Abstract
Introduction
Beta-blocker therapy reduces syncope and sudden death in long-QT syndrome type 1 (LQT1), but the mechanism of protection is incompletely understood. This study tested the hypothesis that beta-blockade reduces QT prolongation and dispersion of repolarization, measured as the T peak-to-end interval (Tpe), during exercise and recovery in LQT1 patients.
Methods and Results
QT and Tpe were measured in 10 LQT1 patients (33 ± 13 yrs) and 35 normal subjects (32 ± 12 yrs) during exercise tests on and off beta-blockade. In LQT1 patients, beta-blockade reduced QT (391 ± 25 msec vs. 375 ± 26 msec, p = 0.04 during exercise; 419 ± 41 msec vs. 391 ± 39 msec, p = 0.02 during recovery) and markedly reduced Tpe (91 ± 26 msec vs. 67 ± 19 msec, p = 0.03 during exercise; 103 ± 26 msec vs. 78 ± 11 msec, p = 0.02 during recovery). In contrast, in normal subjects, beta-blockade had no effect on QT (320 ± 17 msec vs. 317 ± 16 msec, p 0.29 during exercise; 317 ± 13 msec vs. 315 ± 14 msec, p = 0.15 during recovery) and mildly reduced Tpe (69 ± 13 msec vs. 61 ± 11 msec, p = 0.01 during exercise; 77 ± 19 msec vs. 68 ± 14 msec, p < 0.001 during recovery).
Conclusion
In LQT1 patients, beta-blockers reduced QT and Tpe during exercise and recovery, supporting the theory that beta-blocker therapy protects LQT1 patients by reducing dispersion of repolarization during exercise and recovery.
Keywords: Long-QT Syndrome, Beta-Blocker Therapy, Tpeak-end, Dispersion of Ventricular Repolarization, Sudden death
INTRODUCTION
Congenital long-QT syndrome (LQTS) is a genetically heterogeneous group of cardiac repolarization disorders characterized by prolongation of the QT interval and a propensity to develop syncope or sudden cardiac death due to the malignant ventricular arrhythmia known as torsades de pointes (TdP). In long-QT syndrome type 1 (LQT1), the commonest form of LQTS, affected patients often develop TdP during or shortly after exercise. Whereas in normal subjects the QT interval shortens as the heart rate (HR) increases, in LQT1 the QT interval fails to shorten adequately during exercise1–3 or epinephrine infusion4–7. These exercise-induced repolarization responses are thought to play a role in the mechanism of arrhythmogenesis in LQT1.
Beta-blocker therapy (BB) significantly reduces the occurrence of syncope and sudden cardiac death in LQT1 patients8–10, although a small subset of LQT1 patients continues to have syncope or sudden death despite BB. Identification of these high-risk, BB-resistant patients, who could benefit from implantable cardioverter-defibrillator therapy, remains a challenge for the clinician. Several parameters are available to help clinicians assess the response to BB in LQT1 patients, such as resting and peak exercise HR, and recurrence of syncope despite BB. In many centers, serial exercise testing is performed routinely in LQT1 patients, but whether such testing can be used to confirm a protective effect of BB has not been established. Specifically, whether BB results in a measurable change in abnormal repolarization during exercise and recovery (i.e., during greatest susceptibility to arrhythmias) in LQTS patients remains controversial based on conflicting published data11–13. Thus, it is not clear whether BB actually improves abnormal repolarization, or whether BB simply blunts the maximal HR LQT1 patients may achieve during exercise. Furthermore, QT interval duration may be a less specific marker of the risk of developing TdP than measures such as the ECG interval from the peak to the end of the T wave (Tpe)14–20, thought to represent global dispersion of repolarization, a reflection of electrical inhomogeneity and repolarization gradients within the ventricular myocardium.
Deviation from a normal range of the Tpe interval has been shown to be a marker of arrhythmogenesis16,19,21, and the Tpe is prolonged by sympathetic stimulation in both congenital14,22–26 and acquired27 LQTS patients but not in normal subjects28. Shimizu showed that BB prevents Tpe prolongation with epinephrine infusion in LQT1 subjects26. However, whether BB prevents Tpe prolongation during symptom-limited exercise in LQT1 subjects is not known. We hypothesized that BB would protect against exercise-induced prolongation of QT and Tpe in LQT1 subjects, and that BB would have no significant effect on these parameters in normal subjects.
METHODS
Study population
The institutional review board at MetroHealth Medical Center approved the protocol, and all subjects provided written informed consent. Participants included 10 genotype-proven LQT1 subjects from 7 different families and 35 age- and gender-matched normal subjects (Table 1). An effort was made to recruit 3–4 control subjects for each LQT1 subject, matched by gender and within a 5-year age span. Normal subjects were required to complete a screening questionnaire. Subjects were excluded if they had known cardiac disease, diabetes mellitus, hypertension, severe allergic reaction, asthma requiring treatment, pregnancy, inability to provide informed consent, inability to walk on a treadmill, or use of medications other than oral contraceptives, acetaminophen, non-steroidal anti-inflammatory drugs, or thyroid replacement. All control subjects had normal resting electrocardiograms.
Table 1.
| Baseline Characteristic | Normal Subjects (n=35) | LTQ1 (n=10) | P Value |
|---|---|---|---|
| Age (Years) | 32±12 | 33±13 | 0.92 |
| Sex (Male) | 15 (43%) | 4 (40%) | 0.87 |
| History of Syncope | No=35 | Yes=4, No=6 | NA |
| Mean BB dose (mg/day) | Propranolol: 158 | Propranolol (n = 5): 184 Nadolol (n = 3): 60 Metoprolol Succinate (n = 1): 25 Atenolol (n = 1): 100 |
0.06* |
| Resting HR off BB (bpm) | 70±11 | 74±14 | 0.45 |
| Resting QTc off BB (msec) | 401±26 | 477±35 | < 0.001 |
| Resting Tpe off BB (msec) | 74±13 | 82±33 | 0.45 |
Applies to propranolol weight-based dose
Experimental protocol
LQT1 subjects completed a symptom-limited exercise treadmill test (ETT) prior to initiation of BB and again on their maintenance dose of BB. The target dose of BB was 2–3 mg/kg/day of long-acting propranolol or 1–2 mg/kg/day of nadolol. If these were not well-tolerated, metoprolol succinate or atenolol were given. Normal subjects completed an ETT following 2 weeks of treatment with long-acting propranolol, as well as following 2 weeks of treatment with matching placebo, dosed in a random-order, double-blind fashion. Propranolol was dosed 80 mg/day for the first week and, depending on tolerance, increased to 160 mg/day for the second week.
ECG Recordings
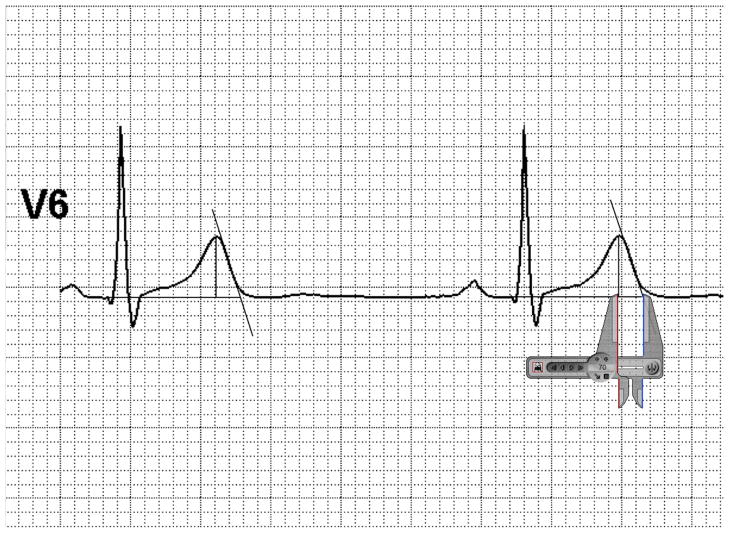
Digital 12-lead ECG data were recorded continuously throughout the rest, exercise, and recovery stages using a CH 2000 or HearTwave system (Cambridge Heart, Inc., Tewksbury, MA). Selected tracings corresponding to rest, peak exercise, and 1 minute into recovery were printed for QT measurements or transferred to a personal computer in pdf format for Tpe measurements. For each tracing, Adobe Photoshop™ was used to perform the following editing functions: (1) enlarging the image 600–700% its original size, (2) cropping the image to include 2.5 seconds in the desired lead only, and (3) de-identification. Because lead V6 may best represent the transmural axis of the heart29, lead V6 was used for measurement of Tpe whenever possible. When the end of the T wave was not discernable in lead V6, Tpe was measured in lead V5, or, if not possible, as in one patient, in lead II. After blinding of the investigators to genotype and BB status, the RR, QT and Tpe intervals were defined by using Adobe Photoshop™ to draw an isoelectric line, a Tend using the standard tangent method29, and a Tpeak line. Using commercially available digital screen calipers (Iconico™), between 2 and 5 measurements were made per ECG tracing, depending on heart rate and number of complexes in the 2.5 second interval (Figure 1).
Figure 1.
Example of Tpe measurement, in which a best-fit tangent line was drawn to the isoelectric line, and Tpe interval was defined by drawing a vertical line from the Tpeak to the isoelectric line.
QT Interval Analysis
Due to the limitations associated with the use of HR correction formulae for QT measurement, we sought to compare QT at matched HR. ECG tracings were selected during exercise and recovery at rates between 100–110 bpm (peak HR limited by maximal achieved HR on BB) and both an isoelectric line and a tangent line were used to define the end of the T wave. The QT interval was manually measured in lead II using standard calipers. Measurements were made by a single observer, blinded to LQTS/control status and to beta-blocker use.
Statistical Analysis
For QT interval analysis, the uncorrected QT interval was compared before and after BB at matched HR, in normal subjects and in LQT1 patients. For Tpe analysis, mean values were plotted against stage of exercise. Paired t-tests were used to compare the means for each group before and after BB. A p-value of <0.05 was considered statistically significant.
RESULTS
Table 1 shows the baseline characteristics of the study subjects. The LQT1 subjects and controls were matched for age and gender. The mean QT interval was longer among LQT1 subjects and 4 of 10 had a history of syncope. Five subjects received long-acting propranolol (mean dose 2.73 mg/kg/day), 3 received nadolol (mean dose 1.4 mg/kg/day), 1 received atenolol (1.5 mg/kg/day), and 1 received metoprolol succinate (0.33 mg/kg/day). Of the 35 normal subjects, 34 were taking long-acting propanolol at 160 mg/day and 1 was taking 80 mg/day (dose limited by tolerance) for a mean dose of 2.23 mg/kg/day.
Effect of beta-blocker therapy on QT interval
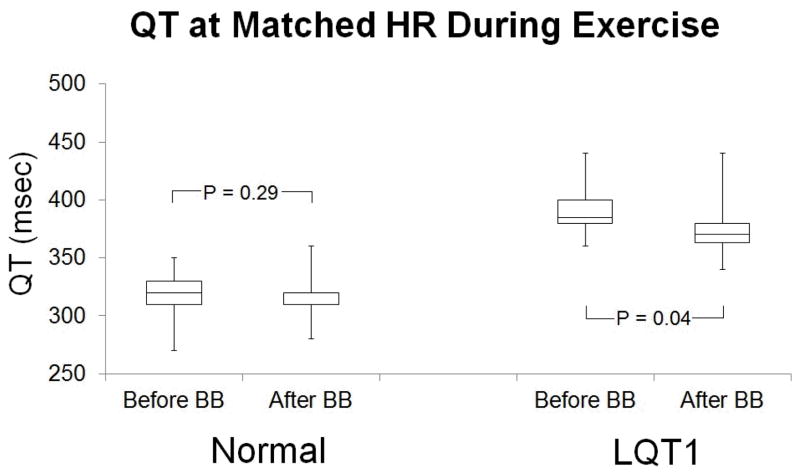
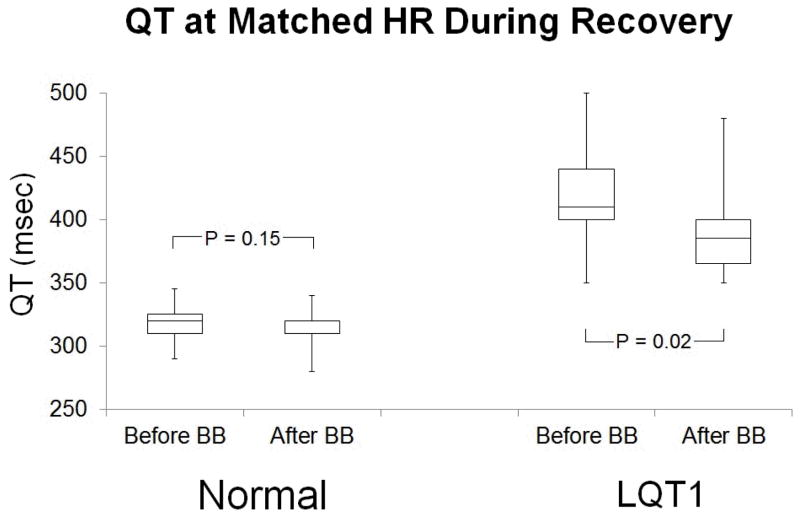
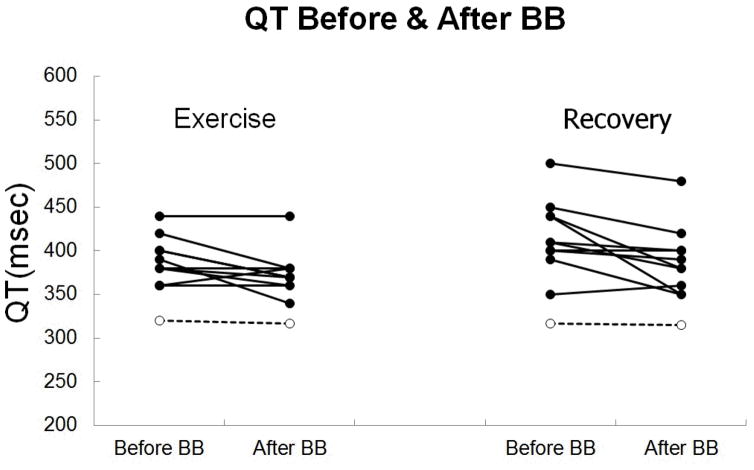
BB reduced QT during exercise and recovery in LQT1 patients but not in normal subjects (Figures 2 and 3). During exercise, normal subjects had QT interval 320 ± 17 msec before BB vs. 317 ± 16 msec after BB at mean HR 106 ± 3 bpm (p = 0.29), and LQT1 subjects had QT interval 391 ± 25 msec before BB vs. 375 ± 26 msec after BB at mean HR 106 ± 4 bpm (p = 0.04). During recovery, normal subjects had QT interval 317± 13 msec before BB vs. 315 ± 14 msec after BB at mean HR 105 ± 2 bpm (p = 0.15), and LQT1 subjects had QT interval 419 ± 41 msec before BB vs. 391 ± 39 msec after BB at mean HR 106 ± 5 bpm (p = 0.02). Although the mean reduction in QT interval (16 msec during exercise and 28 msec during recovery) with BB reached statistical significance, the magnitude of the effect of BB was not uniform across the group of LQT1 subjects, and the QT interval actually increased with BB in 1 subject (figure 4). We observed variability in the QT response to BB even among members of a single family, even when all members of the family had achieved their target dose of BB (Table 2).
Figure 2.
QT at matched HR (106 ± 3 bpm) during exercise in normal subjects = 320 ± 17 msec before BB and 317 ± 16 msec after BB; p = 0.29. QT at matched HR (106 ± 4 bpm) in LQT1 subjects = 391 ± 25 msec before BB and 375 ± 26 msec after BB; p = 0.04.
Figure 3.
QT at matched HR (105 ± 2 bpm) during recovery in normal subjects = 317 ± 13 msec before BB and 315 ± 14 msec after BB; p = 0.15. QT at matched HR (106 ± 5 bpm) = 419 ± 41 msec before BB and 391 ± 39 msec after BB; p = 0.02.
Figure 4.
QT at matched HR during exercise and recovery before and after BB. Solid dots represent individual LQT1 subjects and open dots represent mean values for 35 normal subjects.
Table 2.
| Subject | Syncope (Before BB) | Mutation | Effect of BB on QT During Exercise (msec) |
|---|---|---|---|
| 1 | Yes | S225L | 0 |
| 2 | Yes | S349W | −20 |
| 3 | Yes | S349W | −50 |
| 4 | No | S349W | −30 |
| 5 | No | A150fs+132x | +20 |
| 6 | Yes | G325R | 0 |
| 7 | No | G325R | −10 |
| 8 | No | A344E | 0 |
| 9 | No | T587M | −30 |
| 10 | No | R518x | −40 |
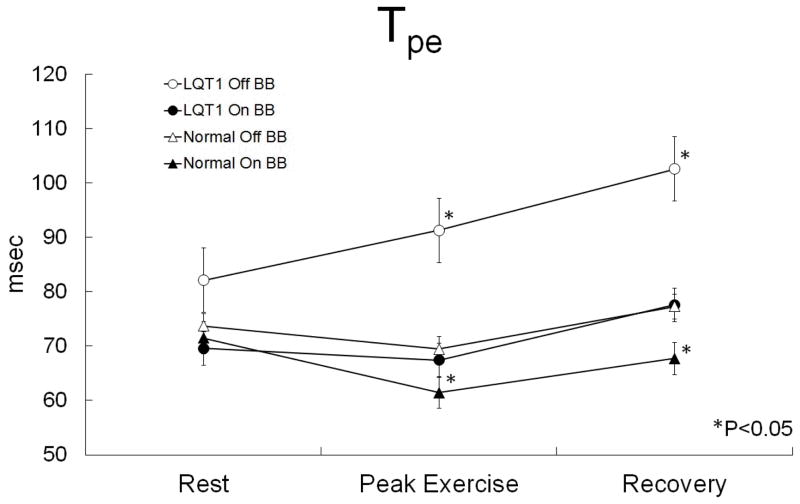
Effect of beta-blocker therapy on Tpe interval
BB markedly reduced Tpe in LQT1 patients and mildly reduced Tpe in normal subjects during exercise and recovery (Figure 5). The peak exercise Tpe was 69 ± 13 msec before BB vs. 61 ± 11 msec after BB (p = 0.01) in normal subjects, and 91 ± 26 msec before BB vs. 67 ± 19 msec after BB (p = 0.03) in LQT1 subjects. At 1 minute into recovery the Tpe was 77 ± 19 msec before BB vs. 68 ± 14 msec after BB (p < 0.001) in normal subjects, and 103 ± 26 msec before BB vs. 78 ± 11 msec after BB (p = 0.02) in LQT1 subjects. Resting Tpe was not significantly different between LQT1 patients and normal subjects, and resting Tpe was not reduced by BB in either group. Resting Tpe was 74 ± 13 msec before BB vs. 72 ± 13 msec after BB (p = 0.18) in normal subjects, and 82 ± 33 msec before BB vs. 70 ± 8 msec after BB (p = 0.27) in LQT1 subjects.
Figure 5.
Tpe at rest, peak exercise, and 1 minute into recovery in normal subjects and LQT1 subjects on and off BB. *p < 0.05 for comparison of off vs. on BB between paired samples of normal or LQT1 subjects.
DISCUSSION
In the current study, we determined the effect of BB on abnormal repolarization during exercise in LQT1 subjects. We found that BB both shortened QT and substantially reduced Tpe, effectively normalizing this measure of dispersion of repolarization. These findings help to clarify the mechanism whereby BB reduces the risk of TdP in LQT1: our findings support the hypothesis that BB reduces abnormal QT prolongation and protects against dispersion of repolarization in LQT1 subjects during exercise and recovery, the time period of greatest arrhythmic risk.
Effect of beta-blocker therapy on QT interval
There are conflicting data in the existing literature as to whether BB results in a shortening of the QT interval at a given HR in LQT1 subjects. Moss et al11 reported a reduction in resting QTc (20 msec) by BB in a cohort of 23 LQT1 patients, but this effect did not reach statistical significance. Kaltman et al12 found no significant difference in QTc during exercise with BB among non-genotyped patients. Wong et al13 reported a decrease in QTc during exercise in LQT1 subjects treated with BB, but this did not reach statistical significance (p = 0.07) and the peak exercise HR was significantly lower in the BB group. Sy30 et al noted that the maximal exercise QTc during exercise was not significantly different in LQT1 and LQT2 patients on BB versus those naïve to BB. Since Bazett’s HR correction formula, which tends to overestimate QTc at elevated HR, is not optimally suited for assessing the effect of BB on QT, we compared QT at matched HR before and after BB, during the ascending and descending limbs of the graded exercise test. This methodology precluded comparing the QT at peak HR before BB to the QT at peak HR after BB, since subjects did not achieve the same HR after BB. By comparing QT at matched HR, we found that BB resulted in QT values that either stayed the same or decreased during exercise and recovery in 90% of LQT1 subjects.
In general, subjects whose QT interval was longer before BB had a greater reduction in QT with BB, and the 1 patient whose QT increased with BB (+20 msec) had the shortest QT interval before BB (360 msec). Thus, although BB appears to reduce QT modestly in the majority of LQT1 subjects, our data illustrate the variability of phenotypic expression of QT prolongation and response to BB even among family members with identical mutations (Table 2). BB did not significantly affect QT in normal subjects, and there was a significantly smaller variability in normal subjects than in LQT1 subjects (reduction of QT by 3 ± 14 msec in normal vs. 16 ± 22 msec in LQT1 during exercise, p = 0.02; reduction of QT by 2 ± 8 msec in normal vs. 28 ± 30 msec in LQT1 during recovery, p < 0.001).
Effect of beta-blocker therapy on Tpe interval in normal subjects
In our cohort of normal subjects, the Tpe did not change significantly during exercise, and Tpe values at rest and during exercise were identical to those previously reported in non-beta-blocked subjects28. Interestingly, although BB did not affect the Tpe in normal subjects at rest, BB shortened the Tpe during exercise and recovery to values significantly lower than those observed in non-beta-blocked normal subjects. These findings suggest that BB results in more efficient terminal repolarization during exercise and recovery even in normal subjects. It is known that the risk of sudden cardiac arrest is increased during and after vigorous exercise31,32, and it is possible that this increased risk might be related to increased global dispersion of repolarization that may reach clinically significant levels in some individuals.
Effect of beta-blocker therapy on Tpe interval in LQT1 subjects
The resting Tpe values in LQT1 subjects did not differ significantly from those seen in normal subjects, but LQT1 subjects exhibited marked Tpe prolongation during exercise and recovery. BB normalized the Tpe response to exercise and recovery in LQT1 subjects to values seen in normal subjects without BB. These findings provide strong evidence to suggest that BB offers a protective effect in LQT1 by decreasing global dispersion of ventricular repolarization to a level seen in normal subjects.
Our observation that LQT1 subjects have abnormal Tpe prolongation during exercise that is prevented by BB extends the findings of Shimizu and Antzelevitch33. In their canine wedge model of LQT1, isoproterenol infusion increased QT, transmural dispersion of repolarization, and inducibility of ventricular arrhythmia, and this effect was completely suppressed in the presence of BB. While it is true that Tpe measured on the surface ECG is not equivalent to transmural dispersion of repolarization17,18,20 there is evidence to support the theory that the Tpe interval reflects global ventricular dispersion of repolarization16–19. Our data provide clinical evidence to support the theory that BB prevents the abnormal exercise-induced increase in global dispersion of repolarization in LQT1 subjects. Our finding has practical application, since both QT and Tpe can be measured easily on clinical exercise test data, and a salutatory effect of BB on abnormal repolarization can be confirmed. We speculate that the dose of BB required to reduce Tpe and QT may vary between patients, and that repeated exercise testing may allow the clinician to judge whether a therapeutic or protective dose has been achieved. It is tempting to speculate that failure of BB to normalize Tpe in LQT1 patients during exercise may be indicative of an inadequate protective effect. Larger studies will be needed to test this hypothesis.
Limitations
The most significant limitation to this study is the limited sample size. Our enrollment of LQT1 patients was limited by the number of patients who underwent a baseline exercise test prior to initiation of beta-blocker therapy and subsequently were found to have LQT1 mutations. We follow other LQT1 patients who were already started on beta-blocker therapy prior to their initial evaluation in our center, but we thought it would be unethical to discontinue their beta-blocker therapy to obtain drug-free exercise test data. Despite the small number of subjects, however, we were able to show a significant effect of BB on QT interval and on Tpe in LQT1 subjects. Another limitation is the potential error that is associated with manual measurement of ECG data. However, with the measurements made by an investigator blinded to subject status (LQT1 vs. control and BB exposure vs. no BB) we showed significant reduction in both QT and Tpe in LQT1 subjects during exercise and recovery. A further limitation was the use of multiple BB agents in the LQT1 patients. This is a reflection of variations in clinical practice style and, in some cases, reflects what medication regimen was best tolerated by the individual patient. The clinical goal was long acting propranolol 2–4 mg/kg/day or nadolol 1–2 mg/kg/day, depending on tolerance. Patients who did not tolerate either of these received alternative beta blockers. When we designed a uniform protocol for our control subjects, we estimated that a daily dose of 160 mg of long acting propranolol would give at least 2 mg/kg/day, the target dose for our patients. Of our 35 control subjects, only one was limited to 80 mg due to symptoms; the other 34 tolerated 160 mg daily. An argument could be made that our control subjects were treated “better” than some of the LQT1 patients who did not tolerate the preferred beta blocker. Yet, despite the use of multiple kinds of BB in the LQT1 patients, we were able to demonstrate normalization of Tpe during exercise.
CONCLUSIONS
In LQT1 subjects, BB reduced the QT interval during exercise, but not to values seen in normal subjects. In contrast, LQT1 subjects displayed marked Tpe prolongation during exercise and BB suppressed this effect, resulting in mean Tpe values identical to those seen in normal subjects without BB. These findings suggest that BB reduces arrhythmogenic dispersion of ventricular repolarization in LQT1 subjects, and that this is likely a key mechanism by which BB reduces the risk of syncope and sudden cardiac arrest in this population. The use of exercise testing to monitor the Tpe response to BB in LQT1 may add useful risk stratification information, as LQT1 subjects with persistent exercise-induced Tpe prolongation despite BB may be at increased risk of syncope or sudden cardiac arrest. A larger sample of patients with long term follow-up data will be necessary to determine whether this is true.
Acknowledgments
This work was supported in part by NIH Grant T35HL082544 and in part by Grant Number M01 RR000080 and Grant Number UL1RR024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Abbreviations
- BB
beta-blocker therapy
- LQT1
long QT syndrome type 1
- Tpe
the interval from the peak of the T-wave to the end of the T-wave
- TdP
torsades de pointes
Footnotes
Dr. Kaufman is the recipient of grant support from Cambridge Heart, Inc. Other authors: No disclosures.
References
- 1.Swan H, Toivonen L, Viitasalo M. Rate adaptation of QT intervals during and after exercise in children with congenital long QT syndrome. Eur Heart J. 1998;19:508–513. doi: 10.1053/euhj.1997.0764. [DOI] [PubMed] [Google Scholar]
- 2.Swan H, Viitasalo M, Piippo K, Laitinen P, Kontula K, Toivonen L. Sinus node function and ventricular repolarization during exercise stress test in long QT syndrome patients with KvLQT1 and HERG potassium channel defects. J Am Coll Cardiol. 1999;34:823–829. doi: 10.1016/s0735-1097(99)00255-7. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu W, Ohe T, Kurita T, Shimomura K. Differential response of QTU interval to exercise, isoproterenol, and atrial pacing in patients with congenital long QT syndrome. Pacing Clin Electrophysiol. 1991;14:1966–1970. doi: 10.1111/j.1540-8159.1991.tb02799.x. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman MJ, Khositseth A, Tester DJ, Hejlik JB, Shen WK, Porter CB. Epinephrine-induced QT interval prolongation: a gene-specific paradoxical response in congenital long QT syndrome. Mayo Clin Proc. 2002;77:413–421. doi: 10.4065/77.5.413. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu W, Noda T, Takaki H, Kurita T, Nagaya N, Satomi K, Suyama K, Aihara N, Kamakura S, Sunagawa K, Echigo S, Nakamura K, Ohe T, Towbin JA, Napolitano C, Priori SG. Epinephrine unmasks latent mutation carriers with LQT1 form of congenital long-QT syndrome. J Am Coll Cardiol. 2003;41:633–642. doi: 10.1016/s0735-1097(02)02850-4. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman ES, Gorodeski EZ, Dettmer MM, Dikshteyn M. Use of autonomic maneuvers to probe phenotype/genotype discordance in congenital long QT syndrome. Am J Cardiol. 2005;96:1425–1430. doi: 10.1016/j.amjcard.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 7.Vyas H, Hejlik J, Ackerman MJ. Epinephrine QT stress testing in the evaluation of congenital long-QT syndrome: diagnostic accuracy of the paradoxical QT response. Circulation. 2006;113:1385–1392. doi: 10.1161/CIRCULATIONAHA.105.600445. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz PJ, Locati E. The idiopathic long QT syndrome: pathogenetic mechanisms and therapy. Eur Heart J. 1985;6(Suppl D):103–114. doi: 10.1093/eurheartj/6.suppl_d.103. [DOI] [PubMed] [Google Scholar]
- 9.Priori SG, Napolitano C, Schwartz PJ, Grillo M, Bloise R, Ronchetti E, Moncalvo C, Tulipani C, Veia A, Bottelli G, Nastoli J. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004;292:1341–1344. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg I, Bradley J, Moss A, McNitt S, Polonsky S, Robinson JL, Andrews M, Zareba W International LQTS Registry Investigators. Beta-blocker efficacy in high-risk patients with the congenital long-QT syndrome types 1 and 2: implications for patient management. J Cardiovasc Electrophysiol. 2010;21:893–901. doi: 10.1111/j.1540-8167.2010.01737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss AJ, Zareba W, Hall WJ, Schwartz PJ, Crampton RS, Benhorin J, Vincent GM, Locati EH, Priori SG, Napolitano C, Medina A, Zhang L, Robinson JL, Timothy K, Towbin JA, Andrews ML. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- 12.Kaltman JR, Ro PS, Stephens P, McBride MG, Cohen MI, Tanel RE, Vetter VL, Rhodes LA. Effects of beta-adrenergic antagonists on the QT measurements from exercise stress tests in pediatric patients with long QT syndrome. Pediatr Cardiol. 2003;24:553–558. doi: 10.1007/s00246-003-0436-0. [DOI] [PubMed] [Google Scholar]
- 13.Wong JA, Gula LJ, Klein GJ, Yee R, Skanes AC, Krahn AD. Utility of treadmill testing in identification and genotype prediction in long-QT syndrome. Circ Arrhythm Electrophysiol. 2010;3:120–125. doi: 10.1161/CIRCEP.109.907865. [DOI] [PubMed] [Google Scholar]
- 14.Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928–1936. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 15.Antzelevitch C. T peak-Tend interval as an index of transmural dispersion of repolarization. Eur J Clin Invest. 2001;31:555–557. doi: 10.1046/j.1365-2362.2001.00849.x. [DOI] [PubMed] [Google Scholar]
- 16.Yan GX, Martin J. Electrocardiographic T wave: a symbol of transmural dispersion of repolarization in the ventricles. J Cardiovasc Electrophysiol. 2003;14:639–640. doi: 10.1046/j.1540-8167.2003.03155.x. [DOI] [PubMed] [Google Scholar]
- 17.Xia Y, Liang Y, Kongstad O, Holm M, Olsson B, Yuan S. Tpeak-Tend interval as an index of global dispersion of ventricular repolarization: evaluations using monophasic action potential mapping of the epi- and endocardium in swine. J Interv Card Electrophysiol. 2005;14:79–87. doi: 10.1007/s10840-005-4592-4. [DOI] [PubMed] [Google Scholar]
- 18.Xia Y, Liang Y, Kongstad O, Liao Q, Holm M, Olsson B, Yuan S. In vivo validation of the coincidence of the peak and end of the T wave with full repolarization of the epicardium and endocardium in swine. Heart Rhythm. 2005;2:162–169. doi: 10.1016/j.hrthm.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Antzelevitch C, Sicouri S, Di Diego JM, Burashnikov A, Viskin S, Shimizu W, Yan GX, Kowey P, Zhang L. Does Tpeak-Tend provide an index of transmural dispersion of repolarization? Heart Rhythm. 2007;4:1114–6. doi: 10.1016/j.hrthm.2007.05.028. author reply 1116–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opthof T, Coronel R, Wilms-Schopman FJ, Plotnikov AN, Shlapakova IN, Danilo P, Jr, Rosen MR, Janse MJ. Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm. 2007;4:341–348. doi: 10.1016/j.hrthm.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe N, Kobayashi Y, Tanno K, Miyoshi F, Asano T, Kawamura M, Mikami Y, Adachi T, Ryu S, Miyata A, Katagiri T. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol. 2004;37:191–200. doi: 10.1016/j.jelectrocard.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Lubinski A, Lewicka-Nowak E, Kempa M, Baczynska AM, Romanowska I, Swiatecka G. New insight into repolarization abnormalities in patients with congenital long QT syndrome: the increased transmural dispersion of repolarization. Pacing Clin Electrophysiol. 1998;21:172–175. doi: 10.1111/j.1540-8159.1998.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 23.Tanabe Y, Inagaki M, Kurita T, Nagaya N, Taguchi A, Suyama K, Aihara N, Kamakura S, Sunagawa K, Nakamura K, Ohe T, Towbin JA, Priori SG, Shimizu W. Sympathetic stimulation produces a greater increase in both transmural and spatial dispersion of repolarization in LQT1 than LQT2 forms of congenital long QT syndrome. J Am Coll Cardiol. 2001;37:911–919. doi: 10.1016/s0735-1097(00)01200-6. [DOI] [PubMed] [Google Scholar]
- 24.Takenaka K, Ai T, Shimizu W, Kobori A, Ninomiya T, Otani H, Kubota T, Takaki H, Kamakura S, Horie M. Exercise stress test amplifies genotype-phenotype correlation in the LQT1 and LQT2 forms of the long-QT syndrome. Circulation. 2003;107:838–844. doi: 10.1161/01.cir.0000048142.85076.a2. [DOI] [PubMed] [Google Scholar]
- 25.Viitasalo M, Oikarinen L, Swan H, Vaananen H, Glatter K, Laitinen PJ, Kontula K, Barron HV, Toivonen L, Scheinman MM. Ambulatory electrocardiographic evidence of transmural dispersion of repolarization in patients with long-QT syndrome type 1 and 2. Circulation. 2002;106:2473–2478. doi: 10.1161/01.cir.0000036369.16112.7d. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu W, Tanabe Y, Aiba T, Inagaki M, Kurita T, Suyama K, Nagaya N, Taguchi A, Aihara N, Sunagawa K, Nakamura K, Ohe T, Towbin JA, Priori SG, Kamakura S. Differential effects of beta-blockade on dispersion of repolarization in the absence and presence of sympathetic stimulation between the LQT1 and LQT2 forms of congenital long QT syndrome. J Am Coll Cardiol. 2002;39:1984–1991. doi: 10.1016/s0735-1097(02)01894-6. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi M, Shimizu M, Ino H, Terai H, Uchiyama K, Oe K, Mabuchi T, Konno T, Kaneda T, Mabuchi H. T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: a new index for arrhythmogenicity. Clin Sci (Lond) 2003;105:671–676. doi: 10.1042/CS20030010. [DOI] [PubMed] [Google Scholar]
- 28.Kannankeril PJ, Harris PA, Norris KJ, Warsy I, Smith PD, Roden DM. Rate-independent QT shortening during exercise in healthy subjects: terminal repolarization does not shorten with exercise. J Cardiovasc Electrophysiol. 2008;19:1284–1288. doi: 10.1111/j.1540-8167.2008.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta P, Patel C, Patel H, Narayanaswamy S, Malhotra B, Green JT, Yan GX. T(p-e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41:567–574. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Sy RW, Chattha IS, Klein GJ, Gula LJ, Skanes AC, Yee R, Bennett MT, Krahn AD. Repolarization dynamics during exercise discriminates between LQT1 and LQT2 genotypes. J Cardiovasc Electrophysiol. 2010;21:1242–1246. doi: 10.1111/j.1540-8167.2010.01788.x. [DOI] [PubMed] [Google Scholar]
- 31.Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343:1355–1361. doi: 10.1056/NEJM200011093431902. [DOI] [PubMed] [Google Scholar]
- 32.Mittleman MA, Siscovick DS. Physical exertion as a trigger of myocardial infarction and sudden cardiac death. Cardiol Clin. 1996;14:263–270. doi: 10.1016/s0733-8651(05)70279-4. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu W, Antzelevitch C. Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome. J Am Coll Cardiol. 2000;35:778–786. doi: 10.1016/s0735-1097(99)00582-3. [DOI] [PubMed] [Google Scholar]