Abstract
Deficits in endothelial cell repair mechanisms are thought to contribute to the etiology of endothelial dysfunction and, subsequently, cardiovascular disease (CVD). CD31+ T cells or so-called “angiogenic T cells” are a newly defined T cell subset that exhibit favorable vascular qualities and show a strong negative relation with atherosclerotic disease severity. Despite growing evidence that CD31+ T cells are important for vascular homeostasis, it is currently unknown if CD31+ T cell number and function are related to endothelial function and CVD risk in healthy adults. To address this question, we studied 24 healthy adult men (ages: 21–70). Endothelial function was assessed by the forearm blood flow (FBF) response to intra-arterial infusion of acetylcholine (ACh) and CVD risk was estimated by Framingham Risk Score (FRS). CD31+ T cell number was determined by fluorescence-activated cell sorting. Magnetic-activated cell sorting was used to isolate CD31+ T cells for Boyden chamber migration. No relation was observed between CD31+ T cell number and FBF response to ACh or FRS. However, CD31+ T cell migration to stromal cell-derived factor (SDF)-1α and vascular endothelial growth factor (VEGF) was positively correlated with FBF response to ACh (r=0.43 for SDF-1α; r=0.38 for VEGF; both P<0.05) and inversely related to FRS (r=−0.53 for SDF-1α; r=−0.48 for VEGF; both P<0.05). These findings demonstrate that CD31+ T cell function, but not number, is associated with in vivo endothelial function and CVD risk in healthy adult men.
Keywords: Vascular Function, Angiogenic T cells, Vascular Repair
Introduction
Beyond their well-established role in regulating the adaptive immune response, T cells have been shown to participate in vascular maintenance and repair [1, 2]. Recently, a novel T cell population characterized by the expression of CD31 antigen has been shown to promote endothelial repair and revascularization [3, 4]. Data from our laboratory [5] and others [3] suggest that these so-called “angiogenic T cells” exhibit a distinct vasculogenic phenotype characterized by enhanced migratory capacity and angiogenic cytokine release compared with CD31− T cells. It was recently reported that circulating CD31+ T cell number is inversely correlated with age and cardiovascular disease (CVD) risk factors in patients undergoing coronary angiography, suggesting that these cells may be used as a novel surrogate biomarker for vascular disease risk [3]. This notion is supported by the observation that circulating CD31+ T cell number is inversely correlated with atherosclerotic lesion size in patients with atherosclerotic abdominal aortic aneurysm [6]. However, a link between CD31+ T cell function and CVD risk has not yet been explored, nor has the relation between CD31+ T cell number and CVD risk in healthy adults. Furthermore, it is currently unknown if CD31+ T cell number and function are associated with endothelium-dependent vasodilation, a hallmark characteristic of endothelial dysfunction that is etiologically linked to the initiation and development of atherosclerotic vascular disease [7]. Accordingly, we tested the hypothesis that CD31+ T cell number and function are associated with endothelium-dependent vasodilation and Framingham Risk Score in healthy adult men.
Methods
Twenty-four healthy adult men were studied. All subjects were non-smokers, non-medicated and free of overt cardiometabolic disease as assessed by medical history, physical examination and fasting blood chemistries. Subjects were excluded from the study if they presented a history or evidence of: hepatic, renal, or hematological disease; peripheral vascular disease; stroke; diabetes (fasting plasma glucose > 7.0 mmol/L) [8]; dyslipoproteinemia (total cholesterol ≥ 6.2 mmol/L, triglycerides ≥ 3.5 mmol/L) [9]; and hypertension (arterial blood pressure ≥ 140/90 mmHg) [10]. Men over the age of 40 years were further evaluated for clinical evidence of coronary artery disease with resting and maximal-exercise electrocardiograms and blood pressure measurements. CD31+ T cells were isolated from peripheral blood samples. Before participation, all of the subjects provided written informed consent according to the guidelines of the University of Colorado at Boulder.
CD31+ T cell number was determined by fluorescence-activated cell sorting analysis in 13 of the 24 adults as previously described [11]. Briefly, non-viable cells were excluded with propidium iodide and the remaining cells were analyzed for events double-positive for CD3 and CD31. Following isolation of CD31+ T cells via magnetic-activated cell sorting (MACS), migratory capacity was measured using a modified Boyden chamber technique with stromal cell-derived factor-1α (SDF-1α, Sigma Aldrich; 20 ng/mL) or vascular endothelial growth factor (VEGF, R&D Systems; 2 ng/mL) as a chemoattractant.
Forearm blood flow (FBF) was measured using venous occlusion plethysmography at baseline and in response to incremental doses of acetylcholine (ACh; 4.0–16.0 μg/100 mL tissue/min) as previously described [12]. Total FBF in response to ACh was calculated as the area under the curve above baseline using a trapezoidal model.
Framingham Risk Score was calculated as previously described [13]. Age, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, blood pressure, diabetes, and smoking status were used as risk variables.
Relations between variables of interest were assessed by linear regression analysis. All values are expressed as mean ± SEM. Statistical significance was set at P < 0.05.
Results
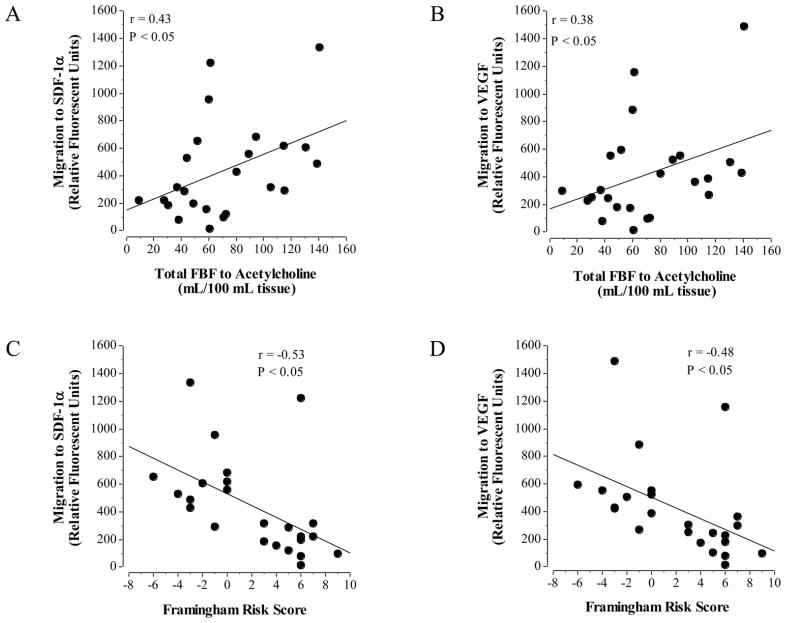
Selected subject characteristics are presented in Table 1. Overall, CD31+ T cell migratory capacity, but not number, was related to endothelial vasodilator function and Framingham Risk Score. A significant relation was observed between total FBF response to ACh and CD31+ T cell migration to both SDF-1α (r = 0.43; P < 0.05; Figure 1A) and VEGF (r = 0.38; P < 0.05; Figure 1B). Framingham risk score was inversely related to CD31+ migration to SDF-1α (r = −0.53; P < 0.05; Figure 1C) and VEGF (r = −0.48; P < 0.05; Figure 1D). There was no relation between CD31+ T cell number (range: 23.0 – 46.7% of CD3+ cells) and total FBF response to ACh (r = 0.30; P = 0.32) or Framingham Risk Score (r = 0.09, P = 0.77).
Table 1.
Selected Subject Characteristics
| Variable | N = 24 |
|---|---|
| Age (yr) | 46 ± 3 |
| Body mass (kg) | 81.0 ± 2.7 |
| BMI (kg/m2) | 25.2 ± 0.7 |
| Body fat (%) | 22.7 ± 1.7 |
| Waist circumference (cm) | 90.3 ± 2.4 |
| Waist-to-hip ratio | 0.90 ± 0.02 |
| Systolic BP (mmHg) | 122 ± 2 |
| Diastolic BP (mmHg) | 76 ± 2 |
| Total Cholesterol (mmol/L) | 4.5 ± 0.2 |
| HDL-Cholesterol (mmol/L) | 1.2 ± 0.1 |
| LDL-Cholesterol (mmol/L) | 2.9 ± 0.2 |
| Triglycerides (mmol/L) | 1.0 ± 0.1 |
| Glucose (mmol/L) | 4.8 ± 0.1 |
| Insulin (pmol/L) | 42.3 ± 6.0 |
| HOMA-IR | 1.6 ± 0.2 |
| CD31+ T Cell Migration to SDF-1α (Relative Fluorescent Units) | 447.9 ± 73.8 |
| CD31+ T Cell Migration to VEGF (Relative Fluorescent Units) | 421.8 ± 70.5 |
| Total FBF to Acetylcholine (mL/100 mL tissue) | 74.1 ± 7.8 |
| Framingham Risk Score | 2.1 ± 0.9 |
|
| |
| Variable measured in 13 of the 24 subjects | |
|
| |
| CD31+ T Cell Number (% of CD3+ cells) | 36.1 ± 1.9 |
BMI = body mass index; BP = blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein, HOMA-IR = homeostasis model assessment of insulin resistance, SDF = stromal cell-derived factor, VEGF = vascular endothelial growth factor
Data presented as mean ± SEM
Figure 1.
Relation between CD31+ T cell migration (to both SDF-1α or VEGF) and endothelium-dependent vasodilation (A & B) and Framingham Risk Score (C & D).
Discussion
The novel finding of the present study is that CD31+ T cell migratory capacity, but not number, is positively related to endothelium-dependent vasodilation and inversely related to Framingham Risk Score in healthy adult men. To our knowledge, this is the first study to demonstrate an association between CD31+ T cell migratory function and endothelial function and CVD risk.
Dysfunctional vascular repair processes are thought to contribute etiologically to the development of endothelial dysfunction and the subsequent progression of CVD [14]. Although T cells have long been known to participate in neovascularization [1, 2], CD31+ T cells have recently emerged as a functionally discrete “angiogenic T cell” subpopulation with distinct vasculogenic qualities [3, 5]. Hur and colleagues [3] demonstrated that CD31+ T cells possess superior tubule formation and transendothelial migration compared with other T cell subsets, as well as a greater ability to enhance endothelial cell proliferation and function in vitro. These angiogenic characteristics have also been observed in CD31+ T cells from healthy adult humans; specifically, CD31+ T cells exhibit greater migratory and angiogenic cytokine release capacity than CD31− T cells [5]. Of note, clinical data indicating an inverse relation between circulating CD31+ T cell number and cardiovascular risk factors and disease severity has fueled interest in these cells as a potential therapeutic target for CVD and speculation that angiogenic T cells may represent a novel biomarker of CVD risk [3, 6]. The present finding that CD31+ T cell migratory capacity is related to both endothelial vasodilator function and Framingham Risk Score in healthy men across the adult age range supports and extends this idea by providing initial evidence of a link between angiogenic T cell migratory function, endothelial vasomotor function, and CVD risk. Because the ability of angiogenic cells to migrate to sites of damage is critical in mediating vascular repair and neovascularization [14], diminished CD31+ T cell migration may impair vascular repair processes, thereby facilitating the development of endothelial dysfunction and subsequent CVD.
In contrast to function, there was no significant relation between CD31+ T cell number and endothelium-dependent vasodilation or Framingham Risk Score. This finding is inconsistent with previous data from Hur et al. [3] who reported a significant inverse relation between CD31+ T cell number and Framingham Risk Score in patients undergoing coronary angiography. However, the contrasting findings between these studies may be explained by differences in the overall health of the populations studied. The subjects in the present study were highly screened, healthy, non-smoking adult men without clinically overt cardiometabolic disease, while the group of patients studied by Hur and colleagues included cigarette smokers and a large proportion (~50%) of patients with hypertension and/or type 2 diabetes [3]. Thus, in the absence of disease and/or cardiometabolic risk factors, CD31+ T cell number is not related to endothelial vasodilator function or cardiovascular risk. It is possible that the number of circulating CD31+ T cells may have a more significant role in influencing endothelial function and CVD risk under pathologic conditions.
In conclusion, CD31+ T cell migratory ability, but not number, is positively related to endothelium-dependent vasodilation and inversely related to Framingham Risk Score in healthy adult men. Although these findings support the notion that CD31+ T cell function may be a novel biomarker for endothelial function and cardiovascular risk in healthy men, larger gender-balanced studies are needed to confirm this postulate.
Acknowledgments
We thank all of the subjects who participated in the study as well as Yoli Casas for her administrative assistance. This study was supported by National Institutes of Health Awards HL076434, HL077450, and RR00051, and American Heart Association Awards 0840167N and 13094097.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, et al. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation. 2003 Jul 15;108(2):205–10. doi: 10.1161/01.CIR.0000079225.50817.71. [DOI] [PubMed] [Google Scholar]
- 2.Stabile E, Kinnaird T, la Sala A, Hanson SK, Watkins C, Campia U, et al. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation. 2006 Jan 3;113(1):118–24. doi: 10.1161/CIRCULATIONAHA.105.576702. [DOI] [PubMed] [Google Scholar]
- 3.Hur J, Yang HM, Yoon CH, Lee CS, Park KW, Kim JH, et al. Identification of a novel role of T cells in postnatal vasculogenesis: characterization of endothelial progenitor cell colonies. Circulation. 2007 Oct 9;116(15):1671–82. doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
- 4.Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007 Dec;27(12):2514–23. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 5.Kushner EJ, MacEneaney OJ, Morgan RG, Van Engelenburg AM, Van Guilder GP, DeSouza CA. CD31+ T cells represent a functionally distinct vascular T cell phenotype. Blood Cells Mol Dis. 2010 Mar-Apr;44(2):74–8. doi: 10.1016/j.bcmd.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Caligiuri G, Rossignol P, Julia P, Groyer E, Mouradian D, Urbain D, et al. Reduced immunoregulatory CD31+ T cells in patients with atherosclerotic abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2006 Mar;26(3):618–23. doi: 10.1161/01.ATV.0000200380.73876.d9. [DOI] [PubMed] [Google Scholar]
- 7.Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998 Jul 6;105(1A):32S–9S. doi: 10.1016/s0002-9343(98)00209-5. [DOI] [PubMed] [Google Scholar]
- 8.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009 Jan;32( Suppl 1):S62–7. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fedder DO, Koro CE, L’Italien GJ. New National Cholesterol Education Program III guidelines for primary prevention lipid-lowering drug therapy: projected impact on the size, sex, and age distribution of the treatment-eligible population. Circulation. 2002 Jan 15;105(2):152–6. doi: 10.1161/hc0202.101971. [DOI] [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 11.Kushner EJ, Weil BR, MacEneaney OJ, Morgan RG, Mestek ML, Van Guilder GP, et al. Human Aging and CD31+ T Cell Number, Migration, Apoptotic Susceptibility and Telomere Length. J Appl Physiol. 2010 Dec;109(6):1756–61. doi: 10.1152/japplphysiol.00601.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Guilder GP, Stauffer BL, Greiner JJ, Desouza CA. Impaired endothelium-dependent vasodilation in overweight and obese adult humans is not limited to muscarinic receptor agonists. Am J Physiol Heart Circ Physiol. 2008 Apr;294(4):H1685–92. doi: 10.1152/ajpheart.01281.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998 May 12;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 14.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med. 2004 Oct;82(10):671–7. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]