Abstract
Purpose
Here we report a phase II clinical trial, which was designed to test a novel hypothesis that treatment with GEM/DOX would be efficacious via reconstitution of C18-ceramide signaling in HNSCC patients for whom first-line platinum-based therapy failed.
Experimental Design
Patients received GEM (1,000 mg/m2) and DOX (25 mg/m2) on days 1 and 8, every 21 days, until disease progression. After completion of 2 treatment cycles, patients were assessed radiographically, and serum samples were taken for sphingolipid measurements.
Results
We enrolled 18 patients in the trial, who were evaluable for toxicity, and 17 for response. The most common toxicity was neutropenia, observed in 9 of 18 patients, and there were no major non-hematological toxicities. Of the 17 patients, 5 patients had progressive disease (PD), 1 had complete response (CR), 3 exhibited partial response (PR), and 8 had stable disease (SD). The median progression-free survival (PFS) was 1.6 months (95% CI, 1.4, 4.2) with a median survival of 5.6 months (95% CI, 3.8, 18.2). Remarkably, serum sphingolipid analysis revealed significant differences in patterns of C18-ceramide elevation in patients with CR/PR/SD in comparison to patients with PD, indicating the reconstitution of tumor suppressor ceramide generation by GEM/DOX treatment.
Conclusions
Our data suggest that the GEM/DOX combination could represent an effective treatment for some patients with recurrent or metastatic HNSCC, and that serum C18-ceramide elevation might be a novel serum biomarker of chemotherapy response.
Keywords: Sphingolipids, ceramide, ceramide synthase, sphingosine 1-phosphate, sphingosine
Introduction
HNSCC is the tenth most common cancer worldwide. In 2011, 39,400 Americans developed HNSCC, and 7,900 died from this disease (1). The primary risk factors for HNSCC in American men and women are tobacco and alcohol use (1). Most recently, exposure to oncogenic human papilloma virus 16 (HPV16) has been implicated in the development of HNSCC (2).
The median survival for patients with recurrent or metastatic HNSCC is about 6 months and the 1-year survival rate is around 20% (1,2). The role of chemotherapy in the treatment of recurrent HNSCC is expanding. Single-agent chemotherapy response rates vary from 6 to 30%, and platinum based combination therapy had response rates from 20 to 60% in phase III trials (3). However, overall survival of patients with HNSCC, even those treated with platinum-based combination regimens, has not improved over several decades. Therefore, alternative strategies to treat HNSCC, and the identification of novel biomarkers to estimate response in advance of chemotherapy are desperately needed.
Gemcitabine (GEM) (dFdC) is an anti-metabolite, whose main mechanism of action is the incorporation of dFdC triphosphate adducts into DNA, resulting in chain termination and inhibition of DNA synthesis. A phase II trial of single-agent GEM in patients with metastatic or recurrent HNSCC produced a response rate of 13% in 54 evaluable patients (4). Doxorubicin (DOX) is an anthracycline, which has 3 mechanisms of action: it induces the formation of covalent topoisomerase-DNA complexes, intercalates DNA, and/or causes oxidative damage. Single agent DOX has a demonstrated response rate of 13–23% in phase II studies (5). However, the efficacy of the GEM plus DOX combination (GEM/DOX) against HNSCC has not been previously studied.
Ceramide, a bioactive tumor suppressor sphingolipid, mediates anti-proliferative effects, such as the induction of apoptosis and/or growth inhibition (6). Ceramide contains a sphingosine backbone which is amide linked to a 12- to 26-carbon containing fatty acyl chain, resulting in the generation C12- to C26-ceramides (7). Endogenous C12- to C26-ceramides and other major sphingolipids, such as sphingosine, and sphingosine 1-phosphate (S1P) can be quantified in human cells and tissues using high performance liquid chromatography/mass spectroscopy (LC/MS) (8-10). Interestingly, recent data suggest that endogenous ceramides with different fatty acyl chain lengths might have biologically distinct functions (11). For example, whereas generation of C18-ceramide, mainly by ceramide synthase 1, CerS1 (12), has a tumor suppressive role (11), C16-ceramide generated by ceramide synthase 6 (CerS6) might induce HNSCC tumor growth (13). To this end, our previous studies have shown that C18-ceramide was decreased in the majority (70%, n=45) of human HNSCC tumors compared to their paired non-cancerous head and neck tissues (14,15). Importantly, decreased C18-ceramide significantly correlated with lymphovascular invasion and nodal disease indicating the clinical relevance of C18-ceramide in the overall prognosis of HNSCC patients (15). In reciprocal studies, reconstitution of C18-ceramide generation via forced expression of CerS1 inhibited HNSCC xenograft-derived tumors in SCID mice compared to controls (11), supporting the tumor suppressive role of CerS1-generated C18-ceramide in HNSCC.
Additionally, it has been previously established that treatment of various human cancer cells with conventional chemotherapeutic agents inhibits growth via ceramide generation (16). Consistent with these data, our studies demonstrated that the GEM/DOX combination, but not 5-fluorouracil, methotrexate, cisplatin, or paclitaxel had a synergistic effect on growth inhibition of UM-SCC-22A cells (IC50 values were between 150-220 nM) in situ (17, 18). Importantly, our pre-clinical studies revealed that GEM/DOX combination reconstituted levels of C18-ceramide via up-regulation of CerS1 expression (mRNA and protein), increasing CerS1 activity by 3.5-fold for C18-ceramide generation, leading to growth inhibition in HNSCC xenografts in vivo (18). Thus, these data show that the combination of GEM/DOX inhibits HNSCC tumor growth via reconstitution of C18-ceramide generation.
Based on these preclinical data, we hypothesized that GEM/DOX might provide a viable treatment option against recurrent or metastatic HNSCC via reconstitution of C18-ceramide-dependent tumor suppressor signaling. To test this novel concept, a single center, phase II clinical trial was initiated and completed to evaluate the toxicity and overall response rate produced by the combination of GEM/DOX in patients with recurrent or metastatic HNSCC for whom prior platinum-based therapy failed, which is the first line chemotherapy treatment for HNSCC (19). Reactivation/reconstitution of C18-ceramide tumor suppressor signaling in these patients in response to GEM/DOX treatment was serially examined by measurements of sphingolipids in the serum samples of patients during therapy by LC/MS. We report here the results of this phase II trial.
Patients and Methods
Eligibility
This phase II, single-center, open-label study enrolled patients with recurrent or metastatic HNSCC who had received prior platinum therapy (cisplatin or carboplatin). Additional criteria for inclusion were measurable disease, >18 years-of-age, Eastern Cooperative Oncology Group (ECOG) performance score ≤2, normal organ and marrow function (absolute neutrophil count ≥1,500/mL, platelets ≥100,000/mL, total bilirubin ≤1.5 mg/dl, AST(sGOT)/ALT(sGPT) ≤2.5 X institutional upper limit of normal, creatinine ≤2 mg/dl or creatinine clearance ≥30mmL/min as calculated by the Cockroft-Gault formula) and lastly, the most recent platinum-based chemotherapy treatment for each patient had to be 3 or more weeks prior (or longer) to the day of entry into this phase II clinical trial. The exclusion criteria were receiving chemotherapy or radiotherapy within the past 3 or 4 weeks, respectively, prior treatment with any other investigational agents, or previous treatment with GEM and/or DOX. Patients with known brain metastases, history of allergic reactions attributed to compounds similar to GEM or DOX, lower than normal cardiac ejection fraction (due to potential cardio-toxic effects of DOX), uncontrolled inter-current illness, clinical AIDS (or known positive HIV serology), and pregnant women were also excluded from the trial. The MUSC Institutional Review Board approved this study protocol and written informed consent was obtained from each patient prior to their enrollment into the trial at the Hollings Cancer Center. The Clinical Trial Registry ID number is NCT00509665.
Treatment plan
Prior to initiating the treatment protocol, eligible patients were assessed by complete physical examination, computed tomography scan (CT Scan) or other imaging modalities of the head and neck region, complete blood count, renal and liver function tests, and a MUGA or echocardiogram. Serum ceramide and sphingolipid were also measured. Eligible patients were treated with GEM 1,000 mg/m2 and DOX 25 mg/m2 on days 1 and 8 every 3 weeks (1 cycle = 21 days). GEM was administered as a 30-min IV infusion. DOX was administered as a slow IV push or rapid IV drip over 5–10 min subsequent to the infusion of GEM. Patients were assessed on day 1 of every 21-day cycle for changes in performance status, and the presence of toxicities. Toxicities were graded according to CTCAE Version 3.0 (Common Toxicity Criteria for Adverse Events, National Cancer Institute). On days 1 and 8 of every 21-day cycle, each patient’s blood was evaluated, and lastly, every 2 cycles (6 weeks), patients had a CT scan for tumor restaging, and serum was collected for ceramide and sphingolipid measurement. Dose reduction or schedule change was allowed based on protocol. Adjustment was based upon clinical laboratory results on the scheduled day of treatment, and upon maximum toxicity encountered during the previous course. Discontinuation of treatment was required if the patient developed grade 1 or higher of cardiac left ventricular function toxicity, grade 2 or higher of pulmonary toxicity, and grade 4 hypersensitivity reactions. In the absence of treatment delays due to adverse events, patients continued on therapy at the discretion of the treating physician until disease progression, inter-current illness, or unacceptable adverse events occurred. The flow chart of the treatment protocol is illustrated in Fig. 1.
Figure 1.
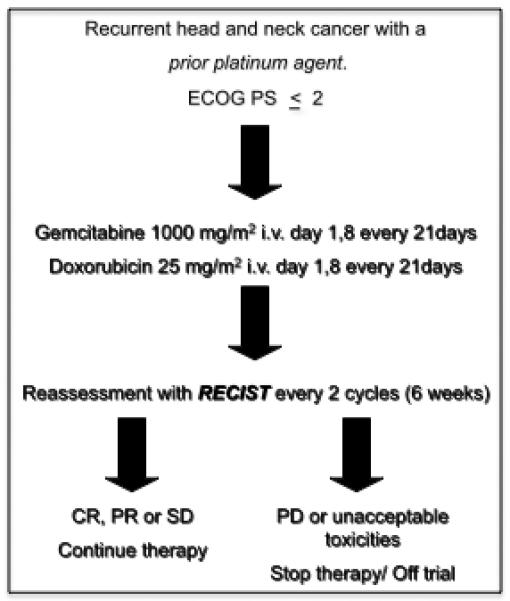
The design of the GEM/DOX phase II clinical trial. This phase II, single-center, open-label study enrolled patients with recurrent or metastatic HNSCC who had received prior cisplatin or carboplatin therapy.
Response assessment and follow-up
Patient response to therapy was assessed by CT scans at every 2 cycles (6 weeks). Response and progression was evaluated using the international criteria proposed by the Response Evaluation Criteria in Solid Tumors (RECIST 1.0) Committee (20). Measurable disease is defined as having at least tumor with a diameter ≥ 20 mm measured with conventional techniques (CT, MRI, X-ray) or as a tumor with a diameter ≥ 10 mm as measured with a spiral CT scan. Evaluation of target lesions was performed every 2 cycles and patients were removed from the study, if restaging scans showed disease progression (Fig. 1).
Measurements of serum ceramides by LC/MS
Blood samples were collected from the patients at every 2 cycles and LC/MS measurements were performed to quantify serum ceramide (C12- to C26-ceramides), C16-dihydro-ceramide, sphingosine, and S1P (Lipidomics) during the course of treatment. In brief, blood was collected from patients and centrifuged at 3,000 rpm for 5 min. Serum was used (0.1 ml) for LC/MS analysis (21). Sphingolipids were extracted via a single phase extraction method using ethyl-acetate:isopropanol:water, and sphingolipid concentrations were calculated as pmol/100 μL of serum (21).
Statistical Analysis and Sample Size Calculations
Simon’s optimal 2-stage design was used to evaluate the efficacy of GEM/DOX. The null and alternative response rates (CR and PR) were 0.20 and 0.45, respectively. Specifically, the first stage enrolled 7 patients with at least 2 responses necessary to move to the second stage. In the second stage, 10 patients were enrolled. At the end of the study, 6 or more responses in 17 patients would provide evidence to reject the null hypothesis with 81% power and an overall one-sided alpha of 0.09. The point estimate for the response rate, p-value and 95% confidence interval for the response rate were calculated adjusting for early stopping design (22). The primary outcome was overall response rate and the secondary outcomes were progression free survival (PFS), overall survival (OS) and toxicity. The Kaplan-Meier method was used to estimate PFS, and OS. Baseline (prior to cycle 1 treatment) and changes from cycle 1 to cycle 3 in ceramide levels were evaluated by response categories (CR/PR, SD, and PD) using graphical displays and Kruskal-Wallis tests. Although many patients had data collected on ceramide markers beyond cycle 3, those who progressed did not have follow-up for ceramide measurement beyond cycle 3. As a result comparisons between response categories (CR, PR, SD compared to PD) were limited to changes from cycle 1 to cycle 3. Moreover, ceramide was measured for patients with CR, PR, or SD until treatment was stopped at subsequent cycles.
RESULTS
Patient Characteristics
Between August 2005 and September 2010, 18 patients were enrolled on protocol at the Hollings Cancer Center, Medical University of South Carolina. Baseline characteristics are summarized in Table 1. The median age was 57 years with a range of 45-77 years. Stage IVa disease (67%) was the most common stage at diagnosis and oropharynx was the most common location of the primary disease (44.4%). 17 out of 18 patients were smokers, with an average of 35 pack years (py), which was defined as smoking 20 cigarettes per day for 1 year. Moreover, 8 patients were heavy drinkers, whereas 6 patients were moderate or social drinkers, and 4 patients did not drink alcoholic beverages.
Table 1.
Patient Characteristics
| Characteristics | No. | % | |
|---|---|---|---|
| Age, years | |||
| Median | 56.5 | ||
| Range | 45-77 | ||
| Initial tumor | |||
| Oropharynx | 8 | 44.4 | |
| Larynx | 6 | 33.3 | |
| Hypopharynx | 1 | 5.6 | |
| Oral cavity | 2 | 11.1 | |
| Multi | 1 | 5.6 | |
| ECOG performance status | |||
| 0 | 3 | 16.7 | |
| 1 | 12 | 66.7 | |
| 2 | 3 | 16.7 | |
| No. of cycles received | |||
| 1 | 2 | 11.1 | |
| 2 | 8 | 44.4 | |
| 3 | 0 | 0.0 | |
| 4 | 4 | 22.2 | |
| 5 | 0 | 0.0 | |
| 6 | 2 | 11.1 | |
| 7 | 0 | 0.0 | |
| 8 | 1 | 5.6 | |
| 9+ | 1 | 5.6 | |
| Overall Response Rate (n=17) | |||
| CR + PR | 4 | 26* | |
| SD | 8 | 47 | |
| PD | 5 | 29 | |
estimated response rate is adjusted for Simon two-stage design which proceeds to stage II if there is sufficient activity in stage I. Sample size is 18 for all variables, except response rate due to inevaluability of one patient.
All patients enrolled in our trial had previously been treated with a platinum based therapy prior to enrollment. Upon enrollment 5/18 patients had locally advanced disease, 9/18 patients had distant metastases only, and 4/18 patients had locally recurrent disease and distant metastases. As primary treatment, 10 patients had definitive chemo-radiation treatment, 7 patients had primary surgery followed by either adjuvant radiation or chemo-radiation treatment, and one patient had neo-adjuvant chemotherapy followed by surgery. Prior to enrollment into this Phase II study, 5 patients had salvage surgery and 3 patients had re-irradiation treatment with platinum agents as local therapy. Ten patients received GEM/DOX as first line, and eight patients had GEM/DOX as second or third line recurrent/metastatic chemotherapy. Patients on our trial received a median of 2 cycles of GEM/DOX (range 1 to 19 cycles), with 8 patients (44.5%) who had 4 or more cycles of GEM/DOX. Post GEM/DOX treatment, 13 patients went on to subsequent chemotherapy including docetaxel (n=6), cetuximab (n=3), cisplatin + cetuximab (n=1), paclitaxel + cetuximab (n=1), carboplatin + docetaxel + cetuximab (n=1), and bortezomib + celebrex (n=1).
Toxicity
Toxicity information was collected for every patient prior to initiating each cycle of therapy while on GEM/DOX treatment and was graded according to CTCAE Version 3.0. A summary of the toxicities can be found in Table 2. In general, the main toxicities seen were hematological. The most common toxicity was neutropenia, which was present as a grade 3 toxicity in 4/18 patients, and as a grade 4 toxicity in 5/18 patents. Also, of significance, thrombocytopenia was observed in 1 patient as grade 3, and 2 patients as grade 4. Twelve of the 18 patients received granulocyte-colony stimulating factor (G-CSF) during the course of the treatment, and 13 patients required dose delay or reduction per protocol. Only one patient had febrile neutropenia. Thus, this regimen had an acceptable non-hematological toxicity profile. For future studies, primary prophylaxis with growth factors would be recommended.
Table 2. Toxicities.
Of note, 8/18 patients (44.4%) did not experience significant (> grade 2) toxicity
| Adverse Event | Grade 3 | % | Grade 4 | % | |
|---|---|---|---|---|---|
| Constitutional | |||||
| Fatigue | 1 | 5.6 | 0 | 0.0 | |
| Hematologic | |||||
| Neutropenia | 4 | 22.2 | 5 | 27.8 | |
| Leukopenia | 3 | 167 | 3 | 167 | |
| Anemia | 1 | 5.6 | |||
| Thrombocytopenia | 1 | 5.6 | 2 | 11.1 | |
| Non-hematologic | |||||
| cough | 1 | 5.6 | 0 | 0 | |
| hypocalcemia | 1 | 5.6 | 0 | 0 | |
| hypophosphotemia | 1 | 5.6 | 0 | 0 | |
| hyponatremia | 2 | 11.1 | 0 | 0 | |
| hypokalemia | 1 | 5.6 | 0 | 0 | |
| nausea | 1 | 5.6 | 0 | 0 | |
| vomiting | 1 | 5.6 | 0 | 0 | |
| infection | 2 | 11.1 | 0 | 0 | |
| DVT | 1 | 5.6 | 0 | 0 | |
| dysphagia | 1 | 5.6 | 0 | 0 | |
Patients were assessed on day 1 of every 21-day cycle for changes in performance status, and the presence of toxicities. Toxicities were graded according to CTCAE Version 3.0 (Common Toxicity Criteria for Adverse Events, National Cancer Institute).
Response and Survival
All patients received at least one cycle of GEM/DOX. However, only 17/18 patients were included in the analysis of objective response rate because one patient did not have week 6 evaluations, and was therefore not evaluable for response. The patient was replaced to complete the required 17 patients for the Simon 2-stage design evaluation. Of the remaining 17 patients, complete or partial responses were observed in 4 patients. This fails to meet the criteria for rejecting the null hypothesis (p=0.26). The adjusted estimated response rate is 0.26 with a 90% confidence interval (0.1, 0.52). In addition, 8 patients had stable disease (47%) for an average of 2.4 months (95% CI 0.97, 3.8), and 5 patients (29%) demonstrated disease progression at the time of first assessment. The median overall survival was 5.6 months (95% CI 3.8, 18.2) for all patients on trial, and the median PFS was 1.6 months (95% CI 1.4, 4.2) (Fig. 2). The overall survival at 1-year was 30% (Fig. 2).
Figure 2.
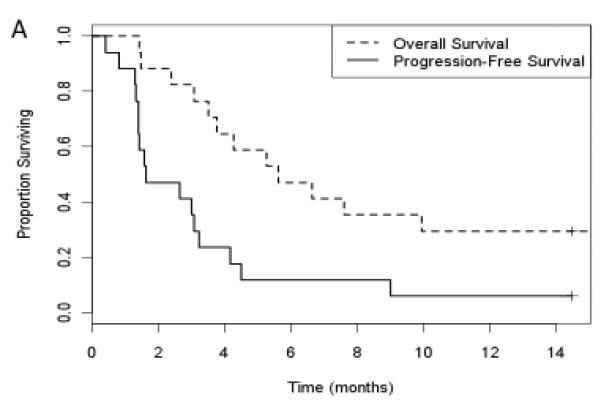
Kaplan-Meier curves for overall survival and progression free survival. Patient response to therapy was assessed by CT scans at every 2 cycles (6 weeks). Response and progression was evaluated using the new international criteria proposed by the RECIST 1.0. Measurable disease is defined as having at least tumor with a diameter ≥ 20 mm measured with conventional techniques (CT, MRI, X-ray) or as a tumor with a diameter ≥ 10 mm as measured with a spiral CT scan. Overall survival and progression free survival were analyzed using Kaplan-Meier curves.
Ceramide Analysis
To determine whether response to GEM/DOX therapy correlated with the elevation of C18-ceramide generation, an indication of ceramide reconstitution, peripheral blood was collected from all patients in the trial after every 2 cycles of treatment for measurement of sphingolipids by LC/MS. C12- to C26-ceramides, sphingosine, S1P, and C16-dihydro-ceramide were measured in serum samples using LC/MS, as described previously (21). A baseline blood sample was collected before the first treatment, and subsequent blood samples were collected prior to cycle 3, and thereafter prior to every 2 cycles, until the patient was taken off the trial. Serum ceramide and responses were then correlated. Baseline sphingolipid measurements and changes from baseline to cycle 3 in these patients are summarized in Fig 3. Baseline ceramides and S1P measurements did not vary by eventual response category (Fig. 3A). Importantly, sphingolipid profiles changed during the course of GEM/DOX treatment in some patients. Of significance, after 2 cycles of treatment there were significant differences in the mean percent increase in C18-ceramide in patients who were classified as CR, PR and SD when compared to PD patients (p = 0.05, Wilcoxon rank sum test) (Fig. 3B). Note that of the 6 patients who progressed, only 3 had ceramide measured at cycle 3. Comparisons in other markers did not yield statistical significance (total ceramide: p = 0.19; S1P: p=0.92; C16-ceramide: p=0.28). There were no significant correlations between the base line levels of ceramides (prior to cycle 1 treatment) and response to GEM/DOX. Ceramide and S1P levels from cycle 1 through 6 are shown in Figure 4 for patients who had CR/PR or SD compared to PD. In most patients with CR, PR or SD, C18-ceramide was elevated above baseline as early as after cycle 2, whereas in the 3 PD patients, C18-ceramide slightly decreased in two patients, or did not change in one patient over 2 cycles of GEM/DOX treatment (Fig. 4, lower left panel), suggesting that increases in C18-ceramide may precede favorable clinical outcomes.
Figure 3.
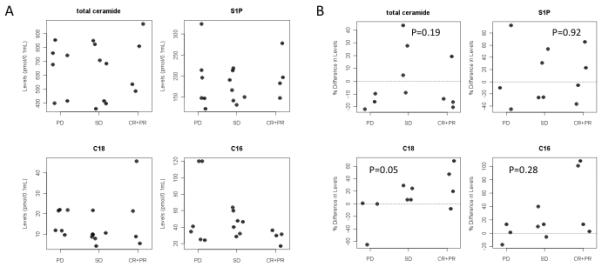
Analyses of serum sphingolipids in HNSCC patients during GEM/DOX treatment using LC/MS. (A) Baseline serum measurements of total ceramide, C18-ceramide, S1P, C16-ceramide, reported as pmol/100 mL of serum. (B) Changes in sphingolipids, presented as % change between baseline and cycle 3 of treatment (p-values were calculated for CR, PR, SD vs. PD).
Figure 4.
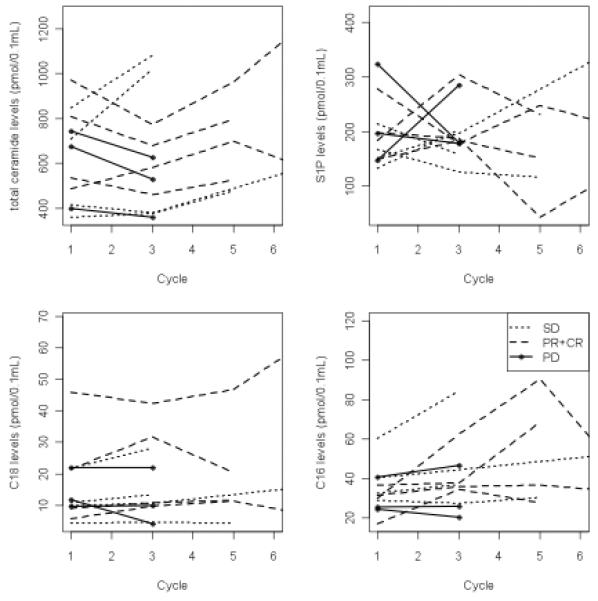
Changes in ceramide and S1P in patients with CR, PR or SD who have repeated measurements beyond cycle 3. The full spectrum of serum ceramide levels in patients with CR, PR and SD was measured at every 2 cycles of GEM/DOX treatment until the therapy was terminated using LC/MS/MS.
Discussion
In this report of our phase II clinical trial, we provide preliminary evidence that the combination of GEM/DOX has activity in some patients with recurrent or metastatic HNSCC. In our trial, 12 of the 17 treated patients either responded to treatment or had stable disease as a best response. Significantly, 1 of the 17 patients had a complete response, and 3 patients exhibited partial response, giving CR/PR rates of 26%. Importantly, our data also suggests that elevation of serum C18-ceramide, but not other ceramides, sphingosine or S1P, which was only observed in patients with CR/PR/SD compared to patients with PD, might provide a novel biomarker, which can be serially measured, to help estimate response during therapy.
Currently platinum-based chemotherapy +/− cetuximab is considered to be the standard of care for patients with unresectable recurrent or metastatic HNSCC despite the fact that most of them have been previously treated with a platinum-containing regimen (19,23). For the majority of patients with recurrence, these responses are not durable and disease progression is inevitable; most patients will eventually succumb to the disease. Hence, development of alternate non-platinum regimens like GEM/DOX, which has not been previously reported in the setting of refractory HNSCC, is critically necessary. Our studies showed that the GEM/DOX combination was reasonably well tolerated in most patients without major non-hematological toxicities. However, because of the observed hematological toxicities, we recommend primary prophylaxis with granulocyte colony stimulating factor (G-CSF). Both the toxicity profile and clinical benefit of this regimen could be better understood in a larger phase II/III trial. To this end, in this phase II trial, CR/PR rate of GEM/DOX treatment was 26%, which is higher than CR/PR rates of single GEM or DOX treatments with around 13% (4,5). Importantly, when compared to various other published combination studies in Phase II clinical trials for the treatment of recurrent or metastatic HNSCC (23-26), CR/PR rate obtained in response to GEM/DOX appears to be more efficacious. For example, recent trials using combinations of pemetrexed plus GEM, or bertozomib plus docetaxel reported response rates of 16% and 5%, respectively, in recurrent or metastatic HNSCC (24,25). Moreover, a recent review of cetuximab therapy reported that the response rate of 5 collective clinical trials in recurrent or metastatic HNSCC was 18.7% (23). In addition, our CR/PR rates of GEM/DOX (26%) was comparable to the response rate obtained using tri-weekly reduced-dose docetaxel and cisplatin for the treatment of HNSCC with 24.4% (26). Thus, our data revealed that GEM/DOX treatment showed limited but important efficacy at least in some recurrent or metastatic HNSCC patients, for whom there are no successful treatment options to date. In fact, in this trial, the 1-year survival was 30%, which is encouraging given that the reported 1-year survival for this patient population in average is about 20% (19,23-26).
Ceramides are a family of bioactive sphingolipids with tumor suppressor functions (13, 27-29). The unique and novel finding of our study is that changes in serum C18-ceramide correlated with response to GEM/DOX treatment, demonstrating that increasing serum C18- ceramide may serve as an estimate of therapeutic response, which has important clinical implications. It has been well established that one of the mechanisms by which treatment with chemotherapeutic agents induce cell death is via induction of ceramide or inhibition of sphingosine kinase in various cancers (30-32). Moreover, previous studies have shown that GEM/DOX inhibits HNSCC tumor growth in SCID mice via induction of C18-ceramide generation (18). The results of this phase II clinical trial reported here corroborate the finding of the previously reported preclinical studies. To our knowledge, ours is the first trial to suggest that serum C18-ceramide could be a potential serum biomarker of therapeutic response in HNSCC (or any other) cancer. To this end, a limitation of this study is the fact that our protocol did not require serum sample collection at the time of progression. This made it difficult to analyze the exact change of lipid profile at progression. The next step is to analyze the timing and degree of C18-ceramide changes in relation to treatment effects. Our future investigations will include a study with HNSCC patients treated with surgery, definitive chemo-radiation, and palliative/salvage chemotherapy to address the abovementioned relationships.
Currently, there are few established biomarkers in use for HNSCC. EGFR and HPV are the main two in use, and confirming their tissue expression is now a part of standard pathology for HNSCC at various institutions (33,34). However, obtaining serial biopsies for biomarker assessment is not practical in the clinical setting. Serum LDH is used in the clinic to determine the metastatic spread of melanoma and non-Hodgkin’s lymphoma; however, it tends to be nonspecific (35). Some potential diagnostic serum markers for HNSCC have been identified, such as IL-6, to monitor the development of secondary primary cancer (36), MMP-13 to diagnose lymphatic metastasis in HNSCC (37), and hypoxia-related factors to predict recurrence in HPV-negative HNSCC patients (38). While advances in serum markers may contribute to better HNSCC diagnosis, our data suggest that elevation of serum C18-ceramide might be a novel biomarker for monitoring response to therapy.
It should be noted also that reconstitution of ceramide generation and/or elevation has been implicated in radiosensitization of fibrosarcoma tumor xenografts (39), and treatment with nanoliposomal ceramide enhanced effectiveness of sorafenib causing synergistic inhibition of growth in situ and in vivo (40). Thus, these studies are also agreement with our data, which implicated GEM/DOX-mediated ceramide generation in the control of tumor progression in some HNSCC patients. More importantly, assessment of an RNA interference screen revealed mitotic and ceramide pathways as potential markers of pathological complete response in primary triple-negative breast cancers in a retrospective analysis of five clinical trials (41), supporting our data regarding the identification of serum C18-ceramide elevation as a potential biomarker for response to GEM/DOX therapy in HNSCC patients.
In summary, our data suggest that the combination of GEM/DOX might add to the therapeutic armamentarium against HNSCC. More importantly, this study suggests for the first time that serum C18-ceramide presents a novel serum biomarker for monitoring treatment response, which could be conveniently and serially measured during therapy. However, these findings need to be confirmed in studies with larger patient cohorts, in which measurement of serum ceramides would be performed earlier and more often to determine the relationship between C18-ceramide changes and timing of response or progression during treatment.
Statement of Translational Relevance.
C18-ceramide is a bioactive sphingolipid with tumor suppressive functions. Here we report the results of an exploratory phase II clinical trial designed to test the hypothesis that treatment with a gemcitabine (GEM)/doxorubicin (DOX) combination will be efficacious via reconstitution of C18-ceramide signaling in head and neck squamous cell carcinoma (HNSCC) patients for whom first-line platinum-based therapy failed. Seventeen evaluable patients were treated. The most common toxicity was neutropenia (grade 3 or 4). No major non-hematological toxicities were observed. Responses were seen in four patients, complete (CR) = 1 and partial (PR) =3, and, eight patients had stable disease (SD). In lipidomics analyses, C18-ceramide elevation patterns were significantly different in patients who exhibited CR, PR, or SD compared to patients who progressed (PD). These novel data suggest that C18-ceramide might be a novel serum marker to estimate response to GEM/DOX, which was efficacious in some refractory HNSCC patients.
Acknowledgements
We thank the members of the Ogretmen Laboratory for helpful discussions. Additionally, we are grateful to the MUSC Lipidomics Core for the sphingolipid analysis. Lastly, we thank the Hollings Cancer Center and the patients who participated on this trial. This work was supported by research grants from the US National Institutes of Health (DE016572, CA088932, and CA097132) to B.O. S.A.S was supported by an F30 grant from the National Institute of Environmental Health Sciences (ES019464).
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011. CA Cancer J Clin. 2010 doi: 10.3322/caac.20121. in press. [DOI] [PubMed] [Google Scholar]
- 2.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 3.Fung C, Grandis JR. Emerging drugs to treat squamous cell carcinomas of the head and neck. Expert Opin Emerg Drugs. 2010;15:355–73. doi: 10.1517/14728214.2010.497754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catimel G, Vermorken JB, Clavel M, de Mulder P, Judson I, Sessa C, et al. EORTC Early Clinical Trials Group A phase II study of Gemcitabine (LY 188011) in patients with advanced squamous cell carcinoma of the head and neck. Ann Oncol. 1994;5:543–7. doi: 10.1093/oxfordjournals.annonc.a058910. [DOI] [PubMed] [Google Scholar]
- 5.Cobleigh MA, Hill JH, Gallagher PA, Kukla LJ, Lad TE, Shevrin DH, Applebaum EL, McGuire WP. A phase II study of Adriamycin in previously untreated squamous cell carcinoma of the head and neck. Cancer. 1985;56:2573–5. doi: 10.1002/1097-0142(19851201)56:11<2573::aid-cncr2820561106>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 6.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–16. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 7.Merrill AH, Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods. 2005;36:207–24. doi: 10.1016/j.ymeth.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Sullards MC, Allegood JC, Kelly S, Wang E, Haynes CA, Park H, et al. Structure-specific, quantitative methods for analysis of sphingolipids by liquid chromatography-tandem mass spectrometry: “inside-out” sphingolipidomics. Methods Enzymol. 2007;432:83–115. doi: 10.1016/S0076-6879(07)32004-1. [DOI] [PubMed] [Google Scholar]
- 9.Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Andreyev AY, Fahy E, Guan Z, Kelly S, Li X, McDonald JG, et al. Subcellular organelle lipidomics in TLR-4-activated macrophages. J Lipid Res. 2010;51:2785–97. doi: 10.1194/jlr.M008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponnusamy S, Meyers-Needham M, Senkal CE, Saddoughi SA, Sentelle D, Selvam SP, et al. Sphingolipids and cancer: ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol. 2010;6:1603–24. doi: 10.2217/fon.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stiban J, Tidhar R, Futerman AH. Ceramide synthases: roles in cell physiology and signaling. Adv Exp Med Biol. 2010;688:60–71. doi: 10.1007/978-1-4419-6741-1_4. [DOI] [PubMed] [Google Scholar]
- 13.Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, Ogretmen B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010;24:296–308. doi: 10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koybasi S, Senkal CE, Sundararaj K, Spassieva S, Bielawski J, Osta W, et al. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J Biol Chem. 2004;279:44311–9. doi: 10.1074/jbc.M406920200. [DOI] [PubMed] [Google Scholar]
- 15.Karahatay S, Thomas K, Koybasi S, Senkal CE, Elojeimy S, Liu X, et al. Clinical relevance of ceramide metabolism in the pathogenesis of human head and neck squamous cell carcinoma (HNSCC): attenuation of C(18)-ceramide in HNSCC tumors correlates with lymphovascular invasion and nodal metastasis. Cancer Lett. 2007;256:101–11. doi: 10.1016/j.canlet.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–80. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 17.Rossi MJ, Sundararaj K, Koybasi S, Phillips MS, Szulc ZM, Bielawska A, et al. Inhibition of growth and telomerase activity by novel cationic ceramide analogs with high solubility in human head and neck squamous cell carcinoma cells. Otolaryngol Head Neck Surg. 2005;132:55–62. doi: 10.1016/j.otohns.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Senkal CE, Ponnusamy S, Rossi MJ, Bialewski J, Sinha D, Jiang JC, et al. Role of human longevity assurance gene 1 and C18-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Mol Cancer Ther. 2007;6:712–22. doi: 10.1158/1535-7163.MCT-06-0558. [DOI] [PubMed] [Google Scholar]
- 19.Gibson MK, Li Y, Murphy B, Hussain MH, DeConti RC, Ensley J, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23:3562–67. doi: 10.1200/JCO.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, et al. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J Lipid Res. 2010;51:3074–87. doi: 10.1194/jlr.D008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koyama T, Chen H. Proper inference from Simon’s two-stage designs. Stat Med. 2008;27:3145–54. doi: 10.1002/sim.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves TD, Hill EG, Armeson KE, Gillespie MB. Cetuximab Therapy for Head and Neck Squamous Cell Carcinoma: A Systematic Review of the Data. Otolaryngol Head Neck Surg. 2011 doi: 10.1177/0194599811399559. in press. [DOI] [PubMed] [Google Scholar]
- 24.Fury MG, Haque S, Stambuk H, Shen R, Carlson D, Pfister D. A phase 2 study of pemetrexed plus gemcitabine every 2 weeks for patients with recurrent or metastatic head and neck squamous cell cancer. Cancer. 2011;117:795–801. doi: 10.1002/cncr.25464. [DOI] [PubMed] [Google Scholar]
- 25.Chung CH, Aulino J, Muldowney NJ, Hatakeyama H, Baumann J, Burkey B, et al. Nuclear factor-kappa B pathway and response in a phase II trial of bortezomib and docetaxel in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2010;21:864–70. doi: 10.1093/annonc/mdp390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang PM, Tzeng CH, Chen MH, Tsao CJ, Su WC, Hwang WS, et al. Triweekly reduced-dose docetaxel combined with cisplatin in recurrent/metastatic head and neck squamous cell carcinoma: a multicenter phase II study. Cancer Chemother Pharmacol. 2011 doi: 10.1007/s00280-011-1645-5. in press. [DOI] [PubMed] [Google Scholar]
- 27.Deng X, Yin X, Allan R, Lu DD, Maurer CW, Haimovitz-Friedman A, et al. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science. 2008;322:110–5. doi: 10.1126/science.1158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menuz V, Howell KS, Gentina S, Epstein S, Riezman I, Fornallaz-Mulhauser M, et al. Protection of C. elegans from anoxia by HYL-2 ceramide synthase. Science. 2009;324:381–4. doi: 10.1126/science.1168532. [DOI] [PubMed] [Google Scholar]
- 29.Mesicek J, Lee H, Feldman T, Jiang X, Skobeleva A, Berdyshev EV, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 2010;22:1300–7. doi: 10.1016/j.cellsig.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park MA, Mitchell C, Zhang G, Yacoub A, Allegood J, Häussinger D, et al. Vorinostat and sorafenib increase CD95 activation in gastrointestinal tumor cells through a Ca(2+)-de novo ceramide-PP2A-reactive oxygen species-dependent signaling pathway. Cancer Res. 2010;70:6313–24. doi: 10.1158/0008-5472.CAN-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Ryland L, Yang J, Liao A, Aliaga C, Watts R, et al. Targeting of survivin by nanoliposomal ceramide induces complete remission in a rat model of NK-LGL leukemia. Blood. 2010;116:4192–201. doi: 10.1182/blood-2010-02-271080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickson MA, Carvajal RD, Merrill AH, Gonen M, Cane LM, Schwartz GK. A Phase I Clinical Trial of Safingol in Combination with Cisplatin in Advanced Solid Tumors. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2323. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharafinski ME, Ferris RL, Ferrone S, Grandis JR. Epidermal growth factor receptor targeted therapy of squamous cell carcinoma of the head and neck. Head Neck. 2010;32:1412–21. doi: 10.1002/hed.21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mydlarz WK, Hennessey PT, Califano JA. Advances and Perspectives in the Molecular Diagnosis of Head and Neck Cancer. Expert Opin Med Diagn. 2010;4:53–65. doi: 10.1517/17530050903338068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egberts F, Hitschler WN, Weichenthal M, Hauschild A. Prospective monitoring of adjuvant treatment in high-risk melanoma patients: lactate dehydrogenase and protein S-100B as indicators of relapse. Melanoma Res. 2009;19:31–5. doi: 10.1097/CMR.0b013e32831993cc. [DOI] [PubMed] [Google Scholar]
- 36.Duffy SA, Taylor JM, Terrell JE, Islam M, Li Y, Fowler KE, et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750–7. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 37.Marcos AC, Martinez DAK, Toyos JR, Iglesias FD, Hermsen M, Guervos MA, et al. The usefulness of new serum tumor markers in head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg. 2009;140:375–390. doi: 10.1016/j.otohns.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 38.Byers LA, Holsinger FC, Kies MS, William WN, El-Naggar AK, Lee JJ, et al. Serum signature of hypoxia-regulated factors is associated with progression after induction therapy in head and neck squamous cell cancer. Mol Cancer Ther. 2010;9:1755–63. doi: 10.1158/1535-7163.MCT-09-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truman JP, García-Barros M, Kaag M, Hambardzumyan D, Stancevic B, Chan M, et al. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS One. 2010;5:e12310. doi: 10.1371/journal.pone.0012310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran MA, Smith CD, Kester M, Robertson GP. Combining nanoliposomal ceramide with sorafenib synergistically inhibits melanoma and breast cancer cell survival to decrease tumor development. Clin Cancer Res. 2008;14:3571–81. doi: 10.1158/1078-0432.CCR-07-4881. [DOI] [PubMed] [Google Scholar]
- 41.Juul N, Szallasi Z, Eklund AC, Li Q, Burrell RA, Gerlinger M, et al. Assessment of an RNA interference screen-derived mitotic and ceramide pathway metagene as a predictor of response to neoadjuvant paclitaxel for primary triple-negative breast cancer: a retrospective analysis of five clinical trials. Lancet Oncol. 2010;11:358–65. doi: 10.1016/S1470-2045(10)70018-8. [DOI] [PubMed] [Google Scholar]


