Abstract
Purpose
To determine whether curcumin would inhibit IKKβ kinase activity and suppress expression of proinflammatory cytokines in head and neck cancer (HNSCC) patients.
Experimental Design
Saliva was collected before and after subjects chewed curcumin tablets. Protein was extracted and IKKβ kinase activity measured. IL-6 and IL-8 levels in the salivary supernatants were measured using ELISA. IL-6, IL-8 and other interleukins were also measured independently with ELISA to confirm the inhibitory effect of curcumin on expression and secretion of salivary cytokines.
Results
Curcumin treatment led to a reduction in IKKβ kinase activity in the salivary cells of HNSCC patients (p<0.05). Treatment of UM-SCC1 cells with curcumin as well as with post curcumin salivary supernatant showed a reduction of IKKβ kinase activity. Significant reduction of IL-8 levels (p<0.05) was seen in post curcumin samples from patients with dental caries. Although there was reduced IL-8 expression in 8 of 21 post curcumin samples of HNSCC patients, the data did not reach statistical significance. Saliva samples from HNSCC patients were also analyzed in a blinded fashion for expression of cytokines. IL-10, IFN-γ, IL-12p70, and IL-2 clustered together, and GM-CSF and TNF-α clustered together. Log10 ratio analysis demonstrated decrease in expression of all nine cytokines in both the salivary supernatant and salivary cells of curcumin treated samples.
Conclusions
Curcumin inhibited IKKβ kinase activity in the saliva of HNSCC patients and this inhibition correlated with reduced expression of a number of cytokines. IKKβ kinase could be a useful biomarker for detecting the effect of curcumin in head and neck cancer.
Keywords: Head and Neck Cancer, Curcumin, IKKβ Kinase, IL-6, IL-8
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is one of the most morbid of human cancers, and affects 600,000 people worldwide annually, including 42,000 people in the United States.1,2 HNSCC includes oral, laryngeal and pharyngeal malignancies, with about 40% of these arising in the oral cavity.1 Over the years, there have been many changes in the way HNSCC is treated. Current treatment methods for advanced head and neck cancer include radiation therapy, chemotherapy, and surgery. Despite medical advancements, the overall survival rates for patients with advanced HNSCC have remained poor. The 5-year survival rate for all patients with head and neck cancers is 57%, and for patients with stage III and IV oral cancers the rates are even lower at 10–20%.1,3 In addition, 30–50% of patients develop local or regional recurrence, and another 10–40% of patients develop second primary tumors of the aerodigestive tract due to field cancerization.4 As a result, there have been ongoing investigations into alternative therapies with reduced morbidity as well as reduced toxicity to minimize the adverse effect on cancer patients.
Curcumin (diferuloylmethane), the yellow pigment that is derived from the East Indian plant Curcuma longa, is the major anti-oxidant and anti-inflammatory substance found in turmeric. For centuries, turmeric has been used as a spice and food-coloring agent and has served as a naturally occurring medicine to treat inflammatory disorders.5,6 Pre-clinical studies have consistently shown that curcumin inhibits cancer proliferation in various cancer cell lines, including breast, cervical, and pancreatic cancers.6–8 One study by Hanif et al7 tested curcumin's effect on colon adenocarcinoma cell lines and saw as much as 96% reduction in the number of colon cancer cells following curcumin administration. Numerous other studies have concluded that curcumin can act as a chemopreventive agent both in vitro and in vivo in animal models. Limtrakul et al9 demonstrated that dietary curcumin administration significantly inhibited the development of skin tumors and also reduced skin tumor volume in mice. Our laboratory has also found that curcumin suppresses cancer cell proliferation of head and neck cancer cell lines in vitro and in vivo in nude mice with xenograft tumors.10–12 Furthermore, we have found curcumin to be nontoxic in the organs of the mice. Even though various mechanisms by which curcumin acts are not completely understood, studies have shown that curcumin down-regulates nuclear factor-κβ (NF-κβ), an inducible transcription factor that is involved in the activation of a number of cell processes, including cell growth, invasion, and metastasis.5,12,13
NF-κB functions in a variety of human diseases, such as asthma, AIDS, septic shock, and cancer. This factor is activated in many cell types in response to a broad range of stimuli, which include mitogens, inflammatory cytokines, and extracellular stress, such as cigarette smoke and UV irradiation.14,15 NF-κB activation occurs as it is transported from the cytoplasm to the nucleus upon phosphorylation/degradation of its inhibitory molecule IκB.15 The nuclear translocation leads to DNA binding and transcription of growth factors and cytokines (VEGF, TNF, MCP-1, Bcl-2, COX-2, cyclin D1). IKK kinase, a complex consisting of three proteins: IKKα, IKKβ, and IKKγ (also known as the NF-κB essential modulator [NEMO]), is responsible for the phosphorylation of the IκBα subunit of IκB, resulting in ubiquitination and rapid degradation.15 Previously we have shown that curcumin inhibits IKK kinase activity, thereby preventing NF-κB translocation to the nucleus.11 Recently, our laboratory has also shown that curcumin binds to IKKβ protein to exert the inhibitory effect.16 Thus, curcumin's modulation of the IKK kinase activity of the NF-κB pathway leads to decreased NF-κB transcription activation and reduced cell growth.
Higher concentrations of proinflammatory and proangiogenic cytokines are correlated with advanced stage, metastatic disease or large tumor burdens of various cancers.17–19 One study17 identified elevated levels of the cytokines interleukin-6 (IL-6), interleukin-8 (IL-8), and VEGF in patients with HNSCC compared to patients with laryngeal papilloma or age-matched controls. Another study18 detected increased concentrations of IL-8 in the saliva of patients with oral cavity and oropharyngeal squamous cell carcinoma (OSCC) compared with age- and sex-matched control subjects. Our laboratory has found that the level of IL-6 and IL-8 expression correlated with the aggressiveness of the HNSCC cell lines and there was dose-dependent inhibition of IL-6 and IL-8 expression with curcumin treatment.11 Since the in vitro studies have shown that curcumin reduced the expression of inflammatory cytokines through the inhibition of IKKβ kinase activity, we wanted to determine whether curcumin would also inhibit IKKβ kinase activity and the expression of IL-6 and IL-8 in vivo when administered to patients with HNSCC.
MATERIAL AND METHODS
Cell Lines
The UM-SCC1 (oral cancer cell line) was grown in Minimum Essential Medium containing Earle's Salts, L-Glutamine, 10% fetal bovine serum and 1% nonessential amino acids.
Patient Selection
Patients were recruited from the Division of Head and Neck Surgery and the Dental Clinic associated with the Veterans Affairs Greater Los Angeles Healthcare System with informed consent under Institutional Review Board-approved protocols over an 8-month period. The sample group consisted of thirty-four subjects (thirteen with dental caries and twenty-one with head and neck cancer) (Table 1). The control group included five disease-free individuals. There were a total of 39 subjects in this study (thirteen with dental caries, twenty-one with head and neck cancer, and five healthy volunteers). The group with dental caries ranged in age from 42–60 years. The group with head and neck cancer ranged in age from 36–90 years.. The group of healthy volunteers ranged in age from 51 −91 years. All subjects in all groups were male.
Table 1
| Oral Cancer Subject Information | |||
|---|---|---|---|
| Sample # | Stage | Differentiation | |
| 2 | tongue | no prior | Moderate |
| 4 | tongue | T2N2 BOT, recurrent, s/p chemo XRT | Poor |
| 5 | oral | T4N2 M1--no prior | Moderate |
| 6 | oral | T2N2B-no prior | Poor |
| 7 | oral | T4N3 no prior | Moderate |
| 8 | oral | T3N0 recurrent, s/p XRT, path only by FNA (fine needle aspiration) | |
| 9 | pharynx | T4N2B--no prior | Poor |
| 10 | oral | T4N0--prior XRT | No note |
| 11 | base of tongue | T4aN2c no prior treatment | Only FNA |
| 12 | floor of mouth | T2N1 | Basaloid |
| 13 | oral | T2N3--no prior | Undiff./ Poor |
| 14 | R tonsil | T4N0--no prior | Poor |
| 15 | tonsil | T2N1--no prior | Moderate |
| 16 | tonsil | T2N1 | Basaloid |
| 17 | tonsil | Recurrent --previous surgery only (no XRT, chemo) | Moderate |
| 18 | oral | T2N1--no prior | Poor |
| 19 | oral | T4N2C--no prior | Poor |
| 20 | oral | T2N2C--no prior | No note, only FNA |
| 21 | tongue | T4N0--no prior | Moderate |
| 22 | tongue | T1N0--recurrent | Moderate |
| 23 | tongue | T1N0--no prior treatment | Moderate |
Saliva Collection
Saliva (5 mL) was collected in 50 mL tubes, without any PBS or media, before and one hour after subjects chewed two curcumin caplets (Jarrow Formulas® Curcumin 95) for five minutes. The total curcumin concentration of the chewed caplets was 1000 mg. All subjects were required to refrain from eating, drinking, smoking, or using oral hygiene products throughout the duration of the experiment. The saliva samples were immediately placed on ice, centrifuged at 3500 rpm for 15 minutes at 4°C. The supernatant was removed from the cell pellets and stored in 1 mL aliquots. Both the cell pellets and the supernatants were stored at −80°C.
Treatment of UM-SCC1 cells with Salivary Samples
The UM-SCC1 cell line was plated in 100 mm tissue culture dishes, and allowed to grow in 10 mL medium for 24 hours to reach 80% confluency. The cells were serum starved for 24 hours and pre-incubated with tumor necrosis factor α (TNF-α) (10 ng/mL) for fifteen minutes at 37°C. The cells were then treated with 2 mL serum-containing media or with 1mL of salivary supernatant and 1mL of medium and incubated at 37°C for 4 hours. Cell extracts were prepared in kinase buffer (50 mM Tris-hydrochloride [pH, 7.5], 20 mM MgCl2, 0.20 mM Na3VO4, 10 mM β-glycerophosphate, 4 mM diethiothreitol) and IKKβ activity was measured using the IKKβ Kinase Assay Kit (Cell Signaling Technology, Danvers, Massachusetts). Cells treated with DMSO alone or curcumin dissolved in DMSO were used as controls.
IKKβ Kinase Activity Assay
IKKβ kinase activity was measured using the protocol provided with the assay kit (HTScan® IKKβ Kinase Assay Kit; Cell Signaling Technology, Danvers, Massachusetts). Proteins isolated from the salivary cells or from the UM-SCC1 cells were mixed with the IKKβ specific substrate, biotinylated Iκβ-α (phosphorylated at serine residue 32 [ser 32]) peptide (3μM), and adenosine triphosphate (400 μM) in a 50-μL reaction. The assay mixture was incubated at room temperature for 30 minutes followed by the addition of the stop buffer (50 mM EDTA). The reaction mixture was then transferred to a 96-well streptavidin-coated plate (Delfia® Streptavidin-coated yellow plate, 96-well plates, PerkinElmer Inc., Waltham, Massachusetts) and incubated at room temperature for 1 hour. The plate was washed 3 times with polysorbate 20-containing phosphate-buffered saline solution and the phosphorylated Iκβ-α (ser 32) rabbit monoclonal antibody (Cell Signaling Technology, Danvers, Massachusetts) was added. The reaction was then incubated for 2 hours at room temperature and washed 3 times with the phosphate-buffered saline solution with 0.02% of Tween-20 (PBST). Then the antirabbit horseradish peroxidase secondary antibody was added and the plate was incubated for an additional 30 minutes at room temperature. After 5 more washes with PBST, the 3,5,3',5'-tetramethylbenzidine substrate (Cell Signaling Technology, Danvers, Massachusetts) was added. Finally, the plate was incubated for 15 minutes at room temperature, and the reaction was stopped using the stop solution. Absorbance of the colored reaction product was measured at 450 nm using a 96-well microplate reader (PowerWave™ XS; BioTek® Instruments, Inc., Winooski, Vermont). The study was performed thrice and each time in triplicates.
Quantification of cytokine Levels in Salivary Supernatants
We initially measured IL-6 and IL-8 concentrations using an enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's protocol (R&D Systems, Minneapolis, Minnesota). The supernatant of each saliva sample (100 μl) was tested in triplicate. After development of the colorimetric reaction, the absorbance at 450 nm was measured using a 96-well microplate reader (PowerWave™ XS; BioTek® Instruments, Inc., Winooski, Vermont). The absorbance readings were then converted to picograms per milliliter based on standard curves obtained with the recombinant cytokine. The lower limits of sensitivity for the IL-6 and IL-8 assays were 3.12 and 31.2 pg/mL, respectively. The ELISA studies were repeated once.
Cytokine levels (GM-CSF, TNFα, IFN-γ, IL-1β, IL-10, IL-12 p70, IL-2, IL-6 and IL-8) in saliva and solubilized saliva pellets were determined using the Human 9-Plex Ultrasensitive Electrochemiluminescent MULTI-SPOT Assay from Meso Scale Discovery (Gaithersburg, MD). Saliva pellet proteins were solubilized through the addition of 100 – 200 μl of a detergent-containing extraction buffer (Clontech, Mountain View, CA). Twenty five microliters of saliva or saliva pellet protein were used for the MULTI-SPOT assays which were performed according to the manufacturer's directions. Cytokines were detected using the SECTOR Imager 6000 CCD camera (Meso Scale Discovery, Gaithersburg, MD).
Statistical Evaluation
Student t test and wilcoxon signed-rank test were performed to determine the significance of IKKβ enzyme activity changes after curcumin treatment in salivary cell samples. Two-sided two-sample t test was carried out to determine the significance of IL-6 and IL-8 expression level changes in curcumin treated versus untreated salivary supernatant samples. Hierarchical clustering was employed using Genepattern (Broad Institute, Cambridge, MA) to identify patient and cytokine expression clusters in both the saliva pellet and supernatant samples. We centered the rows and columns to their respective median values, and used the Euclidean distance measure. Clustering was performed separately on pre curcumin treatment data, post curcumin treatment data, and on the effect of curcumin on cytokine expression levels (using the log of the ratio between post- and pre-curcumin levels).
RESULTS
Inhibition of IKKβ kinase activity by curcumin in the salivary cells of HNSCC patients
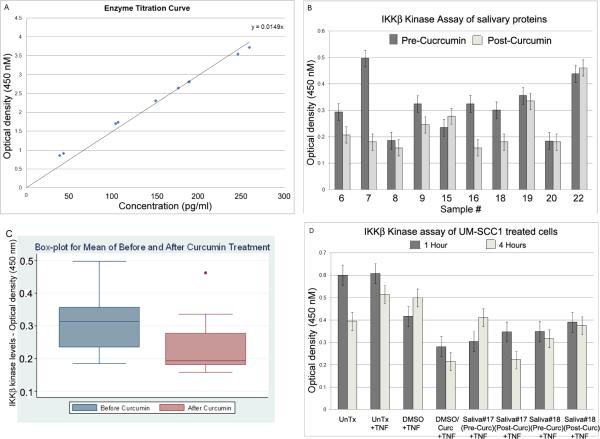
An enzyme titration for IKKβ kinase activity was performed using the IKKβ Kinase Assay Kit in order to derive a standard curve that could be used for the protein measurements in salivary samples. The enzyme activity was linear up to 250 pg/mL protein concentration tested in this investigation (Fig. 1A). Pre-treatment saliva samples had IKKβ concentrations ranging from 12–33.6 pg/mL while post-treatment saliva samples had IKKβ concentrations between 10.6–31 pg/mL (Fig. 1B). We found six of ten samples (60%) to have a reduction in IKKβ kinase activity following one hour of curcumin treatment, with three of the samples showing a 50% reduction. Statistical significance (p<0.05) was observed for the inhibitory effect of curcumin on the IKKβ kinase activity (Fig. 1C). Two samples did not show a change and the remaining two samples had less than a10% increase in enzyme activity post curcumin treatment. These results corroborated our hypothesis that curcumin inhibits IKKβ kinase activity in the salivary cells of patients with head and neck cancer.
Figure 1.
Curcumin inhibits salivary cell IKKβ kinase activity of head and neck cancer patients. A, Dose dependent curve of IKKβ kinase activity was achieved using five IKKβ protein concentrations in increments of 50 pg/mL. The enzyme activity is linear up to 250 pg/mL tested in the present assay. B, Protein was extracted from salivary samples collected from patients with HNSCC before and after a 1-hour curcumin treatment. IKKβ kinase activity was measured using the IKKβ Kinase Assay Kit. Six of 10 samples show a decrease in IKKβ kinase activity, with three samples (#7, #16 and #18) indicating a 50% reduction post curcumin treatment. C, Boxplot showing expression level of IKKβ enzyme activity in pre and post curcumin salivary samples. The results indicate a statistically significant inhibition of IKKβ kinase activity by curcumin with a p value of <0.05. D, The UM-SCC1 cell line was treated with DMSO, curcumin dissolved in DMSO, or saliva samples collected pre and post curcumin treatment. The cells were also pretreated with TNF-α for 15 minutes. As reported earlier11, addition of curcumin inhibits IKKβ kinase activity of UM-SCC1 cells. One of the two post curcumin salivary samples (sample # 17) exhibits an inhibitory effect on IKKβ kinase activity, indicating usefulness of this methodology to determine IKKβ kinase levels in the saliva of HNSCC patients. The IKKβ kinase assay performed at four hours in untreated samples (UnTx) shows a reduction in comparison to 1 hour due to loss of growth factors. The 1 hour treatment of UN-SCC1 cells with pre curcumin saliva:medium (50%:50%) shows reduced IKKβ kinase activity in comparison to untreated controls due to the presence of half the level of growth factors in this mixed medium.
Inhibition of IKKβ kinase activity in curcumin treated saliva samples by the in vitro assay
To determine whether curcumin has IKKβ kinase inhibitory activity on growing tumor cells, the salivary supernatants were used to treat the UM-SCC1 in an in vitro cell assay. TNF-α was also added to increase the enzyme activity. Two samples for which we had sufficient salivary supernatant were tested in the in vitro assay. As reported earlier10, we used DMSO and curcumin dissolved in DMSO as controls. A 4 hour treatment with curcumin showed a 60% reduction in enzyme activity (Fig. 1D). Treatment of the UM-SCC1 cells with one of the post-curcumin salivary samples (#17) resulted in a 50% reduction in enzyme activity (Fig. 1D) reflecting a possible reduction in proinflammatory cytokine concentration in the supernatant sample. It is also possible that the enzyme inhibition was due to curcumin still present in the saliva of this patient. Although inhibition was not observed with sample #18, the salivary cells of this sample have revealed an inhibitory effect on the enzyme activity (see Fig. 1B). It is likely then that a longer oral curcumin treatment might be required to induce a reduction in the level of cytokines released from some of the HNSCC patients.
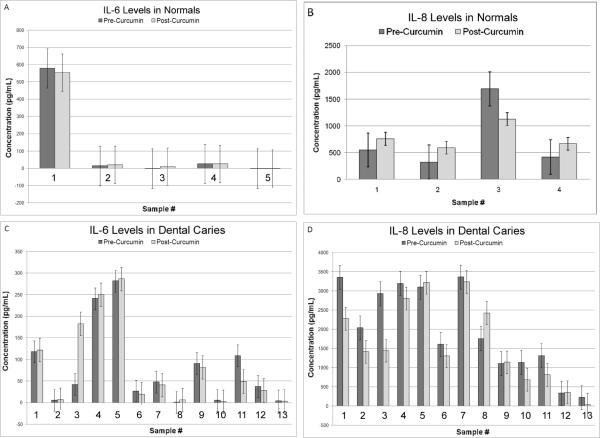
Curcumin treatment decreased IL-8 levels in saliva of patients with dental caries
To demonstrate further that the inhibitory effect can be seen in cytokines secreted in the saliva, we measured the IL-6 and IL-8 levels in the salivary supernatant of the head and neck cancer patients. As a control, we studied IL-6 and IL-8 levels in normal individuals and individuals with an inflammatory condition, dental caries. The IL-6 level in four of five normal individuals was less than 5 pg/mL and the expression did not change after curcumin treatment (Fig. 2A). One sample had a 600 pg/mL concentration that did not vary after curcumin treatment. The IL-8 expression level in four individuals ranged from 300–1700 pg/mL (Fig. 2B). Curcumin treatment resulted in a 30% decrease in one individual and appreciable changes were not seen in the other three samples.
Figure 2.
Inhibitory effect of curcumin on IL-8 expression in the saliva of patients with dental caries. A, The IL-6 expression is less than 5 pg/mL in four of five normal individuals, which is similar to the expression level reported in the literature. Individual #1 has high level expression that could reflect an inflammatory condition. B, IL-8 expression in three individuals resembles the expression range reported in the literature. Individual #3 has three times the expected expression. Appreciable changes were not observed in IL-6 or IL-8 levels after curcumin treatment. C, Of the thirteen patients with dental caries tested, one sample, #11 shows a 50% reduction and another sample #3 has a four-fold increased expression of IL-6. Appreciable changes are not seen in other samples. D, Eight of the dental caries patients show reduced IL-8 expression and four of them show greater than 25% reduction (#1, 3, 11 and 13). One sample, #8, shows a 20% increase in IL-8 expression post curcumin treatment.
The IL-6 and IL-8 expression in many of the samples collected from patients with dental caries were higher than the normal range. The IL-6 level was less than 50 pg/mL in 8 samples, less than 100 pg/mL in three samples, and higher than 200 pg/mL in two samples (Fig. 2C). Curcumin treatment showed a 50% decrease in one sample, #11 and a four-fold increase in another sample, #3 (Fig. 2C). The IL-8 expression was less than 1000 pg/mL in five samples, close to 2000 pg/mL in three samples, and higher than 3000 pg/mL in five other samples (Fig. 2D). Thus, the IL-8 expression in 11 samples was higher than the level observed for the normal individuals reflecting an inflammatory response to dental caries. Curcumin treatment resulted in decreased expression in 8 samples (62%) with greater than a 25% reduced expression in four samples (#1, 3, 11 and 13) (Fig. 2D). One sample (#8) had a 20% increased expression and the remaining four samples did not show a change in expression. Statistical evaluation of the cytokine levels showed that the decrease in IL-8 expression in post curcumin samples was significant (p<0.05).
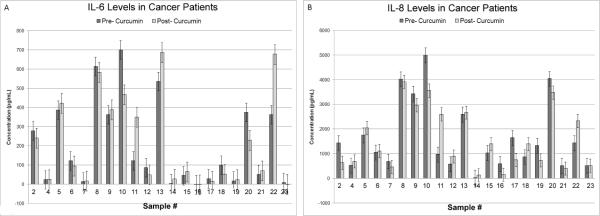
Modest inhibitory effect on IL-8 expression by curcumin in patients with head and neck cancer
The IL-6 and IL-8 levels in patients with head and neck cancer were greater than the levels observed in normal individuals. Expression levels were in the range of 4–700 pg/mL for IL-6 and 30–5000 pg/mL for IL-8 (Fig. 3). Post curcumin treatment, greater than a 25% decrease in IL-6 expression was seen in two samples (#10 and 20) (9%) and a 25% increased expression was noticed in three samples (#11, 13 and 22) (14%) (Fig. 3A). Five samples (#2, 10, 16, 17 and 19) had a 20% reduction in IL-8 expression (24%) and two samples (#11 and 22) had greater than a 20% increased expression (9%) (Fig. 3B). The remaining samples did not show an appreciable change, indicating a modest inhibitory effect on IL-8 post curcumin treatment in these individuals. Based on these findings, we feel that curcumin may need to be administered for a longer time period in order for it to have a stronger inhibitory effect on the IL-6 and IL-8 levels in the saliva of head and neck cancer patients. It is also likely that higher curcumin concentrations coupled with an extended treatment time period may be required for the curcumin effect to be noticeable in HNSCC patients. Further studies are needed in order to confirm this phenomenon.
Figure 3.
Modest inhibitory effect of curcumin on salivary IL-8 expression in head and neck cancer patients. A, Of the twenty-one head and neck cancer patient samples analyzed, two samples (#10 and 20) show decreased expression and three samples (#11, 13 and 22) show increased expression of IL-6 following curcumin treatment. B, Curcumin treatment results in greater than a 20% decreased expression of IL-8 in five samples (#2, 10, 16, 17 and 19). Higher than 20% expression of IL-8 is seen in two samples, # 11 and # 22, the same samples that have shown increased IL-6 expression after curcumin treatment. The modest inhibitory effect on IL-8 suggests that higher dose of curcumin and longer treatment time periods may be required to induce significant inhibitory effect on cytokine expression in the saliva of different head and neck cancer patients.
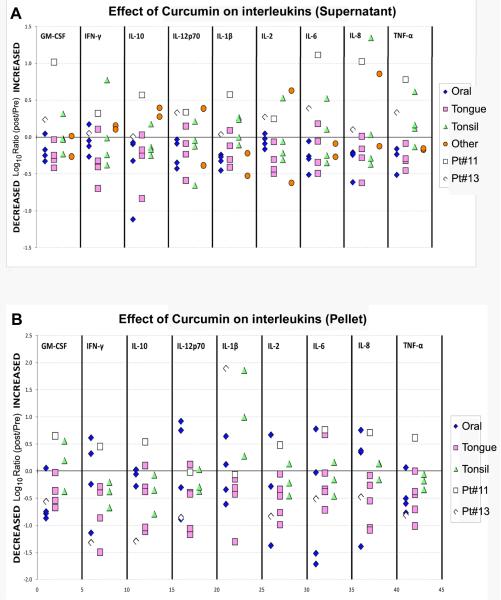
Independent verification of the inhibitory effect of curcumin on cytokine expression in head and neck cancer
As an independent verification of the effect of curcumin on IL-6 and IL-8, and to assess whether curcumin regulates inflammatory response, we measured cytokine levels using mesoscale discovery platform in 14 of the salivary samples. These studies were performed in a blinded fashion in the Institute for Molecular Medicine, Uniformed Services University of Health Sciences (USUHS) School of Medicine, Bethesda, MD. Both the supernatant and cell pellet samples were analyzed. Clustering analysis and statistical significance were determined for the tumor site and recurrence. Clustering of the oral cancers separate from the tongue and tonsil were noticed (supplementary results figure 1). We could further observe clustering of IL-10, IFN-γ, IL-12p70 and IL-2 as a group, and that of GM-CSF and TNF-α as another group in the cluster analysis. We also observed a statistically significant reduction in IL-10, IFN-γ, IL-12p70 expression in the salivary cells of the recurrent tumors in comparison to tumors without a prior tumor history (supplementary results figure 2). Finally, in a majority of samples, post vs pre curcumin log10 ratio analysis demonstrated a clear reduction in the expression (8 to 10 of 14 samples, 57 to 71%) of all nine cytokines in the salivary supernatant as well as in cell pellets (Figure 4). The inhibitory effect was highly noticeable in tongue tumors.
Figure 4.
Inhibitory effect of curcumin on inflammatory cytokines. Analysis of 14 salivary samples using the Human 9-Plex Ultrasensitive Electrochemiluminescent MULTI-SPOT Assay system followed by the cluster analysis shows decreased expression of all nine cytokines in A, supernatant and in B, cellular pellet. While the inhibition is variable as was seen in figure 3, cluster analysis points to enhanced inhibitory activity on tongue tumors indicating the usefulness of curcumin in the treatment of this tumor type. Overexpression of IL-6 and IL-8 observed in the supernatant samples of #11 and #13 confirmed the results obtained with the individual cytokine ELISA method (Figure 3 above), indicating the usefulness of either of the methods to detect interleukin expression in salivary samples.
DISCUSSION
HNSCC encompasses a wide range of tumors, including the nasopharynx, oral cavity, oropharynx, hypopharynx, and/or larynx. It is the fifth most common cancer in the world with one of the lowest 5-year survival rates.1 Current treatment modalities for HNSCC includes surgery, radiation, and chemotherapy; however, these often result in significant morbidity especially for patients with late-stage HNSCC.
Curcumin, a food derivative that is mostly used as a spice and food coloring agent, has been found to possess potent chemopreventive properties.5–8 Many studies have shown that curcumin has antiproliferative and proapoptotic effects against various tumors in vitro as well as in vivo. In addition, curcumin has been proven to be pharmacologically safe. To date clinical trials have not identified a maximum tolerated dose of curcumin in humans.8 Clinical studies have been administering up to 8,000 mg/day of curcumin to patients and have concluded that curcumin is non-toxic and poses minimal adverse effects on humans. One of eight pancreatic cancer patients receiving the oral curcumin showed remission, indicating the usefulness of curcumin in some cancer patients.20
Inflammatory response from cancer is well documented and increased expression of proinflammatory cytokines have been observed in serum and saliva of cancer patients. St.John et al18 and Rhodus et al19 have shown statistically significant higher concentrations of IL-8 in the saliva of oral cancer patients in comparison to age- and sex-matched non cancer individuals. Secretion of cytokines such as IL-6, IL-8 and VEGF are related to increased activation of transcription factor NF-κB in the cancer patients. Therefore it is hypothesized that the inhibition of NF-κB could play an important role in the control of cancer development.
We and others have documented constitutive activation of NF-κB in head and neck cancer cell lines.5,10–12 We have also shown that HNSCC cell lines express IKKβ in the nucleus where the enzyme may be involved in the transcription activation of NF-κB.16 Cell line studies from our laboratory have shown that curcumin inhibits NF-κB activity through the inhibition of IKKβ kinase. In the present investigation, we have demonstrated that curcumin downregulates IKKβ kinase activity of the salivary cells leading to the inhibition of cytokine expression in some cancer patients. The inhibitory effect of curcumin was highly noticeable in recurrent tumors and in tumors of the tongue. It is likely that longer treatment time periods and higher doses of curcumin delivered through increased treatment frequency might be required to achieve beneficial effects in the other cancer patients. Cluster analysis has shown that the curcumin effect may be significant in recurrent tumors. It is also possible that some tumors are refractory to curcumin treatment due to mutations in the curcumin binding site of the IKKβ protein. Since curcumin is shown to act via an AKT independent pathway12, cancers with AKT activation could also be immune to curcumin treatment.
We have found that IKKβ kinase activity in the salivary cells of HNSCC patients could be measured using two different assays described in this investigation. Since IKKβ kinase activation is an early event in the activation of NF-κB, enzyme activity measurements in saliva collected from patients receiving cisplatin treatment would point to the level of resistance developing through this signaling pathway in HNSCC. In such situations, a combination treatment with curcumin will be a valuable option for cancer treatment in HNSCC. We believe that IKKβ kinase activity is a good indicator of the NF-κB mediated cytokine expression and thus this protein will be a useful therapeutic target in patients with head and neck cancer.
In conclusion, we believe that curcumin is a potential cancer therapeutic agent that may be combined with the current HNSCC treatment protocols to help alleviate inflammatory responses and reduce harmful side-effects for patients. In the clinical setting, high doses of curcumin along with a longer treatment time period may be necessary to achieve a suppressive effect on IKKβ kinase activity, and on the expression of inflammatory cytokines. Even though high doses of oral curcumin are well tolerated in humans, there is poor absorption from the gastrointestinal tract. Hence, future studies need to be conducted to not only determine the maximum tolerated dose of curcumin, but to also find optimal delivery methods of curcumin that would more effectively treat patients with head and neck cancer.
Supplementary Material
Translational Relevance.
Curcumin shows promise as a potential adjuvant treatment for patients with head and neck squamous cell carcinoma (HNSCC). Its suppressive activity appears to involve inhibition of IKK kinase activity, preventing NF-κB translocation to the nucleus. In vitro studies have demonstrated that curcumin reduces the expression of inflammatory cytokines through the inhibition of IKKβ kinase activity. In this study we demonstrate that curcumin inhibits IKKβ kinase activity in the saliva of head and neck cancer patients and this inhibition correlates with a reduced expression of a number of cytokines. Our data suggests that IKKβ kinase could be a useful biomarker for detecting the effect of curcumin in head and neck cancer. Our findings also support investigation of a larger set of head and neck cancer patients to determine the clinical utility of curcumin in the suppression of IKKβ kinase activity and the inhibitory effect on the inflammatory cytokines.
Acknowledgement
We thank Zilu Zhang of the Department of Biostatistics, David Geffen School of Medicine at UCLA.
Financial support: The study was supported by funds from VAGLAHS, West Los Angeles Surgical Education Research Center, UCLA Academic Senate grant (M. B. Wang), NIH (R21 CA116826-01 to M. Wang) and Merit grant from the Veterans Administration, Washington, DC (E.S. Srivatsan).
References
- 1.Mignogna MD, Fedele S, Russo LL. The world cancer report and the burden of oral cancer. Eur J Cancer Prev. 2004;13:139–142. doi: 10.1097/00008469-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute. 2007:7–22. 07-6215. [Google Scholar]
- 4.Argiris A, Brockstein BE, Haraf DJ, Stenson KM, Mittal BB, Kies MS, Rosen FR, Jovanovic B, Vokes EE. Competing causes of death and second primary tumors in patients with locoregionally advanced head and neck cancer treated with chemoradiotherapy. Clin Cancer Res. 2004;10:1956–1962. doi: 10.1158/1078-0432.ccr-03-1077. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal S, Takada Y, Singh S, Myers JN, Aggarwal BB. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-kappaB signaling. Int J Cancer. 2004;111:679–692. doi: 10.1002/ijc.20333. [DOI] [PubMed] [Google Scholar]
- 6.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A Review of Anti-Cancer Properties and Therapeutic Activity in Head & Neck Squamous Cell Carcinoma. Molecular Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanif R, Qiao L, Shiff SJ, Rigas B. Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J Lab Clin Med. 1997;130:576–584. doi: 10.1016/s0022-2143(97)90107-4. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170–181. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Limtrakul P, Lipigorngoson S, Namwong O, Apisariyakul A, Dunn FW. Inhibitory effect of dietary curcumin on skin carcinogenesis in mice. Cancer Lett. 1997;116:197–203. doi: 10.1016/s0304-3835(97)00187-0. [DOI] [PubMed] [Google Scholar]
- 10.LoTempio MM, Veena MS, Steele HL, Ramamurthy B, Ramalingam TS, Cohen AN, Chakrabarti R, Srivatsan ES, Wang MB. Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res. 2005;11:6994–7002. doi: 10.1158/1078-0432.CCR-05-0301. [DOI] [PubMed] [Google Scholar]
- 11.Cohen AN, Veena MS, Srivatsan ES, Wang MB. Suppression of interleukin 6 and 8 production in head and neck cancer cells with curcumin via inhibition of Ikappa beta kinase. Arch Otolaryngol Head Neck Surg. 2009;135:190–197. doi: 10.1001/archotol.135.2.190. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Veena MS, Stevenson K, Tang C, Ho B, Suh JD, Duarte VM, Faull KF, Mehta K, Srivatsan ES, Wang MB. Liposome-encapsulated curcumin suppresses growth of head and neck squamous cell carcinoma in vitro and in xenografts through the inhibition of nuclear factor kappaB by an AKT-independent pathway. Clin Cancer Res. 2008;14:6228–6236. doi: 10.1158/1078-0432.CCR-07-5177. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–2362. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- 14.Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 15.Garg A, Aggarwal BB. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia. 2002;16:1053–1068. doi: 10.1038/sj.leu.2402482. [DOI] [PubMed] [Google Scholar]
- 16.Duarte VM, Han E, Veena MS, Salvado A, Suh JD, Liang LJ, Faull KF, Srivatsan ES, Wang MB. Curcumin enhances the effect of cisplatin in suppression of head and neck squamous cell carcinoma via inhibition of IKKβ protein of the NFκB pathway. Mol Cancer Ther. 2010;9:2665–2675. doi: 10.1158/1535-7163.MCT-10-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Malhotra PS, Thomas GR, Ondrey FG, Duffey DC, Smith CW, Enamorado I, Yeh NT, Kroog GS, Rudy S, McCullagh L, Mousa S, Quezado M, Herscher LL, Van Waes C. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5:1369–1379. [PubMed] [Google Scholar]
- 18.St John MA, Li Y, Zhou X, Denny P, Ho CM, Montemagno C, Shi W, Qi F, Wu B, Sinha U, Jordan R, Wolinsky L, Park NH, Liu H, Abemayor E, Wong DT. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:929–935. doi: 10.1001/archotol.130.8.929. [DOI] [PubMed] [Google Scholar]
- 19.Rhodus NL, Ho V, Miller CS, Myers S, Ondrey F. NF-kappaB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect Prev. 2005;29:42–45. doi: 10.1016/j.cdp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.