In the United States 13% to 9% of children (ages 0–17 years) have special healthcare needs (1). Unfortunately, 50–55% of children and their families do not follow treatment plans as prescribed (2). Such high rates of nonadherence (i.e., extent to which a person’s behavior does not correspond with agreed upon recommendations from a healthcare provider (3)) have significant negative consequences, including: greater risk of relapse, increased morbidity and mortality, unnecessary changes to the regimen, development of drug resistance, decreased cost-effectiveness of medical care, and inaccurate clinical trial results (2, 4–7). Thus, documentation of nonadherence rates, identification of barriers to or strategies to improve adherence, and development and integration of adherence interventions are imperative.
Regrettably, despite increased recognition that adherence is a significant issue across pediatric disease groups, there remains a lack of consensus regarding the “best” adherence measure. Patient, parent, and provider-report, pharmacy refill data, pill counts, serum assays, and electronic monitors have all been used to monitor medication adherence with varying success. Of note, even though there remains no clear “gold standard” measure of medication adherence (8), the use of electronic monitoring has increased and is often employed as the standard to which other measures of adherence are compared (e.g., (9–11)). Given increased recognition of the role of adherence in health outcomes, the use of electronic monitors to assess adherence, and the increasing use of electronic measures as key components in interventions to improve adherence, it is critical that objective evaluation of their utility and evidence of their functional capabilities in real world settings be made available. In order to aid decision making related to using electronic adherence monitors in future studies or incorporating such measures into clinical practice in order to improve adherence rates, this systematic review will 1) provide a brief description of currently available electronic measures of medication adherence, 2) discuss the strengths and weaknesses of these measures (and validation information when available), and 3) provide examples of their use and relevant empirical data for a subsample of measures from our own research.
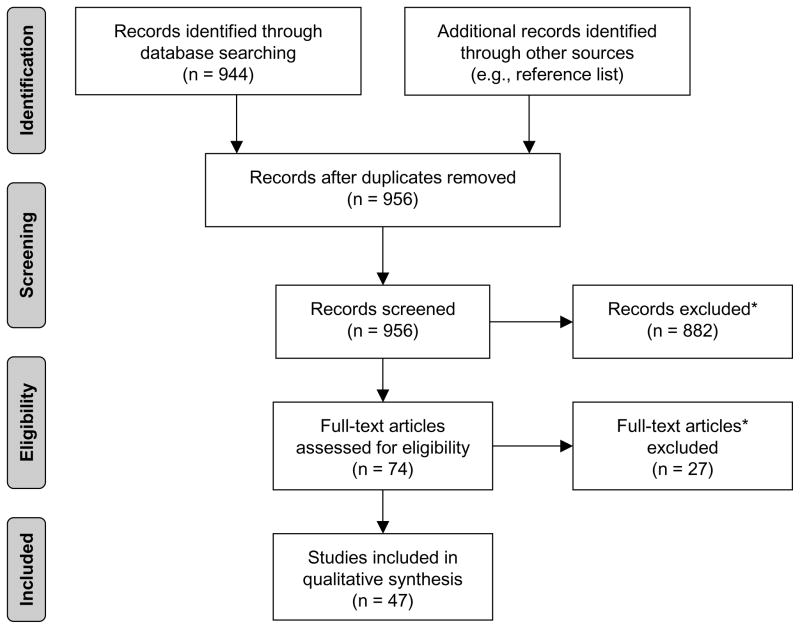
A systematic review was completed using PubMed (12) to identify previous studies that used electronic measures of medication adherence in pediatric populations through February 2010. Search terms included: (adherence OR compliance) AND (electronic, technology, MEMS, Doser, MDIlog, Smartinhaler, Medsignals, Pillphone, Nebulizer, Chronolog, Drug Exposure Monitor). Initial device names were included from the authors’ experiences; additional names were added as identified through other search terms. References of identified articles were examined to identify additional published studies. Final inclusion/exclusion criteria and article selection is further illustrated in the Figure. A summary of monitor use by disease group is provided in the Table.
Figure.
Study selection flow diagram (60). Reasons for exclusion: (1) not written in English; (2) primary or original study data not included; (3) electronic monitor of oral medication adherence not included (not prescribed treatment regiment [eg, blood glucose monitoring, peak flow monitoring]), and (4) sample did not include participants under 18 years of age or included participants above age 25 years.
Table 1.
Electronic monitor by illness group and websites for additional product and pricing information
| Illness group used | Website | |
|---|---|---|
| Oral Medication Monitors | ||
| • Medication Event Monitoring System (MEMS)/Drug Exposure Monitor (eDEM) |
Arthritis (40, 41) Asthma (13) Cystic fibrosis (21, 42) Dermatitis (43, 44) Epilepsy (17) Heart disease (45, 46) Hepatitis (47) HIV (11, 15, 19, 48) Leukemia (20) Liver transplant (49, 50) Renal transplant (23, 24, 50, 51) Sickle cell disease (22) Thalassemia (18) Tuberculosis (16) |
www.aardexgroup.com |
| • MedSignals | Inflammatory bowel disease | www.medsignals.com |
| Inhaled Medication Monitors | ||
| • DOSER | Asthma (9, 13, 25–27, 52–54) Eosinophil-associated gastrointestinal disorders |
www.doser.com |
| • MDI Chronolog a | Asthma (55) | - |
| • MDILog | Asthma (13, 28, 29, 52, 53, 56, 57) | www.westmedinc.com b |
| • Smartinhaler. | Asthma (10, 31) | www.smartinhaler.com |
| Nebulized Medication Monitors | ||
| • I-neb Adaptive Aerosol Delivery (AAD)/HaloLite Nebulizer b |
Asthma (36, 37, 58) Cystic fibrosis (35, 56)(57) |
www.ineb.respironics.com c |
| • Nebulizer Chronolog a | Asthma (32–34, 59) | – |
No website available.
As of July 2010, currently being redesigned and not available for purchase from website.
Information for redesigned model; I-neb Adaptive Aerosol Delivery (AAD) and Patient Logging System (PLS) is a newer model of the HaloLite Nebulizer.
Oral Medication Monitors
Comprising a standard plastic vial and cap with a micro-electric circuit, Medication Event Monitoring System (MEMS) records the date, time, and frequency of vial openings. The device is available in a TrackCap or SmartCap (LCD display of openings and time frame since last opening) model. The Drug Exposure Monitor (eDEM) (13) is a similar, but older, device. Data are transferred to a Windows-based computer (AARDEX Ltd. Union City, CA).
Strengths
MEMS is the most prevalent electronic monitor for oral medication adherence. By recording date, time, and frequency of pill bottle openings, MEMS provides a long-term measure of patient adherence in real-time. The device is strongly correlated with serum assays and pharmacy refill data (11, 14), with up to 100% specificity between MEMS and pharmacy refill data (11) reported. Researchers have also described significant correlations between higher adherence rates and lower viral loads in HIV (15). Data output from MEMS can reveal detailed medication-taking patterns, such as under-dosing, over-dosing, delayed-dosing, drug holidays (16), and/or “white coat compliance” (i.e., patient taking medication more frequently directly before clinic appointments) (17).
Weaknesses
As with all electronic monitors, MEMS does not confirm the number of medications ingested (16–20) (e.g., patient does not remove medication or removes medication from bottle but does not ingest). Patients may not consume removed doses or open the vial once to take out several doses. Second, several studies suggested MEMS may not be feasible for routine clinical use or is only feasible in research-driven clinical settings due to its expense (19, 21). Third, malfunction rates ranged from 5–20% (19, 22, 23), resulting in loss of data. One study reported 59% of patients with unexplained bottle openings (e.g., illogical times, families reported that medications were not taken) that were subsequently deleted from data (15). Due to the lack of water resistant caps and vials, studies using liquid medications reported malfunctions and loss of data (19, 22, 23), limiting use in young patients who cannot ingest pills (24). Other complaints included poor device durability, misplacement of caps, difficulty in covert use, not conducive to travel, and interference with family’s existing pill organization (8, 11, 14, 15, 21, 22, 24).
Example
As part of an ongoing longitudinal study examining oral medication adherence in pediatric new onset epilepsy, children (2–12 years) received a MEMS Trackcap and bottle to monitor adherence to antiepileptic drug (AED) therapy over two years. To date, of the 53 children who completed the two year study, 13% had unexplained bottle openings, 2% had incorrect time recordings, 6% lost caps and 6% had broken caps. Data download difficulties were experienced by 13% of patients and 26% of families forgot to bring their bottle to at least one scheduled visit. Overall, 15% of caps needed to be replaced during the course of the study; however, AARDEX was very willing to work with investigators to solve such issues. Despite some difficulties with the software program used to download MEMS data (i.e., PowerView) (e.g., incorrect phase dates, incorrect medication saved) the program provides several ways to examine data (e.g., calendar view, non-monitored periods, drug holidays). Customer support is extremely responsive to device-related difficulties and technical support requests.
MedSignals® is an electronic pill box consisting of four plastic programmable bins and a LCD screen. The device communicates through analog telephone lines and uploads daily bin openings to an internet server. A secured website enables remote set-up and management of the device. Each bin can be programmed to display visual and/or verbal reminders to take a medication and related details (e.g., take with food).
Strengths
MedSignals® tracks up to four medications and provides reminders to take medications at correct times (e.g., voice activation, blinking lights, text, beeping). Each bin is programmable through the website and can be updated as the patient’s medications change. The box is portable and holds a charge for several days. When placed back on the cradle to recharge, the device automatically uploads data to the server and website. Due to daily automatic uploads, the website provides real-time feedback on patient medication adherence and lessens family burden by eliminating trips to the research/clinical site for downloads. Data output provides graphs, charts, adherence statistics, and date/time-stamped bin openings.
Weaknesses
High device expense and additional monthly upload fees may limit MedSignals® use in routine clinical settings (see website for current pricing). Similar to MEMS, the device does not confirm number of medications ingested and lacks the ability to detect omitted doses or openings where several pills are extracted. Bin size is small and might not hold certain medications in sufficient quantity.
Example
In a randomized controlled trial to improve adherence in pediatric inflammatory bowel disease (IBD), 24 youth (11–17 years) received MedSignals® to monitor oral medication adherence. One family chose not to use the device and 53% of families reported device-related errors (e.g., spontaneous time reset, data upload failure, unregistered or phantom bin openings, incompatibility with digital phone line). Additional reported difficulties included: difficulty fitting medication in bin, inability to record daily number of pills prescribed (device limited one medication to 9 pills), spontaneous return to default settings, and need for manual upload of data. Technical support was responsive to device-related difficulties, usually responding to requests within 48 hours. Of note, device-related errors substantially decreased during the study as requests to customer support were addressed (53% versus 18%).
Inhaled Medication Monitors
The DOSER™ (MEDITRACK Products Easton, MA) and DOSER Clinical Trials (CT) consist of a circular LCD screen that attaches to the top of a metered dose inhaler (MDI) and tracks medication exhausted from the MDI. The device displays the number of daily inhalations and the number remaining in the canister, storing 30 days of data. When firmly pressed, the device beeps to signal an occurrence and the screen documents inhalation. No feedback is provided in masked or blinded studies.
Strengths
The DOSER™ enables discrimination of inhaler use patterns across time (25). Three studies found a high correlation between canister weight and raw DOSER™ data (9, 26, 27). For example, Bender et al reported DOSER™ adherence was 50% compared with 69% via canister weight (9). Similarly, Jentzsch et al reported 51.5% DOSER™ adherence compared with 46.3% canister weight adherence (26). Other researchers reported accuracy as high as 94.3%, with reliability data ranging from 52–58% (8, 9). The DOSER™ is sensitive to treatment effects. For example, Bartlett et al described an increase in medication use from 29% to 54% following a five-week intervention (25).
Weaknesses
Recurrent mechanical failure limits DOSER™ data collection. From 8–21% failure rates were reported across studies (e.g., blank/unreadable display, battery failure, error messages, display recording only a few days of use) (8, 9, 27). Young children have difficulty pressing the device with enough force to register a puff (27) and, because the device must have one second between puffs, the device cannot register double puffing (i.e., patient quickly takes multiple puffs directly after another). Lastly, the DOSER™ does not record actual inhalation of medication and data cannot be directly downloaded to a computer software program (8).
Example
A subsample of 14 children (4–17 years) from a larger study examining the psychosocial functioning of children with eosinophil-associated gastrointestinal disorders (EGID) and their families were given standard DOSER™ devices to monitor three-month adherence to an inhaled corticosteroid. During the study, one family decided not to use the device, and 46% reported errors (e.g., device stopped recording, failed to register or recorded extra puffs, recorded over data after 30 days, plastic ring on bottom of device prevented full dose of medication from being dispensed). In addition, the device could not be sterilized and reused. Device training (observed practice of installation and use of device with placebo inhaler) at the time of consent was instituted with recruitment of the sixth participant and substantially decreased reported errors (60% versus 38%). One family, noting the DOSER™ facilitated medication monitoring, asked for information for continued personal use following participation.
MDILog is a device that contains a computer chip and attaches to the top of a MDI. The patient presses down on the top of the device during each inhalation, and the date and time of each MDI actuation is recorded and displayed on an LCD screen (Westmed Technologies Inc, Englewood, Colorado).
Strengths
The MDILog is able to detect medication dumping (i.e., the rapid expelling of multiple actuations in approximately one minute) (28, 29). The device also performs a self-check and battery test nightly to ensure data integrity (28). Weinstein et al also noted the favorable reliability data evident for the most recent version of the MDILog (30).
Weaknesses
Like most electronic monitoring devices, mechanical errors (e.g., faulty calibration, battery failure, device loss) are the most prevalent weaknesses. McQuaid et al reported that data from approximately 9% of devices were excluded due to systematic errors (28), and Walders et al reported that 9% of devices produced unrecoverable data due to device loss, failure, or damage (29). Although MDILog can detect when medication dumping occurs, like other monitors it cannot confirm actual inhalation (29, 30) or prevent medication dumping (13, 29). Of note, the MDILog II is now able to document improper inhalation. Lastly, the device cannot be sterilized and reused with multiple patients (30) and cannot be used with medication samples.
The Smartinhaler replaces the plastic case of a MDI and records the date and time the medication is actuated (10, 31). Data is downloaded to a computer (Nexus 6, Auckland, New Zealand).
Strengths
The Smartinhaler can alert researchers/clinicians to dumping patterns in patient adherence. For example, Burgess et al discovered one patient actuated three doses in one month followed by a month where 213 doses were actuated within minutes (31).
Weaknesses
Burgess et al reported that contact with water led to unrecoverable data for two devices used by the same patient (31). Similar to other monitors, the inability to confirm medication ingestion and whether effective techniques are used (10, 31) are also significant limitations.
Nebulized Medication Monitors
The Nebulizer Chronolog replaces the standard plastic mouthpiece and records the date and time of each actuation (32–34). Data can be downloaded to a computer (Forefront Technologies Inc, Lakewood, Colorado).
Strengths
Similar to other electronic monitors, the Nebulizer Chronolog can detect medication dumping (32, 33), patterns of missed doses over time (34), and can be used with a variety of inhaled medications (32). Coutts et al found that one patient was dumping medication directly before clinic visits, as many as 77 times in 13 minutes (32).
Weaknesses
Like its counterparts, the Nebulizer Chronolog does not confirm medication inhalation (32, 33). Gibson et al reported that patients had difficulty fitting the device onto a MDI, problems where an actuation was recorded but the patient did not take medication, and that the electronic switch on some devices stuck during actuations (34).
The I-neb Adaptive Aerosol Delivery (AAD) is the newer version of the Halolite Nebulizer, an aerosol delivery system that releases medication when inhalation is detected. The device records the date, time, and duration of each actuation and can be used with multiple forms of nebulized medications (35, 36).
Strengths
The device only releases medication upon detection of inhalation and can be used with multiple nebulized medication (35). Patients can also take breaks or pauses during treatment (36).
Weaknesses
Butz et al reported 17 monitors (8%) failed due to technical malfunctions and the device does not account for a patient’s potentially inaccurate flow rate or technique (37).
DISCUSSION
This review focused exclusively on electronic monitors of medication adherence in use across pediatric populations and updates the review provided by Quittner et al (8). As such, it provides an important resource regarding strengths and weaknesses of particular electronic monitors in several pediatric disease groups. These data are especially relevant for individuals considering incorporation of these devices into research protocols or clinical practice. There are several advantages inherent in electronic monitors, which is likely responsible for their increased use and frequent reference as the “gold standard” to which other measures of adherence (e.g., pill count, self-report) are compared (e.g., (9–11)). Clinicians can use electronic monitors with their patients to address a number of clinical issues and initiatives. These may include, among others, 1) dosing regimen complexity (e.g., multiple doses per day may be too difficult for some patients), 2) dose timing, which can affect drug concentration in blood, 3) white coat compliance or other problematic patterns of medication taking, and 4) ongoing objective monitoring of adherence behavior, which is superior to current practice habits which rely on self-report, clinician ratings, or perhaps no assessment. In conjunction with continuing advancements in software development, electronic monitors enable insight into medication taking patterns (e.g., white coat compliance, drug holidays), help identify barriers to medication adherence, and are sensitive to treatment effects (e.g., (25)). These features allow for testing of various hypotheses regarding adherence patterns, which is critical to ultimately designing and optimizing interventions to promote treatment adherence.
However, electronic monitors are not without their limitations. Difficulties related to mechanical malfunctions are evident across all electronic monitors. In addition, the high cost and training necessary to effectively use current monitors cannot be understated and restricts their use in primary or specialized clinical care. However, as additional data regarding the relationship between monitoring, adherence, and health outcomes emerges, health management organizations and other insurance companies may be more likely to assume their cost. Similar to other adherence measures (e.g., serum assays, self-report), inability to confirm the number of medications ingested restricts their reliability and validity. Use of monitors that include feedback (e.g., Trackcap) and introduction of novel devices (e.g., unfamiliar pill container) into the family routine also limit their function as naturalistic measures. Consequently, this review supports previous recommendations for inclusion of other methods of adherence measurement (e.g., serum assay, pill count, self-report) to complement electronic data (8). Although review of these other methods goes beyond the scope of the current review, previous research suggests that the use of these measures also incorporates particular strengths/weaknesses.
Regarding specific recommendations in selecting monitors for future research and/or clinical use, several conclusions can be drawn. Despite the tremendous potential offered by newer devices, available studies strongly support the reliability and validity of MEMS as the electronic device of choice in monitoring oral medication adherence. Future, additional data for other oral medication monitors will better allow investigators/clinicians to make informed decisions regarding their use. Concerning inhaled medication monitors, the MDILog and Doser have been widely used in pediatric asthma. Given their similar strengths and weaknesses, individuals are encouraged to examine the needs of their study and/or clinical practice and weigh the advantages/disadvantages of each monitor in best meeting their requirements. Finally, that the I-neb AAD system has been redesigned to address some of the weaknesses in its earlier model should be encouraging in measuring nebulized medication adherence.
Although this review summarizes available data regarding electronic adherence measures and their use in pediatric disease groups, these data are meant to suggest some issues that may be encountered when using these devices, not to dissuade researchers or clinicians from any particular device. Of note, many of the studies included in this review relied on small samples, and/or were pilot studies. Studies varied widely in regard to the information provided concerning device function. For example, even though validation data may be available in adult studies, data was not always provided for the pediatric population. Standardization of such data provided in future studies will help further advance research in this area. In addition, some of the measures are not currently available or have been updated to address earlier technological issues. However, given the small number of articles, they were still included to illustrate possible difficulties that may arise. It is anticipated that the information provided in this review will aid individuals in selecting appropriate electronic adherence monitors for research and/or clinical purposes and effectively address potential weaknesses of measures through careful planning and monitoring.
Despite these limitations, this review provides several key directions for future research and development in this area. First, improvements in technology will increase electronic adherence measures’ reliability and validity and may help to decrease their cost. Second, use of a cellular device signal will enable remote downloading and relieve the burden associated with home visits or downloading at clinic appointments. Several companies are developing and testing these technologies now. Third, research and clinic friendly servers that enable direct downloading and interpretation of data will enhance their usefulness for analysis and efficient incorporation into routine clinical visits. For example, clinicians can use electronic monitoring data to identify and problem-solve with families around possible adherence-related difficulties at the time of clinic visits. Fourth, patient-friendly devices (e.g., pill boxes that hold a full week of medication doses, portable devices) will help to foster their acceptability to families. Finally, investigation of how best to combine measures of adherence is imperative across chronic conditions. For example, researchers have used correction factors or composite scores calculated from electronic adherence measures to address inflation in self-report and pill count data (e.g., (38, 39)). With advances in medicine, the continued awareness of adherence as a significant health care issue in pediatric populations, and demonstrated relationships between adherence and health outcomes (e.g., (15)), the use of electronic monitors may become a permanent fixture in adherence-related research and clinical practice.
Acknowledgments
Supported in part by NIH K23 DK079037 (to K.H.) and NIH K23 HD057333 (to A.M.).
Abbreviations
- MEMS
Medication Event Monitoring System
- eDEM
Drug Exposure Monitor
- AAD
Adaptive Aerosol Delivery
Footnotes
Lisa M. Ingerski, Elizabeth A. Hente, Avani C. Modi, and Kevin A. Hommel report no disclosures relevant to this study.
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lisa M. Ingerski, Email: lisa.ingerski@cchmc.org, Cincinnati Children’s Hospital Medical Center, Division of Behavioral Medicine and Clinical Psychology
Elizabeth A. Hente, Email: Elizabeth.hente@cchmc.org, Cincinnati Children’s Hospital Medical Center, Division of Behavioral Medicine and Clinical Psychology
Avani C. Modi, Email: avani.modi@cchmc.org, Cincinnati Children’s Hospital Medical Center, Division of Behavioral Medicine and Clinical Psychology.
Kevin A. Hommel, Cincinnati Children’s Hospital Medical Center, Division of Behavioral Medicine and Clinical Psychology
References
- 1.Bethell C, Read D, Blumberg S, Newacheck P. What is the prevalence of children with special health care needs? Toward an understanding of variations in findings and methods across three national surveys. Matern Child Health J. 2008;12:1–14. doi: 10.1007/s10995-007-0220-5. [DOI] [PubMed] [Google Scholar]
- 2.Rapoff MA. Adherence to Pediatric Medical Regimens. 2. New York: Springer; 2010. [Google Scholar]
- 3.Sabaté E. Adherence to long-term therapies: evidence for action. World Health Organization; 2003. [PubMed] [Google Scholar]
- 4.Kennard BD, Stewart SM, Olvera R, Bawdon RE, OhAilin A, Lewis CP, et al. Nonadherence in adolescent oncology patients: Preliminary data on psychological risk factors and relationships to outcome. J Clin Psychol Med Settings. 2004;11:31–9. [Google Scholar]
- 5.Cloutier MM, Wakefield DB, Sangeloty-Higgins P, Delaronde S, Hall CB. Asthma guideline use by pediatricians in private practices and asthma morbidity. Pediatrics. 2006 Nov;118:1880–7. doi: 10.1542/peds.2006-1019. [DOI] [PubMed] [Google Scholar]
- 6.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: A meta-analysis. Med Care. 2002 Sep;40:794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Dunbar-Jacob J, Schlenk E. Handbook of Health Psychology. Mahwah: Erlbaum; 2001. Patient adherence to treatment regimens; pp. 571–80. [Google Scholar]
- 8.Quittner AL, Modi AC, Lemanek KL, Ievers-Landis CE, Rapoff MA. Evidence-based assessment of adherence to medical treatments in pediatric psychology. J Pediatr Psychol. 2008 Oct;33:916–36. doi: 10.1093/jpepsy/jsm064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bender B, Wamboldt FS, O’Connor SL, Rand C, Szefler S, Milgrom H, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol. 2000 Nov;85:416–21. doi: 10.1016/s1081-1206(10)62557-4. [DOI] [PubMed] [Google Scholar]
- 10.Burgess SW, Sly PD, Morawska A, Devadason SG. Assessing adherence and factors associated with adherence in young children with asthma. Respirology. 2008 Jun;13:559–63. doi: 10.1111/j.1440-1843.2008.01292.x. [DOI] [PubMed] [Google Scholar]
- 11.Farley J, Hines S, Musk A, Ferrus S, Tepper V. Assessment of adherence to antiviral therapy in HIV-infected children using the Medication Event Monitoring System, pharmacy refill, provider assessment, caregiver self-report, and appointment keeping. J Acquir Immune Defic Syndr. 2003 Jun 1;33:211–8. doi: 10.1097/00126334-200306010-00016. [DOI] [PubMed] [Google Scholar]
- 12.PubMed [database on the Internet] Available from: http://www.ncbi.nlm.nih.gov/pubmed/
- 13.McNally KA, Rohan J, Schluchter M, Riekert KA, Vavrek P, Schmidt A, et al. Adherence to combined montelukast and fluticasone treatment in economically disadvantaged african american youth with asthma. J Asthma. 2009 Nov;46:921–7. doi: 10.3109/02770900903229651. [DOI] [PubMed] [Google Scholar]
- 14.Gerson AC, Furth SL, Neu AM, Fivush BA. Assessing associations between medication adherence and potentially modifiable psychosocial variables in pediatric kidney transplant recipients and their families. Pediatr Transplant. 2004 Dec;8:543–50. doi: 10.1111/j.1399-3046.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 15.Martin S, Elliott-DeSorbo DK, Wolters PL, Toledo-Tamula MA, Roby G, Zeichner S, et al. Patient, caregiver and regimen characteristics associated with adherence to highly active antiretroviral therapy among HIV-infected children and adolescents. Pediatr Infect Dis J. 2007 Jan;26:61–7. doi: 10.1097/01.inf.0000250625.80340.48. [DOI] [PubMed] [Google Scholar]
- 16.Starr M, Sawer SM, Carlin JB, Powell CVE, Newman RG, Johnson PDR. A novel approach to monitoring adherence to preventive therapy for tuberculosis in adolescence. J Paediatr Child Health. 1999;35:350–4. doi: 10.1046/j.1440-1754.1999.00371.x. [DOI] [PubMed] [Google Scholar]
- 17.Modi AC, Morita DA, Glauser TA. One-month adherence in children with new-onset epilepsy: white-coat compliance does not occur. Pediatrics. 2008 Apr;121:e961–6. doi: 10.1542/peds.2007-1690. [DOI] [PubMed] [Google Scholar]
- 18.Olivieri NF, Matsui D, Hermann C, Koren G. Compliance assessed by the Medication Event Monitoring System. Arch Dis Child. 1991 Dec;66:1399–402. doi: 10.1136/adc.66.12.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller AD, Bode S, Myer L, Roux P, von Steinbuchel N. Electronic measurement of adherence to pediatric antiretroviral therapy in South Africa. Pediatr Infect Dis J. 2008 Mar;27:257–62. doi: 10.1097/INF.0b013e31815b1ad4. [DOI] [PubMed] [Google Scholar]
- 20.Lau RC, Matsui D, Greenberg M, Koren G. Electronic measurement of compliance with mercaptopurine in pediatric patients with acute lymphoblastic leukemia. Med Pediatr Oncol. 1998 Feb;30:85–90. doi: 10.1002/(sici)1096-911x(199802)30:2<85::aid-mpo3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Modi AC, Lim CS, Yu N, Geller D, Wagner MH, Quittner AL. A multi-method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros. 2006 Aug;5:177–85. doi: 10.1016/j.jcf.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Berkovitch M, Papadouris D, Shaw D, Onuaha N, Dias C, Olivieri NF. Trying to improve compliance with prophylactic penicillin therapy in children with sickle cell disease. Br J Clin Pharmacol. 1998 Jun;45:605–7. doi: 10.1046/j.1365-2125.1998.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blowey DL, Hebert D, Arbus GS, Pool R, Korus M, Koren G. Compliance with cyclosporine in adolescent renal transplant recipients. Pediatr Nephrol. 1997 Oct;11:547–51. doi: 10.1007/s004670050335. [DOI] [PubMed] [Google Scholar]
- 24.Shellmer DA, Zelikovsky N. The challenges of using medication event monitoring technology with pediatric transplant patients. Pediatr Transplant. 2007 Jun;11:422–8. doi: 10.1111/j.1399-3046.2007.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartlett SJ, Lukk P, Butz A, Lampros-Klein F, Rand CS. Enhancing medication adherence among inner-city children with asthma: Results from pilot studies. J Asthma. 2002 Feb;39:47–54. doi: 10.1081/jas-120000806. [DOI] [PubMed] [Google Scholar]
- 26.Jentzsch NS, Camargos PA, Colosimo EA, Bousquet J. Monitoring adherence to beclomethasone in asthmatic children and adolescents through four different methods. Allergy. 2009 Oct;64:1458–62. doi: 10.1111/j.1398-9995.2009.02037.x. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor SL, Bender BG, Gavin-Devitt LA, Wamboldt MZ, Milgrom H, Szefler S, et al. Measuring adherence with the Doser CT in children with asthma. J Asthma. 2004 Sep;41:663–70. doi: 10.1081/jas-200026434. [DOI] [PubMed] [Google Scholar]
- 28.McQuaid EL, Kopel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: Reasoning, responsibility, and behavior. J Pediatr Psychol. 2003 July 1;28:323–33. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- 29.Walders N, Kopel SJ, Koinis-Mitchell D, McQuaid EL. Patterns of quick-relief and long-term controller medication use in pediatric asthma. J Pediatr. 2005 Feb;146:177–82. doi: 10.1016/j.jpeds.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein AG. Asthma treatment and noncompliance. Del Med J. 2000 May;72:209–13. [PubMed] [Google Scholar]
- 31.Burgess SW, Sly PD, Cooper DM, Devadason SG. Novel spacer device does not improve adherence in childhood asthma. Pediatr Pulmonol. 2007 Aug;42:736–9. doi: 10.1002/ppul.20647. [DOI] [PubMed] [Google Scholar]
- 32.Coutts JA, Gibson NA, Paton JY. Measuring compliance with inhaled medication in asthma. Arch Dis Child. 1992 Mar;67:332–3. doi: 10.1136/adc.67.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.da Costa IG, Rapoff MA, Lemanek K, Goldstein GL. Improving adherence to medication regimens for children with asthma and its effect on clinical outcome. J Appl Behav Anal. 1997 Winter;30:687–91. doi: 10.1901/jaba.1997.30-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson NA, Ferguson AE, Aitchison TC, Paton JY. Compliance with inhaled asthma medication in preschool children. Thorax. 1995 Dec;50:1274–9. doi: 10.1136/thx.50.12.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNamara PS, McCormack P, McDonald AJ, Heaf L, Southern KW. Open adherence monitoring using routine data download from an adaptive aerosol delivery nebuliser in children with cystic fibrosis. J Cyst Fibros. 2009 Jul;8:258–63. doi: 10.1016/j.jcf.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Nikander K, Arheden L, Denyer J, Cobos N. Parents’ adherence with nebulizer treatment of their children when using an sdaptive serosol delivery (AAD™) system. J Aerosol Med. 2003;16:273–81. doi: 10.1089/089426803769017640. [DOI] [PubMed] [Google Scholar]
- 37.Butz AM, Donithan M, Bollinger ME, Rand C, Thompson RE. Monitoring nebulizer use in children: comparison of electronic and asthma diary data. Ann Allergy Asthma Immunol. 2005 Mar;94:360–5. doi: 10.1016/S1081-1206(10)60988-X. [DOI] [PubMed] [Google Scholar]
- 38.Jasti S, Siega-Riz AM, Cogswell ME, Hartzema AG. Correction for errors in measuring adherence to prenatal multivitamin/mineral supplement use among low-income women. J Nutr. 2006 Feb;136:479–83. doi: 10.1093/jn/136.2.479. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001 May 15;134:968–77. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 40.Rapoff MA, Belmont JM, Lindsley CB, Olson NY. Electronically monitored adherence to medications by newly diagnosed patients with juvenile rheumatoid arthritis. Arthritis Care Res. 2005;53:905–10. doi: 10.1002/art.21603. [DOI] [PubMed] [Google Scholar]
- 41.Rapoff MA, Belmont J, Lindsley C, Olson N, Morris J, Padur J. Prevention of nonadherence to nonsteroidal anti-inflammatory medications for newly diagnosed patients with juvenile rheumatoid arthritis. Health Psychol. 2002;21:620–3. doi: 10.1037//0278-6133.21.6.620. [DOI] [PubMed] [Google Scholar]
- 42.Zindani GN, Streetman DD, Streetman DS, Nasr SZ. Adherence to treatment in children and adolescent patients with cystic fibrosis. J Adolesc Health. 2006 Jan;38:13–7. doi: 10.1016/j.jadohealth.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Conde JF, Kaur M, Fleischer AB, Jr, Tusa MG, Camacho F, Feldman SR. Adherence to clocortolone pivalate cream 0.1% in a pediatric population with atopic dermatitis. Cutis. 2008 May;81:435–41. [PubMed] [Google Scholar]
- 44.Krejci-Manwaring J, Tusa MG, Carroll C, Camacho F, Kaur M, Carr D, et al. Stealth monitoring of adherence to topical medication: Adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007 Feb;56:211–6. doi: 10.1016/j.jaad.2006.05.073. [DOI] [PubMed] [Google Scholar]
- 45.Matsui D, Hermann C, Braudo M, Ito S, Olivieri N, Koren G. Clinical use of the Medication Event Monitoring System: a new window into pediatric compliance. Clin Pharmacol Ther. 1992 Jul;52:102–3. doi: 10.1038/clpt.1992.108. [DOI] [PubMed] [Google Scholar]
- 46.Rogan JW, Lyszkiewicz DA, Blowey D, Khattak S, Arbus GS, Koren G. A randomized prospective crossover trial of amlodipine in pediatric hypertension. Pediatr Nephrol. 2000;14:1083–7. doi: 10.1007/s004670000400. [DOI] [PubMed] [Google Scholar]
- 47.Kerkar N, Annunziato RA, Foley L, Schmeidler J, Rumbo C, Emre S, et al. Prospective analysis of nonadherence in autoimmune hepatitis: A common problem. J Pediatr Gastroenterol Nutr. 2006;43:629–34. doi: 10.1097/01.mpg.0000239735.87111.ba. [DOI] [PubMed] [Google Scholar]
- 48.Steele RG, Anderson B, Rindel B, Dreyer ML, Perrin K, Christensen R, et al. Adherence to antiretroviral therapy among HIV-positive children: Examination of the role of caregiver health beliefs. AIDS Care. 2001;13:617 – 29. doi: 10.1080/09540120120063241. [DOI] [PubMed] [Google Scholar]
- 49.Shemesh E. Non-adherence to medications following pediatric liver transplantation. Pediatr Transplant. 2004 Dec;8:600–5. doi: 10.1111/j.1399-3046.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 50.Maikranz JM, Steele RG, Dreyer ML, Stratman AC, Bovaird JA. The relationship of hope and illness-related uncertainty to emotional adjustment and adherence among pediatric renal and liver transplant recipients. J Pediatr Psychol. 2007 June 1;32:571–81. doi: 10.1093/jpepsy/jsl046. [DOI] [PubMed] [Google Scholar]
- 51.Gerson AC, Furth SL, Neu AM, Fivush BA. Assessing associations between medication adherence and potentially modifiable psychosocial variables in pediatric kidney transplant recipients and their families. Pediatr Transplant. 2004 Dec;8:543–50. doi: 10.1111/j.1399-3046.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 52.Bender B, Bartlett S, Rand C, Turner C, Wamboldt F, Zhang L. Impact of interview mode on accuracy of child and parent report of adherence with asthma-controller medication. Pediatrics. 2007 Sep;120:e471–7. doi: 10.1542/peds.2006-3457. [DOI] [PubMed] [Google Scholar]
- 53.Bender B, Zhang L. Negative affect, medication adherence, and asthma control in children. J Allergy Clin Immunol. 2008 Sep;122:490–5. doi: 10.1016/j.jaci.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 54.Fiese BH, Wamboldt FS, Anbar RD. Family asthma management routines: Connections to medical adherence and quality of life. J Pediatr. 2005 Feb;146:171–6. doi: 10.1016/j.jpeds.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 55.Bender B, Milgrom H, Rand C, Ackerson L. Psychological factors associated with medication nonadherence in asthmatic children. J Asthma. 1998;35:347–53. doi: 10.3109/02770909809075667. [DOI] [PubMed] [Google Scholar]
- 56.Modi AC, Quittner AL. Utilizing computerized phone diary procedures to assess health behaviors in family and social contexts. Child Health Care. 2006;35:29–45. [Google Scholar]
- 57.Modi AC, Quittner AL. Barriers to treatment adherence for children with cystic fibrosis and asthma: What gets in the way? P Pediatr Psychol. 2006 January 9;31:846–58. doi: 10.1093/jpepsy/jsj096. [DOI] [PubMed] [Google Scholar]
- 58.Iqbal S, Ritson S, Prince I, Denyer J, Everard ML. Drug delivery and adherence in young children. Pediatr Pulmonol. 2004;37:311–7. doi: 10.1002/ppul.10435. [DOI] [PubMed] [Google Scholar]
- 59.Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol. 1996 Dec;98:1051–7. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]