Abstract
The reduction of plasma low-density lipoprotein levels by HMG-CoA reductase inhibitors, or statins, has had a revolutionary impact in medicine, but muscle-related side effects remain a dose-limiting toxicity in many patients. We describe a chemical epistasis approach that can be useful in refining the mechanism of statin muscle toxicity, as well as in screening for agents that suppress muscle toxicity while preserving the ability of statins to increase the expression of the low-density lipoprotein receptor. Using this approach, we identified one compound that attenuates the muscle side effects in both cellular and animal models of statin toxicity, likely by influencing Rab prenylation. Our proof-of-concept screen lays the foundation for truly high-throughput screens that could help lead to the development of clinically useful adjuvants that can one day be co-administered with statins.
3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, or statins, are important drugs for lowering plasma low-density lipoprotein (LDL) levels and decreasing the risk of cardiac events and mortality. They are taken by tens of millions of people worldwide (1). A common side effect of statin use is muscle toxicity, ranging from patient-reported muscle weakness and cramps to myopathy requiring hospitalization and life-threatening rhabdomyolysis (2), which can occur in 0.1–0.5% of patients (3). Vigorous exercise is a risk factor for these side effects (2). Although rhabdomyolysis itself is rare (3, 4), complaints of muscle-related symptoms necessitate a lowering in dose, a change in statin, or even complete cessation (5), thereby preventing the optimal lowering of plasma LDL levels. The etiology of this side effect is not fully understood, but is generally thought to be on-target, that is, related to statins’ effects on HMG-CoA reductase. Indeed, knockdown of HMG-CoA reductase in zebrafish phenocopies the effects of statins on muscle (6). Genome-wide association studies for statin myopathy have pointed to polymorphisms in the hepatic organic anion transporter that may influence circulating levels of statins (7). Identifying the cellular basis of statin-induced myopathy, and targeting it chemically, could in principle allow us to fully harness the therapeutic potential of these drugs.
We previously identified a cellular signature of statin-induced muscle toxicity in the C2C12 myotube. Specifically, we reported that a subset of statins tend to decrease cellular ATP and MTT levels, while leaving intact the mitochondrial gene expression and membrane potential (8). Other clinically used drugs exhibiting the same signature have been reported to be associated with myopathy, helping to credential this cellular signature as a surrogate for myopathy. Notably, one of the clinically used drugs sharing this signature was propranolol, not traditionally associated with myopathy. Subsequent epidemiological studies have confirmed this hypothesis (9). Collectively, these studies suggest that myotube ATP levels could serve as a cellular surrogate of drug-induced myopathy. Here, we use this assay to further explore the mechanism of statin myopathy and perform a proof-of-concept chemical screen to identify agents that suppress this toxicity without compromising its efficacy in cultured cells.
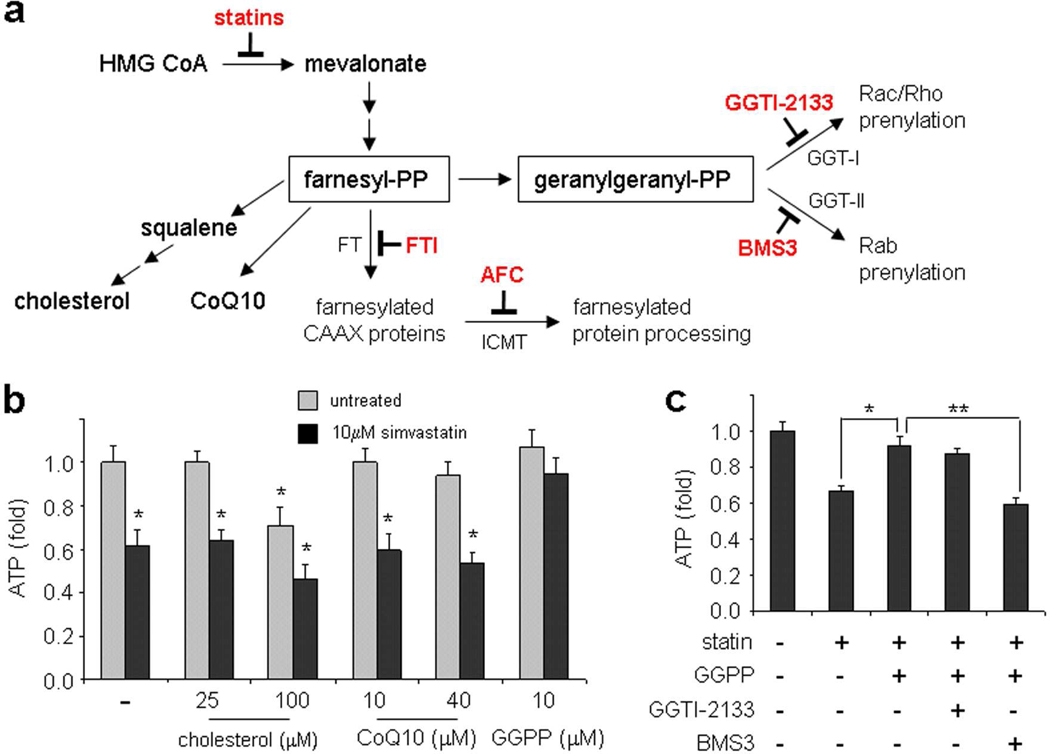
The mevalonate pathway produces biosynthetic precursors for cholesterol, steroids, terpenoids, and isoprenoids required for protein prenylation (Figure 1, panel a). It is well known that inhibiting HMG-CoA reductase results in a homeostatic change that preserves the synthesis of mevalonate, so it has been suggested that a decrease in other products of this pathway, such as ubiquinone (CoQ) may underlie muscle toxicity (10, 11). To determine which of these branches may be contributing to statin toxicity, we treated C2C12 myotubes for 48 hours with 10 µM simvastatin, while simultaneously adding back various pathway intermediates. Simultaneous addition of cholesterol or CoQ was unable to prevent the simvastatin-mediated decrease in ATP levels (Figure 1, panel b). We then tested the effects of the isoprenoid intermediates farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which are incorporated into cells (12), to assess the importance of protein prenylation events on ATP levels. While FPP had no efficacy (Supplementary Figure 1), GGPP completely preserved ATP levels in the presence of simvastatin (Figure 1, panel b), suggesting that this branch of the mevalonate pathway downstream of HMG-CoA reductase is responsible for the major side effect of statin treatment. We also observed that GGPP suppressed the effects of simvastatin on caspase-3/7 and MTT activities, indicating a role for apoptosis as well (Supplementary Figure 2). Collectively, these results are consistent with previous reports (12–15), which have suggested that the relatively small pool of GGPP in skeletal muscle may underlie its unique sensitivity to statins (12).
Figure 1. Chemical epistasis analysis links statin muscle toxicity to protein prenylation.
(a) Cholesterol biosynthesis pathway. Small-molecule inhibitors of enzymatic reactions used in this study are indicated in red. FTI, farnesyltransferase (FT) inhibitor; AFC, acetylfarnesylcysteine; GGTI-2133, inhibitor of geranylgeranyltransferase-I (GGT-I); BMS-3, inhibitor of geranylgeranyltransferase-II (GGT-II). (b) Addition of pathway intermediates to determine enhancement of cellular ATP levels. C2C12 cells were differentiated for 4–6 days into myotubes in 384-well plates, and treated for 48 hours with cholesterol, CoQ10, or geranylgeranylpyrophosphate (GGPP) in the absence (gray bars) or presence (black bars) of 10 µM simvastatin. CoQ10 was conjugated to methylcyclodextrin to ensure cell permeability (see Methods). Cellular ATP levels were measured with a commercial luciferase-based kit, and normalized to the no-treatment condition. *, p < 0.01 (t-test) relative to the untreated state. (c) The increase in ATP levels by GGPP is abolished by inhibiting geranylgeranyltransferase-II (GGT-II). C2C12 myotubes were treated with 10 µM simvastatin, 10 µM GGPP, and 10 µM each of chemical inhibitors of GGT-I (GGTI-2133) or GGT-II (BMS3). Cellular ATP levels were normalized to the no-treatment condition. *, p < 0.01 (t-test) relative to statin treatment; **, p < 0.01 (t-test) relative to statin + GGPP treatment.
Next, we used chemical suppressor studies to further determine the prenyl transfer steps responsible for muscle toxicity. Twenty-carbon geranylgeranyl groups are transferred to recipient proteins by two enzymes: geranylgeranyltransferase I (GGT-I), which is responsible for prenylation of Rac and Rho family proteins; and geranylgeranyltransferase II (GGT-II), which prenylates the Rab family of proteins. To determine if one or both pathways are important for statin muscle toxicity, we treated C2C12 myotubes with both 10 µM simvastatin and 10 µM GGPP, a condition which should restore normal cellular ATP levels. Simultaneously, we added specific small-molecule inhibitors of either GGT-I or GGT-II; the inability to transfer geranylgeranyl groups to the relevant protein(s) involved in statin toxicity should therefore abolish GGPP's beneficial effects. We observed that inhibition of GGT-I by GGTI-2133 had no effect on the system, while inhibition of GGT-II by BMS3 (16, 17) resulted in lower cellular ATP levels, similar to statin treatment alone (Figure 1, panel c). These results suggest that blockade of a Rab prenylation event by statins contributes to muscle toxicity.
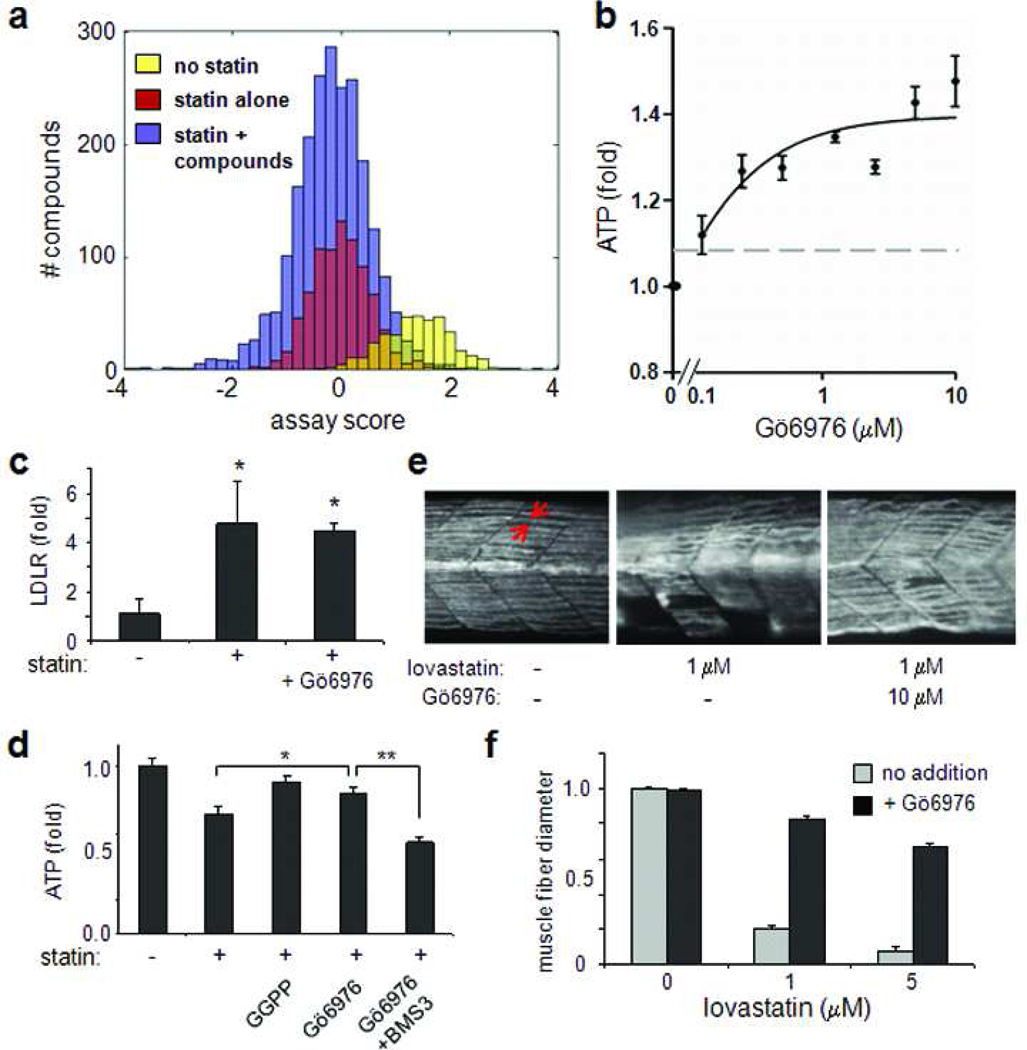
We sought to identify additional novel agents capable of suppressing the cell-based surrogate of muscle toxicity. To that end, we treated C2C12 myotubes with 10 µM simvastatin and a small-molecule library of 2,240 diverse compounds, including known bioactives, commercially available compounds, natural products, and a set of internally synthesized compounds, and assessed ATP levels after 48 hours (Figure 2, panel a; screening data provided as Supplementary Table 1). We identified four compounds that, upon retesting, exhibited a dose-dependent preservation of cellular ATP levels in the presence of simvastatin (Supplementary Figure 3), including one protein kinase C inhibitor, one VEGF receptor kinase inhibitor, one farnesyltransferase inhibitor (FTI), and one farnesyl analog, acetylfarnesylcysteine (AFC). Interestingly, the last two compounds are inhibitors of protein farnesylation (Figure 1, panel a).
Figure 2. Screening for suppressors of simvastatin-induced muscle toxicity.
(a) Histogram of screening results. C2C12 cells were differentiated in 384-well plates, and 2,240 compounds were pin-transferred into plates containing media with 10 µM simvastatin (blue bars). Wells containing DMSO with simvastatin were included as negative controls (purple bars), and one plate containing no simvastatin treatment was included as a positive control (yellow bars). Cellular ATP levels were measured with a commercial luciferase-based kit. Each well was scored relative to the distribution of DMSO control wells (see Methods). (b) Dose-response analysis of the kinase inhibitor Gö6976 in the presence of simvastatin. C2C12 myotubes were treated 48 hours with 10 µM simvastatin and the indicated concentration of Gö6976. Cellular ATP levels were normalized to the untreated state; gray dashed line indicates the standard deviation of DMSO-treated wells. (c) Gene-expression analysis of LDLR in C2C12 myotubes after 48-hour treatment with DMSO (vehicle control), 10 µM simvastatin, or simvastatin with 5 µM Gö6976. Quantitative real-time PCR results were normalized to the vehicle control. *, p < 0.01 (t-test) relative to the vehicle control. (d) The suppression of statin-induced toxicity in muscle cells by Gö6976 is eliminated by inhibition of GGT-II. C2C12 myotubes were treated for 48 hours with DMSO or 10 µM simvastatin in the presence of 10 µM GGPP, 5 µM Gö6976, or 5 µM Gö6976 with 10 µM BMS3 as indicated. ATP levels were normalized to the vehicle control. *, p < 0.01 (t-test) relative to statin treatment; **, p < 0.01 (t-test) relative to statin + Gö6976 treatment. (e) Zebrafish embryos were treated for 12 h with combinations of 1 or 5 µM lovastatin and 10 µM Gö6976. Embryos were fixed and stained for myosin heavy chain (see Methods) for muscle fiber size determination (red arrows). Representative somite phenotypes are shown, using 1 µM lovastatin; anterior, left. (f) Quantification of muscle fiber diameter, using one hundred embryos per group. Results are graphed as the ratio of the mean experimental fiber size to the control fiber size.
We focused on Gö6976, annotated as an indolocarbazole kinase inhibitor of CHK1 and protein kinase C (PKC) alpha and beta, which had the most potent suppressive effects on statin muscle toxicity (Figure 2, panel b). Although this compound has not been used clinically, the structurally and functionally similar compound UCN-01 has been tested in humans for treating cancer (18, 19). Immunostaining of myotubes for myosin heavy chain revealed a clear improvement in the condition of cells after co-treatment with simvastatin and Gö6976 (Supplementary Figure 4), with a preservation of muscle fiber size and striation. Importantly, this compound had no effect on the statin-induced increase in LDL receptor gene expression in either myotubes or in human HepG2 cells (Figure 2, panel c, and Supplementary Figure 5). The increase in LDLR expression in the liver is thought to be the primary mechanism by which statins decrease plasma LDL (20). Thus, in cell culture, Gö6976 is able to suppress the myotoxic effects of statins, while preserving their therapeutic effect.
To determine whether Gö6976 is suppressing toxicity through the prenylation defined above, we added back inhibitors of GGT-I and GGT-II to determine if they suppress the rescue. Addition of BMS3 to the combination of simvastatin and Gö6976 resulted in a reduction in cellular ATP, to levels similar to statin treatment alone (Figure 2, panel d). Further, treatment with BMS3 alone phenocopies the effects of simvastatin, while GGTI-2133 had no effect on myotubes (Supplementary Figure 6). Thus, we can infer that the suppressive effects of Gö6976 on statin-reduced ATP levels may be mediated through prenylation of one or more members of the Rab family of proteins, perhaps the same event that is restored by the addition of GGPP. It is notable that in previous reports focused on zebrafish models, knock-down of either GGT-I or GGT-II mimicked statin-induced muscle toxicity (21). Although our results are consistent with a previous report in isolated rat myotubes (15), it is possible that, for example, inhibitor specificity or differences in the model sytems used are responsible for these differing results.
Finally, we sought to determine whether Gö6976 might be useful to prevent statin myopathy in an animal model. Recently, zebrafish have been used to study the mechanism of statin muscle toxicity, and to assess its suppression by geranylgeraniol supplementation (6, 21). The successful suppression of statin muscle toxicity in zebrafish by geranylgeraniol indicates that this model is a good one for testing our compounds of interest. In a blinded study, we added either 1 or 5 µM lovastatin and 10 µM Gö6976 to zebrafish for 48 hours, and stained for myosin heavy chain to assess myofiber size and integrity. While lovastatin treatment alone resulted in a ragged appearance and a decrease in myofiber size, the addition of Gö6976 in combination with lovastatin resulted in an increase in striated fibers (Figure 2, panel e) and muscle fiber size (Figure 2, panel f), and a decrease in the percentage of embryos with appearance of muscle damage (Supplementary Figure 7, 8).
Our results suggest that myotube ATP levels may serve as a sensitive, cellular surrogate of myopathy that can be useful for understanding the mechanism of statin muscle toxicity as well as for preventing it. It is notable that two of the four screening positives (FTI and AFC) directly inhibit farnesylation (Figure 1, panel a); it is possible that these compounds may increase the isoprenoid pool available for protein geranylgeranylation. We have confirmed that Gö6976, a commercially available protein kinase C inhibitor, is capable of suppressing toxic effects of simvastatin in C2C12 myotubes and in zebrafish. These results demonstrate the suitability of this in vitro screen to identify compounds with in vivo activity. Our data suggest that myotoxic effects of statins, as well as their suppression by Gö6976, are mediated by a geranylgeranylation event. Further study is required to determine the specificity of the two GGT enzymes downstream of this pathway. Moreover, identifying these prenylation targets represents an important next step in understanding and preventing statin muscle toxicity. Given that statins are taken by millions of patients worldwide, a clinically useful suppressor of myopathy would itself would need to have an extremely safe toxicity profile. As such, we do not anticipate that Gö6976 itself is likely to represent such an agent. However, our results provide proof-of-concept that it should be possible to screen large libraries of compounds to discover adjuvants that can in principle safely suppress statin myopathy while preserving their efficacy.
Methods
Cell culture and reagents
C2C12 myoblasts (ATCC) were grown in Dulbecco’s Modified Eagle Medium (DMEM, Mediatech) supplemented with 10% (vol/vol) fetal bovine serum and antibiotics (100 mg/ml penicillin/streptomycin mix) in a humidified atmosphere at 37 C with 5% CO2. Differentiation into myotubes was induced at 80–100% density on ‘day 0’ by changing the medium to DMEM supplemented with 2% (vol/vol) horse serum. Simvastatin, GGPP, FPP, cholesterol, CoQ10, GGTI-2133, and Gö6976 were purchased from Sigma. Coenzyme Q10 conjugation was accomplished by adding in small aliquots to 0.05 g of methylcyclodextrin dissolved in 1 M ammonium hydroxide. The solution was heated at 75 C with occasional vortexing until solution was clear. The solution was lyophilized and resuspended at the desired concentration for cell treatments. BMS3 was synthesized, purified and characterized as described (22, 23). The sample was analytically pure according to LCMS and 1H-NMR. BMS3 is a potent inhibitor of cellular isoprenylation by farnesyl transferase (FT) and GGT-II, but does not inhibit isoprenylation by GGT-I (17).
High-throughput cell-based assay for cellular ATP levels
C2C12 cells were seeded at 5,000 cells/well using a Multidrop Combi (Thermo Labsystems) in white optical 384-well plates (Corning Life Sciences). Differentiation occurred over 4–6 days. Myotubes were treated with 10 µM simvastatin; using libraries of compounds dissolved in DMSO and a CyBi-Well pin-transfer robot (CyBio Corp.), 0.1 µL of each compound was added to the wells. After incubation for 48hr, 20 µL per well CellTiter-Glo reagent (Promega) was added to 20 mL per well of cell-culture medium. Luminescence was measured after 10-min incubation using an EnVision plate reader (PerkinElmer).
Screening data analysis
Instrument output files were processed using Pipeline Pilot (Accelrys) and input to a MATLAB (The MathWorks) routine for data normalization. Compound performance scores relative to a distribution of mock-treated (DMSO) wells were calculated using version of the scoring system underlying ChemBank (24), revised as follows. The role of replicate treatments was further developed: first, mock-treatment distributions were modeled using all mock-treated wells measured on a single day, regardless of their nominal replicate; second, per-compound scores weighted each in-plate background-subtracted measurement by the uncertainty in that measurement, using the method of maximum likelihood. The uncertainty in a single background-subtracted measurement was estimated using the number of mock-treated wells on the plate and, as a measure of the assay noise, the standard deviation of the per-day mock-treatment distribution. The signal, a weighted average of differences, was scaled by the noise, the standard deviation of the mock-treatment distribution.
Gene expression
We extracted RNA using an RNeasy kit (Qiagen) and synthesized cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems) with random hexamers, as described by the manufacturer. The cDNA was then used for real-time PCR quantification of products for mouse Ldlr (MmLdlr_1_SG, Qiagen), with Actb (MmActb_2_SG) serving as an internal control, using SYBR green quantitation (Applied Biosystems).
Immunofluorescence
To stain for myosin heavy chain (MHC) expression, C2C12 cells were cultured and differentiated in 96-well plates as described. After compound treatment, cells were fixed with ice-cold methanol for 5 minutes, washed with PBS three times, and blocked for 1 hour with PBS containing 0.01% Tween-20 and 2% BSA (PBSTB2). Cells were incubated in primary antibody (mouse anti-MHC, Upstate Biotechnology) at 1:1000 in PBSTB2 for 1 hour, washed three times with PBSTB2, and secondary antibody (anti-mouse IgG conjugated to Alexa Fluor 488, Invitrogen) at 1:500 in PBSTB2 for 1 hour, washed three times with PBSTB2, and stored sealed at 4 C for imaging. Images were captured with a Zeiss Axiovert at 40× magnification; the size and brightness of images were adjusted with Adobe Photoshop. Identical exposure times and brightness settings were applied to each image.
Zebrafish maintenance and treatment
Zebrafish maintenance, treatment with compounds, staining for myosin heavy chain, and image quantification was performed as described(6, 21). All compound additions were performed in a blinded fashion.
Supplementary Material
Acknowledgements
We thank N.Doro, Z.Gauhar, and K.Hartland for technical assistance; M.Freeman, R.Gould, T.Kitami, and S.Shaw for helpful comments, and the Broad Chemical Biology Platform for compound arraying. This work was supported by fellowships or grants from the Howard Hughes Medical Institute (V.K.M.), the Alexander von Humboldt Stiftung (to R.S.B.), and the National Institutes of Health (R24 DK-080261 to V.K.M. and DP2 DK-083048 to B.K.W.).
Footnotes
Conflict of interest statement
The Broad Institute has filed a patent application related to work presented in this paper.
Supporting Information Available: This material is available free of charge via the Internet.
References
- 1.Wenner Moyer M. The search beyond statins. Nat Med. 2010;16:150–153. doi: 10.1038/nm0210-150. [DOI] [PubMed] [Google Scholar]
- 2.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 3.Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, Gurwitz JH, Chan KA, Goodman MJ, Platt R. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–2590. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- 4.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–1790. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqi SA, Thompson PD. How do you treat patients with myalgia who take statins? Curr Atheroscler Rep. 2009;11:9–14. doi: 10.1007/s11883-009-0002-1. [DOI] [PubMed] [Google Scholar]
- 6.Hanai J, Cao P, Tanksale P, Imamura S, Koshimizu E, Zhao J, Kishi S, Yamashita M, Phillips PS, Sukhatme VP, Lecker SH. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest. 2007;117:3940–3951. doi: 10.1172/JCI32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 8.Wagner BK, Kitami T, Gilbert TJ, Peck D, Ramanathan A, Schreiber SL, Golub TR, Mootha VK. Large-scale chemical dissection of mitochondrial function. Nat Biotechnol. 2008;26:343–351. doi: 10.1038/nbt1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Setoguchi S, Higgins JM, Mogun H, Mootha VK, Avorn J. Propranolol and the risk of hospitalized myopathy: translating chemical genomics findings into population-level hypotheses. Am Heart J. 2010;159:428–433. doi: 10.1016/j.ahj.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49:2231–2237. doi: 10.1016/j.jacc.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 11.Paiva H, Thelen KM, Van Coster R, Smet J, De Paepe B, Mattila KM, Laakso J, Lehtimaki T, von Bergmann K, Lutjohann D, Laaksonen R. High-dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clin Pharmacol Ther. 2005;78:60–68. doi: 10.1016/j.clpt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Flint OP, Masters BA, Gregg RE, Durham SK. HMG CoA reductase inhibitor-induced myotoxicity: pravastatin and lovastatin inhibit the geranylgeranylation of low-molecular-weight proteins in neonatal rat muscle cell culture. Toxicol Appl Pharmacol. 1997;145:99–110. doi: 10.1006/taap.1997.8174. [DOI] [PubMed] [Google Scholar]
- 13.Johnson TE, Zhang X, Bleicher KB, Dysart G, Loughlin AF, Schaefer WH, Umbenhauer DR. Statins induce apoptosis in rat and human myotube cultures by inhibiting protein geranylgeranylation but not ubiquinone. Toxicol Appl Pharmacol. 2004;200:237–250. doi: 10.1016/j.taap.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Mullen PJ, Luscher B, Scharnagl H, Krahenbuhl S, Brecht K. Effect of simvastatin on cholesterol metabolism in C2C12 myotubes and HepG2 cells, and consequences for statin-induced myopathy. Biochem Pharmacol. 2010;79:1200–1209. doi: 10.1016/j.bcp.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto K, Honda T, Yokoya S, Waguri S, Kimura J. Rab-small GTPases are involved in fluvastatin and pravastatin-induced vacuolation in rat skeletal myofibers. FASEB J. 2007;21:4087–4094. doi: 10.1096/fj.07-8713com. [DOI] [PubMed] [Google Scholar]
- 16.Lackner MR, Kindt RM, Carroll PM, Brown K, Cancilla MR, Chen C, de Silva H, Franke Y, Guan B, Heuer T, Hung T, Keegan K, Lee JM, Manne V, O'Brien C, Parry D, Perez-Villar JJ, Reddy RK, Xiao H, Zhan H, Cockett M, Plowman G, Fitzgerald K, Costa M, Ross-Macdonald P. Chemical genetics identifies Rab geranylgeranyl transferase as an apoptotic target of farnesyl transferase inhibitors. Cancer Cell. 2005;7:325–336. doi: 10.1016/j.ccr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen UT, Guo Z, Delon C, Wu Y, Deraeve C, Franzel B, Bon RS, Blankenfeldt W, Goody RS, Waldmann H, Wolters D, Alexandrov K. Analysis of the eukaryotic prenylome by isoprenoid affinity tagging. Nat Chem Biol. 2009;5:227–235. doi: 10.1038/nchembio.149. [DOI] [PubMed] [Google Scholar]
- 18.Edelman MJ, Bauer KS, Jr., Wu S, Smith R, Bisacia S, Dancey J. Phase I and pharmacokinetic study of 7-hydroxystaurosporine and carboplatin in advanced solid tumors. Clin Cancer Res. 2007;13:2667–2674. doi: 10.1158/1078-0432.CCR-06-1832. [DOI] [PubMed] [Google Scholar]
- 19.Kummar S, Gutierrez ME, Gardner ER, Figg WD, Melillo G, Dancey J, Sausville EA, Conley BA, Murgo AJ, Doroshow JH. A phase I trial of UCN-01 and prednisone in patients with refractory solid tumors and lymphomas. Cancer Chemother Pharmacol. 65:383–389. doi: 10.1007/s00280-009-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown MS, Goldstein JL. Lowering plasma cholesterol by raising LDL receptors. N Engl J Med. 1981;305:515–517. doi: 10.1056/NEJM198108273050909. [DOI] [PubMed] [Google Scholar]
- 21.Cao P, Hanai J, Tanksale P, Imamura S, Sukhatme VP, Lecker SH. Statin-induced muscle damage and atrogin-1 induction is the result of a geranylgeranylation defect. FASEB J. 2009;23:2844–2854. doi: 10.1096/fj.08-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen B-C, Sundeen JE, Guo P, Bednarz MS, Zhao R. Novel triethylsilane mediated reductive N-alkylation of amines: improved synthesis of 1-(4-imidazolyl)methyl-4-sulfonylbenzodiazepines, new farnesyltransferase inhibitors. Tetrahedron Lett. 2001;42:1245–1246. [Google Scholar]
- 23.Ding CZ, Batorsky R, Bhide R, Chao HJ, Cho Y, Chong S, Gullo-Brown J, Guo P, Kim SH, Lee F, Leftheris K, Miller A, Mitt T, Patel M, Penhallow BA, Ricca C, Rose WC, Schmidt R, Slusarchyk WA, Vite G, Yan N, Manne V, Hunt JT. Discovery and structure-activity relationships of imidazole-containing tetrahydrobenzodiazepine inhibitors of farnesyltransferase. J Med Chem. 1999;42:5241–5253. doi: 10.1021/jm990391w. [DOI] [PubMed] [Google Scholar]
- 24.Seiler KP, George GA, Happ MP, Bodycombe NE, Carrinski HA, Norton S, Brudz S, Sullivan JP, Muhlich J, Serrano M, Ferraiolo P, Tolliday NJ, Schreiber SL, Clemons PA. ChemBank: a small-molecule screening and cheminformatics resource database. Nucleic Acids Res. 2008;36:D351–D359. doi: 10.1093/nar/gkm843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.