Abstract
Thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS) are clinically similar disorders characterized by microvascular thrombosis, hemolysis, thrombocytopenia, and end organ damage. Although they may present with overlapping symptoms, multiple etiologies have been proposed for these thrombotic microangiopathies (TMAs). Chemotherapy-induced TMA has been described with the use of mitomycin, gemcitabine, and others and has a poor prognosis. Recently, reports of TMA associated with targeted cancer agents have surfaced in the literature. We discuss the clinical presentation, outcome, and etiology of TMA reported with the use of immunotoxins, monoclonal antibodies, and tyrosine kinase inhibitors. A search of PubMed and meeting abstracts was conducted for cases of TMA with the use of targeted cancer agents. The defining symptoms, laboratory values, time to onset, and patient outcomes were compiled. Consistent definitions of TMA and grading of severity in these cases are lacking. However, presentation of TMA in these cases revealed the importance of monitoring for renal toxicity, hemolysis, and thrombocytopenia. Patient outcomes appear to differ from those seen in cases of chemotherapy-induced TMA and may reflect a different underlying etiology. Little is known about the pathogenesis of TMA with targeted cancer agents. In contrast to chemotherapy-induced TMA, partial to full reversibility may be a common outcome. However, further research is warranted into optimal management of patients diagnosed with TMA following treatment with targeted agents.
Keywords: Hemolytic Uremic Syndrome, Thrombotic Thrombocytopenic Purpura, Thrombotic Microangiopathy, Immunotoxin
Introduction
The thrombotic microangiopathies (TMAs) are a group of disorders characterized by occlusive microvascular thrombosis, thrombocytopenia, and end organ damage. The principal subtypes of TMA are thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). In turn, both TTP and HUS may each have several distinct subtypes caused by differing pathophysiologic mechanisms.
TTP is classically described by a pentad of clinical symptoms including thrombocytopenia, microangiopathic hemolytic anemia (MAHA), neurologic abnormalities, renal failure, and fever. HUS may also present with many overlapping signs and symptoms1. However, a diagnosis of TTP is usually reserved for cases in which neurologic abnormalities such as seizure and vision loss predominate, while HUS is diagnosed in cases where renal failure is the most prominent sign.
The differentiation between TTP and HUS, their classification, etiologies, and prognoses has been the subject of much discussion. Recent research has focused on identifying the causes of TTP and HUS at the molecular level. Of particular interest is the relationship between severely low levels of the serum metalloprotease ADAMTS13 with TTP. Bianchi et al. reported that ADAMTS13 activity less than 5% of normal is specific for TTP2. It should be noted, however, that reduced ADAMTS13 levels have also been observed in patients diagnosed with HUS3. .
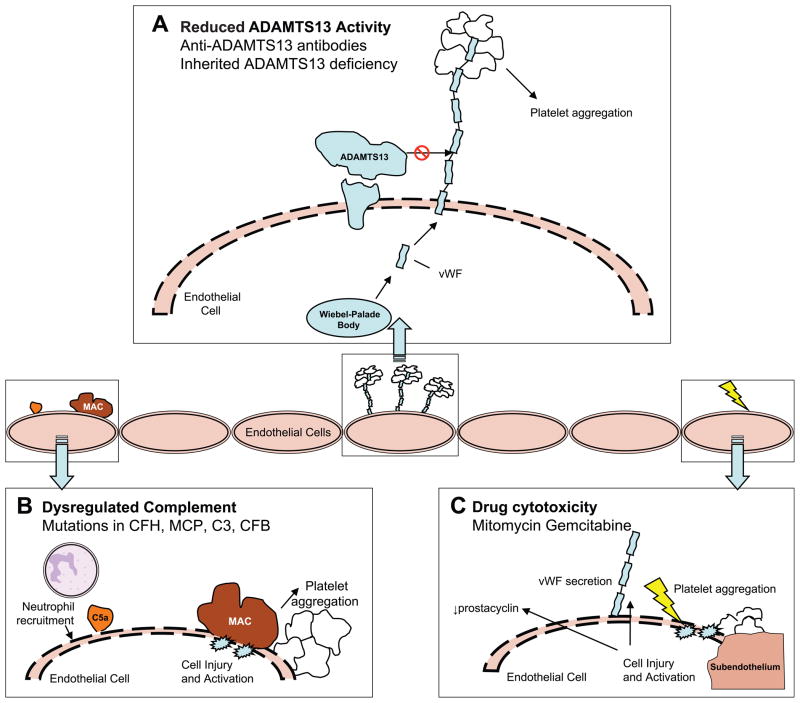
As extensively reviewed4,5, Von Willebrand’s factor (vWF) is initially secreted from Wiebel-Palade bodies as multimers tethered to the endothelial cell surface where they provide glycoprotein Ibα receptor sites for platelet adhesion and thrombus formation. ADAMTS13 cleaves the multimeric vWF, regulating thrombus formation. However, a significant decrease in ADAMTS13 or ADAMTS13 activity allows pathogenic thrombus formation as seen in TTP (Figure 1). Familial TTP is a rare and recurrent disorder caused by an inherited deficiency in ADAMTS136. Development of autoantibodies against this same enzyme may lead to a more common form of acquired TTP.
Figure 1. Proposed pathophysiology of thrombotic microangiopathies.
Formation of pathogenic thrombi in TMA may arise from reduced ADAMTS13 activity as shown in Panel A. ADAMTS13 bound to the surface of endothelial cells can cleave ultralarge multimers of Von Willebrand’s Factor (vWF). When the activity of ADAMTS13 is reduced due to anti-ADAMTS13 antibodies or an inherited deficiency, the uncleaved multimers of vWF induce platelet aggregation. In atypical HUS (Panel B), mutations in complement proteins lead to unregulated formation of C5a and C5b-9 (membrane-attack complex). Recruitment of neutrophils, endothelial cell injury, and exposure of the subendothelium results in a prothrombotic state. Drug cytotoxicity (Panel C) can also result in direct injury to the endothelium and lead to TMA. The exact pathogenesis of TMA in these cases is unknown, but may involve decreased levels of prostacyclin, secretion of vWF, or exposure of the subendothelium leading to thrombus formation.
The majority (~90%) of HUS cases appear in children infected with verocytotoxin-producing bacteria7. Following infection of the colon, the Shiga-like toxins (Stx1 and Stx2) enter the bloodstream and bind to cells displaying globotriaosylceramide receptors (Gb3 and Gb4), including the glomerular endothelial and epithelial cells1,8. The toxins result in cell death due to inhibition of protein formation, and result in the release of TNF-α, IL-1, IL-6, and IL-8. These inflammatory cytokines may potentiate renal injury secondary to infiltration by neutrophils and lead to upregulation of the Gb3 receptor. In addition, TNF-α and IL-8 have been shown to cause vWF secretion, while IL-6 inhibits cleavage of ultralarge multimers in vitro9. Other direct effects of Stx1 include stimulation of endothelial cells to express tissue factor and secrete vWF, as well as activation of platelets1, 10.
The etiology of the remaining 10% of “atypical” HUS (aHUS) cases is heterogeneous and largely linked to mutations in factors of the complement system11,12. The end result of these mutations is an overactive complement system causing endothelial cell damage, detachment, and eventual activation of the coagulation cascade. The alternative pathway of complement may play a role in typical HUS as well. Orth, et al. demonstrated in vitro activation of complement by Stx2 and propose that complement may contribute to kidney damage in typical HUS 8.
Finally, both TTP and HUS have been associated with malignancy, hematopoietic stem cell transplantation, and with specific medications. Historically, review articles of drug-induced TMA have focused on immunosuppressants, anti-aggregating agents, and cytotoxic chemotherapy 13,14,15,16. Among cytotoxic chemotherapy agents, mitomycin and gemcitabine (Table 1) are particularly associated with TMA and the FDA-approved labeling warns of this risk 17,18
Table 1.
| Chemotherapy | Incidence | Clinical Presentation | Onset | Prognosis |
|---|---|---|---|---|
| Mitomycin | 2–15% | Severe MAHA Thrombocytopenia Renal dysfunction Elevated LDH Elevated bilirubin Pulmonary Edema | Cumulative doses > 30 mg/m2 and > 1 year of treatment | Mortality ~ 75% related to renal failure |
| Gemcitabine | 0.25–0.4% | 2000–48000 mg/m2 and 5–8 months of treatment | Mortality ~60%; Renal failure 34–69%; Reversal of anemia and thrombocytopenia may be common |
The etiology of chemotherapy-induced TMA is thought to be non-specific, toxic insult to the microvasculature. Direct endothelial cell injury has been reproduced in an animal model of mitomycin-induced HUS and most likely plays a central role 14. Following endothelial injury and exposure of the subendothelium, platelet activation and subsequent clotting within the microvasculature may occur.
Thrombotic Microangiopathy induced by Targeted Agents
Immunotoxins
Immunotoxins are proteins comprised of a cell-selective ligand chemically conjugated or genetically fused to a toxin 19,20. The cell-selective portion of the immunotoxin is commonly a monoclonal antibody, antibody fragment, growth factor, or cytokine which binds to specific cell surface receptors. Once bound to a surface antigen, immunotoxins enter the target cell through endocytosis and undergo processing to release the toxin into the cytosol 21. Several of these agents have shown promising activity in clinical trials, however TMA has been reported with their use and the mechanism behind this adverse effect is not completely understood.
CAT-3888, formerly called BL22, is an immunotoxin which targets CD22 and has been investigated for the treatment of Hairy Cell Leukemia (HCL), NHL, and CLL22–24. During phase I/II testing of CAT-3888, 9 cases of grade 1 - 4 HUS were reported in 8 of the 82 subjects treated 22–24. In addition, HUS was reported in 1 of 2 HCL patients treated by special exemption prior to the opening of the phase II trial 22. Subjects in the phase I study were treated with 6 – 12 days of plasmapheresis, while those on the phase II study were given only supportive care. HUS was completely reversible in 9 of the 10 cases, regardless of treatment, with up to 57 months of follow-up in the phase I study. Note that the 1st of the 10 cases was not evaluable for reversibility because the patient had an aggressive lymphoma and refused additional treatment for rapidly progressive disease. However, this patient who became anuric with HUS resumed normal urination prior to dying of progressive lymphoma. ADAMTS13 was reported to be adequate in all cases, suggesting that ultra-large multimers of vWF were not circulating in these patients.
Moxetumomab pasudotox, formerly known as CAT-8015 or HA22, is an affinity-matured recombinant anti-CD22 immunotoxin which offers enhanced binding affinity compared to CAT-388825. A preliminary report of an ongoing phase I trial in HCL suggests that HUS may occur with lower frequency in patients treated with moxetumomab pasudotox as compared to CAT-3888 26. Two of 28 subjects treated had experienced reversible, grade 2 HUS following moxetumomab pasudotox administration. The clinical presentation of HUS appears to be similar to that seen in subjects treated with CAT-3888. However, in both of these cases, the peak creatinine was < 2.0 mg/dL and the nadir platelet count was > 100,000/uL.
Combotox® is an investigational combination of two deglycosylated ricin A chain (dgA) immunotoxins directed against CD19 and CD2227. This combination has been evaluated in a phase I study (N = 22) in which 2 cases of HUS were observed 27. Both subjects were reported to have followed a similar clinical course of vascular leak, respiratory insufficiency, HUS, and eventual death. The severe morbidity and mortality in these subjects may have been related to complicating vascular leak syndrome (VLS) rather than HUS. In fact, VLS has proven to be a prominent toxicity for large, conjugated immunotoxins due either to their long plasma half-lives or residues which bind to endothelial cells 28. The authors of this study suggest that HUS in these patients may be associated with low or absent levels of circulating tumor cells and possibly with high serum concentrations of the immunotoxins.
DAB486IL-2 is a recombinant immunotoxin targeting IL-2 with the membrane translocation and ADP-ribosylation domains of diphtheria toxin (DT) and is the precursor of DAB389IL-2 (Ontak®, denileukin diftitox) 29. During a phase I trial (N = 23) of DAB486IL-2, the maximum tolerated dose was determined by HUS toxicity in 2 patients 30. Similar toxicity was noted in one patient in a later phase II study (N = 14)31. The HUS experienced by this patient was described as “mild”, reversible, and did not recur upon retreatment at a reduced dose. Recurrent HUS may have been avoided in this case due to the presence of anti-toxin antibodies, but the authors do not report on levels of neutralizing antibodies in this study. To our knowledge, there are no published case reports of HUS associated with denileukin diftitox.
The mechanism behind TMA induced by immunotoxins is not well understood. Animal models of ricin-induced HUS provide evidence toward a pathway for renal injury 32,33. Administration of unconjugated ricin to mice and rats leads to the development of a syndrome closely resembling HUS in humans. The animals develop glomerular thrombosis, renal failure, hemolysis, and thrombocytopenia. Accompanying these pathologic changes are rapid increases in the proinflammatory cytokines MCP-1, TNF-α, IL-1β, and IL-6. Upregulation of these cytokines may promote an inflammatory environment and subsequent infiltration of the glomeruli with macrophages and stimulate secretion of vWF.
Each of the above mentioned immunotoxins utilizes different protein toxins and targets different cellular receptors. Messman et al. speculate that both targeted and non-targeted endothelial cell binding of toxins may play an integral role in the pathogenesis of HUS with the use of Combotox®. Identification of low levels of CD19, CD22, and IL-2 receptor on glomerular endothelial cells would support the theory of targeted cell death in immunotoxin-induced TMA. However, the presence of CD22 on glomerular endothelium seems unlikely to be a major mechanism as one would expect moxetumomab pasudotox to cause HUS at lower concentrations than CAT-3888 as it binds with 15-fold higher affinity to this cell surface receptor. Other mechanisms, such as cross-reactivity to a different target, or nonspecific uptake into cells by pinocytosis, may possibly be related to immunotoxin-induced HUS 21.
Immunotherapy
Apolizumab (Hu1D10) is a humanized monoclonal antibody against an antigen on the β chain of HLA-DR called 1D10. HUS was observed to be a dose limiting toxicity in one subject during a phase I/II trial (N = 23) of apolizumab 34. There were no schistocytes observed by peripheral blood smear.
HUS has also been reported in a phase I study of apolizumab plus rituximab35. In this study, subjects were administered weekly doses of 375 mg/m2 rituximab followed by increasing doses of apolizumab. The dose limiting toxicity was reported to be HUS, but the number of subjects experiencing HUS and their clinical presentation was not described. The authors reported that 1D10 was found on endothelial cells and may play a role in the pathogenesis of HUS in these patients.
Alemtuzumab (Campath®) is a monoclonal antibody which targets CD52 on the surface of B and T lymphocytes. During an experimental study (N = 41) in rheumatoid arthritis, a 49 year old female developed HUS after receiving her second dose of alemtuzumab36. Following 2 days of plasmapheresis, renal function returned to normal and the patient made a complete recovery. Alemtuzumab has been noted to cause elevation of serum TNF-α and IL-6. Similar to one of the proposed mechanisms behind TMA following treatment with cytotoxic chemotherapy, cytokine release may be partly responsible for HUS in this subject.
Anti-VEGF Therapy
Of the targeted oncology drugs associated with TMA, those which inhibit the VEGF pathway are the most widely studied. A series of 21 case reports of TMA associated with VEGF inhibition have been published in the last 5 years 37–44. These cases have been observed with the use of multiple anti-VEGF agents, suggesting a potential class-wide effect.
Eremina et al. describe six biopsy-documented cases of TMA resembling HUS following bevacizumab administration 39. All of the patients described in this series developed proteinuria or increased serum creatinine following the initiation of bevacizumab, ultimately leading to renal biopsy. However, beyond these indications of renal injury the clinical presentation was not uniform across all patients. Following discontinuation of bevacizumab, improvement in renal function was noted, suggesting at least partial reversibility.
Reports of TMA are not limited to bevacizumab, as they also appear for agents that target the VEGF pathway by different mechanisms. Three cases of renal TMA have been reported with the use of sunitinib (Sutent®)37,41,42. One case of TMA associated with afilbercept (VEGF Trap) has also been reported 44.
Combination therapy of bevacizumab and sunitinib in a phase I trial resulted in TMA of greater severity. Feldman et al. report 5 cases of MAHA along with 3 additional cases of “MAHA-like features” in a trial of 25 patients 43. MAHA was graded according to CTCAE version 3.0 guidelines for TMA. Two patients with grade 3 MAHA also developed neurologic symptoms.
Those patients described as having “MAHA-like features” presented similarly, but without schistocytosis or neurological symptoms. Following discontinuation of anti-VEGF therapy, all subjects showed improvement. The high rate of TMA in this trial (8 of 25 subjects) and the development of extra-renal complications in 2 subjects indicate a more severe manifestation of TMA with combined blockade of the VEGF pathway.
Eremina et al. have provided a detailed proposal for a mechanism of VEGF-induced TMA. They propose that inhibition of VEGF in the glomerular microvasculature prevents the formation and maintenance of a healthy, fenestrated endothelium 39. Without active VEGF signaling, the endothelium is compromised along with the filtration barrier of the glomerulus. The author’s proposed pathway to TMA is supported by elegant animal models in which only the renal podocytes are genetically deprived of the VEGF gene. In this model, the knockout mice developed features in-line with those seen in human cases of bevacizumab-induced TMA.
Imatinib
Imatinib (Gleevec®) inhibits multiple TKIs including bcr-abl, and those for PDGF, SCF, and c-kit and has been associated with TMA. Al Aly et al. report on a 22 year old female with hypereosinophilic syndrome who developed TMA after receiving imatinib 45. ADAMTS13 activity was below the normal range, while the ADAMTS13 inhibitor level was markedly elevated. The patient was treated with plasma exchange and hemodialysis leading to a hematologic recovery, but persistent renal impairment.
Discussion
Diagnosing TMA
While a diagnosis of TMA is centered on recognizing microvascular thrombosis, red blood cell destruction, and platelet consumption, no guidelines currently exist to completely define TMA or its severity. The difficulty in evaluating TMA is reflected in the similar CTCAE definitions of HUS and TTP46,47. Moreover, the CTCAE fails to provide more than a cursory outline of severity grading for TMA. Further delineation of critical signs and symptoms of HUS and TTP are warranted considering their heterogeneous causes and prognoses. Severity defined by laboratory value cut offs would be particularly beneficial. An example of a more detailed scheme for grading TMA is shown in Table 2.
Table 2.
Proposed Detailed Grading of Thrombotic Microangiopathies
| Grade | Description |
|---|---|
| Grade 1: Evidence of RBC destruction, without clinical consequences |
|
| Grade 2: Laboratory findings without clinical consequences |
|
| Grade 3: Laboratory findings with clinical consequences (e.g. renal insufficiency, petechiae) |
|
| Grade 4: Laboratory findings with life- threatening or disabling consequences (e.g. CNS hemorrhage/bleeding, or thrombosis/embolism, or renal failure) |
|
| Grade 5: Death |
|
The clinical presentation of TMA appears to have some common characteristics for the cases described in this review (Table 3). Renal dysfunction was almost universal as manifested by elevations in creatinine and/or worsening proteinuria. Similarly, evidence of hemolysis was frequent and laboratory values for hemoglobin, reticulocyte count, LDH, haptoglobin, and bilirubin were outside of normal limits. Interestingly, identification of fragmented red blood cells by peripheral smear was not documented in all TMA cases described. In fact, even in cases of biopsy-proven TMA following anti-VEGF therapy, schistocytes were only noted in 4 of 10 cases. However, performing biopsies to evaluate TMA is not appropriate for all patients. Thrombocytopenia was noted in most cases of TMA, but with varying severity. In the cases reviewed, consequences of thrombocytopenia such as petechiae and hemorrhage were not discussed. Strict monitoring of renal function, platelet count, and for signs of non-autoimmune hemolytic anemia are recommended for patients receiving these targeted therapies.
Table 3.
Comparison of Thrombotic Microangiopathy associated with Targeted Cancer Agents
| Targeted Therapy | CAT-3888 | Moxetumomab pasudotox | Combotox | DAB486IL- 2 | Apolizumab | Alemtuzumab | Bevacizumab | Sunitinib | Afilbercept | Bevacizumab + sunitinib | Imatinib |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Cases | 10 | 2 | 2 | 3 | ≥3 | 1 | 9 | 3 | 1 | 8 | 1 |
| Time to Onset | 2–3 cycles | 3–5 cycles | 3–5 days | NR | 11 doses | 2 doses | 4 – 29 doses | 2 weeks -7 months | 1 cycle | NR | 5 doses |
| Clinical Presentation | |||||||||||
| thrombocytopenia | + | + | + | + | + | + | +/− | + | + | + | |
| decreased Hgb | + | + | + | + | + | +/− | + | + | + | ||
| hemolysis | + | + | + | + | + | + | + | ||||
| elevated reticulocyte count | + | ||||||||||
| elevated bilirubin | + | + | |||||||||
| decreased haptoglobin | + | + | + | + | |||||||
| schistocytes | + | +/− | +/− | +/− | + | ||||||
| elevated LDH | + | + | + | + | + | + | |||||
| elevated creatinine | + | + | + | + | + | + | + | + | + | ||
| proteinuria | + | + | + | + | |||||||
| decreased urine output | + | +/− | |||||||||
| decreased albumin | + | + | |||||||||
| pulmonary s/s | + | ||||||||||
| edema | + | + | |||||||||
| hypertension | + | + | + | + | |||||||
| neurologic s/s | +/− | +/− | |||||||||
| Biopsy Confirmation | + | + | + | + | |||||||
| Outcome | 9/10 fully reversible, 1 death due to progressive disease | 2/2 fully reversible | 2/2 deaths | 1 fully reversible (others NR) | 1 death (others NR) | fully reversible | 1 fully reversible, 7 partially reversible with persistent proteinuria, 1 death due to progressive disease | 3/3 fully reversible | partially reversible | 3 fully reversible, 5 NR | partially reversible (hematologic recovery; dialysis dependent) |
NR: Not Reported
Treatment and Outcome
Many interventions for TMA have been described (Table 4). Optimal treatment of drug-induced TMA, and especially TMA induced by targeted agents, has yet to be proven. In the cases summarized in this review, plasma exchange and plasmapheresis were both used, but they may not be necessary if ADAMTS13 activity is unaltered in these patients. As noted for the cases of HUS associated with CAT-3888 and moxetumomab pasudotox, supportive care alone resulted in full recovery for several subjects. An advantage of these molecules compared to larger immunotoxins is their smaller size (~62 kDa), leading to relatively rapid (2–3 hour) half-life and fewer complications due to vascular leak syndrome. Until more is known about the mechanism of TMA following the use of targeted agents, immediate discontinuation of the offending drug may be the most important step in treatment. For patients with aHUS refractory to plasma exchange, eculizumab has resulted in clinical remission in select cases49,50. Likewise, rituximab has shown a high response rate without severe toxicity in a trial of refractory TTP (n = 24) and should be considered for these patients51.
Table 4.
| TMA | Intervention | Proposed Mechanism of Action | Comment |
|---|---|---|---|
| Familial TTP | Infusions of Fresh Frozen Plasma | Source of exogenous ADAMTS13 | Reverses or prevents episodes of TTP in these cases |
| Acute, Acquired TTP | Plasma Exchange | Removal of ultra large multimers of vWF, immune complexes, and autoantibodies to ADAMTS13 | Survival improved from ~10% to 70–90% |
| Acute, Acquired TTP | Glucocorticoids or Splenectomy | Decreased formation of immune complexes | Has been used in combination with plasma exchange in severe cases |
| Acute, Acquired TTP | Rituximab | Depletion of B-cells responsible for production of anti- ADAMTS13 antibodies | Has been successfully used in TTP refractory to plasma exchange and in relapsed TTP |
| “Atypical” HUS | Eculizumab | Binds complement protein C5, preventing formation of C5a and C5b-9 (the Membrane- Attack Complex) | Has been used in aHUS refractory to plasma exchange and following renal transplant to prevent recurrent aHUS |
| “Typical” HUS | Dialysis | Renal replacement therapy | Dialysis may not be necessary in mild cases |
Reintroducing the drug at lower dose levels may be a strategy to avoid recurrent TMA, while allowing for continued treatment. Retreatment following TMA has been safely carried out with CAT-3888, DAB486IL-2, and bevacizumab 24,31,39. However, it may be difficult to choose an appropriate dose for reintroduction and careful consideration of the potential risks and benefits to the patient should take place.
TMA with several of the targeted cancer agents appears to be reversible. HUS was fully reversible in 11 of the 12 subjects administered the immunotoxins CAT-3888 and moxetumomab pasudotox, at least 1 of the subjects given DAB486IL-2, and in the 1 casereport involving alemtuzumab. Partial to full reversibility was also noted for anti-VEGF treated patients, however persistent proteinuria was common. Deaths were reported for both HUS cases seen with Combotox® and in 1 of the cases of apolizumab treatment.
Unlike TMA induced by mitomycin and gemcitabine, there may not be a correlation between dose, cumulative dose, or time to onset with the use of targeted therapy. Perhaps any relationship between dose or time on therapy will become more clear as additional case reports are compiled.
Conclusions
As strategies to treat cancer begin to incorporate more targeted therapeutics such as monoclonal antibodies, immunotoxins, and small molecule inhibitors it will be important to recognize the unique toxicities that accompany their use. TMA is one such toxicity that has been associated with these targeted agents and research into its pathogenesis is in its infancy. Detailed evaluation of biopsy results, laboratory values, and ADAMTS13 levels of future cases may be warranted. In addition, animal models such as those performed by Eremina et al. may help to further define the mechanisms of these disorders.
Preliminary analysis appears to show a reversible course of TMA in the majority of patients described in this review. However, until preferable methods of prevention and treatment are determined, management of TMA should focus on strict monitoring for and early recognition of the signs and symptoms of HUS or TTP.
Translational Relevance.
Thrombotic Microangiopathies (TMAs) are known to occur in malignancy and have been linked to cytotoxic chemotherapy including mitomycin and gemcitabine. The pathogenesis of chemotherapy-induced TMA is likely due to direct endothelial cell injury. Recently, case reports of TMA related to the use of targeted cancer agents have also surfaced in the literature. Little is known about the etiology of TMA in these cases. Herein, we review the clinical presentation, outcome, and proposed mechanisms of TMA associated with the use of monoclonal antibodies, immunotoxins, and tyrosine kinase inhibitors.
Acknowledgments
None
Footnotes
Authorship Contributions: J.A.B.H. contributed to concept design and writing the article, R.J.L. edited and reviewed the manuscript, R.J.K. contributed to concept design, editing, and review of the manuscript.
Conflict of Interest: J.A.B.H. is a post-doctoral fellow sponsored by MedImmune, LLC. R.J.L. is a former employee of MedImmune, LLC.
References
- 1.Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347:589–600. doi: 10.1056/NEJMra020528. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi V, Robles R, Alberio L, Furlan M, Lammle B. Von Willebrand factor-cleaving protease (ADAMTS13) in thrombocytopenic disorders: a severely deficient activity is specific for thrombotic thrombocytopenic purpura. Blood. 2002;100:710–3. doi: 10.1182/blood-2002-02-0344. [DOI] [PubMed] [Google Scholar]
- 3.Remuzzi G. Is ADAMTS-13 deficiency specific for thrombotic thrombocytopenic purpura? No J Thromb Haemost. 2003;1:632–4. doi: 10.1046/j.1538-7836.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- 4.Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res. 2007;120:S5–S9. doi: 10.1016/j.thromres.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 6.Zheng XL, Sadler JE. Pathogenesis of thrombotic microangiopathies. Annu Rev Pathol. 2008;3:249–77. doi: 10.1146/annurev.pathmechdis.3.121806.154311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besbas N, Karpman D, Landau D, Loirat C, Proesmans W, Remuzzi G, et al. A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney Int. 2006;70:423–31. doi: 10.1038/sj.ki.5001581. [DOI] [PubMed] [Google Scholar]
- 8.Orth D, Khan AB, Naim A, Grif K, Brockmeyer J, Karch H, et al. Shiga Toxin Activates Complement and Binds Factor H: Evidence for an Active Role of Complement in Hemolytic Uremic Syndrome. J Immunol. 2009;182:6394–400. doi: 10.4049/jimmunol.0900151. [DOI] [PubMed] [Google Scholar]
- 9.Bernardo A, Ball C, Nolasco L, Moake J, Dong J. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104:100–6. doi: 10.1182/blood-2004-01-0107. [DOI] [PubMed] [Google Scholar]
- 10.Karpman D, Manea M, Vaziri-Sani F, Stahl A, Kristoffersson A. Platelet Activation in Hemolytic Uremic Syndrome. Semin Thromb Hemost. 2006;32:128–45. doi: 10.1055/s-2006-939769. [DOI] [PubMed] [Google Scholar]
- 11.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–87. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 12.Kavanagh D, Goodship TH, Richards A. Atypical haemolytic uraemic syndrome. Br Med Bull. 2006;77–78:5–22. doi: 10.1093/bmb/ldl004. [DOI] [PubMed] [Google Scholar]
- 13.Pisoni R, Ruggenenti P, Remuzzi G. Drug-induced thrombotic microangiopathy: Incidence, prevention and management. Drug Saf. 2001;24:491–501. doi: 10.2165/00002018-200124070-00002. [DOI] [PubMed] [Google Scholar]
- 14.Dlott JS, Danielson CF, Blue-Hnidy DE, McCarthy L. Drug-induced thrombotic thrombocytopenic purpura/hemolytic uremic syndrome: A concise review. Ther Apher Dial. 2004;8:102–11. doi: 10.1111/j.1526-0968.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 15.Izzedine H, Isnard-Bagnis C, Launay-Vacher V, Mercadal L, Tostivint I, Rixe O, et al. Gemcitabine-induced thrombotic microangiopathy: a systematic review. Nephrol Dial Transplant. 2006;21:3038–45. doi: 10.1093/ndt/gfl507. [DOI] [PubMed] [Google Scholar]
- 16.Lesesne JB, Rothschild N, Erickson B, Korec S, Sisk R, Keller J, et al. Cancer-associated hemolytic-uremic syndrome: Analysis of 85 cases from a national registry. J Clin Oncol. 1989;7:781–9. doi: 10.1200/JCO.1989.7.6.781. [DOI] [PubMed] [Google Scholar]
- 17.Eli Lilly and Company. Gemzar® Prescribing Information. Indianapolis: 2007. [Google Scholar]
- 18.Bristol-Myers Squibb Company. MutamycinR Prescribing Information. Princeton: 2000. [Google Scholar]
- 19.Kreitman RJ. Immunotoxins for Targeted Cancer Therapy. AAPS J. 2006;8:E532–51. doi: 10.1208/aapsj080363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathew M, Verma RS. Humanized immunotoxins: A new generation of immunotoxins for targeted cancer therapy. Cancer Sci. 2009;100:1359–65. doi: 10.1111/j.1349-7006.2009.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastan I, Hassan R, FitzGerald DJ, Kreitman R. Immunotoxin Treatment of Cancer. Ann Rev Med. 2007;58:221–37. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]
- 22.Kreitman RJ, Squires DR, Stetler-Stevenson M, Noel P, FitzGerald D, Wilson W, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol. 2005;23:6719–29. doi: 10.1200/JCO.2005.11.437. [DOI] [PubMed] [Google Scholar]
- 23.Kreitman RJ, Wilson WH, Bergeron K, Raggio M, Stetler-Stevenson M, FitzGerald D, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med. 2001;345:241–7. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 24.Kreitman RJ, Stetler-Stevenson M, Margulies I, Noel P, FitzGerald D, Wilson W, et al. Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol. 2009;27:2983–90. doi: 10.1200/JCO.2008.20.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alderson RF, Kreitman RJ, Chen T, Yeung P, Herbst R, Fox J, et al. CAT-8015: A Second-Generation Pseudomonas Exotoxin A-Based Immunotherapy Targeting CD22-Expressing Hematologic Malignancies. Clin Cancer Res. 2009;15:832–9. doi: 10.1158/1078-0432.CCR-08-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreitman RJ, Tallman MS, Coutre S, Robak T, Wilson W, Stetler-Stevenson M, et al. Phase I Dose-Escalation Study of CAT-8015 (HA22), A CD22-Specific Targeted Immunotoxin, in Relapsed or Refractory Hairy Cell Leukemia. Blood (ASH Annual Meeting Abstracts) 2009:114. (abstract 888) [Google Scholar]
- 27.Messmann RA, Vitetta ES, Headlee D, Senderowicz A, Figg W, Schindler J, et al. A phase I study of combination therapy with immunotoxins IgG-HD37-deglycosylated ricin A chain (dgA) and IgG-RFB4-dgA (combotox) in patients with refractory CD19(+), CD22(+) B cell lymphoma. Clin Cancer Res. 2000;6:1302–13. [PubMed] [Google Scholar]
- 28.Kreitman RJ. Recombinant immunotoxins for the treatment of hematological malignancies. Expert Opin Biol Ther. 2004;4:1115–28. doi: 10.1517/14712598.4.7.1115. [DOI] [PubMed] [Google Scholar]
- 29.Manoukian G, Hagemeister F. Denileukin diftitox: a novel immunotoxin. Expert Opin Biol Ther. 2009;9:1445–51. doi: 10.1517/14712590903348135. [DOI] [PubMed] [Google Scholar]
- 30.LeMaistre CF, Craig FE, Meneghetti C, McMullin B, Parker K, Reuben J, et al. Phase I trial of a 90-minute infusion of the fusion toxin DAB486IL-2 in hematological cancers. Cancer Res. 1993;53:3930–4. [PubMed] [Google Scholar]
- 31.Foss FM, Borkowski TA, Gilliom M, Stetler-Stevenson M, Jaffe E, Figg W, et al. Chimeric fusion protein toxin DAB486IL-2 in advanced mycosis fungoides and the sezary syndrome: Correlation of activity and interleukin-2 receptor expression in a phase II study. Blood. 1994;84:1765–74. [PubMed] [Google Scholar]
- 32.Taylor CM, Williams JM, Lote CJ, Howie A, Thewles A, Wood J, et al. A laboratory model of toxin-induced hemolytic uremic syndrome. Kidney Int. 1999;55:1367–74. doi: 10.1046/j.1523-1755.1999.00387.x. [DOI] [PubMed] [Google Scholar]
- 33.Korcheva V, Wong J, Corless C, Iordanov M, Magun B. Administration of Ricin Induces a Severe Inflammatory Response via Nonredundant Stimulation of ERK, JNK, and P38 MAPK and Provides a Mouse Model of Hemolytic Uremic Syndrome. Am J Pathol. 2005;166:323–39. doi: 10.1016/S0002-9440(10)62256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin TS, Stock W, Xu H, Phelps M, Lucas M, Guster S, et al. A phase I/II dose escalation study of apolizumab (Hu1D10) using a stepped-up dosing schedule in patients with chronic lymphocytic leukemia and acute leukemia. Leuk Lymphoma. 2009;50:1958–63. doi: 10.3109/10428190903186486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunleavy K, White T, Grant N, Shovlin M, Stetler-Stevenson M, Pittaluga S, et al. Phase 1 study of combination rituximab with apolizumab in relapsed/refractory B-cell lymphoma and chronic lymphocytic leukemia. J Clin Oncol. 2005;23(16S):6607. (suppl) [Google Scholar]
- 36.Isaacs JD, Manna VK, Rapson N, Bulpitt K, Hazleman B, Matteson E, et al. CAMPATH-1H in rheumatoid arthritis--an intravenous dose-ranging study. Br J Rheumatol. 1996;35:231–40. doi: 10.1093/rheumatology/35.3.231. [DOI] [PubMed] [Google Scholar]
- 37.Frangie C, Lefaucheur C, Medioni J, Jacquot C, Hill G, Nochy D. Renal thrombotic microangiopathy caused by anti-VEGF-antibody treatment for metastatic renal-cell carcinoma. Lancet Oncol. 2007;8:177–8. doi: 10.1016/S1470-2045(07)70037-2. [DOI] [PubMed] [Google Scholar]
- 38.Stokes MB, Erazo MC, D'Agati VD. Glomerular disease related to anti-VEGF therapy. Kidney Int. 2008;74:1487–91. doi: 10.1038/ki.2008.256. [DOI] [PubMed] [Google Scholar]
- 39.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–36. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roncone D, Satoskar A, Nadasdy T, Monk J, Rovin B. Proteinuria in a patient receiving anti-VEGF therapy for metastatic renal cell carcinoma. Nat Clin Pract Nephrol. 2007;3:287–93. doi: 10.1038/ncpneph0476. [DOI] [PubMed] [Google Scholar]
- 41.Kapiteijn E, Brand A, Kroep J, Gelderblom H. Sunitinib induced hypertension, thrombotic microangiopathy and reversible posterior leukencephalopathy syndrome. Ann Oncol. 2007;18:1745–7. doi: 10.1093/annonc/mdm454. [DOI] [PubMed] [Google Scholar]
- 42.Bollee G, Patey N, Cazajous G, Robert C, Goujon J, Fakhouri F, et al. Thrombotic microangiopathy secondary to VEGF pathway inhibition by sunitinib. Nephrol Dial Transplant. 2009;24:682–5. doi: 10.1093/ndt/gfn657. [DOI] [PubMed] [Google Scholar]
- 43.Feldman DR, Baum MS, Ginsberg MS, Hassoun H, Flombaum C, Velasco S, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1432–9. doi: 10.1200/JCO.2008.19.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izzedine H, Brocheriou I, Deray G, Rixe O. Thrombotic microangiopathy and anti-VEGF agents. Nephrol Dial Transplant. 2007;22:1481. doi: 10.1093/ndt/gfl565. [DOI] [PubMed] [Google Scholar]
- 45.Al Aly Z, Philocet Ashley JM, Gellens ME, Gonzalez E. Thrombotic Thrombocytopenic Purpura in a Patient Treated With Imatinib Mesylate: True Association or Mere Coincidence? Am J Kidney Dis. 2005;45:762–8. doi: 10.1053/j.ajkd.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 46.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0. 2006. [Google Scholar]
- 47.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009. [Google Scholar]
- 48.Scheiring J, Rosales A, Zimmerhackl LB. Today’s understanding of the haemolytic uraemic syndrome. Eur J Pediatr. 2010;169:7–13. doi: 10.1007/s00431-009-1039-4. [DOI] [PubMed] [Google Scholar]
- 49.Nurnberger J, Philipp T, Witzke O, Saez A, Vester U, Baba H, et al. Eculizumab for Atypical Hemolyitic-Uremic Syndrome. N Engl J Med. 2009;360:542–4. doi: 10.1056/NEJMc0808527. [DOI] [PubMed] [Google Scholar]
- 50.Gruppo R, Rother R. Eculizumab for Congenital Atypical Hemolytic-Uremic Syndrome. N Engl J Med. 2009;360:544–6. doi: 10.1056/NEJMc0809959. [DOI] [PubMed] [Google Scholar]
- 51.de la Rubia J, Moscardo F, Gomez M, Guardia R, Rodriguez P, Sebrango A, et al. Efficacy and safety of rituximab in adult patients with idiopathic relapsing or refractory thrombotic thrombocytopenic purpura: Results of a Spanish multicenter study. Transfus Apher Sci. 2010;43:299–303. doi: 10.1016/j.transci.2010.09.018. [DOI] [PubMed] [Google Scholar]