Abstract
Attenuation of the growth hormone (GH)/ insulin-like growth factor-1 (IGF-1) axis results in extended lifespan in many organisms including mice. Conversely, GH transgenic mice have excess GH action and die prematurely. We have studied bovine (b) GH transgenic mice (n = 9) and their wild type (WT) littermates (n = 8) longitudinally and have determined several age-related changes. Compared to WT mice, bGH mice lost fat mass, became hypoglycemic and had lower insulin levels at older ages despite being hyperinsulinemic when young. To examine plasma protein differences in bGH mice relative to controls, samples at 2, 4, 8, 12 and 16 months of age were analyzed by two-dimensional gel electrophoresis followed by identification using mass spectrometry. We found several differences in plasma proteins of bGH mice compared to controls, including increased apolipoprotein E (five isoforms), haptoglobin (four isoforms) and mannose-binding protein-C (one out of three isoforms), and decreased transthyretin (six isoforms). In addition, clusterin (two out of six isoforms) and haptoglobin (four isoforms) were up-regulated in bGH mice as a function of age. Finally, alpha-2 macroglobulin (seven isoforms) was altered in an isoform-specific manner with two isoforms increased and two decreased in bGH mouse plasma compared to controls. In conclusion, identification of these proteins suggests that bGH mice exhibit an increased inflammatory state with an adverse lipid profile, possibly contributing to their diminished life expectancy. Also, these newly discovered plasma proteins may be indicative or ‘biomarkers’ of a shortened lifespan.
Keywords: Proteomics, Growth hormone, Plasma, Two-dimensional gel electrophoresis, Aging, Inflammation
Introduction
Transgenic animals are widely used to study functions of specific genes. They can provide models of human diseases (Dunn et al. 2005) and have applications in many diverse fields ranging from production of pharmaceuticals (Dunn et al. 2005; Houdebine 2009) to conferring disease resistance in cattle (Wall et al. 2005).
Acromegaly is a condition caused by excessive production of growth hormone (GH), which is a hormone regulating longitudinal growth and metabolism. GH induces expression of insulin-like growth factor-1 (IGF-1), a potent growth factor that mediates many of GH’s actions. Acromegalic characteristics include increased lean mass, decreased fat mass, enlarged bones and swelling of soft tissues, with complications including cardiovascular abnormalities, insulin resistance and respiratory problems (Chanson and Salenave 2008; Paisley et al. 2009; Berg et al. 2010; Melmed et al. 2009). If left untreated, these abnormal physical features would result in death. GH transgenic mice (Hammer et al. 1985; McGrane et al. 1988), which have markedly elevated serum levels of IGF-1, are gigantic, lean, insulin-resistant and die prematurely (Wolf et al. 1993; Steger et al. 1993) partly due to liver, kidney and heart problems (Quaife et al. 1989; Doi et al. 1990; Bollano et al. 2000; Izzard et al. 2009). These mice are particularly valuable to study because they share many characteristics with human acromegalic individuals. Additionally, the reduced lifespan of GH transgenic mice makes them a useful model system to study aging and age-related diseases.
Serum/plasma samples are routinely used for disease diagnoses. Blood contains subsets of tissue proteins that can provide indicators or biomarkers of the physiological state of the organism. Protein separation and identification by two-dimensional gel electrophoresis (2-DE) has been used to uncover biomarkers in diseases states including cancer, Alzheimer’s disease, diabetes, liver and heart diseases (Huang et al. 2006; Zhang et al. 2004; Villanueva et al. 2006). Because GH transgenic mice represent a mouse model of premature aging, we set up a longitudinal study to examine the plasma proteomes of bovine (b) GH transgenic mice over their lifespan. Plasma proteins that showed significant genotype differences as well as aging differences from wild type (WT) littermates were identified. These proteins may be indicative of an accelerated aging phenotype.
Materials and methods
Animals
bGH transgenic mice were generated by injection of a linearized plasmid encoding the metallothionein 1 transcriptional regulatory region (promoter) driving bGH cDNA expression into the pronucleus of C57BL/6 J embryos as previously described (Hammer et al. 1985; Berryman et al. 2004). Male bGH mice (n = 9) and their WT littermates (n = 8) in the C57BL/6 J background were analyzed at 2, 4, 8, 12 and 16 months of age. Since five bGH mice died between 16 and 18 months of age, which was consistent with the previously reported shortened lifespan of this mouse line (Berryman et al. 2004; Wolf et al. 1993), 16 months was the last age included in the analyses. Mice were housed 2–3 per cage in a temperature controlled room (22°C) with a 14-h light, 10-h dark cycle. Mice were fed ad libitum with a standard chow diet (ProLab RMH 3000 PMI Nutrition International, Brentwood, MO; calories proportion is 14% from fat, 26% from protein and 60% from carbohydrates). Animal protocols were approved by Ohio University’s Institutional Animal Care and Use Committee.
Body composition
Body composition was measured at 9, 13 and 16 months using the Bruker Minispec (The Woodlands, TX) as described previously (Palmer et al. 2009; List et al. 2009; Ding and Kopchick 2010). Measurement of earlier ages was not performed as a previous longitudinal study had already followed body composition up to 12 months of age on a separate cohort of the same strain (bGH) of these mice (Palmer et al. 2009). The current study focused on changes occurring at later ages. The data are presented as absolute mass (gram) for lean, fat and fluid mass. Measurements were performed in duplicates and mean values were reported.
Plasma collection
All bleedings were performed at approximately 3 PM to eliminate potential diurnal variation. Blood was collected in heparinized capillary tubes following tail tip clipping. Whole blood was centrifuged at 7000 g for 10 min at 4°C and the resulting plasma was stored at −80°C until additional analyses were performed. Plasma for proteomics and IGF-1 analysis was collected at 2, 4, 8, 12, and 16 months of age. Because of insufficient volume of plasma for all assays, separate bleedings were performed 2−4 weeks after each time point and used for glucose and insulin measurements. These mice were fasted for 4 h before the bleedings.
Fasting glucose, plasma insulin and IGF-1
Blood glucose was measured on whole blood using a ONE TOUCH glucometer (Lifescan, Milpitas, CA). Plasma insulin levels were measured using an ultrasensitive mouse insulin EIA kit (ALPCO, Windham, NH), following the manufacturer’s instructions. The sensitivity of the insulin EIA was 0.115 ng/ml and the intraassay coefficient of variation was 9.3%. Plasma IGF-1 levels were determined using the DSL-10–29200 mouse/rat IGF-1 ELISA kit (Diagnostic Systems Laboratories, Inc., Webster, TX) following the manufacturer’s instructions. The sensitivity of the IGF-1 ELISA was 1.3 ng/ml and the intraassay coefficient of variation was 4.83%.
2-DE and quantification of proteins
2-DE was carried out within a week after plasma collection. The 2-DE procedure was described in detail previously (List et al. 2007b; Qiu et al. 2005; Sackmann-Sala et al. 2009; Okada et al. 2010; Ding and Kopchick 2010; Christensen et al. 2010; Cruz-Topete et al. 2010). Briefly, plasma total protein concentration was measured by the Bradford method (Bradford 1976) using a protein assay reagent (Bio-Rad, Hercules, CA). For each sample, 750 µg plasma proteins were treated with 8 M urea, 1.8 M thiourea, 4% zwitterionic detergent (CHAPS), 5 mM reducing agent tributylphosphine, a protease inhibitor cocktail (Sigma–Aldrich, Inc., St. Louis, MO) and 15 mM iodoacetamide. The sample was loaded onto a 17 cm immobile pH gradient gel (IPG) strip with a linear pI range of 3–10 (Bio-Rad, Hercules, CA). After actively rehydrating (50 V) for 12 h at 20°C using a Protean IEF cell (Bio-Rad), the strips were subjected to first dimensional isoelectric focusing (IEF) at 10,000 V for 60,000 V-h. Strips were then incubated in 2% (w/v) sodium dodecyl sulfate (SDS), 0.5 M Tris/HCl (pH 6.8), 20% (v/v) glycerol for 25 min and the middle section (pI 5–8) possessing the majority of plasma proteins, as previously determined (List et al. 2007a), was removed and subjected to second dimension SDS polyacrylamide gel electro-phoresis (PAGE) with 15% acrylamide. The gels were fixed overnight in 40% ethanol and 2% acetic acid followed by washing in 2% acetic acid. Gels were then stained with SYPRO Orange (1:5000) (Molecular Probes, Eugene, OR) for 2 h.
Gel images were captured using Pharos FX plus laser-scanner (Bio-Rad) with an excitation wavelength of 488 nm and an emission wavelength of 604 nm, as previously described (Sackmann-Sala et al. 2009; Okada et al. 2010; Ding and Kopchick 2010; Christensen et al. 2010; Cruz-Topete et al. 2010). Proteins were matched across all gel images using PDQuest (Bio-Rad) software and manually checked. For quantification, the intensity of each protein spot was determined according to the fluorescent signal strength and normalized to the total density of each image. The data were exported, log-transformed and then subjected to statistical analysis.
Protein identification by mass spectrometry (MS) and MS/MS (performed at Protea Biosciences, Inc.)
Proteins of interest were excised manually from the SDS–PAGE gels and shipped to Protea Biosciences, Inc. (Morgantown, WV) for MS and MS/MS analyses using matrix assisted-laser desorption ionization (MALDI)-time of flight (TOF) and MALDI-TOF-TOF. The details of MS and MS/MS were described previously (List et al. 2007b; Qiu et al. 2005; Sackmann-Sala et al. 2009; Okada et al. 2010; Ding and Kopchick 2010; Christensen et al. 2010; Cruz-Topete et al. 2010).
Manual confirmation of protein identification from the MS and MS/MS data (performed at Ohio University)
MS and MS/MS data were manually submitted to MASCOT as described previously (List et al. 2007b; Qiu et al. 2005; Sackmann-Sala et al. 2009; Okada et al. 2010; Ding and Kopchick 2010; Christensen et al. 2010; Cruz-Topete et al. 2010) for confirmation of the results reported by Protea Biosciences. For MS data, the searching criteria were as follows: SwissProt as the database, mouse as the species, trypsin digestion, maximum one missed cleavage, fixed carbamidomethylation of Cys, variable modifications of oxidation-M (methionine), pyro-Glu, monoisotopic, and 50 ppm of peptide mass or parent tolerance. For MS/MS ion search, in addition to the above conditions, a peptide charge of +1 and a fragment mass tolerance of 0.5 Da were used.
Western blotting
Plasma samples were used for 1-D and 2-D Western blotting analysis as described previously (Ding and Kopchick 2010). Western blots were performed for apolipoprotein E (APOE), haptoglobin (HP), transthyretin (TTR) and mannose-binding protein C (MBP-C) based on antibody availability. All primary antibodies were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA) diluted 1:1000, including goat anti-mouse apoE, rabbit anti-mouse HP α-chain, rabbit anti-mouse TTR and rat anti-mouse MBP-C. For all Western blots, samples at an intermediate age (8 months) were used. For each sample, 50 µg plasma proteins were loaded for 1-D Western blotting and 750 µg plasma proteins for 2-D Western blotting. Equal loading of samples was confirmed by PageBlue protein staining (Fermentas, Glen Burnie, MA) of the gels after protein transfer to PVDF membranes (data not shown). After blocking with 5% milk in tris-buffered saline, primary antibody incubation and washing, the membrane was incubated in horseradish peroxidase (HRP)-linked secondary antibodies diluted 1:5000 (goat anti-rabbit from Millipore, Temecula, CA; donkey anti-goat and goat anti-rat from Santa Cruz Biotechnology), followed by Pierce® ECL Western blotting substrate (Thermo Scientific, Rockford, IL) for 1 min, and finally exposed to HyBlot CL™ autoradiography film (Denville Scientific Inc., Metuchen, NJ) for 1–6 min depending on the signal strength.
Statistical analysis
All statistical analyses were performed using SPSS 14.0 software (Chicago, IL). Data for IGF-1 were subjected to Student’s t-test with a significance value P < 0.05. Data for body weight, body composition, fasting glucose and insulin were subjected to two-way repeated measures ANOVA with genotype as a fixed factor. For genotypic differences at each age, an independent t-test was performed. For protein intensity, the log-transformed data were subjected to two-way repeated measures ANOVA for five different age points (2, 4, 8, 12 and 16 months) with genotype as a fixed factor. The significance threshold used for proteomic data was set to <0.01 to reduce false positives (Biron et al. 2006). All data were presented as mean ± SEM.
Results
Body weight and body composition
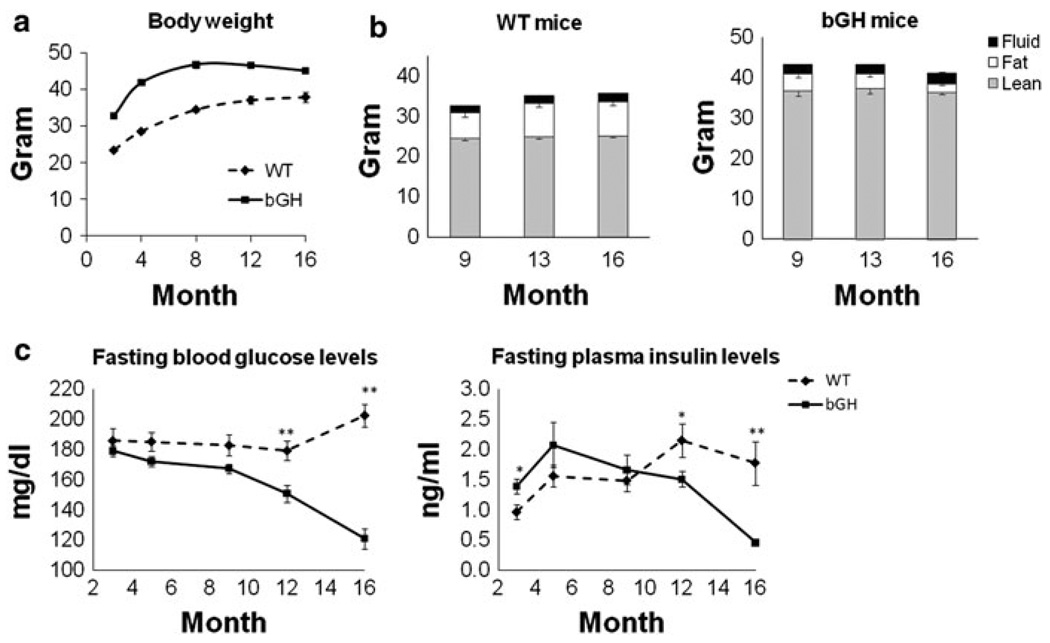
As shown in Fig. 1a, bGH mice had significantly greater weights than WT mice throughout their lifespan as has been reported previously (Palmer et al. 2009). Interestingly, body weight showed a significant interaction between genotype and age, with WT mice gaining weight as they aged while bGH mice reached a maximum weight at 8 months, remained constant from 8 to 12 months, then lost weight from 12 months onward.
Fig. 1.
Body weight, body composition, fasting glucose and insulin levels. a Body weight of bGH and WT mice during aging. Two-way repeated measures ANOVA revealed a significant effect of genotype (F(1,15) = 151.7, P = 3 × 10−9) and a significant interaction between genotype and age (F(1.7,25.4) = 9.1, P = 0.002). b Absolute body composition of WT and bGH mice at 9, 13 and 16 months. Black, fluid mass; white, fat mass; gray, lean mass. Two-way repeated measures ANOVA revealed a significant interaction between genotype and age for fat mass (F(2,30) = 32.7, P = 3 × 10−7). c Fasting glucose and insulin levels of WT and bGH mice during aging. Two-way repeated measures ANOVA revealed a significant effect of genotype (F(1,15) = 32.2, P = 4 × 10−5) for glucose; a significant interaction between genotype and age for glucose (F(3.194, 47.91) = 16.3, P = 0.003) and insulin (F(4,44) = 5.5, P = 0.001). * means significant difference between genotypes (* P < 0.05 and ** P < 0.01)
Body composition was examined at 9, 13 and 16 months of age and were in agreement with previous studies (Berryman et al. 2006; Berryman et al. 2004; Palmer et al. 2009). In terms of absolute mass, bGH mice had greater lean and fluid mass but smaller fat mass than WT controls (Fig. 1b). Interestingly, fat mass showed a significant interaction between genotype and age with an increase as WT mice aged but a decrease as bGH mice aged. bGH mice had significantly larger normalized percent lean mass (70.7 ± 1.6% versus 80.9 ± 1.4% at 9 months; 67.0 ± 1.9% versus 81.8 ± 0.5% at 13 months; and 66.3 ± 2% versus 82.9 ± 0.5% at 16 months for WT and bGH mice respectively), smaller percent fat mass (18.2 ± 1.8% versus 8.5 ± 0.7% at 9 months; 22.4 ± 2.3% versus 6.5 ± 0.5% at 13 months; 22.1 ± 2.4% versus 4.7 ± 0.6% at 16 months for WT and bGH mice, respectively), but similar percent fluid mass compared to WT mice (5.3 ± 0.2% versus 5.5 ± 0.2% at 9 months; 5.2 ± 0.2% versus 5.3 ± 0.1% at 13 months; 5.4 ± 0.1% versus 6.1 ± 0.5% at 16 months for WT and bGH mice, respectively).
IGF-1, fasting blood glucose and plasma insulin levels
Plasma IGF-1 levels were measured at 6 months. IGF-1 levels for bGH mice were 820.9 ± 57.2 compared to 373.9 ± 18.0 ng/ml for WT mice (P = 3 × 10−5), similar to a previous report (Mathews et al. 1988). Fasting blood glucose and plasma insulin levels were measured longitudinally. Surprisingly, bGH mice had significantly lower glucose levels than WT mice (Fig. 1 c). In fact, glucose levels were similar at younger ages but significantly different at older ages (P = 0.005 for 12 months and P = 8 × 10−7 for 16 months). Thus, there was a significant interaction between genotype and age with WT mice maintaining a similar glucose level through aging while bGH mice had decreased glucose levels at 12 and 16 months. Insulin levels (Fig. 1c) also showed a significant interaction between genotype and age with higher levels in bGH mice at 2 months (P = 0.014) but similar levels at 4 and 8 months, and lower levels at 12 (P = 0.035) and 16 months (P = 0.007) compared to WT mice.
Detection of plasma proteins in bGH versus WT mice by 2-DE
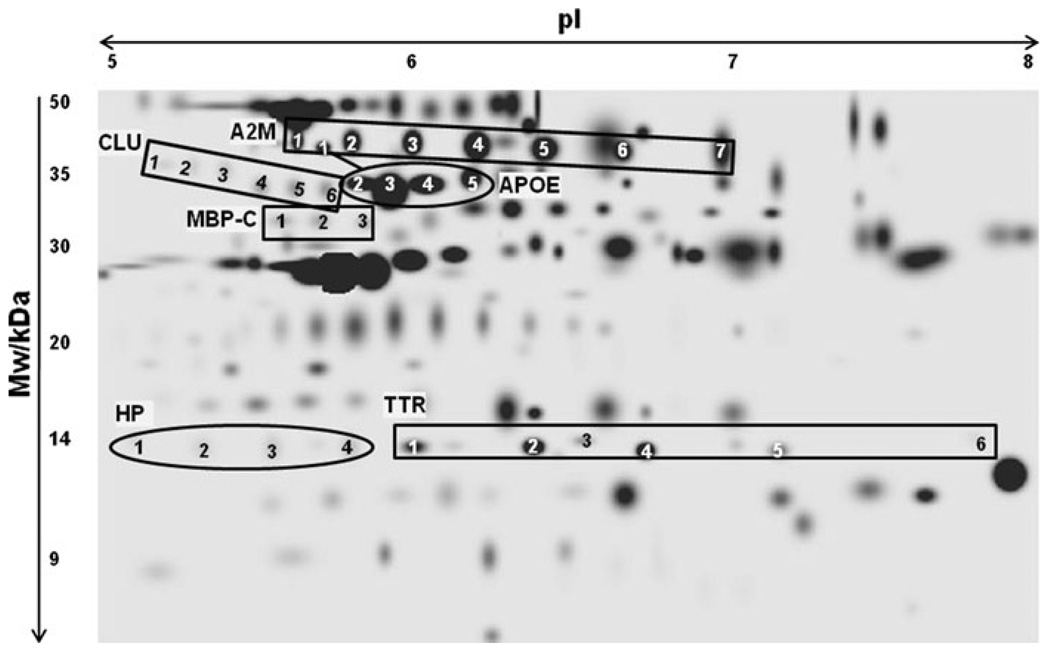
Plasma proteins were analyzed at 2, 4, 8, 12 and 16 months of age. On average 150 protein spots were detected per gel. Figure 2 is a reference gel image of identified proteins that changed significantly in bGH versus WT mice. MS and MS/MS identification, details on theoretical and experimental molecular weight (Mw), isoelectric focusing points (pIs) and known post-translational modifications (PTMs) can be found in the Online Resource 1. For these proteins, more than one spot on the 2-D gel were identified as the same protein (Fig. 2). These different versions of the same protein are termed ‘isoforms’. For example, seven spots corresponded to seven distinct isoforms of α-2 macroglobulin (A2M). The different isoforms may be a result of PTMs that alter the Mw and/or pI of a given protein. For some proteins, all isoforms increased or decreased; however, for others, only a subset of isoforms changed significantly.
Fig. 2.
2-D gel image and identities of mouse plasma proteins. The same protein with multiple isoforms has been identified with different numbers (i.e. 1, 2, etc.). Abbreviations: Mw: molecular weight; pI: isoelectric point; A2M: α-2 macroglobin; APOE: apolipoprotein E; CLU: Clusterin; HP: haptoglobin; MBP-C: mannose binding protein-C; TTR: transthyretin
APOE and MBP-C were up-regulated in bGH mice
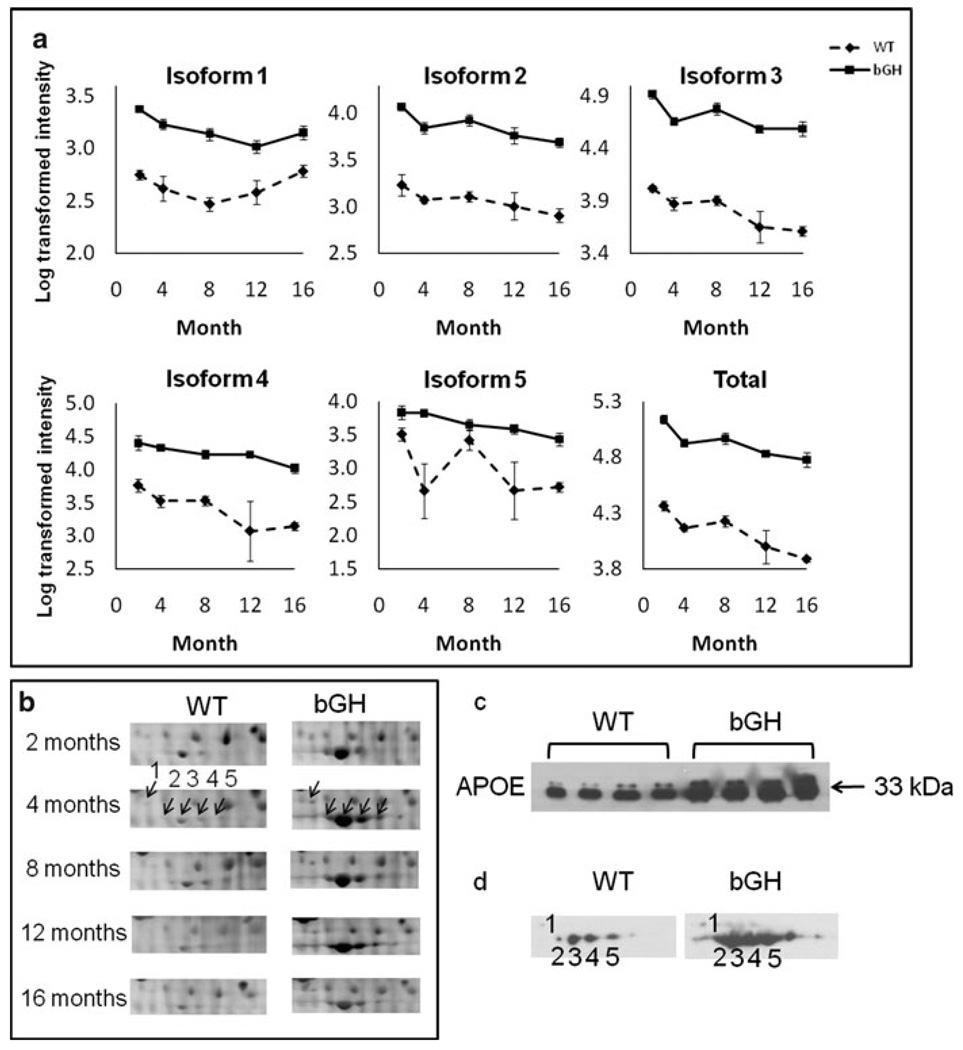
The five isoforms of APOE increased dramatically in bGH mice at all ages compared to age-matched WT controls (Fig. 3a) with total APOE levels (defined as the sum of all APOE isoform intensities) being significantly higher in bGH mice. Figure 3b shows the gels in which the intensities of the five APOE isoforms were more intense in bGH than WT mice at all ages. Western blotting confirmed that total APOE levels were higher in bGH than WT mice (Fig. 3c). The isoform distribution pattern and the expression differences were also confirmed by 2-D Western blotting (Fig 3d).
Fig. 3.
APOE was up-regulated in bGH compared to WT mice. a Quantification of individual isoforms as well as total APOE. The isoform number corresponds to those in Fig. 2. Total APOE is calculated as the sum intensity of all APOE isoforms. The Y axis represents log-transformed intensity. Two-way repeated measures ANOVA revealed a significant effect of genotype: isoform 1, F(1,15) = 159.1, P = 2 × 10−9; isoform 2, F(1,15) = 320.7, P = 2 × 10−11; isoform 3, F(1,15) = 443.3, P = 2 × 10−12; isoform 4, F(1,15) = 65.9, P = 7 × 10−7; isoform 5, F(1,15) = 25.5, P = 0.0001; total apoE, F(1,15) = 442.1, P = 2 × 10−12. b Cropped image of one representative gel from WT and bGH mice at each age. The arrows point to individual apoE isoforms at 4 months, with the numbers corresponding to isoform numbers. c 1-D Western blotting of APOE using 8-month old mouse plasma samples (n = 4 for each genotype). d 2-D Western blotting of APOE from one WT and one bGH mouse at 8 months of age. Isoforms 1–5 labeled on the X-ray film images correspond to APOE isoforms 1–5 in Fig. 2. Equal loading of protein samples was confirmed by PageBlue staining of gels (not shown)
Similar with APOE, MBP-C isoform 1 increased significantly in bGH as compared to controls (Online Resource 2). 2-D Western blotting confirmed this; in addition, two other isoforms of MBP-C also appeared more abundant in bGH than WT mice (Online Resource 2), although they did not reach statistical significance (data not shown).
TTR was down-regulated in bGH mice
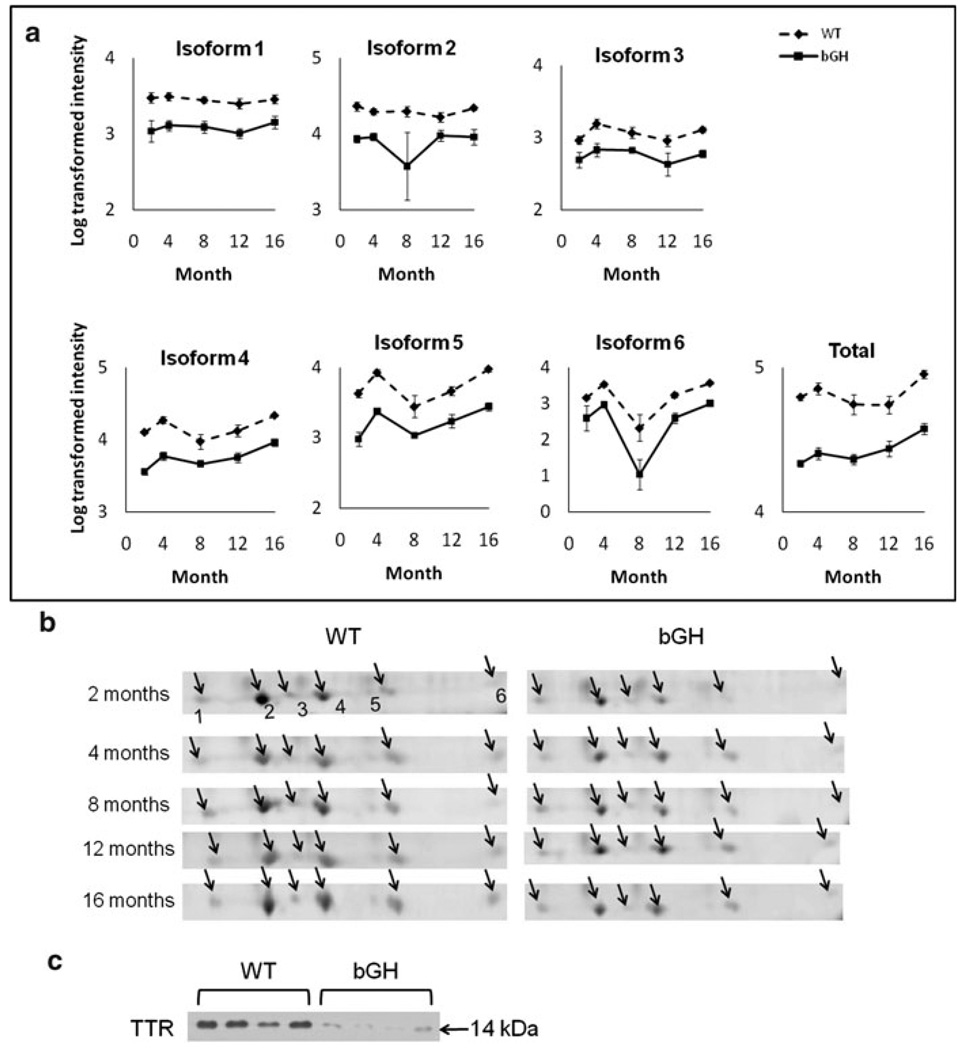
Six TTR isoforms decreased in bGH mice compared to controls (Fig. 4a, b), as did the total level of TTR. As can be seen in Fig. 4b, these isoforms had lower spot intensities in bGH than WT mice at all ages. This decrease in total TTR levels was also confirmed by 1-D Western blotting (Fig. 4c).
Fig. 4.
TTR was down-regulated in bGH compared to WT mice. a Quantification of individual isoforms as well as total TTR. Isoform # corresponds to Fig. 2. Total TTR is calculated as the sum intensity of all TTR isoforms. The Y axis represents log-transformed intensity. Two-way repeated measures ANOVA revealed a significant effect of genotype: isoform 1, F(1,15) = 59.2, P = 1 × 10−6; isoform 2, F(1,15) = 15.1, P = 0.001; isoform 3, F(1,15) = 54.4, P = 2 × 10−6; isoform 4, F(1,15) = 179.3, P = 1 × 10−9; isoform 5, F(1,15) = 106.8, P = 3 × 10−8; isoform 6, F(1,15) = 26.5, P = 0.0001; total TTR, F(1,15) = 188.1, P = 7 × 10−10. b Cropped image of one representative gel from WT and bGH mice at each age. The arrows point to isoforms 1–6. c 1-D Western blotting of TTR using 8-month old mouse plasma samples (n = 4 for each genotype). Equal loading of protein samples was confirmed by PageBlue staining of the gel (not shown)
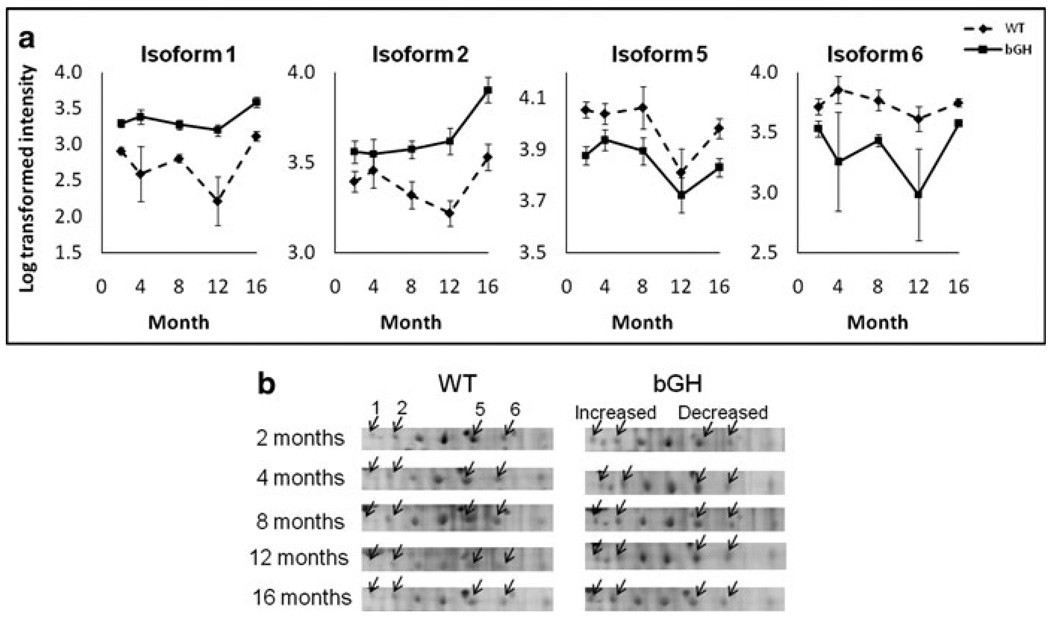
A2M showed isoform-specific changes in bGH mice
Of the seven isoforms identified as A2M, isoforms 1 and 2 increased, whereas isoforms 5 and 6 decreased in bGH as compared to controls (Fig. 5). The other isoforms as well as total A2M did not change between genotypes.
Fig. 5.
Isoform-specific changes of A2M in bGH compared to WT mice, with isoforms 1 and 2 up-regulated while isoforms 5 and 6 down-regulated. a Quantification of A2M isoforms. The isoform number corresponds to Fig. 2. The Y axis represents log-transformed intensity. Two-way repeated measures ANOVA revealed a significant effect of genotype: isoform 1, F(1,15) = 46.7, P = 6 × 10−6; isoform 2, F(1,15) = 17.8, P = 0.007; isoform 5, F(1,15) = 11.2, P = 0.004; isoform 6, F(1,15) = 9.8, P = 0.007. b cropped image of one representative gel from WT and bGH mice at each age. The arrows point to A2M isoforms 1, 2, 5 and 6
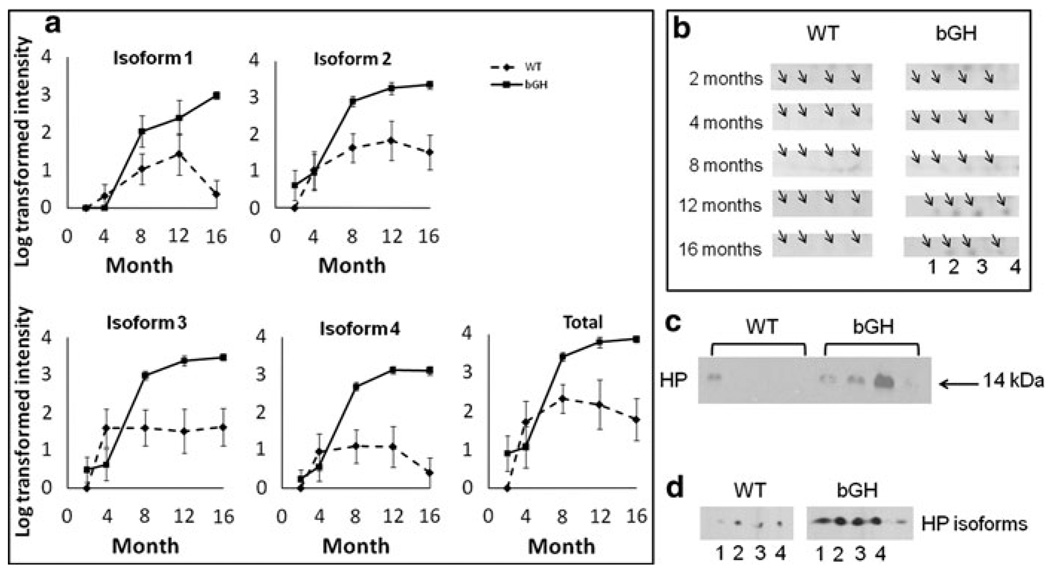
HP and clusterin (CLU) showed a significant interaction between genotype and age
Four HP alpha chain isoforms were up-regulated in bGH mice and showed a rapid increase during aging compared to WT mice (Fig. 6a). HP alpha was barely detectable in WT or bGH mice at 2 and 4 months, but became detectable after 8 months (Fig. 6b, c), and continued to increase as a function of age. This age-dependent increase has been reported previously for WT mice (Ding and Kopchick 2010). However, a more dramatic increase was found in bGH mice (Fig. 6a). Total HP showed a significant increase in bGH mice and a significant interaction between genotype and age. The increase of total HP alpha was confirmed by 1-D Western blotting (Fig. 6d). The HP alpha isoform pattern, as well as up-regulation in the bGH mice, were also confirmed by 2-D Western blotting (Fig. 6e).
Fig. 6.
Rapid increase of HP as a function of age in bGH compared to WT mice. a Quantification of individual isoforms as well as total Hp. The isoform number corresponds to Fig. 2. Total HP is calculated as the sum intensity of all HP isoforms. The Y axis represents log-transformed intensity. Two-way repeated measures ANOVA revealed a significant effect of age, genotype and a significant interaction between genotype and age. Effect of age: isoform 1, F(2.843, 42.647) = 18.6, P = 1 × 10−7; isoform 2, F(4,60) = 16.7, P = 3 × 10−9; isoform 3, F(4,60) = 20.5, P = 1 × 10−10; isoform 4, F(4,60) = 18.7, P = 5 × 10−10; total HP, F(4,60) = 18.1, P = 8 × 10−10. Effect of genotype: isoform 1, F(1,15) = 10.0, P = 0.006; isoform 2, F(1,15) = 13.5, P = 0.002; isoform 3, F(1,15) = 8.3, P = 0.011; isoform 4, F(1,15) = 17.8, P = 0.0007; total HP, F(1,15) = 10.4, P = 0.006. Interaction between genotype and age: isoform 1, F(2.843, 42.647) = 7.5, P = 0.0005; isoform 2, F(4,60) = 2.3, P = 0.068; isoform 3, F(4,60) = 7.2, P = 8 × 10−5; isoform 4, F(4,60) = 10.6, P = 1 × 10−6; total HP, F(4,60) = 3.8, P = 0.008. b cropped image of one representative gel from WT and bGH mice at each age. The arrows point to individual HP isoforms at 8, 12 and 16 months, with the numbers corresponding to isoform numbers. c 1-D Western blotting of HP using 8-month old mouse plasma samples (n = 4 for each genotype). d 2-D Western blotting of HP from one WT and one bGH mouse at 8 months of age. Isoforms 1–4 labeled on the X-ray film images correspond to HP isoforms 1–4 in Fig. 2. Equal loading of protein samples was confirmed by PageBlue staining of gels as well as non-specific bands and spots on X-ray films (not shown)
Similar to HP, isoforms 1 and 2 of CLU as well as total CLU revealed a progressive increase as a function of age in bGH mice but did not change in WT mice, showing a significant interaction between genotype and age (Online Resource 3). A similar trend was found in the other four CLU isoforms but did not reach statistical significance (data not shown). Thus, CLU is differentially expressed as a function of age in bGH mice versus WT mice.
Discussion
Body composition, glucose and insulin
Due to a shortened lifespan of bGH mice, many studies have analyzed them at relatively early ages (up to 12 months of age). Our study has followed the same cohort of bGH mice with their littermates up to 16 months of age. In this study, bGH mice have increased IGF-1, body weight and lean mass, but decreased fat mass as reported earlier (Mathews et al. 1988; Berryman et al. 2006; Berryman et al. 2004; Palmer et al. 2009). Our longitudinal data indicate that bGH mice are leaner than WT mice, and this phenotype is progressively more exaggerated with advanced age due to losses in fat mass. This was observed in a previous longitudinal study that followed the bGH mice up to 1 year of age (Palmer et al. 2009). The early-onset weight/fat loss of bGH mice appears to reflect their shortened lifespan. Indeed, the mean lifespan of this mouse line is 409 ± 45 days (about 14 months) versus 602 ± 34 days (about 21 months) for WT in a C57Bl/6 J genetic background (Berryman et al. 2004).
Previous studies have shown that bGH mice have increased insulin levels (Dominici et al. 1999; Olsson et al. 2005; Wang et al. 2007). These studies are conducted on relatively young mice (younger than 7 months of age). Similarly, we report that bGH mice are hyperinsulinemic at 3 months of age. However, by 5 months of age, the insulin levels in bGH mice are not significantly different from WT mice, which has also been reported (Berryman et al. 2006). Surprisingly, at 12 and 16 months of age, bGH mice are hypoinsulinemic relative to controls. The reason for this phenomenon is not known. One possibility is failure of beta cells to secrete insulin as bGH mice age. In this regard, GH/IGF-1 is known to stimulate beta cell proliferation and insulin secretion (Parsons et al. 1995; Olsson et al. 2005; Okuda et al. 2001). However, chronic exposure to high GH leads to accelerated aging (Wolf et al. 1993) and this ‘aging’ phenotype is manifested in multiple organs (Quaife et al. 1989), perhaps also affecting the pancreas. Unfortunately, no data has examined the impact of aging on pancreatic beta cell function in bGH mice.
bGH mice are known to have normal glucose levels. This is the case in our study from 3 to 9 months of age. However, at 12 and 16 months, bGH mice have significantly reduced fasting blood glucose levels compared to WT mice. In previous studies, GH transgenic mice have been reported to have lower, although not always significant, glucose levels at 3 months (Bartke et al. 2004), 8 months (Frick et al. 2001a) and 11 months of age (Rocha et al. 2007). Our data show that bGH mice have lower fasting glucose levels at 12 and 16 months of age. One possible explanation is that the prematurely ‘old’ bGH mice have reduced liver function that cannot adequately support sufficient gluconeogenesis, since fasting blood glucose is mainly derived from hepatic output (Sheehan 2004). A recent study shows that bGH mice have suppressed gluconeogenic capacity (Boparai et al. 2010). In addition, key enzymes in gluconeogenesis are found to be lower in bGH versus control mice (Valera et al. 1993). A possible mechanism is that PGC-1α, a transcriptional coactivator regulating hepatic glucose production that normally is induced by fasting in mouse liver to up-regulate gluconeogenesis-related enzymes (Yoon et al. 2001), is lower in bGH relatlive to WT mice (Al-Regaiey et al. 2005). Consistent with this observation is the fact that, liver expression of PGC-1α is elevated in GH receptor null (GHR−/−) mice versus WT control littermates (Rocha et al. 2007).
Human acromegalic patients have hyperinsulinemia and insulin resistance that can advance to glucose intolerance and type 2 diabetes (Chanson and Salenave 2008). As for the effect of aging, older acromegalic patients are more likely to have glucose intolerance and type 2 diabetes than younger patients (Gierach et al. 2010). For example, in two cohorts of acromegalic patients, 94% of the elderly (> 61 yr) suffer from glucose intolerance and 63% from diabetes versus 33% and 11% in young patients (< 30 yr), respectively (Tanimoto et al. 2008). In comparison to human acromegaly, bGH mice also have hyperinsulinemia when young. However, bGH mice do not seem to develop glucose intolerance or diabetes. In fact, recent data show that GH transgenic mice have enhanced glucose tolerance and increased glucose-stimulated insulin secretion (Boparai et al. 2010). The discrepancy between GH transgenic mice and acromegalic humans is perhaps due to species differences, the timing of GH excess (entire lifespan including early development in bGH mice versus adult or childhood-onset in acromegalic patients), or the significantly shorter lifespan in bGH mice, which means they may die before becoming diabetic.
Abnormal levels of plasma proteins in bGH mice
We hypothesized that the plasma proteome of bGH mice would be different from WT mice. In this regard, we have found several proteins and/or protein isoforms that were up- or down-regulated in bGH mice, as well as proteins that changed differently as a function of age in bGH mice versus controls. Online Resource 4 discusses the Mw, pI and possible PTMs for the protein isoforms found in this study. Below, we briefly describe the functions and the known effects of GH on each protein.
It is well known that GH regulates lipid metabolism through lipases and lipoproteins (Vijayakumar et al. 2010). APOE is associated with lipoproteins which transport cholesterol and triglyceride in blood. By mediating the clearance of different lipoproteins from blood, APOE plays a significant role not only in cardiovascular disease, but also neurological disease, with the ε4 allele of APOE being the largest known genetic risk factor for Alzheimer’s disease in humans (Huang 2010). It is thought that APOE affects amyloid beta protein aggregation and clearance in an allele-specific manner, therefore, contributing to the pathogenesis of Alzheimer’s disease (Kim et al. 2009). It is not known if inbred C57BL/6 J mice have different alleles of the apoe gene. Since five isoforms of APOE were observed on 2-D gels for all mice regardless of genotype and age (observed also in other cohorts of C57BL/6 J mice not discussed in this paper), it is unlikely that these isoforms are a result of different alleles in mice. The difference in charge and size of the five isoforms probably arise via PTMs, which will be a subject of future studies.
APOE−/− mice exhibit hypercholesterolemia and develop spontaneous atherosclerosis (Nakashima et al. 1994). Interestingly, GH exacerbates the atherosclerotic lesions in these mice even further, whether fed with chow or a high fat diet (Andersson et al. 2006). The present study showed higher levels of APOE in bGH mice compared to controls. This is consistent with bGH mice having increased serum total cholesterol (2.75–4.5 mmol/l for bGH versus 1.75–2 mmol/l for WT controls) (Frick et al. 2001b; Olsson et al. 2005). There is also evidence that GH is needed to maintain normal APOE levels in rats (Oscarsson et al. 1991) by stimulating APOE mRNA translation and subsequent protein secretion from hepatocytes (Sjoberg et al. 1994). Also, acromegalic patients have increased circulating APOE levels (Wildbrett et al. 1997). Finally, GH treatment in adult GH deficient patients for one year increases serum APOE levels (Johannsson et al. 1995). Thus, our data further supports the mounting evidence that GH influences this protein although how this alteration influences aging, cardiovascular and neurological diseases remains to be established.
CLU may also be involved in aging, as it accumulates in atherosclerotic plaques (Jordan-Starck et al. 1994; Witte et al. 1993) and increases in aged human pituitary glands (Ishikawa et al. 2006). Thus, increased plasma levels of CLU at later ages may indicate an aging phenotype of bGH mice. A role of CLU in kidney disease is also known. Secreted CLU is higher in the kidney of patients with renal cell carcinoma than healthy subjects (Kurahashi et al. 2005). In fact, CLU has been used as a marker for severity of kidney tubular damage in rats (Hidaka et al. 2002). bGH mice are known to develop progressive glomerulosclerosis as they age (Quaife et al. 1989) and glomerulosclerosis is detected as early as 7 months (Doi et al. 1990), which interestingly coincides with the up-regulation of CLU in bGH mice from 8 months of age (Online Resource 3).
GH modulates innate immunity (Hansen 2003) and stimulates acquired immunity (Postel-Vinay et al. 1997; Blazar et al. 1995). GH has been shown to affect T and B cell development, cytokine production, antibody production and priming neutrophils and monocytes for superoxide anion secretion (Hattori 2009). However, elevated levels of GH do not necessarily result in a superior ability to fight infection. On the contrary, bGH mice produce less specific antibodies when challenged with tetanus toxoid while GH deficient Ames dwarf mice produce normal levels of antibodies (Hall et al. 2002). Thus, a complete role of GH in immune function is still not clear. Inflammation is a component of innate immunity and increased inflammation is associated with aging (Ferrucci et al. 2005; Brüünsgaard and Pedersen 2003; Roubenoff et al. 1998). Interestingly, several of the proteins identified in the present study are involved in immunity and/or inflammation, including MBP-C, HP, TTR and A2M.
MBP-C is primarily synthesized by the liver and is classified as an acute phase protein that is induced in the liver by inflammation, infection or injury. It is involved in defense against pathogens by activating the classical complement pathway (Turner 2003). Different human alleles of MBP-C have been identified to affect immune functions, e.g., genetic variations in MBP-C are associated with susceptibility to hepatitis B virus infection (Thio et al. 2005). Also, low levels of MBP-C are associated with susceptibility to frequent and chronic infections (Sumiya et al. 1991; Turner 2003). A positive relationship of MBP-C with GH has been reported in humans (Gravholt et al. 2004; Hansen et al. 2001). The present study found one MBP-C isoform to be upregulated in bGH mice, consistent with data from human studies, suggesting increased inflammation in bGH mice. Similar to the rational for APOE above, the isoforms observed in mouse plasma are most likely the result of PTMs rather than different alleles.
HP is also an acute phase protein indicative of acute insult. For example, HP is upregulated following whole-body exposure to gamma-rays in mice (Rithidech et al. 2009). Interestingly, HP seems to be related to GH status. HP is increased in response to GH treatment in Turner’s syndrome (TS) patients (Gravholt et al. 2004). GH treatment also results in HP production in a human hepatoma cell line (Derfalvi et al. 2000). In pigs and lambs treated with acute insults, serum HP as well as GH levels increase (Spurlock et al. 1998; Moore et al. 1995). Previously, it has been shown that WT mice have an increase in HP with advancing age (Ding and Kopchick 2010). The present study confirms this phenomenon and shows that the up-regulation of HP during aging is more exaggerated in bGH compared to WT mice, suggesting an enhanced inflammatory state in bGH mice.
TTR is a negative acute phase protein that is suppressed during inflammation, infection or malnutrition (Lasztity et al. 2002). The lower levels of TTR are associated with higher rate of morbidity/mortality and recovery from acute and chronic disease (Fuhrman et al. 2004). TTR is decreased in type 1 diabetes (Itoh et al. 1992), which is interestingly associated with higher levels of GH (Edge et al. 1990). It has been reported that serum TTR is decreased by GH treatment in hypophysectomized male rats at 2 months of age (Vranckx et al. 1994). bGH mice have decreased levels of thyroxine (T4) (Bohlooly et al. 2001), which is transported by TTR in blood. Consistent with the literature, the present study showed markedly lower levels of TTR in bGH mouse plasma, perhaps indicating higher inflammation and also explaining lower T4 in bGH mice.
A2M is a proteinase inhibitor that is mainly secreted by liver (Van Leuven et al. 1994). Regarding immune modulation, A2M −/− mice are normal under standard conditions but have an increased mortality rate when subjected to acute pancreatitis (Umans et al. 1999). In terms of interaction with hormones, A2M binds GH although with a weaker affinity than GH binding protein (BP) (Kratzsch et al. 1996). A2M also binds to IGFBP-1 and modifies IGF-I/IGFBP-1 actions resulting in enhanced IGF effects (Westwood et al. 2001). Moreover, a positive relationship of A2M with GH has been reported, i.e., as GH goes up so does A2M (Derfalvi et al. 2000; Pozzilli et al. 1978; Zargarova and Konnova 1983). The present study has found no change in total A2M, but up-regulation in isoforms 1 and 2 and down-regulation in isoforms 5 and 6 throughout lifespan in bGH mice, suggesting that GH differentially regulates the PTMs of plasma A2M. Our results indicate a novel regulation of A2M by GH through PTM, rather than at the total protein level.
Many of the aforementioned proteins are acute phase proteins, which are markers of inflammation and important for combating acute infection and injury. Under standard mouse housing conditions, little infection or injury occur to the mice. Thus, the abnormality of these proteins in bGH mice may reflect an inflammation-prone phenotype. In this regard, bGH mice are known to exhibit inflammation in the liver and kidney (Hardy et al. 1997; Quaife et al. 1989; Doi et al. 1990), as well as increased levels of tissue necrosis factor-α (TNF-α) and inter-leukin-6 (IL-6) (Wang et al. 2007). Studies suggest that GH is induced under acute inflammatory conditions. For example, serum GH levels are elevated in children and adults suffering from septic shock (de Groof et al. 2002; Lang et al. 1997). It seems that during infection, the up-regulation of GH serves as a beneficial signal for the induction of acute phase proteins, which, together with other pro-inflammatory cytokines, are necessary to fight infection. However, at high levels, GH is detrimental to critically ill patients (Takala et al. 1999; Chen et al. 2007). Moreover, animals under sepsis become GH resistant with suppressed JAK2/STAT5 signaling and STAT5 DNA binding (Chen et al. 2007). It is not clear how GH induces acute phase proteins, although it is likely by the JAK2/STAT5 pathway (Dajee et al. 1996), the same intracellular pathway used by many cytokines (Hennighausen and Robinson 2008). Finally, nuclear factor κB (NFκB) is a central player regulating inflammation. GH has been shown to activate NFκB via the phosphoinositide-3 kinase-Akt pathway (Jeay et al. 2002), thereby providing a potential cross-talk mechanism between GH and inflammation. Further studies are needed to unravel the mechanism of the interaction of GH and inflammation.
In summary, bGH mice experience weight loss, fat mass loss, and a decline in fasting insulin and glucose levels starting at ~12 months of age, in contrast to no change or an increase of these parameters in WT mice during aging. Additionally, the changes in the plasma proteome of bGH versus control mice throughout their lifespan suggests an altered lipoprotein profile and an increased inflammatory state. Whether or not these changes impact the shortened lifespan of these mice is unknown. Finally, the tissues that secrete these proteins, the molecular mechanisms that regulate their production and how they are related to the aging phenotype will be subjects of future studies.
Supplementary Material
Acknowledgments
This work was supported by World Anti-Doping Agency, Diabetes Research Initiative, the State of Ohio’s Eminent Scholars Program that includes a gift by Milton and Lawrence Goll, and by the AMVETS organization. JJK also is supported by the following grants: National Institute of Health (NIH) R15DK075436, R01AG019899, and 1P01AG031736-01A1.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11248-011-9499-5) contains supplementary material, which is available to authorized users.
Conflict of interest There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Contributor Information
Juan Ding, Edison Biotechnology Institute, Ohio University, Athens, OH 45701, USA.
Darlene E. Berryman, Edison Biotechnology Institute, Ohio University, Athens, OH 45701, USA School of Applied Health Sciences and Wellness, Ohio, University, Athens, OH 45701, USA.
John J. Kopchick, Email: kopchick@ohio.edu, Edison Biotechnology Institute, Ohio University, Athens, OH 45701, USA; Department of Biomedical Sciences, Ohio University, Athens, OH 45701, USA; Edison Biotechnology Institute, Ohio University, 1 Water Tower Drive, The Ridges, Athens, OH 45701, USA.
References
- Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005;146(2):851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- Andersson IJ, Ljungberg A, Svensson L, Gan L-M, Oscarsson J, Bergström G. Increased atherosclerotic lesion area in apoE deficient mice overexpressing bovine growth hormone. Atherosclerosis. 2006;188(2):331–340. doi: 10.1016/j.atherosclerosis.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Bartke A, Peluso MR, Moretz N, Wright C, Bonkowski M, Winters TA, Shanahan MF, Kopchick JJ, Banz WJ. Effects of soy-derived diets on plasma and liver lipids, glucose tolerance, and longevity in normal, long-lived and short-lived mice. Horm Metab Res. 2004;36(08):550–558. doi: 10.1055/s-2004-825796. [DOI] [PubMed] [Google Scholar]
- Berg C, Petersenn S, Lahner H, Herrmann BL, Buchfelder M, Droste M, Stalla GK, Strasburger CJ, Roggenbuck U, Lehmann N, Moebus S, Jockel KH, Mohlenkamp S, Erbel R, Saller B, Mann K. Cardiovascular risk factors in patients with uncontrolled and long-term acromegaly: comparison with matched data from the general population and the effect of disease control. J Clin Endocrinol Metab. 2010;95(8):3648–3656. doi: 10.1210/jc.2009-2570. [DOI] [PubMed] [Google Scholar]
- Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14(4):309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology. 2006;147(6):2801–2808. doi: 10.1210/en.2006-0086. [DOI] [PubMed] [Google Scholar]
- Biron DG, Brun C, Lefevre T, Lebarbenchon C, Loxdale HD, Chevenet F, Brizard JP, Thomas F. The pitfalls of proteomics experiments without the correct use of bioinformatics tools. Proteomics. 2006;6(20):5577–5596. doi: 10.1002/pmic.200600223. [DOI] [PubMed] [Google Scholar]
- Blazar BR, Brennan CA, Broxmeyer HE, Shultz LD, Vallera DA. Transgenic mice expressing either bovine growth hormone (bGH) or human GH releasing hormone (hGRH) have increased splenic progenitor cell colony formation and DNA synthesis in vitro and in vivo. Exp Hematol. 1995;23(13):1397–1406. [PubMed] [Google Scholar]
- Bohlooly YM, Olsson B, Gritli-Linde A, Brusehed O, Isaksson OG, Ohlsson C, Soderpalm B, Tornell J. Enhanced spontaneous locomotor activity in bovine GH transgenic mice involves peripheral mechanisms. Endocrinology. 2001;142(10):4560–4567. doi: 10.1210/endo.142.10.8444. [DOI] [PubMed] [Google Scholar]
- Bollano E, Omerovic E, Bohlooly-y M, Kujacic V, Madhu B, Tornell J, Isaksson O, Soussi B, Schulze W, Fu ML, Matejka G, Waagstein F, Isgaard J. Impairment of cardiac function and bioenergetics in adult transgenic mice overexpressing the bovine growth hormone gene. Endocrinology. 2000;141(6):2229–2235. doi: 10.1210/endo.141.6.7486. [DOI] [PubMed] [Google Scholar]
- Boparai RK, Arum O, Khardori R, Bartke A. Glucose homeostasis and insulin sensitivity in growth hormone-transgenic mice: a cross-sectional analysis. Biol Chem. 2010;391(10):1149–1155. doi: 10.1515/BC.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brüünsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin N Am. 2003;23(1):15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis. 2008;3(1):17. doi: 10.1186/1750-1172-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sun D, Krishnamurthy VMR, Rabkin R. Endotoxin attenuates growth hormone-induced hepatic insulin-like growth factor I expression by inhibiting JAK2/STAT5 signal transduction and STAT5b DNA binding. Am J Physiol Endocrinol Metab. 2007;292(6):E1856–E1862. doi: 10.1152/ajpendo.00581.2006. [DOI] [PubMed] [Google Scholar]
- Christensen B, Sackmann-Sala L, Cruz-Topete D, Jorgensen JO, Jessen N, Lundby C, Kopchick JJ. Novel serum biomarkers for erythropoietin use in humans: a proteomic approach. J Appl Physiol. 2010 doi: 10.1152/japplphysiol.00665.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Topete D, Christensen B, Sackmann-Sala L, Okada S, Jorgensen JO, Kopchick JJ. Serum proteome changes in acromegalic patients following transsphenoidal surgery: novel biomarkers of disease activity. Eur J Endocrinol. 2010 doi: 10.1530/EJE-10-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajee M, Kazansky AV, Raught B, Hocke GM, Fey GH, Richards JS. Prolactin induction of the alpha 2-macroglobulin gene in rat ovarian granulosa cells: stat 5 activation and binding to the interleukin-6 response element. Mol Endocrinol. 1996;10(2):171–184. doi: 10.1210/mend.10.2.8825557. [DOI] [PubMed] [Google Scholar]
- de Groof F, Joosten KFM, Janssen JAMJL, de Kleijn ED, Hazelzet JA, Hop WCJ, Uitterlinden P, van Doorn J, Hokken-Koelega ACS. Acute stress response in children with meningococcal sepsis: important differences in the growth hormone/insulin-like growth factor I axis between nonsurvivors and survivors. J Clin Endocrinol Metab. 2002;87(7):3118–3124. doi: 10.1210/jcem.87.7.8605. [DOI] [PubMed] [Google Scholar]
- Derfalvi B, Igaz P, Fulop KA, Szalai C, Falus A. Interleukin-6-induced production of type II acute phase proteins and expression of junB gene are downregulated by human recombinant growth hormone in vitro. Cell Biol Int. 2000;24(2):109–114. doi: 10.1006/cbir.1999.0454. [DOI] [PubMed] [Google Scholar]
- Ding J, Kopchick JJ. Plasma biomarkers of mouse aging. Age (Dordr) 2010 doi: 10.1007/s11357-010-9179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Striker LJ, Gibson CC, Agodoa LY, Brinster RL, Striker GE. Glomerular lesions in mice transgenic for growth hormone and insulinlike growth factor-I. I. Relationship between increased glomerular size and mesangial sclerosis. Am J Pathol. 1990;137(3):541–552. [PMC free article] [PubMed] [Google Scholar]
- Dominici FP, Cifone D, Bartke A, Turyn D. Alterations in the early steps of the insulin-signaling system in skeletal muscle of GH-transgenic mice. Am J Physiol Endocrinol Metab. 1999;277(3):E447–E454. doi: 10.1152/ajpendo.1999.277.3.E447. [DOI] [PubMed] [Google Scholar]
- Dunn DA, Pinkert CA, Kooyman DL. Foundation review: transgenic animals and their impact on the drug discovery industry. Drug Discov Today. 2005;10(11):757–767. doi: 10.1016/S1359-6446(05)03452-5. [DOI] [PubMed] [Google Scholar]
- Edge JA, Dunger DB, Matthews DR, Gilbert JP, Smith CP. Increased overnight growth hormone concentrations in diabetic compared with normal adolescents. J Clin Endocrinol Metab. 1990;71(5):1356–1362. doi: 10.1210/jcem-71-5-1356. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick F, Bohlooly-Y M, Linden D, Olsson B, Tornell J, Eden S, Oscarsson J. Long-term growth hormone excess induces marked alterations in lipoprotein metabolism in mice. Am J Physiol Endocrinol Metab. 2001a;281(6):E1230–E1239. doi: 10.1152/ajpendo.2001.281.6.E1230. [DOI] [PubMed] [Google Scholar]
- Frick F, Bohlooly YM, Linden D, Olsson B, Tornell J, Eden S, Oscarsson J. Long-term growth hormone excess induces marked alterations in lipoprotein metabolism in mice. Am J Physiol Endocrinol Metab. 2001b;281(6):E1230–E1239. doi: 10.1152/ajpendo.2001.281.6.E1230. [DOI] [PubMed] [Google Scholar]
- Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104(8):1258–1264. doi: 10.1016/j.jada.2004.05.213. [DOI] [PubMed] [Google Scholar]
- Gierach M, Gierach J, Pujanek M, Skowronska A, Rutkowska E, Junik R. Aberrations in carbohydrate metabolism in patients with diagnosed acromegaly, hospitalized in the Endocrinology and Diabetology Department of Collegium Medicum University of Nicolaus Copernicus in Bydgoszcz in the years 2001–2009. Endokrynol Pol. 2010;61(3):260–263. [PubMed] [Google Scholar]
- Gravholt CH, Leth-Larsen R, Lauridsen AL, Thiel S, Hansen TK, Holmskov U, Naeraa RW, Christiansen JS. The effects of GH and hormone replacement therapy on serum concentrations of mannan-binding lectin, surfactant protein D and vitamin D binding protein in Turner syndrome. Eur J Endocrinol. 2004;150(3):355–362. doi: 10.1530/eje.0.1500355. [DOI] [PubMed] [Google Scholar]
- Hall MA, Bartke A, Martinko JM. Humoral immune response in mice over-expressing or deficient in growth hormone. Exp Biol Med. 2002;227(7):535–544. doi: 10.1177/153537020222700719. [DOI] [PubMed] [Google Scholar]
- Hammer RE, Brinster RL, Palmiter RD. Use of gene transfer to increase animal growth. Cold Spring Harb Symp Quant Biol. 1985;50:379–387. doi: 10.1101/sqb.1985.050.01.048. [DOI] [PubMed] [Google Scholar]
- Hansen TK. Growth hormone and mannan-binding lectin: emerging evidence for hormonal regulation of humoral innate immunity. Minerva Endocrinol. 2003;28(1):75–84. [PubMed] [Google Scholar]
- Hansen TK, Thiel S, Dall R, Rosenfalck AM, Trainer P, Fly-vbjerg A, Jorgensen JO, Christiansen JS. GH strongly affects serum concentrations of mannan-binding lectin: evidence for a new IGF-I independent immunomodulatory effect of GH. J Clin Endocrinol Metab. 2001;86(11):5383–5388. doi: 10.1210/jcem.86.11.8009. [DOI] [PubMed] [Google Scholar]
- Hardy CL, Bhathal PS, Snibson KJ, Adams TE. Comparison of intrahepatic lymphocytes from normal and growth hormone transgenic mice with chronic hepatitis and liver cancer. Immunology. 1997;90(3):412–420. doi: 10.1111/j.1365-2567.1997.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N. Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Horm IGF Res. 2009;19(3):187–197. doi: 10.1016/j.ghir.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008;22(6):711–721. doi: 10.1101/gad.1643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka S, Kranzlin B, Gretz N, Witzgall R. Urinary clusterin levels in the rat correlate with the severity of tubular damage and may help to differentiate between glomerular and tubular injuries. Cell Tissue Res. 2002;310(3):289–296. doi: 10.1007/s00441-002-0629-5. [DOI] [PubMed] [Google Scholar]
- Houdebine LM. Production of pharmaceutical proteins by transgenic animals. Comp Immunol Microbiol Infect Dis. 2009;32(2):107–121. doi: 10.1016/j.cimid.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. Mechanisms linking apolipoprotein E isoforms with cardiovascular and neurological diseases. Curr Opin Lipidol. 2010;21(4):337–345. doi: 10.1097/MOL.0b013e32833af368. [DOI] [PubMed] [Google Scholar]
- Huang X, Wei Y, Li L, Wen Y, Yang J, Liu B, Song X, Zhao J. Serum proteomics study of the squamous cell carcinoma antigen 1 in tongue cancer. Oral Oncol. 2006;42(1):25–30. doi: 10.1016/j.oraloncology.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Zhu BL, Li DR, Zhao D, Michiue T, Maeda H. Age-dependent increase of clusterin in the human pituitary gland. Leg Med (Tokyo) 2006;8(1):28–33. doi: 10.1016/j.legalmed.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Itoh N, Hanafusa T, Miyagawa J, Tamura S, Inada M, Kawata S, Kono N, Tarui S. Transthyretin (prealbumin) in the pancreas and sera of newly diagnosed type I (insulin-dependent) diabetic patients. J Clin Endocrinol Metab. 1992;74(6):1372–1377. doi: 10.1210/jcem.74.6.1592883. [DOI] [PubMed] [Google Scholar]
- Izzard AS, Emerson M, Prehar S, Neyses L, Trainer P, List EO, Kopchick JJ, Heagerty AM. The cardiovascular phenotype of a mouse model of acromegaly. Growth Horm IGF Res (in press, corrected proof) 2009 doi: 10.1016/j.ghir.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Jeay S, Sonenshein GE, Postel-Vinay M-C, Kelly PA, Baixeras E. Growth hormone can act as a cytokine controlling survival and proliferation of immune cells: new insights into signaling pathways. Mol Cell Endocrinol. 2002;188(1–):1. doi: 10.1016/s0303-7207(02)00014-x. [DOI] [PubMed] [Google Scholar]
- Johannsson G, Oscarsson J, Rosen T, Wiklund O, Olsson G, Wilhelmsen L, Bengtsson B-A. Effects of 1 year of growth hormone therapy on serum lipoprotein levels in growth hormone–deficient adults: influence of gender and Apo(a) and ApoE phenotypes. Arterioscler Thromb Vasc Biol. 1995;15(12):2142–2150. doi: 10.1161/01.atv.15.12.2142. [DOI] [PubMed] [Google Scholar]
- Jordan-Starck TC, Lund SD, Witte DP, Aronow BJ, Ley CA, Stuart WD, Swertfeger DK, Clayton LR, Sells SF, Paigen B. Mouse apolipoprotein J: characterization of a gene implicated in atherosclerosis. J Lipid Res. 1994;35(2):194–210. [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63(3):287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzsch J, Selisko T, Birkenmeier G. Transformed alpha 2-macroglobulin as a low-affinity growth hormone-binding protein. Acta Paediatr Suppl. 1996;417:108–110. doi: 10.1111/j.1651-2227.1996.tb14315.x. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Muramaki M, Yamanaka K, Hara I, Miyake H. Expression of the secreted form of clusterin protein in renal cell carcinoma as a predictor of disease extension. BJU Int. 2005;96(6):895–899. doi: 10.1111/j.1464-410X.2005.05733.x. [DOI] [PubMed] [Google Scholar]
- Lang CH, Pollard V, Fan J, Traber LD, Traber DL, Frost RA, Gelato MC, Prough DS. Acute alterations in growth hormone-insulin-like growth factor axis in humans injected with endotoxin. Am J Physiol. 1997;273(1 Pt 2):R371–R378. doi: 10.1152/ajpregu.1997.273.1.R371. [DOI] [PubMed] [Google Scholar]
- Lasztity N, Biro L, Nemeth E, Pap A, Antal M. Protein status in pancreatitis-transthyretin is a sensitive biomarker of malnutrition in acute and chronic pancreatitis. Clinical Chem Lab Med. 2002;40(12):1320–1324. doi: 10.1515/CCLM.2002.227. [DOI] [PubMed] [Google Scholar]
- List EO, Berryman DE, Palmer AJ, Gosney E, Okada S, Kelder B, Lichtenberg J, Welch LR, Kopchick JJ. Application of bioinformatics and scalable computing to perform proteomic analysis of stomach tissue from diabetic mice. Scalable Comput Pract Exp. 2007a;8(2):173–183. [Google Scholar]
- List EO, Berryman DE, Palmer AJ, Qiu L, Sankaran S, Kohn DT, Kelder B, Okada S, Kopchick JJ. Analysis of mouse skin reveals proteins that are altered in a diet-induced diabetic state: a new method for detection of type 2 diabetes. Proteomics. 2007b;7(7):1140–1149. doi: 10.1002/pmic.200600641. [DOI] [PubMed] [Google Scholar]
- List EO, Palmer AJ, Berryman DE, Bower B, Kelder B, Kopchick JJ. Growth hormone improves body composition, fasting blood glucose, glucose tolerance and liver triacylglycerol in a mouse model of diet-induced obesity and type 2 diabetes. Diabetologia. 2009;52(8):1647–1655. doi: 10.1007/s00125-009-1402-z. [DOI] [PubMed] [Google Scholar]
- Mathews LS, Hammer RE, Brinster RL, Palmiter RD. Expression of insulin-like growth factor I in transgenic mice with elevated levels of growth hormone is correlated with growth. Endocrinology. 1988;123(1):433–437. doi: 10.1210/endo-123-1-433. [DOI] [PubMed] [Google Scholar]
- McGrane MM, de Vente J, Yun J, Bloom J, Park E, Wynshaw-Boris A, Wagner T, Rottman FM, Hanson RW. Tissue-specific expression and dietary regulation of a chimeric phosphoenolpyruvate carboxykinase/bovine growth hormone gene in transgenic mice. J Biol Chem. 1988;263(23):11443–11451. [PubMed] [Google Scholar]
- Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, Clemmons D, Chanson P, Laws E, Schlechte J, Vance ML, Ho K, Giustina A. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab. 2009;94(5):1509–1517. doi: 10.1210/jc.2008-2421. [DOI] [PubMed] [Google Scholar]
- Moore LG, Pfeffer A, Chie WN, Miller HA, Rogers KM, O’Keeffe LE. Induction of an acute phase response in lambs causes an increase in plasma levels of GH and IGF-I. J Endocrinol. 1995;144(2):243–250. doi: 10.1677/joe.0.1440243. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14(1):133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Okada S, List E, Sankaran S, Kopchick J. Plasma protein biomarkers correlated with the development of diet-induced type 2 diabetes in mice. Clin Proteomics. 2010 doi: 10.1007/s12014-009-9040-5. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda Y, Peña J, Chou J, Field JB. Acute effects of growth hormone on metabolism of pancreatic hormones, glucose and ketone bodies. Diabetes Res Clin Pract. 2001;53(1):1–8. doi: 10.1016/s0168-8227(01)00232-7. [DOI] [PubMed] [Google Scholar]
- Olsson B, Bohlooly-Y M, Fitzgerald SM, Frick F, Ljungberg A, Ahren B, Tornell J, Bergstrom G, Oscarsson J. Bovine growth hormone transgenic mice are resistant to diet-induced obesity but develop hyperphagia, dyslipidemia, and diabetes on a high-fat diet. Endocrinology. 2005;146(2):920–930. doi: 10.1210/en.2004-1232. [DOI] [PubMed] [Google Scholar]
- Oscarsson J, Olofsson S-O, Vikman K, Edén S. Growth hormone regulation of serum lipoproteins in the rat: different growth hormone regulatory principles for apolipoprotein (apo) B and the sexually dimorphic apo E concentrations. Metabolism. 1991;40(11):1191–1198. doi: 10.1016/0026-0495(91)90215-i. [DOI] [PubMed] [Google Scholar]
- Paisley AN, Izzard AS, Gemmell I, Cruickshank K, Trainer PJ, Heagerty AM. Small vessel remodeling and impaired endothelial-dependent dilatation in subcutaneous resistance arteries from patients with acromegaly. J Clin Endocrinol Metab. 2009;94(4):1111–1117. doi: 10.1210/jc.2008-0948. [DOI] [PubMed] [Google Scholar]
- Palmer AJ, Chung MY, List EO, Walker J, Okada S, Kopchick JJ, Berryman DE. Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology. 2009;150(3):1353–1360. doi: 10.1210/en.2008-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JA, Bartke A, Sorenson RL. Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology. 1995;136(5):2013–2021. doi: 10.1210/endo.136.5.7720649. [DOI] [PubMed] [Google Scholar]
- Postel-Vinay M-C, de Mello Coelho V, Gagnerault M-C, Dardenne M. Growth hormone stimulates the proliferation of activated mouse T lymphocytes. Endocrinology. 1997;138(5):1816–1820. doi: 10.1210/endo.138.5.5108. [DOI] [PubMed] [Google Scholar]
- Pozzilli P, Tamburrano G, Mercalli ME, Javicoli M. Raised alpha-2-macroglobulin levels in acromegaly. Horm Metab Res. 1978;10(4):359. doi: 10.1055/s-0028-1095834. [DOI] [PubMed] [Google Scholar]
- Qiu L, List EO, Kopchick JJ. Differentially expressed proteins in the pancreas of diet-induced diabetic mice. Mol Cell Proteomics. 2005;4(9):1311–1318. doi: 10.1074/mcp.M500016-MCP200. [DOI] [PubMed] [Google Scholar]
- Quaife CJ, Mathews LS, Pinkert CA, Hammer RE, Brinster RL, Palmiter RD. Histopathology associated with elevated levels of growth hormone and insulin-like growth factor I in transgenic mice. Endocrinology. 1989;124(1):40–48. doi: 10.1210/endo-124-1-40. [DOI] [PubMed] [Google Scholar]
- Rithidech KN, Honikel L, Rieger R, Xie W, Fischer T, Simon SR. Protein-expression profiles in mouse blood-plasma following acute whole-body exposure to 137Cs γ rays. Int J Radiat Biol. 2009;85(5):432–447. doi: 10.1080/09553000902820390. [DOI] [PubMed] [Google Scholar]
- Rocha JS, Bonkowski MS, de Franc¸a LR, Bartke A. Effects of mild calorie restriction on reproduction, plasma parameters and hepatic gene expression in mice with altered GH/IGF-I axis. Mech Ageing Dev. 2007;128(4):317–331. doi: 10.1016/j.mad.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998;53(1):M20–M26. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- Sackmann-Sala L, Ding J, Frohman LA, Kopchick JJ. Activation of the GH/IGF-1 axis by CJC-1295, a long-acting GHRH analog, results in serum protein profile changes in normal adult subjects. Growth Horm IGF Res. 2009;19(6):471–477. doi: 10.1016/j.ghir.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan JP. Fasting hyperglycemia: etiology, diagnosis, and treatment. Diabetes Technol Ther. 2004;6(4):525–533. doi: 10.1089/1520915041705910. [DOI] [PubMed] [Google Scholar]
- Sjoberg A, Oscarsson J, Eden S, Olofsson SO. Continuous but not intermittent administration of growth hormone to hypophysectomized rats increases apolipoprotein-E secretion from cultured hepatocytes. Endocrinology. 1994;134(2):790–798. doi: 10.1210/endo.134.2.8299573. [DOI] [PubMed] [Google Scholar]
- Spurlock ME, Ranalletta MA, Cornelius SG, Frank GR, Willis GM, Ji S, Grant AL, Bidwell CA. Leptin expression in porcine adipose tissue is not increased by endotoxin but is reduced by growth hormone. J Interferon Cytokine Res. 1998;18(12):1051–1058. doi: 10.1089/jir.1998.18.1051. [DOI] [PubMed] [Google Scholar]
- Steger RW, Bartke A, Cecim M. Premature ageing in transgenic mice expressing different growth hormone genes. J Reprod Fertil Suppl. 1993;46:61–75. [PubMed] [Google Scholar]
- Sumiya M, Tabona P, Arai T, Summerfield JA, Super M, Levinsky RJ, Turner MW. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337(8757):1569–1570. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341(11):785–792. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- Tanimoto K, Hizuka N, Fukuda I, Takano K, Hanafusa T. The influence of age on the GH-IGF1 axis in patients with acromegaly. Eur J Endocrinol. 2008;159(4):375–379. doi: 10.1530/EJE-08-0243. [DOI] [PubMed] [Google Scholar]
- Thio CL, Mosbruger T, Astemborski J, Greer S, Kirk GD, O’Brien SJ, Thomas DL. Mannose binding lectin genotypes influence recovery from hepatitis B virus infection. J Virol. 2005;79(14):9192–9196. doi: 10.1128/JVI.79.14.9192-9196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MW. The role of mannose-binding lectin in health and disease. Mol Immunol. 2003;40(7):423. doi: 10.1016/s0161-5890(03)00155-x. [DOI] [PubMed] [Google Scholar]
- Umans L, Serneels L, Overbergh L, Stas L, Van Leuven F. alpha2-macroglobulin- and murinoglobulin-1-deficient mice. A mouse model for acute pancreatitis. Am J Pathol. 1999;155(3):983–993. doi: 10.1016/s0002-9440(10)65198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera A, Rodriguez-Gil JE, Yun JS, McGrane MM, Hanson RW, Bosch F. Glucose metabolism in transgenic mice containing a chimeric P-enolpyruvate carboxykinase/ bovine growth hormone gene. FASEB J. 1993;7(9):791–800. doi: 10.1096/fasebj.7.9.8330686. [DOI] [PubMed] [Google Scholar]
- Van Leuven F, Umans L, Lorent K, Hilliker C, Serneels L, Overbergh L, Stas L, Raymakers L. Molecular analysis of the human and mouse alpha 2 M family. Ann N Y Acad Sci. 1994;737:163–171. doi: 10.1111/j.1749-6632.1994.tb44310.x. [DOI] [PubMed] [Google Scholar]
- Vijayakumar A, Novosyadlyy R, Wu Y, Yakar S, LeRoith D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm IGF Res. 2010;20(1):1–7. doi: 10.1016/j.ghir.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva J, Shaffer DR, Philip J, Chaparro CA, Erdjument-Bromage H, Olshen AB, Fleisher M, Lilja H, Brogi E, Boyd J, Sanchez-Carbayo M, Holland EC, Cordon-Cardo C, Scher HI, Tempst P. Differential exoprotease activities confer tumor-specific serum peptidome patterns. J Clin Invest. 2006;116(1):271. doi: 10.1172/JCI26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranckx R, Rouaze-Romet M, Savu L, Mechighel P, Maya M, Nunez EA. Regulation of rat thyroxine-binding globulin and transthyretin: studies in thyroidectomized and hypophysectomized rats given tri-iodothyronine or/ and growth hormone. J Endocrinol. 1994;142(1):77–84. doi: 10.1677/joe.0.1420077. [DOI] [PubMed] [Google Scholar]
- Wall RJ, Powell AM, Paape MJ, Kerr DE, Bannerman DD, Pursel VG, Wells KD, Talbot N, Hawk HW. Genetically enhanced cows resist intramammary Staphylococcus aureus infection. Nat Biotechnol. 2005;23(4):445–451. doi: 10.1038/nbt1078. [DOI] [PubMed] [Google Scholar]
- Wang Z, Masternak MM, Al-Regaiey KA, Bartke A. Adipocytokines and the regulation of lipid metabolism in growth hormone transgenic and calorie-restricted mice. Endocrinology. 2007;148(6):2845–2853. doi: 10.1210/en.2006-1313. [DOI] [PubMed] [Google Scholar]
- Westwood M, Aplin JD, Collinge IA, Gill A, White A, Gibson JM. α2-Macroglobulin: a new component in the insulin-like growth factor/insulin-like growth factor binding protein-1 axis. J Biol Chem. 2001;276(45):41668–41674. doi: 10.1074/jbc.M102793200. [DOI] [PubMed] [Google Scholar]
- Wildbrett J, Hanefeld M, Fucker K, Pinzer T, Bergmann S, Siegert G, Breidert M. Anomalies of lipoprotein pattern and fibrinolysis in acromegalic patients: relation to growth hormone levels and insulin-like growth factor I. Exp Clin Endocrinol Diabetes. 1997;105(6):331–335. doi: 10.1055/s-0029-1211774. [DOI] [PubMed] [Google Scholar]
- Witte DP, Aronow BJ, Stauderman ML, Stuart WD, Clay MA, Gruppo RA, Jenkins SH, Harmony JA. Platelet activation releases megakaryocyte-synthesized apolipoprotein J, a highly abundant protein in atheromatous lesions. Am J Pathol. 1993;143(3):763–773. [PMC free article] [PubMed] [Google Scholar]
- Wolf E, Kahnt E, Ehrlein J, Hermanns W, Brem G, Wanke R. Effects of long-term elevated serum levels of growth hormone on life expectancy of mice: lessons from transgenic animal models. Mech Ageing Dev. 1993;68(1–3):71–87. doi: 10.1016/0047-6374(93)90141-d. [DOI] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413(6852):131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- Zargarova OP, Konnova LA. Proteinase inhibitor activity of blood plasma and cerebrospinal fluid in patients with pituitary adenomas before and after proton therapy. Vopr Med Khim. 1983;29(6):96–98. [PubMed] [Google Scholar]
- Zhang R, Barker L, Pinchev D, Marshall J, Rasamoelisolo M, Smith C, Kupchak P, Kireeva I, Ingratta L, Jackowski G. Mining biomarkers in human sera using proteomic tools. Proteomics. 2004;4(1):244–256. doi: 10.1002/pmic.200300495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.