Abstract
Purpose
The variable regions of Ig (idiotype, Id) expressed by malignant B cells can be used as tumor-specific antigens that induce humoral and cellular immunity. However, epitopes derived from Id that stimulate human CD8+ T-cell immunity are incompletely characterized.
Experimental design
The clonal Ig VL of human myeloma cell line U266 and five primary B-cell tumors were sequenced and peptides corresponding to the Ig VL region were tested for their ability to stimulate cytotoxic T lymphocytes (CTLs) from ten HLA-A* 0201 positive normal donors. The CTLs thus generated were tested against peptide-pulsed T2 cells and autologous tumor cells.
Results
14 peptides derived from Ig light chain (VL) of U266 and primary B-cell tumors were used to generate 68 Cytotoxic T lymphocytes (CTLs) lines that specifically produced IFN-γ when co-cultured with peptide-pulsed T2 cells. These CTLs lysed peptide-pulsed T2 cell as well as U266 or autologous tumor targets in an HLA class I-dependent manner. Sequence analysis revealed shared VL T-cell epitopes in U266 and primary B-cell tumors, not previously reported within Ig heavy chain (VH) sequences.
Conclusion
This study thus identifies novel immunogenic CTLs epitopes from Id VL, suggests that they are naturally presented on the surface of B-cell malignancies, and supports their inclusion in next generation Id vaccines. The ability to prime T cells derived from normal HLA-matched donors, rather than patients, also may have direct application to current strategies, designed to generate allogeneic tumor-specific T cells for adoptive transfer.
Keywords: myeloma, plasma cell leukemia, peptide, allogeneic T cells, immunotherapy, donor lymphocyte infusion, vaccine, stem cell transplantation
Introduction
B-cell malignancies express unique variable region determinants in their surface Ig receptor (Id) that can serve as tumor-specific antigens. Studies in mice and humans showed that humoral and cellular immune responses were induced following Id vaccination (1–4). We have previously demonstrated that autologous Id protein can be formulated into an immunogenic antigen in lymphoma patients, by conjugation with a carrier protein, keyhole limpet hemocyanin (KLH), and administration with GM-CSF as adjuvant. Lymphoma-specific CD8+ T-cell responses were associated with achievement of molecular remissions (3). In human myeloma patients, T-cell responses specific for Id protein have generally been demonstrated, suggesting immunogenicity of this tumor antigen (5). Finally, a randomized Phase III clinical trial of an Id protein vaccine recently demonstrated prolonged remission duration in follicular lymphoma (FL) patients in first remission(6). However, the immunogenic epitopes derived from Id that stimulate CD8+ T-cell responses have been incompletely characterized, especially Id light chain (VL) determinants.
Despite the availability of new proteosome inhibitors and other targeted agents, disease relapse still remains a major problem for myeloma patients, and even high dose therapy followed by autologous stem cell transplantation (SCT) in tandem does not appear to be curative for this disease (7). In contrast, allogeneic SCT following either myeloablative or reduced-intensity conditioning has been shown to induce prolonged disease-free survival in a small percentage of patients suggesting a possible graft versus myeloma (GVM) effect(8). Attempts to enhance the GVM effect by donor lymphocyte infusions (DLI) have resulted in an increased incidence of graft versus host disease (GVHD)(9). Therefore, strategies to enhance the specific antitumor effect of the graft without increasing the risk of GVHD are needed to improve outcome in allotransplant recipients.
One novel strategy is to transfer highly-enriched populations of tumor antigen-specific T cells from donor to recipient (i.e., educated donor lymphocyte infusions, DLI) to enhance the antitumor effect of the allograft without exacerbating GVHD. The approach of allogeneic marrow donor immunization in myeloma has been tested clinically in a small number of HLA-matched donor-recipient pairs and donor immunization with Id protein has proved safe (10, 11). As an alternative to vaccinating donors in vivo in future clinical studies, we develop here a method to prime and expand donor idiotype light chain-specific T cells in vitro with the goal of using Id-specific DLI as the transfer element against B-cell malignancies in future clinical studies.
Materials and Methods
Human tumors
U266 myeloma cell line (HLA-A*0201/A3+) was obtained from ATCC. HLA-A*0201 primary FL or chronic lymphocyte leukemia (CLL) tumors were purified from patient’s blood or spleen with HISTOPAQUE-1077 (Sigma) and B-cell isolation kit (Miltenyi Biotec). HLA-A*0201 primary plasma cell leukemia cells (PL) were isolated with CD138+ cell isolation kit (Miltenyi Biotec). All patients’ samples were collected before the administration of high does therapy or idiotype vaccination. This study was approved by the Institutional Review Board Committee and informed consent was obtained in accordance with the Declaration of Helsinki.
RT-PCR of idiotype light chain cDNA
3µg RNA extracted from U266, primary tumors was reverse- transcripted into cDNA with Superscript III kit from Invitrogen (cat# 11745100). The highly variable region of idiotype light chain region was PCR amplified with primers from published paper (12). The PCR conditions are: 94°C 5min, followed by 94°C, 30sec, 58°C, 30sec, 72°C, 45sec for 35 cycles, 72°C, 9 minute. The PCR product was cloned into PCR2.1 TOPO vector (Invitrogen, cat #K2000-01) and sequenced in the DNA core facility of MD.Anderson Cancer Center.
Peptide synthesis and T2 binding
Peptides predicted to bind to HLA-A*0201 were synthesized to greater than 70% purity, dissolved in 100% dimethyl sulfoxide (Sigma), and the binding affinity to HLA-A*0201 molecules was measured with T2 cells according to published methods (13). In brief, T2 cells were incubated with 50µg/ml peptides and 3 µg/ml of β2-microglobulin (Sigma, cat # M4890) for overnight. The cells were then washed and incubated with PE-labeled anti–HLA-A0201 mAb (clone BB7.2, BD Biosciences) for 30 minutes at 4°C. After washes and fixation, cells were analyzed for the levels of HLA-A2 expression by flow cytometer. The binding affinity of peptide was quantified according to the formula [(mean fluorescence with peptide − mean fluorescence without peptide)/mean fluorescence without peptide] × 100%. Influenza A virus M1 58–66 (GILGFVFTL) peptide was used as a positive control.
Generation of peptide-specific CTLs
CTLs were generated from HLA-A*0201 normal donor PBMCs using reported methods (14). Briefly, PBMCs (1 × 105 cells/well) were incubated with 10 µg/ml peptide in quadruplicate in 96-well U-bottom-microculture plates in 200 µl culture medium (50% AIM-V, 50% RPMI-1640, 10% human AB serum, 100 IU/ml of IL-2) and restimulated with peptide every 3 days. After 5 stimulations, T cells were stimulated with peptide-pulsed T2 cells and interferon (IFN)-γ production was determined in supernatants by ELISA. CD8+ T cells were isolated from IFN-γ-producing cultures by MACS and expanded by rapid expansion protocol(15). Influenza A virus M1 58–66 (GILGFVFTL) peptide was used as a positive control.
Cytotoxicity
U266 and primary tumor cells were labeled with 51Cr and standard 4-hour cytotoxicity assay was performed. Anti-human HLA-ABC (clone W6/32, eBiosciences), anti- HLA-DR, DP, DQ (clone TÜ39, BD Biosciences), and Mouse IgG2a Isotype Control (eBiosciences, cat# 16-4724-81) were used to determine HLA restriction. All assays were performed in triplicate and repeated three times.
Intracellular cytokine staining assay
Effector T cells were mixed with T2 or antigen presenting cells (APCs) loaded with 10µg/ml peptide at 1: 1 ratio. 2 hour later, 5µg/ml Brefeldin A (Sigma) was added to block the transfer of Gogi part. The staining of intracellular cytokine was performed with BD Cytofix/Cytoperm™ Plus Fixation/Permeabilization kit 12 hour later. 10µl mouse anti-human IFN-γ (clone 25723.11, BD Biosciences), mouse anti-human TNF-a (clone MAb11 BD Biosciences), mouse anti-human GM-CSF(clone 4H1, eBiosciences), mouse anti-human IL-4 (clone 8D4,BD Biosciences), mouse anti-human IL-10 (clone: JES3-9D7, eBiosciences), mouse anti-human IL-17 (clone eBio64DEC17, eBiosciences), mouse anti-human CD8 (clone HIT8a, BD Biosciences) were added to the 100µl effector T cells and stained for 30minutes at 4°C in the dark. After 2 times washes in 1×Perm buffer, the samples were analyzed by flow cytometer and the data was analyzed with cell Quest software or Flowjo.
Statistical analysis
The Student t test was used to compare various experimental groups. Unless otherwise indicated, the mean average and standard deviations (SD) of triplicate wells are shown.
Results
Generation of Id VL-specific T-cell lines from normal donors
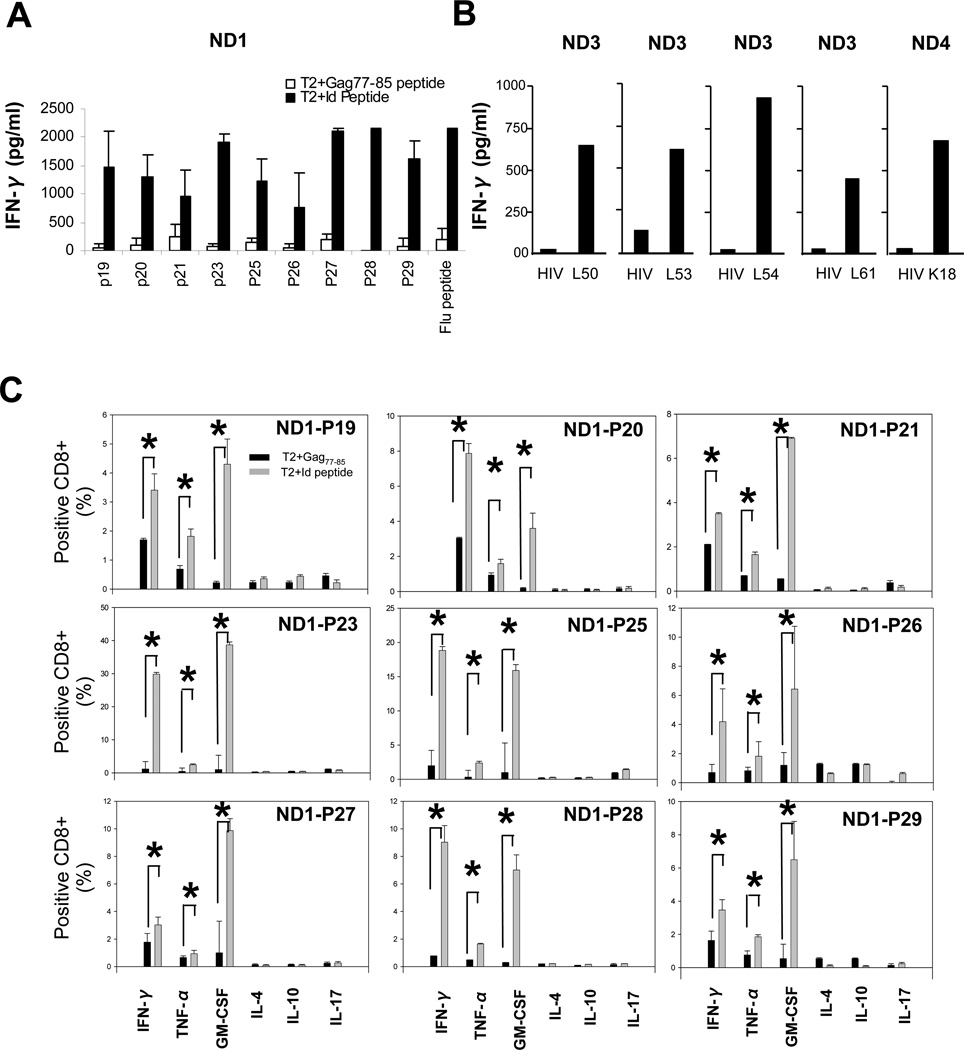
As a model, we used peptides derived from the VL of human U266 myeloma cell line to generate CTLs. Eighteen peptides from lambda VL of U266 were selected based on the predicted binding affinity to HLA-A*0201 (http://www-bimas.cit.nih.gov/molbio/hla_bind/). Most peptides showed modest binding capacity to HLA-A*0201 molecules (T2 binding index: 0.15–1.29) (Supplementary Table 1). As peptides of low affinity are capable of inducing CTLs (16), all were used to generate CTLs from HLA-A* 0201 normal donors (14). Nine U266 peptides (P19, P20, P21, P23, P25, P26, P27, P28, and P29) induced CTLs that specifically secreted IFN-γ when co-cultured with peptide-pulsed T2 cells (Figure 1A). These peptides induced 54 CTLs in ten HLA-A* 0201 donors (Table.2), confirming their immunogenicity in general normal donors. Using intracellular cytokine assay, we demonstrated that CTLs produced IFN-γ, TNF-α, and GM-CSF but not IL-4, IL-10, or IL-17 cytokines (Figure.1C). Next, we used 105 candidate peptides from VL of CLL (n=1), FL (n=3), and PL (n=1) tumor cells and demonstrated that five primary Id VL-derived peptides are also immunogenic to generate 14 CTLs from ten normal donors (Figure 1B and Table.2).
Figure 1.
Generation of Id VL peptide-specific T cells from 10 normal donors (ND1–10). (A) IFN-γ secretion by U266 myeloma cell line VL peptide-specific CTLs after stimulation with peptide-pulsed T2 cells or HIV-Gag77 peptide-pulsed T2 cells (negative control). Influenza A virus M1(58–66) peptide was used as a positive control. Id VL peptide sequences and characteristics are shown in Table.1 (B) IFN-γ secretion by primary B-cell tumor-derived VL peptide specific CTLs against peptide pulsed or HIV-Gag77 peptide-pulsed T2 cells. (C) Intracellular cytokine staining assay of peptide-specific CTLs in response to VL peptide or HIV-Gag77 peptide-pulsed T2 cells. * indicates P<0.05.
Table 2.
Immunogenicity of Id VL peptides derived from human U266 myeloma cell line and primary B-cell tumors
| Peptide specific IFN-γ (pg/ml) secretionb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Petides | ND1a | ND2 | ND3 | ND4 | ND5 | ND6 | ND7 | ND8 | ND9 | ND10 |
| P19 | 1431.9 | 904.4 | 2039.7 | 1339.2 | 2095.9 | 0.0c | 0.0 | 1245.6 | 1563.8 | 0.0 |
| P20 | 1203.9 | 333.0 | 2208.1 | 0.0 | 1432.5 | 0.0 | 0.0 | 1023.5 | 0.0 | 0.0 |
| P21 | 710.2 | 0.0 | 0.0 | 0.0 | 1028.7 | 0.0 | 0.0 | 4512.2 | 0.0 | 0.0 |
| P23 | 1817.3 | 107.1 | 1976.4 | 1649.5 | 1382.4 | 1542.3 | 0.0 | 3215.3 | 0.0 | 1046.7 |
| P25 | 1091.1 | 2075.4 | 2292.2 | 0.0 | 2035.1 | 2365.2 | 1423.2 | 3351.2 | 0.0 | 0.0 |
| P26 | 716.0 | 1239.0 | 1303.5 | 1856.0 | 1241.9 | 2548.2 | 1122.5 | 0.0 | 0.0 | 0.0 |
| P27 | 1931.2 | 117.4 | 2297.3 | 0.0 | 2248.5 | 0.0 | 0.0 | 2534.9 | 1333.5 | 0.0 |
| P28 | 2126.6 | 1870.5 | 2071.9 | 1176.6 | 2640.2 | 0.0 | 0.0 | 3081.5 | 0.0 | 0.0 |
| P29 | 1527.9 | 1186.7 | 2256.6 | 1785.8 | 2677.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| L50 | 0.0 | 0.0 | 605.0 | 0.0 | 585.0 | 487.3 | 0.0 | 569.8 | 0.0 | 0.0 |
| L53 | 0.0 | 0.0 | 401.3 | 0.0 | 0.0 | 455.6 | 0.0 | 399.6 | 0.0 | 0.0 |
| L54 | 0.0 | 0.0 | 863.5 | 0.0 | 578.2 | 0.0 | 0.0 | 0.0 | 601.3 | 0.0 |
| L61 | 0.0 | 0.0 | 348.6 | 0.0 | 0.0 | 0.0 | 0.0 | 123.5 | 0.0 | 0.0 |
| K18 | 0.0 | 0.0 | 0.0 | 598.4 | 0.0 | 0.0 | 0.0 | 232.5 | 0.0 | 0.0 |
ND = normal donor
Peptide specific IFN-γ secretion = IFN-γ from T cells stimulated with Id VL peptide pulsed T2 cells - IFN-γ from T cells stimulated with control peptide (HIV Gag77–85) pulsed T2 cells.
no peptide specific IFN-γ secretion detected
Cytotoxicity of VL peptide-specific donor T cells
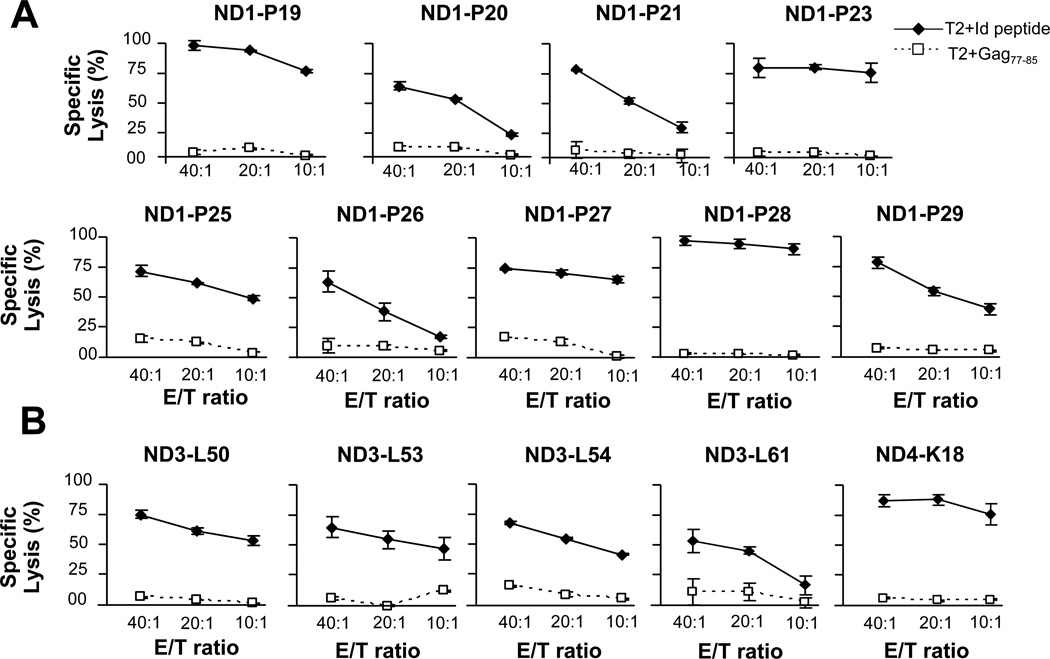
We expanded peptide-specific CTLs and assayed for cytotoxicity against peptide-pulsed T2 cells and primary tumors. Both U266 peptide-specific CTLs (Figure 2A) and CTLs generated against VL peptides from primary tumors (Figure 2B) efficiently lysed Id, but not control peptide-pulsed, T2 targets.
Figure 2.
The cytotoxic function of VL peptide-specific CTLs against Id peptide or control HIV peptide pulsed T2 cells. (A)The cytotoxic function of U266 derived VL peptide-specific CTLs was tested against T2 cells pulsed with U266 or control HIV peptide. (B) The cytotoxic function of donor CTL raised against primary B-cell tumor-derived VL peptides was tested against T2 cells pulsed with the respective human tumor-derived or control HIV peptide. Results are representative of three independent experiments.
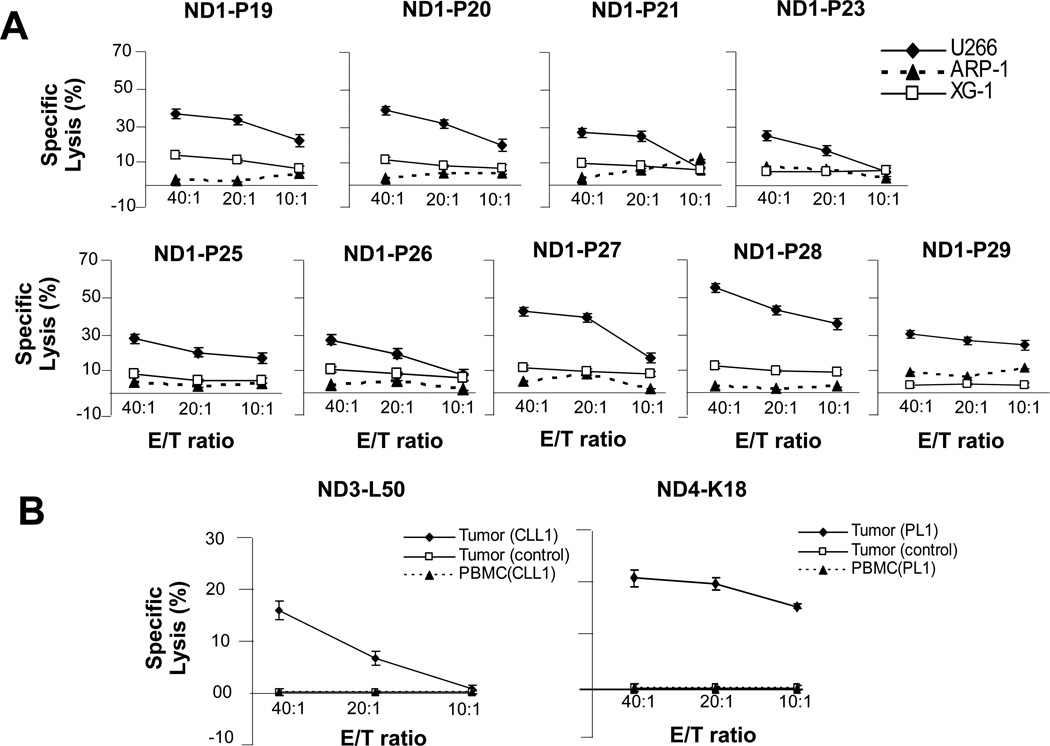
Peptide-specific CTLs raised against multiple U266 peptides also lysed U266 as target efficiently. CTLs did not lyse myeloma targets expressing irrelevant Id (ARP-1, HLA-A2−/Id-; XG1, HLA-A2+/Id-), indicating their specificity (Figure 3A). Donor-derived CTLs raised against primary tumor-derived VL peptides also specifically lysed primary tumors but not tumors expressing irrelevant Ids, or the respective patients’ PBMC (Figure 3B) or K562 cells (not shown).
Figure 3.
Cytotoxic function of VL peptide-specific normal donor CTLs against U266 and primary human B-cell tumor targets. (A) U266 VL peptide-specific (P19, P20, P21, P23, P25, P26, P27, P28, P29) CTLs generated from a normal donor (ND1) lysed U266 myeloma cells but not ARP-1 (HLA A2−, irrelevant Id) or XG-1 (HLA A2+, irrelevant Id) myeloma cell lines. (B) Human tumor-derived VL peptide-specific (L50 and K18) CTLs lysed the respective primary tumor cells: Tumor (CLL1) and Tumor (PL1), but not tumors expressing irrelevant Id: Tumor (control), or autologous patient’s tumor-free PBMC: PBMC(CLL1) and PBMC(PL1).
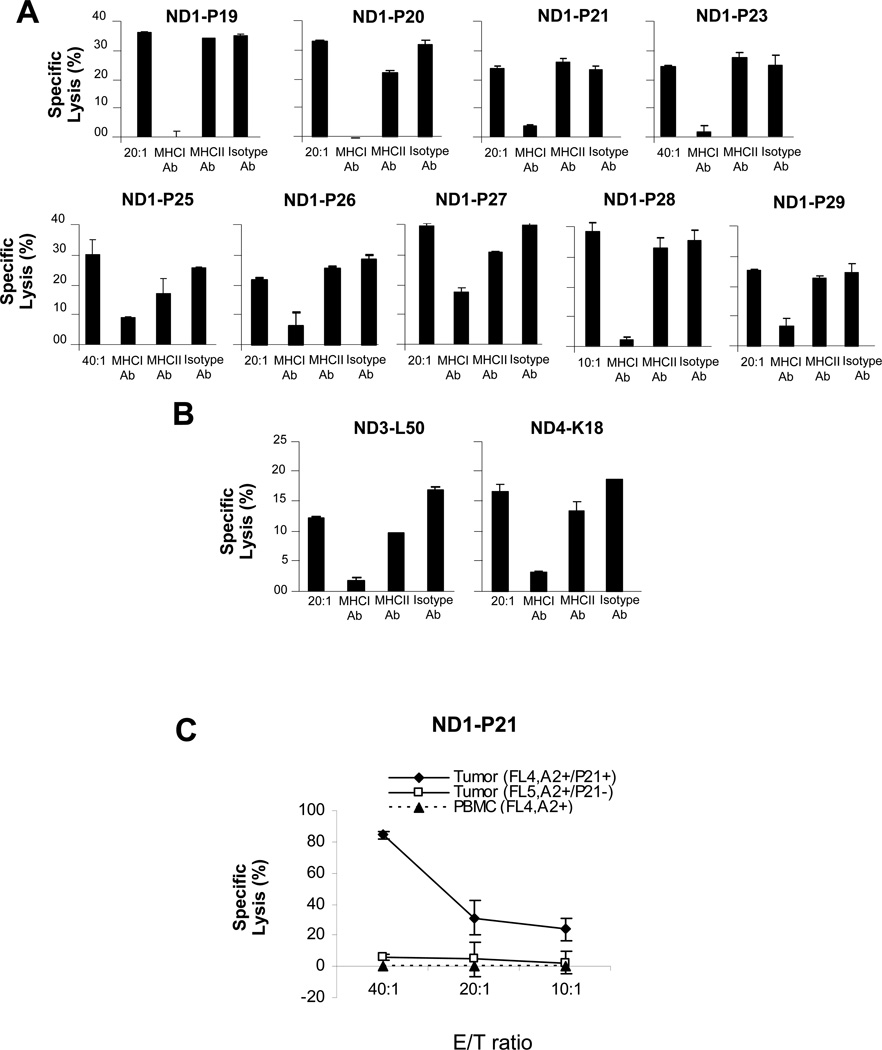
To further characterize effector T cells, we assayed for inhibition of tumor cell lysis by HLA-blocking antibodies. We observed that HLA class I, but not class II or isotype control antibodies inhibited cytolysis by U266 peptide-specific CTL lines (Figure 4A). HLA class I antibodies also inhibited the lysis of primary tumor cells by CTL raised against their respective VL peptides, suggesting that VL peptides were processed and presented on HLA class I molecules by both U266 and primary B-cell tumors (Figure 4B).
Figure 4.
HLA restriction of of VL peptide specific CTLs. (A) HLA class I but not HLA class II specific antibody or isotype control antibody (10 µg/ml) blocked the cytolysis of U266 tumor cells by U266 VL peptide-specific CTLs. (B) HLA class I but not HLA class II antibody or isotype control antibody (10 µg/ml) blocked the cytolysis of primary human CLL and PL tumor cells, respectively by VL peptide-specific CTLs. (C) U266 P21 peptide-specific CTLs specifically lysed HLA-A*0201+ primary FL cells that shared the P21 epitope: Tumor (FL4, P21+), but not the same patients’ PBMC: PBMC(FL4) or HLA-A*0201 tumor cells that did not express P21(FL5,P21−).
Candidate VL epitopes shared by human FL
A current limitation of Id vaccines is the requirement for individualized manufacture which could be partially overcome by identification of universal T-cell epitopes shared by multiple patients. To determine whether the immunogenic U266 and primary tumor VL peptides were shared by other human B-cell tumors, we compared them to 90 lambda and 123 kappa L-chain sequences from a panel of FL tumors (D. Gold, unpublished data). Interestingly, we observed that 12 FL tumors shared the P21 sequence while one FL each shared P19 and P25 epitopes. Seven FL shared the L50 epitope, two FL each shared the L53, L54 and L61 epitopes, and K18 and L10 epitope were shared by one other primary FL each (Table 1). The two most frequently shared peptides (P21 and L50) localized to the framework regions of VL and consisted of germ line sequences, without somatic mutation. Overall, 27 out of 90 (30%) FL tumors expressing lambda L-chain shared at least one of the 13 epitopes we identified. Analysis of shared kappa epitopes will require additional studies, as only a single immunogenic kappa epitope was identified here.
Table 1.
Characteristics of VL T-cell epitopes from U266 and primary B-cell tumors
| Tumor source |
Peptide | Variable Regiona |
Subfamilyb | start positionc |
Sequenced | Length | Predictive bindinge |
Mean Fluorescencef |
shared tumorsg |
|---|---|---|---|---|---|---|---|---|---|
| U266 Myeloma | P19 | FR1 | IGLV1 | 5 | TQPPSTSET | 9 | 0.76 | 0.40 | 1/90 |
| P20 | FR2 | IGLV1 | 39 | HLPGKAPKL | 9 | 0.73 | 0.26 | 0/90 | |
| P21 | FR3 | IGLV1 | 71 | SASLAISGL | 9 | 0.68 | 0.15 | 12/90 | |
| P23 | CDR1 | IGLV1 | 26 | TSNIGSNSV | 9 | 0.45 | 0.24 | 0/90 | |
| P25 | CDR1 | IGLV1 | 32 | NSVNWYQHL | 9 | 0.21 | 1.29 | 1/90 | |
| P26 | CDR3 | IGLV1 | 90 | ASWDDRLNGL | 10 | 10.92 | 0.26 | 0/90 | |
| P27 | CDR1 | IGLV1 | 9 | STSETPGQGV | 10 | 3.96 | 0.53 | 0/90 | |
| P28 | FR1 | IGLV1 | 17 | GVTISCSGST | 10 | 0.09 | 0.15 | 0/90 | |
| P29 | FR1 | IGLV1 | 11 | SETPGQGVTI | 10 | 0.20 | 0.45 | 0/90 | |
| Human CLL1 | L50 | FR1 | IGLV2 | 29 | SVSGSPGQSI | 10 | 0.913 | 0.07 | 7/90 |
| Human FL1 | L53 | FR1 | IGLV1 | 28 | SASATPGQRV | 10 | 0.96 | 0.07 | 2/90 |
| Human FL2 | L54 | FR1 | IGLV4 | 29 | SASASLGASV | 10 | 0.96 | 0.06 | 2/90 |
| Human FL3 | L61 | FR1 | IGLV1 | 16 | KVTISCSGST | 10 | 0.29 | 0.06 | 2/90 |
| Human PL1 | K18 | CDR2 | IGKV3 | 50 | LIYGTSSRA | 9 | 3.7 | 0.52 | 1/123 |
| Lambda | 27/90 | ||||||||
| kappa | 1/123 | ||||||||
Analysis and blast by IMGT website (http://imgt.cines.fr/IMGT_vquest/vquest?livret=0&Option=humanIg)
Somatically mutated amino acids are bolded
Estimate of half-time of disassociation and calculated score in arbitrary units.
Mean fluorescence Index = (Mean fluorescence with peptide-mean fluorescence without peptide)/(mean fluorescence without peptide).
Individual peptide sequences were compared with Ig V-region sequences available from either 90 FL patients with lambda or 123 patients with kappa L-chain isotype, respectively.
CLL, chronic lymphocytic leukemia; FL, follicular lymphoma; PL, plasma cell leukemia
To determine whether CTLs generated against a U266 peptide could lyse primary tumor cells expressing a shared epitope, we incubated donor-derived, P21-specific CTLs with HLA-A*0201 FL that expressed the P21 epitope (FL4, HLA-A2+/P21+). We observed significant lysis of FL target cells, but not PBMC from this patient or FL that did not express P21 (FL5,HLA-A2+/P21−), suggesting specificity for Id (Figure 4C).
Discussion
Increasing data suggest that human T-cell immunity plays an important role in anti-tumor effects induced by Id vaccination with whole protein (17–19). Studies have identified T-cell epitopes harbored by Ig VH of human and Ig VL of murine B-cell tumors (20–29), but whether human Id VL harbor important T cell epitope remains unclear(25, 26). Two previous publications reported that Ig light chains contained few T cell epitopes. In Dabadghao’s study, PBMC from five myeloma patients were incubated with fragment F(ab)2, Fab, HC (heavy chain) or LC (light chain) of autologous idiotype protein to determine idiotype specific T cell proliferation. They found idiotype fragments of F(ab)2, Fab, HC can stimulate PBMC proliferation. However, none of the patients’ PBMC proliferated to LC of idiotype protein (25). In the study by Fagerberg et al., peptides corresponding to the heavy chain and light chain of one myeloma idiotype protein were synthesized and incubated with one myeloma patient’s PBMC. Using ELISPOT assay, they found only peptides derived from HC but none from LC of idiotype protein could stimulate T cells to secret IFN-γ (26). In our study, we have identified 14 peptides from Ig light chain (VL) of U266 and primary B-cell tumors that can be used to generate 68 CTLs lines in vitro. These VL CTLs lysed the peptide pulsed-T2 cell as well as tumor cells, indicating that VL immunogenic epitopes are processed and presented by tumor cells. The differences between our results and previous studies may have resulted from several reasons. First, the patient’s PBMC is not ideal to study protective T cell immunity, as the immune balance is skewed towards immune suppression (30). Second, the whole idiotype protein, may harbor immune regulatory, as well as stimulatory sequences, thus the identification of immunogenic epitope may be hampered by regulatory epitopes on the whole idiotype protein(31) Lastly, significantly more peptides from patients were synthesized and tested in our study. Overall, our study suggests that immunogenic epitopes are present in VL CDR and FWR regions, are processed and presented by primary tumor cells, and supports strategies for targeting VL epitopes on B-cell tumors. CTLs targeting Id VL may also have specific clinical usage against L-chain-only secreting plasma cell dyscrasias (27).
Id vaccination significantly extended the disease-free survival of follicular lymphoma patients as demonstrated in a recent controlled Phase III trial, consistent with suggestions from prior pilot clinical trials (6, 32). The ability to prime HLA-A2 + donor CD8+ T cells against Ig VL epitopes may provide a strategy for improving adoptive therapy against B-cell malignancies. Previous work in human SCT, demonstrating that the transfer of humoral, and to a lesser extent cellular, antigen-specific immunity to clinically important viral antigens from immune donors to recipients can occur, provides a rationale for transferring tumor antigen-specific immunity induced in donors(33–35). Vaccination of HLA-matched sibling donors with myeloma Id protein, with subsequent transfer of Id-specific immunity by stem cell transplantation, has been shown to be feasible in a limited number of patients with myeloma (10, 11). Six donors were vaccinated with Id proteins (conjugated to KLH) isolated from the plasma of the myeloma patients prior to marrow harvest, and respective recipients were administered booster Id immunizations following transplantation. The vaccine was well-tolerated by all donors and recipients. With median follow-up of eight years, no long-term toxicity has been observed in any immunized donor. Vaccination induced specific cellular and/or humoral immune responses against Id and the vaccine carrier, KLH, in all donors. Two patients died within 30 days of SCT due to transplant-related complications, but Id-specific and KLH-specific T-cell responses were detected in all four remaining patients post-, but not pre-BMT. All four surviving patients converted from partial to complete responses following SCT. Taken together, these preliminary results suggest that 1) idiotype protein can elicit a specific immune response in a healthy donor, 2) direct transfer of idiotype specific T-cell immunity can occur from donor to recipient, and 3) donor-derived T-cell responses may not be blocked by circulating idiotype protein present in the patients during and after SCT, or by iatrogenic immunosuppression for GVHD prophylaxis. Our current data suggest that as an alternative to donor vaccination, immunogenic peptides may be used to selectively expand Id-specific T cells ex vivo to generate “educated” donor lymphocyte infusions (DLI). Such primed T cells might enhance tumor specificity and limit graft-vs.-host disease complications of current DLI strategies.
Using a recently described method (14), we completed multiple T-cell stimulations within 2 weeks, such that the T cells are not exhausted at that time. Instead, these T cells are at much younger stage compared to T cells traditionally stimulated 3 to 4 times weekly. Even after these multiple stimulations, we observed that the T cells retained effector function and were able to lyse peptide-pulsed and tumor cell targets (Figures 2–3). Furthermore, as described, using an established rapid expansion protocol, we demonstrated feasibility of expanding such idiotype-specific T cells in large numbers. We are thus now in a position to perform future in vivo experiments to determine whether adoptively transferred T cells recognizing shared epitopes would persist and be active.
Finally, although outside of the scope of the current study, one of the important questions in human tumor immunology is to determine whether autologous tumor-specific CTLs pre-exist in patients. In light of the immunogenicity of idiotype light chain peptides found in this study, specifically, it will be important to determine whether Ig light chain specific CTLs pre-exist in the blood of patients with B cell tumors and whether such precursors can be expanded by vaccination or adoptive transfer. Previous studies have suggested the existence and function of such tumor specific T cells in B cell tumor patients (36, 37). Experiments testing the ability of human tumor VL peptides to generate autologous Id-specific T cells are under active investigation in our laboratory.
Translational Relevance.
Despite improvements in high dose therapy followed by autologous stem cell transplantation (SCT), disease relapse still remains a major problem for B cell malignancy patients, and even tandem autografts do not appear to be curative for these diseases. Allogeneic SCT following either myeloablative or reduced-intensity conditioning has been shown to induce prolonged disease-free survival in a small percentage of patients suggesting a possible graft versus myeloma (GVM) effect. The ability to prime tumor-specific T cells derived from normal HLA-matched donors, rather than autologous patients, may have direct application to current adoptive transfer strategies, already in clinical testing.
Acknowledgements
This study was support by the Leukemia and Lymphoma Society Specialized Center of Research Grant #7262-08(LWK) and the Biospecimens Core of SPORE Grant P50CA142509
Footnotes
Authorship Contributions
J. W. and L.W. K. designed experiments; J.W, S.C, and M. P. performed experiments; S M, G A, JJ M, QY, MW, and S.N provided critical reagents or suggestions; J. W, S. N, and L.W.K analyzed data and wrote the paper.
References
- 1.Lynch RG, Graff RJ, Sirisinha S, Simms ES, Eisen HN. Myeloma proteins as tumor-specific transplantation antigens. Proc. Natl. Acad Sci USA. 1972;69:1540–1544. doi: 10.1073/pnas.69.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwak LW. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992;327:1209–1215. doi: 10.1056/NEJM199210223271705. [DOI] [PubMed] [Google Scholar]
- 3.Bendandi M, Gocke CD, Kobrin CB, Benko FA, Sternas LA, Pennington R, et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999 Oct;5(10):1171–1177. doi: 10.1038/13928. [DOI] [PubMed] [Google Scholar]
- 4.Neelapu SS, Kwak LW, Kobrin CB, Reynolds CW, Janik JE, Dunleavy K, et al. Vaccine-induced tumor-specific immunity despite severe B-cell depletion in mantle cell lymphoma. Nat Med. 2005;11(9):986–991. doi: 10.1038/nm1290. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Bendandi M, Deng Y, Dunbar C, Munshi N, Jagannath S, et al. Tumor-specific recognition of human myeloma cells by idiotype-induced CD8+ T cells. Blood. 2000 October 15;96(8):2828–2833. 2000. [PubMed] [Google Scholar]
- 6.Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, et al. Vaccination With Patient-Specific Tumor-Derived Antigen in First Remission Improves Disease-Free Survival in Follicular Lymphoma. Journal of Clinical Oncology. 2011 May 31; doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giaccone L, Storer B, Patriarca F, Rotta M, Sorasio R, Allione B, et al. Long-term follow-up of a comparison of nonmyeloablative allografting with autografting for newly diagnosed myeloma. Blood. Jun 16;117(24):6721–6727. doi: 10.1182/blood-2011-03-339945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maloney DG, Molina AJ, Sahebi F, Stockerl-Goldstein KE, Sandmaier BM, Bensinger W, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003 Nov 1;102(9):3447–3454. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 9.Lokhorst HM, Schattenberg A, Cornelissen JJ, van Oers MH, Fibbe W, Russell I, et al. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. J Clin Oncol. 2000 Aug;18(16):3031–3037. doi: 10.1200/JCO.2000.18.16.3031. [DOI] [PubMed] [Google Scholar]
- 10.Kwak LW. Transfer of myeloma idiotype-specific immunity from an actively immunized marrow donor. Lancet. 1995;345:1016–1020. doi: 10.1016/s0140-6736(95)90757-2. [DOI] [PubMed] [Google Scholar]
- 11.Neelapu SS, Munshi NC, Jagannath S, Watson TM, Pennington R, Reynolds C, et al. Tumor antigen immunization of sibling stem cell transplant donors in multiple myeloma. Bone Marrow Transplant. 2005 Aug;36(4):315–323. doi: 10.1038/sj.bmt.1705057. [DOI] [PubMed] [Google Scholar]
- 12.Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008 Jan 1;329(1–2):112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nijman HW, Houbiers JG, Vierboom MP, van der Burg SH, Drijfhout JW, D'Amaro J, et al. Identification of peptide sequences that potentially trigger HLA-A2.1-restricted cytotoxic T lymphocytes. Eur J Immunol. 1993 Jun;23(6):1215–1219. doi: 10.1002/eji.1830230603. [DOI] [PubMed] [Google Scholar]
- 14.Hida N, Maeda Y, Katagiri K, Takasu H, Harada M, Itoh K. A simple culture protocol to detect peptide-specific cytotoxic T lymphocyte precursors in the circulation. Cancer Immunol Immunother. 2002 Jun;51(4):219–228. doi: 10.1007/s00262-002-0273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of Tumor-Infiltrating Lymphocytes and Interleukin-2 in the Immunotherapy of Patients with Metastatic Melanoma. New England Journal of Medicine. 1988;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 16.Pavelko KD, Hansen MJ, Pease LR. CTL Activation Using the Natural Low-Affinity Epitope 222–229 from Tyrosinase-Related Protein 1 Leads to Tumor Rejection. Cancer Res. 2009 April 1;69(7):3114–3120. doi: 10.1158/0008-5472.CAN-08-2448. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Kwak LW, Young HA, Pennington RW, Weeks SD. Vaccination with syngeneic, lymphoma-derived immunoglobulin idiotype combined with granulocyte/macrophage colony-stimulating factor primes mice for a protective T-cell response. Proc Natl Acad Sci USA. 1996;93:10972–10977. doi: 10.1073/pnas.93.20.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neelapu SS, Lee S-T, Qin H, Cha S-C, Woo AF, Kwak LW. Therapeutic lymphoma vaccines: importance of T-cell immunity. Expert Review of Vaccines. 2006;5(3):381–394. doi: 10.1586/14760584.5.3.381. [DOI] [PubMed] [Google Scholar]
- 19.Strothmeyer AM, Papaioannou D, Duhren-von Minden M, Navarrete M, Zirlik K, Heining-Mikesch K, et al. Comparative analysis of predicted HLA binding of immunoglobulin idiotype sequences indicates T cell-mediated immunosurveillance in follicular lymphoma. Blood. Sep 9;116(10):1734–1736. doi: 10.1182/blood-2010-02-270199. [DOI] [PubMed] [Google Scholar]
- 20.King CA, Wills MR, Hamblin TJ, Stevenson FK. Idiotypic IGM on a B-Cell Surface Requires Processing for Recognition by Anti-idiotypic T Cells. Cellular Immunology. 1993;147(2):411–424. doi: 10.1006/cimm.1993.1080. [DOI] [PubMed] [Google Scholar]
- 21.Baskar S, Kobrin CB, Kwak LW. Autologous lymphoma vaccines induce human T cell responses against multiple, unique epitopes. J Clin Invest. 2004;113:1498–1510. doi: 10.1172/JCI20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansson L, Rabbani H, Fagerberg J, Osterborg A, Mellstedt H. T-cell epitopes within the complementarity-determining and framework regions of the tumor-derived immunoglobulin heavy chain in multiple myeloma. Blood. 2003 Jun 15;101(12):4930–4936. doi: 10.1182/blood-2002-04-1250. [DOI] [PubMed] [Google Scholar]
- 23.Trojan A, Schultze JL, Witzens M, Vonderheide RH, Ladetto M, Donovan JW, et al. Immunoglobulin framework-derived peptides function as cytotoxic T-cell epitopes commonly expressed in B-cell malignancies. Nat Med. 2000;6(6):667–672. doi: 10.1038/76243. [DOI] [PubMed] [Google Scholar]
- 24.Curti A, Tosi P, Comoli P, Terragna C, Ferri E, Cellini C, et al. Phase I/II clinical trial of sequential subcutaneous and intravenous delivery of dendritic cell vaccination for refractory multiple myeloma using patient-specific tumour idiotype protein or idiotype (VDJ)-derived class I-restricted peptides. Br J Haematol. 2007 Nov;139(3):415–424. doi: 10.1111/j.1365-2141.2007.06832.x. [DOI] [PubMed] [Google Scholar]
- 25.Dabadghao S, Bergenbrant S, Anton D, He W, Holm G, Yi Q. Anti-idiotypic T-cell activation in multiple myeloma induced by M-component fragments presented by dendritic cells. Br J Haematol. 1998 Mar;100(4):647–654. doi: 10.1046/j.1365-2141.1998.00633.x. [DOI] [PubMed] [Google Scholar]
- 26.Fagerberg J. T-cell-epitope mapping of the idiotypic monoclonal IgG heavy and light chains in multiple myeloma. Int J Cancer. 1999;80:671–680. doi: 10.1002/(sici)1097-0215(19990301)80:5<671::aid-ijc7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S, Haimovich J, Hollander N. Dendritic cell-based therapeutic vaccination against myeloma: vaccine formulation determines efficacy against light chain myeloma. J Immunol. 2009 Feb 1;182(3):1667–1673. doi: 10.4049/jimmunol.182.3.1667. [DOI] [PubMed] [Google Scholar]
- 28.Wen YJ, Ling M, Lim SH. Immunogenicity and cross-reactivity with idiotypic IgA of VH CDR3 peptide in multiple myeloma. Br J Haematol. 1998;100:464–468. doi: 10.1046/j.1365-2141.1998.00592.x. [DOI] [PubMed] [Google Scholar]
- 29.Jorgensen T, Bogen B, Hannestad K. T helper cells recognize an idiotope located on peptide 88–114/117 of the light chain variable domain of an isologous myeloma protein (315) J Exp Med. 1983 Dec 1;158(6):2183–2188. doi: 10.1084/jem.158.6.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsay AG, Clear AJ, Kelly G, Fatah R, Matthews J, Macdougall F, et al. Follicular lymphoma cells induce T-cell immunological synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood. 2009 Sep 28; doi: 10.1182/blood-2009-04-217687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P, et al. Activation of natural regulatory T cells by IgG Fc-derived peptide "Tregitopes". Blood. 2008 Oct 15;112(8):3303–3311. doi: 10.1182/blood-2008-02-138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoges S. Clinical benefit associated with idiotypic vaccination in patients with follicular lymphoma. J Natl Cancer Inst. 2006;98:1292–1301. doi: 10.1093/jnci/djj358. [DOI] [PubMed] [Google Scholar]
- 33.Lum LG, Seigneuret MC, Storb R. The transfer of antigen-specific humoral immunity from marrow donors to marrow recipients. J Clin Immunol. 1986 Sep;6(5):389–396. doi: 10.1007/BF00915378. [DOI] [PubMed] [Google Scholar]
- 34.Wimperis JZ, Brenner MK, Prentice HG, Reittie JE, Karayiannis P, Griffiths PD, et al. Transfer of a functioning humoral immune system in transplantation of T-lymphocyte-depleted bone marrow. Lancet. 1986 Feb 15;1(8477):339–343. doi: 10.1016/s0140-6736(86)92315-9. [DOI] [PubMed] [Google Scholar]
- 35.Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994 Apr 28;330(17):1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 36.Wen Y-J, Barlogie B, Yi Q. Idiotype-specific cytotoxic T lymphocytes in multiple myeloma: evidence for their capacity to lyse autologous primary tumor cells. Blood. 2001 March 15;97(6):1750–1755. doi: 10.1182/blood.v97.6.1750. 2001. [DOI] [PubMed] [Google Scholar]
- 37.Wen YJ, Min R, Tricot G, Barlogie B, Yi Q. Tumor lysate-specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood. 2002 May 1;99(9):3280–3285. doi: 10.1182/blood.v99.9.3280. [DOI] [PubMed] [Google Scholar]