol. 2010;28:e236–7. doi: 10.1200/JCO.2009.26.6866. [DOI] [PubMed] [Google Scholar]