Abstract
Although there are 55 serotypes of adenovirus that infect humans, Ad5 is the most widely studied due to the availability of commercial kits for its genetic manipulation. In fact, engineered adenovirus serotype 5 is currently being used in all of the 87 global clinical trials utilizing adenovirus for the treatment of cancer. Unfortunately, Ad5 is one of the most seroprevalent serotypes, meaning that this virus has to confront additional immunological barriers to be effective in Ad5-immune patients. In this work, we compare Ad5 to 13 other adenoviral serotypes from species B, C, D, and E for oncolytic potential in both immunodeficient mouse and immunocompetent hamster models. Our results indicate that species D adenoviruses are not effective oncolytics against most solid tumors. Conversely, lower seroprevalence Ad6 and Ad11 hade anti-cancer activity comparable to Ad5. This work strongly supports the consideration of Ad6-based oncolytic therapies for the treatment of breast, ovarian, kidney, and liver tumors.
Keywords: adenovirus, oncolytic, cancer
Introduction
Adenoviruses (Ads) are non-enveloped, double-stranded DNA viruses that cause respiratory, ocular, digestive, and other types of infections 1. Wild-type Ads have been tested as oncolytic agents against human cancers since the mid-1950s. The premise of this work was that the lytic nature of these viruses could be exploited to kill malignant cells in patients. Indeed, based on their ability to kill HeLa cervical carcinoma cells in vitro, several Ads were tested soon after their discovery in clinical trials against cervical cancer where they showed some efficacy after local or systemic therapy 2, 3. Since then, most adenoviral research has focused on using and manipulating adenovirus serotype 5 (Ad5) as an oncolytic virus or as a cancer gene therapy vector. In fact, every one of the 87 current adenoviral-based cancer clinical trials utilize Ad5 or an Ad5 derivative (www.clinicaltrials.gov).
While Ad5 is potent in a number of solid cancers, from an immunologic perspective, it may be difficult to translate into humans. 27 to 100% of humans are already immune to this serotype of Ad 4, 5. Moreover, Ad5 uses coxsackie and adenovirus receptor (CAR) as a receptor and this receptor can be down-regulated on certain cancers 6, 7.
Given these issues, recent efforts have been directed at screening human and non-human adenoviruses for better activity against different cancers 8-13. We and other groups have recently focused on screening viruses that have lower seroprevalence in humans than Ad5 to avoid neutralization of these vectors in patients with pre-existing immunity 11-13.
Testing against solid cancers including breast, ovarian, prostate, and hepatocellular carcinomas in vitro revealed that species B viruses Ad11 and Ad35 and species C viruses Ad5 and Ad6 could kill cells to varied degrees 11, 12. In vivo testing against human prostate cancer xenografts in nude mice showed that Ad5, Ad6, and Ad11 were effective by intravenous (i.v.) or intratumoral (i.t.) injection, but Ad35 was not 12. In vitro and in vivo testing against ovarian cancers showed that Ad3, Ad7, Ad11, and Ad14 were more effective than CAR-binding or CD46-alone binding viruses 11. While these viruses were potent, they also induced epithelial to mesenchymal transition (EMT) that is associated with increased metastasis.
Given the identification of new Ads with lower seroprevalence and equal or better oncolytic activity against prostate and ovarian cancers, we set out to screen a wider variety of Ad serotypes against human breast and ovarian cancer and against animal tumors that might allow testing in immunocompetent models. This study compares Ad5 to other species C adenoviruses as well as those from species B, D, and E to address these questions. In addition to helping to identify viruses that are more infectious and more oncolytic than Ad5, this work also provides alternatives to Ad5 that may be less immunogenic and less liver tropic.
Materials and Methods
Cell culture
Human breast carcinoma cell lines MDA-MB-231, MDA-MB-468, SKBr3, BT-474; human ovarian carcinoma SKOV3; human hepatocellular carcinoma Hep3B; Chinese hamster ovary (CHO); and Syrian hamster (HaK) cells were purchased from the American Type Culture Collection (ATCC) (Manassas, VA). 293 Human embryonic kidney cells were purchased from Microbix (Toronto, Canada). All cell lines were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) (HyClone: Rockford, IL) and penicillin/streptomycin (Hyclone).
Stable CHO cell lines
CHO-CAR and CHO-CD46 cells were generated by transient transfection of CHO cells with plasmids expressing the two receptors using Lipofectamine 2000 (Invitrogen: Carlsbad, CA) and following manufacturer protocols. Positively transfected cells were selected for using G418 antibiotic at a concentration of 900 ng/mL. Expression of the coxsackie and adenoviral receptor (CAR), and CD46 were confirmed using flow cytometry (data not shown).
Viruses
Ad4, Ad5, Ad6, Ad7, Ad11, Ad17, Ad24, Ad26, Ad28, Ad30, Ad35, Ad45, and Ad48 were obtained from the ATCC. Viruses were propagated in 293 cells. Viruses were purified by CsCl purification and quantitated by determining the optical density at 260nm (OD260).
Cell Viability Assay
4×104 of the indicated cells were plated in each well of a 96-well plate and grown overnight. Cells were then infected with 4×107 viral particles and left to incubate for the indicated time, either 0, 3, or 7 days. On the indicated day, 40 μl of 2.5 μg/ml 3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyl tetrazolium bromide (MTT, Sigma: St. Louis, MO) in PBS was added to each well of the plate and left to incubate at 37°C for four hours. After 4 hours, wells were aspirated and 50 μl of 0.01N HCl in isopropanol was added to the wells to dissolve the crystals. After a five minute room temperature incubation, the plates were read for absorbance at 595 nm using a Beckman-Coulter Multimode DTX 880 plate reader. Percent viability was determined by comparing the absorbance intensity to that of non-infected cells. N = 4.
Viability at Multiple Multiplicities of Infection
4×104 of the indicated cells were plated in each well of a 96-well plate and grown overnight. Cells were then infected at the indicated multiplicity of infection for seven days. Cells were then assayed by standard MTT assay as described above. N = 3.
Human Breast and Ovarian Carcinoma Xenografts
3×106 of either MDA-MB-468 human breast or SKOV3 human ovarian carcinoma cells were injected subcutaneously into the hind flank of 4-6 week-old nude mice (Harlan: Indianapolis, IN). After palpable tumors formed (14 days), the mice were injected intratumorally with 3×1010 viral particles of wild-type virus in 100 μl total volume of Hank's buffered saline solution (HBSS). Control groups were injected with buffer alone. Tumors were measured every three days. Volume was calculated as ½ length × width × width. Animals were sacrificed when tumor volume exceeded 2000 μl, were found with ulcerated tumors, or had weight loss greater than 20%. N = 8 mice for SKOV3 model. N = 10 mice for MDA-MB-468 model.
Hamster Tumor Model
Four- to five-week-old female Syrian (Golden) hamsters (Mesocricetus auratus) were obtained from Harlan Sprague Dawley (Indianapolis, IN). Hamsters were injected with 2×107 HAK (hamster renal cell line) cells subcutaneously into the hind flank. Total injection volume was 200 μl. Cells were diluted in HBSS. When tumor volume was between 100 and 200 μl, the tumors were injected intratumorally with 100 μl of HBSS with or without 3×1010 viral particles. Tumors were measured every seven days. Volume was calculated as ½ length × width × width. Animals were sacrificed when tumor volume exceeded 2000 μl or were observed with ulcerated tumors. N = 6 hamsters/group.
Statistical Analysis
Unless otherwise stated all statistical values for cell viability assays represent p-values from repeated measures ANOVA analysis with Bonferroni post-test. For tumor measurements, p-values shown are from two-tailed T-test. Analysis was performed using the statistical package within the GraphPad PRISM 4 software. P ≤ 0.05 was considered significant.
Results
Virus Selection
Adenoviral serotypes were selected based largely on having low seroprevalence in humans as well as to represent most species (Figure 1). Viruses from species A were excluded because of their possible association with tumorigenesis 4, 5. Species F Ads were excluded because these viruses are “fastidious” in their specificity for cells of the digestive tract and have proven ineffective as oncolytics in our past testing 13. Ad5 was included as the benchmark oncolytic virus despite having high seroprevalence.
Figure 1. Phylogenetic dendrogram of adenoviral serotypes grouped by fiber protein homology.
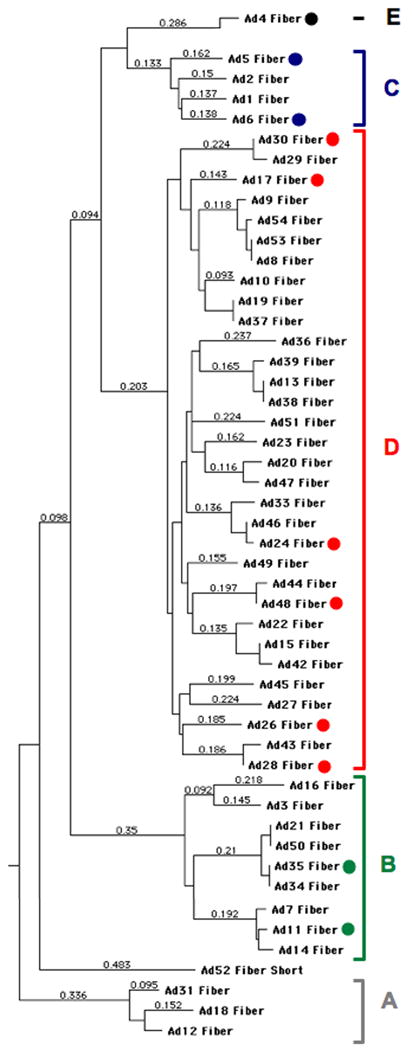
Serotypes marked with a dot were tested in this work. Species designations are shown as letters to the right of the dendrogram.
Cell infection in vitro by Ads is largely dependent on the interaction between cellular receptors and the adenoviral fiber and penton base proteins. High affinity “docking” with cells is generally thought to be mediated by interactions of the fiber protein of a given Ad with its receptor, either the coxsackie and adenovirus receptor (CAR), CD46, or other receptors (reviewed in 14). Species B viruses use CD46, desmoglein 2 15 and perhaps CD80 and CD86 as receptors. Species C and E viruses use CAR as their primary receptor and perhaps also bind heparin sulfate proteoglycans, MHC I, and VCAM-1. Species D viruses can bind CAR, CD46, and sialic acid, but their fibers are typically too short to use CAR and CD46 as efficient receptors 5, 16, 17. The viruses that were chosen for this study are indicated by color-coded circles on the phylogenetic dendrogram of their fiber proteins in Figure 1. Most Ads bear RGD motifs on their penton bases allowing them to bind αv integrins 14. If high affinity fiber-receptor interactions are available, these integrin interactions may be involved mostly in cell entry rather than cell docking 18. In the absence of high affinity interactions, penton base-integrin interactions can become the primary docking mechanism of viruses to cells 14, 18. The expression of CAR and CD46 on MDA-MB-231, MDA-MB-468, and SKBr3, Hep3B, and SKOV3 cells has previously been published12.
Oncolytic Screen on Human Cancer Cells
To compare the oncolytic efficacy of wild-type adenoviruses from different species against human cancers, selected viruses were incubated at a fixed multiplicity of infection (MOI) of 1,000 virus particles (vp) per cell. MOIs were performed with vp/cell rather than plaque forming units (PFU) per cell as the most conservative comparison between viruses with diverse biologies. This particular important since PFU on a given cell may vary due to virus tropism. For example, the vp/PFU 293 ratio of Ad5 on 293 cells is typically 10 whereas it is approximately 75 for Ad26. Were comparisons performed based on equal PFU, 7.5 times virus would be tested for Ad26 than Ad5.
Based on this, each of the viruses were incubated with human breast carcinoma, ovarian carcinoma, and hepatocellular carcinoma cell lines at 1,000 vp/cell. Viability of these cells was monitored over the course of seven days by MTT assay (Figure 2). Hep3B hepatocellular carcinomas were tested as positive controls for the oncolytic activity of Ad5 and the other serotypes. In these cells, all Ads showed strong cell killing with the exception of Ad35 and Ad48. In SKOV-3 ovarian cancer, all viruses killed the cells except species E Ad4. However, species D viruses had only low activity in contrast to robust killing by Ad7 and species C viruses Ad5 and Ad6 (p ≤ 0.05). Testing on four human breast cancer cell lines revealed that three of the four (MDA-MB-231, MDA-MB-468, and SkBr-3) were relatively resistant to all of the viruses with the exception of Ad5 and Ad6. This is an observational comparison, since viability differences in these breast cancer cell lines did not produce a p-value less than 0.05. In contrast, in the human breast cell line BT-474, many of the viruses showed activity. For these cells, the CAR utilizing viruses Ad5 and Ad6 were most effective (p ≤ 0.05). Ad4 also seemed to have a moderate affect; however the oncolytic cytotoxicity of this virus was not quite statistically significant from the buffer group. In summary, a range of oncolytic activity was observed when 13 Ads were screened. Interestingly, species C viruses Ad5 and its lower seroprevalence family member Ad6 had the greatest oncolytic effect in all of the cell lines that were tested.
Figure 2. Effects of adenoviruses on human cancer cell viability in vitro.
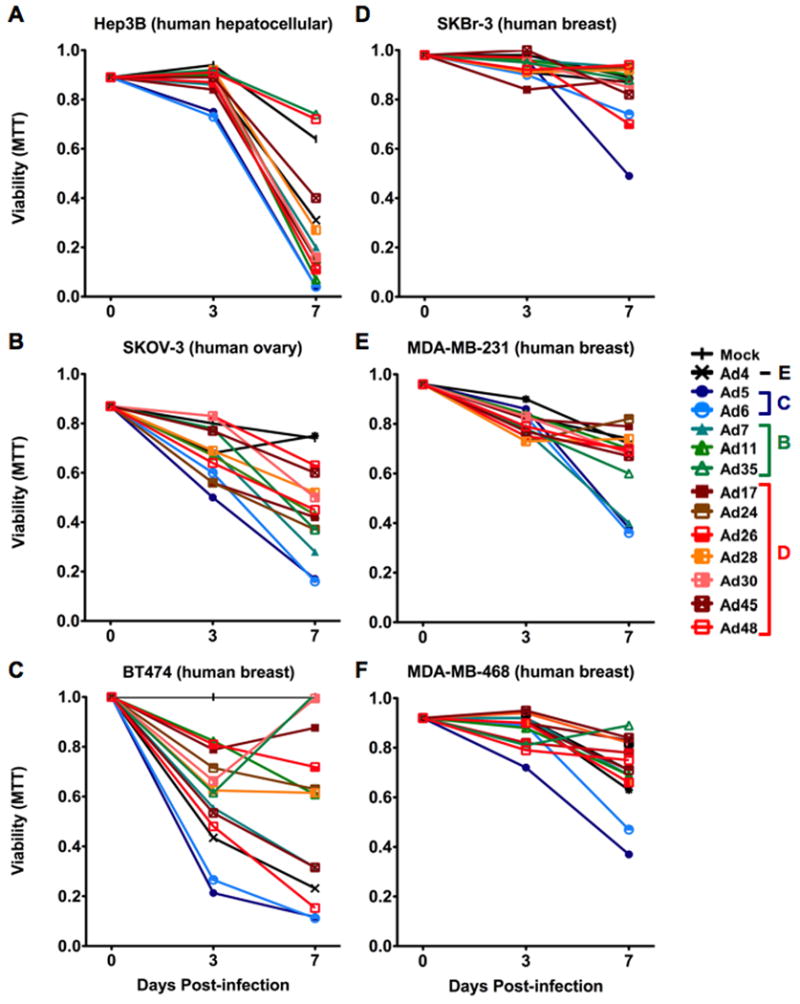
The indicated cells were treated with the indicated Ads and cellular viability was assessed over 7 days by MTT assay. Related viruses are coded with similar colors (species B = green, species C = Blue, Species D = red).
To further explore oncolytic activities, the viruses were tested on four of the cell lines above at MOIs of 0, 102, 104, and 106 vp/cell (Supplemental Figure 1). In these cell lines, trends in viability patterns were similar to those seen in the single MOI time course study (Figure 2). Of note, at varied MOIs, Ad11 demonstrated oncolytic activity that rivalled that of Ad5 and Ad6 that was not as clearly demonstrated in MDA-MD-468 and BT-474 cell single MOI results.
In Vivo Testing of Ad Serotypes Against Human Breast and Ovarian Cancer Xenografts in Immunodeficient Mice
To determine whether the in vitro toxicity study would predict in vivo tumor killing, SKOV3 ovarian and MDA-MB-468 breast cancer tumors were initiated subcutaneously in nude mice. The tumors were then treated with select Ad serotypes by single intratumoral injection with 3×1010 virus particles (vp) of each of the viruses and tumor growth was monitored over time.
For the MDA-MB-468 breast cancer tumors, the greatest inhibition of tumor growth was mediated by single injection of Ad5 (p=0.0002), Ad6 (p=0.0006), Ad11 (p=0.0041), and Ad48 (p=0.0012)(Figure 3A). Ad26 was not effective in treating these tumors (p=.4541), and Ad35 (p=0.0350) had only intermediate level of efficacy. In the SKOV-3 ovarian tumors, single injection of Ads 4 (p ≤ 0.01), 5 (p ≤ 0.05), and 11 (p ≤ 0.0001) were most effective in slowing tumor growth (Figure 3B). Ads 6, 7, 26, and 48 had intermediate levels of killing ability, although none of this cytotoxicity reached statistical significance. Ad35 had the weakest observed effect on SKOV-3 tumor size.
Figure 3. In vivo oncolytic activity of selected adenoviruses against human breast and ovarian cancer xenografts.
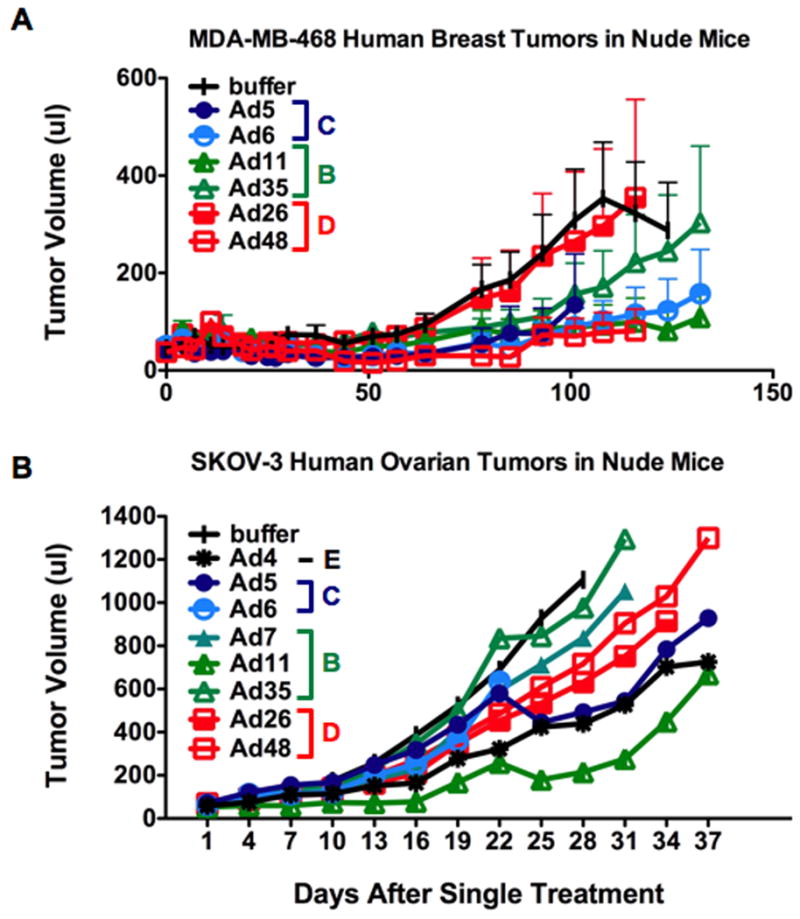
Tumors were implanted subcutaneously in nude mice. Once tumors formed, they were treated a single time by intratumoral injection with 3×1010 viral particles of the indicated viruses and tumor sizes were measured over time. Average tumor size for the group is plotted. Tumor size line terminate when an animal must be sacrificed due to tumor size, tumor hemorrhage, or for other care issues to avoid the artifactual appearance that tumor size for the group have reduced when one animal is removed. A) MDA-MB-468 human breast carcinoma tumors (N = 10 mice per group). B) SKOV3 human ovarian carcinoma tumors (N = 8 mice per group).
Adenovirus Serotype Testing in Non-human Cells
The data in Figure 3 suggests different Ad serotypes may have activity in female cancers in vivo even after single treatment. While useful, in vivo testing against human tumors can only performed in immunodeficient models that remove immune responses against the human cells, but also unfortunately remove immune responses against the viruses as well. To test the viruses in an immunocompetent model, one must have animal cancer cells that will also support viral replication and killing. Ad5 is not thought to efficiently infect and kill most non-human cells. Syrian hamsters are one exception, and their HaK kidney tumor cell line is thought to be a suitable immunocompetent model to test Ad5 19-22.
Given this, the 13 Ad serotypes were tested for oncolysis of HaK cells in vitro. Unlike Ad5, most of the viruses were markedly less effective against HaK cells (Figure 4A). Ad4, Ad11, Ad17 Ad24 mediated transient reductions in MTT activity at day 3, but this rebounded by day 7 under the dynamic conditions of cell killing countered by cell re-growth. Only the species C viruses Ad 5 (p < 0.01) and Ad6 (p < 0.05) efficiently killed the cells without cell re-growth.
Figure 4. Effects of adenoviruses on hamster cancer cells in vitro and in vivo.
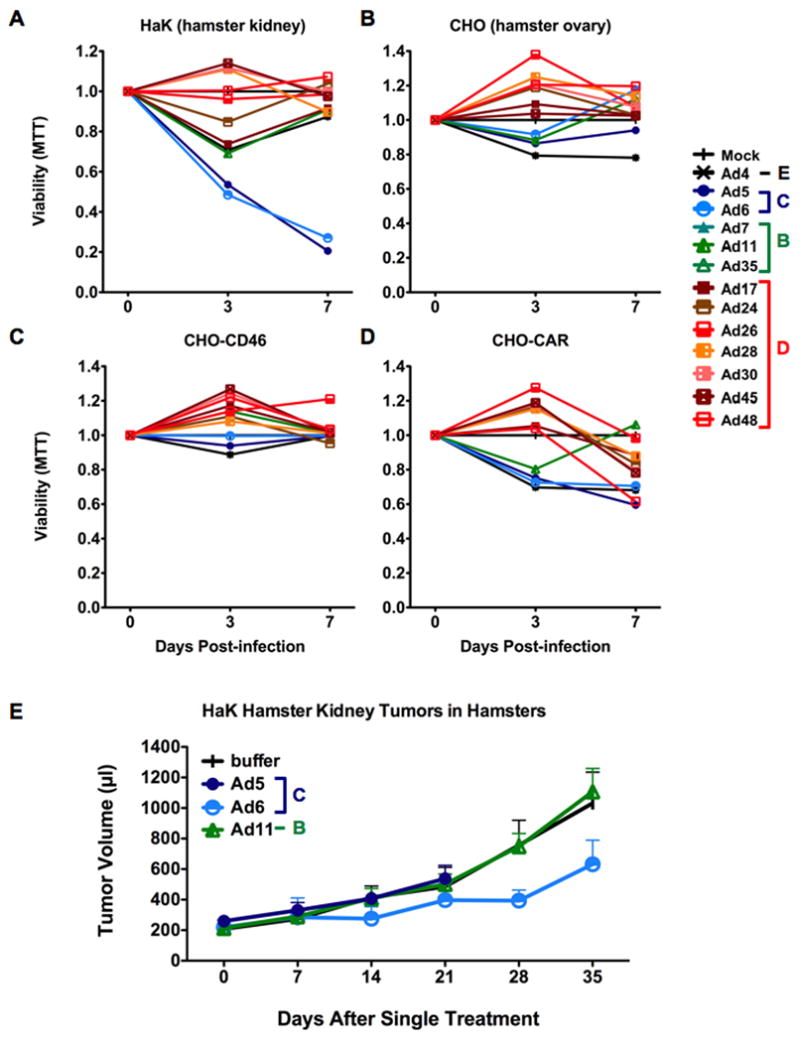
One week cell viability of several hamster cell lines was monitored by trypan blue uptake upon infection with adenovirus (A-D). CHO-CD46 and CHO-CAR cells were derived by transient transfection of CHO cells with CD46 or CAR cellular receptor expression plasmids. E) HaK hamster kidney cell tumor growth was monitored in Syrian Hamsters following intratumoral injection of 3×1010 viral particles in immunocompetent Syrian Hamsters. Average tumor size for the group is plotted as in Figure 3. N = 6 hamsters per group.
These data in HaK cells suggested that Ad5 and Ad6 could infect and kill kidney cancer cells from Syrian hamsters. To test if the Ad serotype panel might have activity against other hamster cells, particularly relevant to female cancers, the 13 viruses were tested against Chinese hamster ovary (CHO) cells (Figure 4B). In this case, CHO cells were not killed by any of the adenoviruses that were tested. This was not surprising for viruses that use CAR or CD46, because CHO cells do not express these receptors. One might have expected the sialic acid-utilizing viruses to kill CHO cells, since sialic acid is a common modification on carbohydrates on cells.
To test whether the presence of natural Ad receptors would increase CHO cell killing, the cells were transfected with CAR or CD46 and the viruses were tested again. Under these conditions, expression of CD46 had little effect on any of the serotypes (Figure 4C). In contrast, the addition of CAR did allow killing by Ads 4, 5, 6, and 48 up to 40% (Figure 4D). These data indicate that some hamster cells are naturally permissive or can be rendered permissive to CAR utilizing Ads. In contrast, these cells do not appear permissive to infection by species B or D adenoviruses.
Oncolytic Testing in Immunocompetent Hamsters
Application of the viruses in female patients will expose them to adaptive immune responses that are not present in the immunodeficient mouse models. Therefore, these viruses need to be tested in an immunocompetent cancer model as a precursor to clinical translation. While CHO cells are ovarian and are more relevant to breast or ovarian cancer treatment, these cells were largely resistant to the viruses. In addition, CHO and CHO-CAR cells are spontaneously rejected from Chinese hamsters after subcutaneous implantation (data not shown).
We therefore tested virus efficacy in the immunocompetent HaK tumor model since it is a current benchmark for Ad5 testing. HaK tumors were initiated subcutaneously in the flanks of Syrian hamsters and they were injected a single time with 3 × 1010 vp of selected Ad serotypes and tumor size was monitored over time (Figure 4E). Injection of either Ad5 or Ad11 failed to reduce tumor growth in the animals. In contrast, one dose of Ad6 delayed tumor growth for over 30 days (p-value for one-tailed T-test was 0.04). These data suggest that species C Ad6 can function as an oncolytic virus in the context of an intact immune system.
Discussion
Although there are over 50 known human adenoviral serotypes, most oncolytic testing has been focused on adenovirus serotype 5. This is largely a result of the vector development of the Ad5 genome and commercial cloning kits designed to allow easy expression of transgenes from replication defective Ad5 vectors. To compare the usefulness of different adenovirus serotypes and species to that of species C adenoviruses, and more specifically Ad5, several wild-type viruses were selected and tested in vitro and in vivo for oncolytic potential. Species A viruses were excluded due to an association between these viruses and oncogenesis. Species F viruses were excluded due their relatively inactivity in previous tests. Ad36 and Ad37 were also excluded because of a possible correlation with human obesity.
Four human breast cancer cell lines were tested, SKBr3, MDA-MB-468, MDA-MB-231, and BT-474. Breast cancers are commonly categorized by the presence or absence of progesterone receptor, estrogen receptor, and HER2 (epidermal growth factor receptor 2) on their cell surface. The presence of any of these receptors is predictive of the patient's response to receptor-targeted treatments. BT-474 cells are positive for all three receptor types. SKBr3 cells are only positive HER2 expression. MDA-MB-231 and MDA-MB-468 are triple-negative for the three receptors, a phenotype that is generally the most difficult to treat. When 13 Ad serotypes were tested against these breast cancer cell lines, Ad5 and Ad6 were the most potent at killing these cell lines. Previous receptor testing on SkBr-3, MDA-MB-231, and MDA-MB-468 indicated that these cells express both CD46 and CAR, although CAR levels are relatively low 12. These data suggest that Ad5 and Ad6 may function well as an adjuvant therapy in breast cancer. This data also indicates that these viruses are good oncolytic platforms that will likely perform better if they are engineered to target receptors other than CAR.
In contrast to the other cell lines tested in vitro, Hep3B and BT-474 cells were readily killed by many of the viruses tested. Killing by species B and C viruses was consistent highly likely due to high expression of cognate receptors CAR and CD46 12. When the viruses were tested at multiple MOIs (Supplemental Figure 1), it was interesting to note that 70-99% killing could be obtained for the CAR positive cell lines BT-474 and Hep3B (data not shown) within seven days at an MOI of just 100 viral particles/cell. For CAR negative cell lines the similar killing required an MOI of 1,000 or greater.
The ability of in vitro studies to predict in vivo activity is variable. In the MDA-MB-468 breast carcinoma xenografts, all of the adenoviruses except Ad26 were effective at reducing tumor growth after single injection. For Ad5 and Ad6, the in vitro study was a reasonable predictor of efficacy. However, for most of the non-subgroup C adenoviruses, in vivo efficacy was better than the in vitro prediction.
For SKOV3 ovarian carcinoma xenografts, Ad4, Ad5, and Ad11 had the greatest reduction in tumor growth. Aside from Ad5, the predictive value of the in vitro studies was poor. The results with Ad11 are consistent with previous work demonstrating that some species B viruses are particularly effective against ovarian cancer in vivo 11. This efficacy appears to be due to the ability of Ad11 to induce epithelial to mesenchymal transition (EMT) to ovarian cancer cells making them more permissive to infection in vivo. While this phenotypic change may make tumor cells more permissive to oncolytics, it may also generate tumor stem cells leading eventually to metastasis. Further study is needed to rule out this possibility before pursuing Ad11 as a safe oncolytic and to determine if Ad4 or Ad5 are viable instead.
Results in immunodeficient nude mice may misrepresent the persistence of viral effects in an immunocompetent human. When select viruses were tested in immunocompetent hamsters, single treatment of HaK tumors with Ad6 effectively reduced tumor growth, but Ad5 and Ad11 did not. Ad11 was not expected to be effective based on in vitro data, but Ad5 was. These data suggest that in vitro infection, spread, and oncolysis of the tested cell lines may not mimic the in vivo process. These results may also suggest, but certainly do not prove, an advantage of using Ad6 in the presence of an intact immune system.
While Ad5 and Ad6 are both species C viruses with high homology, they actually have substantial variation in their fiber and hexon proteins 23. Differences in the hypervariable regions (HVRs) of the hexons Ad5 and Ad6 improves the ability of Ad6 to infect cells after intravenous injection 23, 24 apparently due to reduced uptake of Ad6 by macrophages and Kupffer cells 24. This reduced phagocytosis of Ad6 versus Ad5 may allow the virus to persist longer in tumors in the immunocompetent hamster model or reduce adaptive immune responses against the virus. The Ad6 fiber is surprisingly shorter than all other species C Ads including Ad5 23. This reduction in shaft length may increase virus spread through tumors by reducing the affinity of Ad6 for CAR or by increasing interactions between penton base and cellular integrins. Alternately, a shorter, and perhaps less effective fiber may explain why Ad5 is more effective on some cancers and Ad6 is more effective on others. In reality, both of these species C viruses appear similar in oncolytic efficacy. For example, when tested on prostate and hepatocellular cancer cell lines, Ad5 and Ad6 generated nearly identical large burst sizes 12. However, Ad6 has the immunological advantage of lower seroprevalence in humans.
In summary, screening of 13 adenoviruses on solid human cancers revealed that species D viruses were largely ineffective, except in a subset of tumor types. In contrast, species B virus Ad11 and species C viruses Ad5 and Ad6 had better activities against many of the cancer cell lines in vitro and in vivo. Ad6 and Ad11 hold promise based on their lower seroprevalence than Ad5. However, the potential for Ad11 to induce EMT may hinder its application until this side effect is mitigated. It is ironic that Ad5 remains one of the most potent oncolytic agents in solid tumors, but most humans are already immune to this virus. Since Ad6 is less prevalent than Ad5 in humans, this suggests that Ad6 may be a better choice for clinical translation than Ad5 to reduce neutralization of the virus in patients without sacrificing efficacy. Ad6 may have utility as is. Or for cells with lower CAR expression like breast or ovarian cancer, Ad6 will likely be rendered more effective by targeting the virus to receptors other than CAR.
Supplementary Material
MTT based viability of cell lines after one week at several multiplicities of infection. A) MDA-MB-231. B) MDA-MB-468. C) SKOV3. D) BT-474.
Acknowledgments
We would like to thank Mary Barry for helpful technical assistance. This work was supported by a grant to M.A.B. from NIH (R01-CA136945) and by the Mayo Clinic Breast Cancer SPORE (P50-CA116201).
Footnotes
The authors assert that the material presented in this research article is original, has not been previously published, and has not been submitted for publication elsewhere.
Authors have no competing financial interests with regard to the content of the article.
Competing interests: The authors have no competing interests in regards to the work presented.
References
- 1.Davison AJ, Benko M, Harrach B. Genetic content and evolution of adenoviruses. J Gen Virol. 2003;84(Pt 11):2895–2908. doi: 10.1099/vir.0.19497-0. [DOI] [PubMed] [Google Scholar]
- 2.Pereira HG, Kelly B. Dose-response curves of toxic and infective actions of adenovirus in HeLa cell cultures. Journal of general microbiology. 1957;17(2):517–524. doi: 10.1099/00221287-17-2-517. [DOI] [PubMed] [Google Scholar]
- 3.Huebner RJ, Rowe WP, Schatten WE, Smith RR, Thomas LB. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956;9(6):1211–1218. doi: 10.1002/1097-0142(195611/12)9:6<1211::aid-cncr2820090624>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Piedra PA, Poveda GA, Ramsey B, McCoy K, Hiatt PW. Incidence and prevalence of neutralizing antibodies to the common adenoviruses in children with cystic fibrosis: implication for gene therapy with adenovirus vectors. Pediatrics. 1998;101(6):1013–1019. doi: 10.1542/peds.101.6.1013. [DOI] [PubMed] [Google Scholar]
- 5.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81(9):4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krasnykh VN, Mikheeva GV, Douglas JT, Curiel DT. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. Journal of Virology. 1996;70(10):6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72(12):9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. stably expressing the adenovirus type 9705 fibre protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemminki A, Kanerva A, Kremer EJ, Bauerschmitz GJ, Smith BF, Liu B, et al. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol Ther. 2003;7(2):163–173. doi: 10.1016/s1525-0016(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann D, Bayer W, Heim A, Potthoff A, Nettelbeck DM, Wildner O. Evaluation of Twenty-One Human Adenovirus Types and One Infectivity-Enhanced Adenovirus for the Treatment of Malignant Melanoma. J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5701131. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann D, Heim A, Nettelbeck DM, Steinstraesser L, Wildner O. Evaluation of twenty human adenoviral types and one infectivity-enhanced adenovirus for the therapy of soft tissue sarcoma. HumGene Ther. 2007;18(1):51–62. doi: 10.1089/hum.2006.132. [DOI] [PubMed] [Google Scholar]
- 11.Strauss R, Sova P, Liu Y, Li ZY, Tuve S, Pritchard D, et al. Epithelial phenotype confers resistance of ovarian cancer cells to oncolytic adenoviruses. Cancer research. 2009;69(12):5115–5125. doi: 10.1158/0008-5472.CAN-09-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shashkova EV, May SM, Barry MA. Characterization of human adenovirus serotypes 5, 6, 11, and 35 as anticancer agents. Virology. 2009 doi: 10.1016/j.virol.2009.08.038. Epub Sep 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senac JS, Doronin K, Russell SJ, Jelinek DF, Greipp PR, Barry MA. Infection and killing of multiple myeloma by adenoviruses. Hum Gene Ther. 2010;21(2):179–190. doi: 10.1089/hum.2009.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnberg N. Adenovirus receptors: implications for tropism, treatment and targeting. Reviews in medical virology. 2009;19(3):165–178. doi: 10.1002/rmv.612. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Li ZY, Liu Y, Persson J, Beyer I, Moller T, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nature medicine. 17(1):96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnberg N, Edlund K, Kidd AH, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor. J Virol. 2000;74(1):42–48. [PMC free article] [PubMed] [Google Scholar]
- 17.Burmeister WP, Guilligay D, Cusack S, Wadell G, Arnberg N. Crystal structure of species D adenovirus fiber knobs and their sialic acid binding sites. J Virol. 2004;78(14):7727–7736. doi: 10.1128/JVI.78.14.7727-7736.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins avb3 or avb5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 19.Shashkova EV, Spencer JF, Wold WS, Doronin K. Targeting Interferon-alpha Increases Antitumor Efficacy and Reduces Hepatotoxicity of E1A-mutated Spread-enhanced Oncolytic Adenovirus. Mol Ther. 2007;15(3):598–607. doi: 10.1038/sj.mt.6300064. [DOI] [PubMed] [Google Scholar]
- 20.Thomas MA, Spencer JF, Wold WS. Use of the Syrian hamster as an animal model for oncolytic adenovirus vectors. Methods Mol Med. 2007;130:169–183. doi: 10.1385/1-59745-166-5:169. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein DL, Spencer JF, Doronin K, Patra D, Meyer JM, Shashkova EV, et al. An acute toxicology study with INGN 007, an oncolytic adenovirus vector, in mice and permissive Syrian hamsters; comparisons with wild-type Ad5 and a replication-defective adenovirus vector. Cancer Gene Ther. 2009;16(8):644–654. doi: 10.1038/cgt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying B, Toth K, Spencer JF, Meyer J, Tollefson AE, Patra D, et al. INGN 007, an oncolytic adenovirus vector, replicates in Syrian hamsters but not mice: comparison of biodistribution studies. Cancer Gene Ther. 2009;16(8):625–637. doi: 10.1038/cgt.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weaver EA, Turner MA, Khare R, Borowski W, Barry MA. Characterization of Human Species C Adenovirus Serotype 6. Virology. 2010 doi: 10.1016/j.virol.2010.10.041. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khare R, May SM, Vetrini F, Ng P, Weaver EA, Barry MA. Increased Liver Gene Therapy by a Kupffer Cell-evading Adenovirus Vector. Molecular Therapy. 2010 doi: 10.1038/mt.2011.71. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MTT based viability of cell lines after one week at several multiplicities of infection. A) MDA-MB-231. B) MDA-MB-468. C) SKOV3. D) BT-474.


