Abstract
Growing evidence suggests that amyloid beta (Aβ) and tau pathologies are strongly associated with mitochondrial dysfunction and neuronal damage in Alzheimer’s disease (AD). Extensive research of AD postmortem brains, mouse and fly models, including triple transgenic AD mice and mutant tau mice, and cell culture studies revealed that tau hyperphosphorylation is caused by multiple factors, including intraneuronal Aβ-oligomers, chronic oxidative stress, reduced insulin-like growth factor 1, and astrocytic mediated-Aβ and caspase activation. Overexpressed and phosphorylated tau appears to impair axonal transport of organelles causing synapse starvation, depletion of ATP, and ultimately neuronal damage. This article evaluates the role of tau in mitochondrial dysfunction and assesses how hyperphosphorylated tau impairs axonal transport of organelles in AD neurons.
Introduction
Alzheimer’s disease (AD) is a common age-related mental disorder characterized by memory loss and multiple cognitive impairments (Selkoe, 2001; Mattson, 2004). Currently, 5.4 million Americans suffer from AD (Alzheimer Association 2011). With increasing lifespan in humans, AD is a growing health concern in the society. In addition to the personal and family hardships that AD creates, these numbers translate into extremely high health-care costs. Two major pathological features have been observed in postmortem brains from AD patients: extracellular amyloid beta (Aβ) deposits and intracellular neurofibrillary tangles (NFTs) in the regions of the learning and memory, respectively (Selkoe, 2001; Mattson, 2004; LaFerla et al., 2007; Reddy et al., 2010). AD is also associated with the loss of synapses, synaptic function, mitochondrial structural and functional abnormalities, inflammatory responses, and neuronal loss (Selkoe, 2001; Mattson, 2004; Barsoum et al., 2006; LaFerla et al., 2007; Reddy and Beal, 2008).
Familial AD is caused by genetic mutations in 3 genetic loci: amyloid precursor protein (APP), presenilin 1 (PS1), and presenilin 2 (PS2). Recent genetic discoveries have revealed that DNA changes in a genetic variants of the sortilin-related receptor 1 gene, clusterin, complement the component receptor 1, MS4A4/MS4A6E, CD2AP, CD33, and EPHA1 genes contribute to late-onset AD, in addition to well established that apolipoprotein allele E4 as a risk factor (Goate et al., 1991; Scellenberg et al., 1992; Lavy-Lahad et al., 1995; Strittmatter et al., 1993; Rogaeva et al., 2007; Lambert et al., 2009; Harold et al., 2009; Hollingworth et al., 2011; Naj et al., 2011). Aging has been considered the ‘number one’ risk factor in AD pathogenesis.
Increasing evidence suggests that hyperphosphorylated tau is critically involved in AD pathogenesis, particularly in impairing axonal transport of APP and subcellular organelles including mitochondria in neurons affected by AD (Ittner and Gotz, 2011; Vossel et al., 2010). Further, N-terminal fragmented tau is associated with mitochondria and causing mitochondrial dysfunction and synaptic damage (Amadoro et al., 2011; Atlante et al., 2008). Recent reports demonstrated that Aβ-induced oxidative stress is a critical factor for hyperphosphorylation of tau in AD neurons (Melov et al., 2007; Su et al., 2010; Garwood et al., 2011).
The purpose of this article is to critically evaluate the role of tau in AD pathogenesis. Further, we assess research that identifies the link between tau and mitochondrial dysfunction and oxidative stress, and between hyperphosphorylated tau and the impairment of axonal transport of organelles in AD neurons.
Amyloid beta pathology in AD
The production and accumulation of intraneuronal Aβ precede Aβ deposition and NFT formation in the brains of AD patients (Gouras et al., 2000; Moreira et al., 2004; Golde and Janus, 2005; Gomez-Ramos et al., 2007; LaFerla et al., 2007), of persons with Down’s syndrome (Gyure et al., 2001) and of AD mouse models (Wirths et al., 2001; Oddo et al., 2003; Shie et al., 2003; Casas et al., 2004; Oakley et al., 2006; Lord et al., 2006; Van Broeck et al., 2006; Zerbineeti et al., 2006; Pigino et al., 2009; Pierrot et al., 2009; Rebeck et al., 2010; Tomiyama et al., 2010; Umeda et al., 2011). Aβ is a major component of neuritic plaques that are found in brain regions known to be responsible for learning and memory. Aβ is a cleaved product of the Aβ precursor protein (AβPP) via sequential proteolysis of β secretase and γ secretase in AD brains. Cleavage by γ secretase can generate 2 major forms of Aβ: a shorter form with 40 amino acid residues and a longer form with 42 amino acids. The longer form is toxic and has the capability to self-aggregate, form oligomers, participate in fibrillogenesis, and accumulate into Aβ deposits (Querfurth and LaFerla, 2010; Reddy et al., 2010). Levels of Aβ in brains from AD patients are controlled by the production, clearance, and degradation of Aβ. Recent studies revealed that Aβ production triggers and facilitates hyperphosphorylation of tau in AD brains (LaFerla, 2010).
The biology of tau
NTFs have been identified as a major pathological hallmark of AD. However, the structural and functional relevance of NFT to AD is not completely understood. Tau is a major microtubule-associated protein that plays a large role in the outgrowth of neuronal processes and the development of neuronal polarity. Tau promotes microtubule assembly, stabilizes microtubules, and affects the dynamics of microtubules in neurons (Lee et al 2001, Avila et al., 2004; Iqbal et al., 2005). Tau is abundantly present in the central nervous system and is predominantly expressed in neuronal axons (Brandt et al., 2005). Recent studies reported that tau is also expressed in glia and astrocytes.
The human tau gene is located on chromosome 17 (Neve et al., 1986, Iqbal et al 2005). Tau has six isoforms. Tau gene contains 15 exons, and the tau exons 2, 3, and 10 are alternatively spliced, resulting in 6 isoforms, in the adult human brain (see Fig. 1). Four tandem repeats are encoded by tau exons 9 to 12. The alternative splicing of exon 10 produces tau isoforms with (4R-tau) or without (3R-tau) exon 10. The alternative splicing of exons 2 and 3 produces tau variants containing zero (0N), one (1N), or two (2N) at the N-terminus (Fig. 1). The presence or absence of exons 2, 3 and 10 results in 6 different possible isoforms: 3R0N, 3R1N, 3R2N, 4R0N, 4R1N, and 4R2N (Fig. 1). The proportion of 3R to 4R is 1:1 in the adult human brain. Biochemical analysis of postmortem AD brains revealed that the amount of 4R-tau is higher than 3R in isolated NFTs (Iseki et al., 2006, Togo et al., 2004; Avila et al., 2004).
Figure 1.
Illustration represents human tau gene. Six isoforms are generated by alternative splicing of 2, 3 and 10 exons in human tau gene.
Normal tau stabilizes microtubules in the cytoskeleton of neurons. There are 79 potential serine and threonine phosphate acceptor residues in the longest isoform of tau (Billingsley and Kinciad, 1997), and tau has more than 30 phosphorylated sites. The phosphorylation of tau regulates microtubule binding and assembly (Wang and Liu, 2008). In contrast, pathological tau becomes hyperphosphorylated, which destabilizes microtubules by decreased binding to microtubules, resulting in the aggregation of hyperphosphorylated tau. Tau hyperphosphorylation and NFT pathology are associated with several late-onset neurodegenerative diseases, such as AD, amyotrophic lateral sclerosis, argyrophilic grain dementia, corticobasal degeneration, Cruetfedt-Jacob disease, dementia pugilistica, diffuse NFTs with calcification, Down’s syndrome, fronto-temporal dementia, Gerstmann-Straussler-Scheinker disease, Hallervorden-Spatz disease, myotonic dystrophy, Neimann-Pick disease - type C, non-Guamanian motor neuron disease with NFTs, Pick’s disease, postencephalitic parkinsonism, prion protein cerebral angiopathy, progressive cortical gliosis, progressive supranuclear palsy, subacute sclerosing panencephalitis, and tangle-only dementia (Lee et al., 2001; Iqbal et al., 2005).
Recently, Bonda and colleagues (2011) challenged the classical view on tau (that phosphorylated tau protein is a central mediator of disease pathogenesis) by proposing that tau phosphorylation represents a compensatory response mounted by neurons against oxidative stress that serves a protective function and this concept provides a better understanding of the mechanisms underlying disease pathophysiology and also provides a window for therapeutic intervention.
Mutations in the tau gene are not involved in familial AD. Because of the prominent tau pathology observed in the brains of AD patients, to understand the role of tau in AD, several investigators have generated transgenic mouse models for the tau gene (reviewed in Wang and Liu, 2011). Several lines of tau transgenic mice showed hyperphosphorylated tau in the form of paired helical filaments in the neurons and also motor and behavioral deficits (Lewis et al., 2001, Gotz et al., 2001; Oddo et al., 2003; Santa Cruz et al., 2005; Ramsden et al., 2005; Roberson et al., 2007; Berger et al., 2007; Bolmont et al., 2007; Terwell et al., 2008; Ittner et al., 2010; Coomaraswamy et al., 2010; Dawson et al., 2010; Grueninger et al 2010).
Aβ and tau pathologies in 3xTg-AD mice
LaFerla and Oddo (2003) generated mice that developed both Aβ and tau pathologies, similar to humans with AD. These researchers co-microinjected APPswe and tau p301L transgenes (both under the control of the Thy-1 promoter) into single-cell embryos harvested from PS1M146V knock-in mice (Oddo et al., 2003). The resulting mice harbored 3 mutant transgenes; however, APP and tau were overexpressed in the offspring. The 3xTg-AD mice developed Aβ (5 months of age) and tau (12–13 months of age) pathology that was similar to human AD in terms the brain regions in which the Aβ and tau developed. Although both mutant transgenes were expressed at same time, similar to the Aβ and tau genes in humans with AD, the Aβ deposits developed before tau hyperphosphorylation and before NFT aggregation, indicating that Aβ precede tau pathology. Findings from 3xTg-AD mice suggested that Aβ triggers or facilitates the accumulation of tau pathology (LaFerla and Oddo, 2005). However, recently, this notion has been challenged by Winton et al (2011). Using immunohistochemical, biochemical, and ultrastructural analyses, Winton and colleagues (2011) investigated intraneuronal Aβ that was reported in 3xTg-AD mice. They found that intraneuronal APP and its derivatives shared epitopes with full-length APP but not with free Aβ. Further, to determine unequivocally that this intraneuronal material did not include free Aβ, they crossed 3xTg-AD mice deficient in β-secretase (BACE), the protease required for Aβ generation from APP. In the absence of Aβ production, robust intraneuronal APP immunostaining was detected in the 3xTg-AD/BACE(−/ −) mice. The formation of tau lesions was not different between 3xTg-AD mice versus 3xTg-AD/BACE(−/ −) mice, and these authors conclude that tau pathology forms independently from Aβ in this mouse model. However, overwhelming evidence support against this notion. Further research is needed to resolve this issue.
Other studies also revealed that Aβ and/or chronic oxidative stress is critical to the development of tau pathology, including hyperphosphorylation of tau and NFT formation (Milo et al., 2008; Su et al., 2010). Further, hyperphosphorylation of tau and NFTs have been found in later stages (Braak stages V/VI) of AD (Braak and Braak, 1991). These observations indicate that tau pathology is secondary, but important in understanding and developing therapeutic strategies of AD.
Aβ and tau pathologies in fly models
Several groups investigated the toxic effects of Aβ and tau and mitochondrial function in neurons from Drosophila (fly) that express Aβ42 and/or mutant tau and found reduced mitochondria behavioral deficits and altered synaptic activities in flies that express Aβ and tau (Iijima-Ando et al., 2009; Zhao et al., 2010; Mudher et al., 2004).
Using Drosophila model that express Aβ42, Iijima and colleagues studied mitochondrial content, localization and behavioral changes. They found reduced mitochondria in axons and dendrites, and accumulated mitochondria in the soma. Aβ42-induced behavioral defects were enhanced by genetic reductions in mitochondrial transport, and were modulated by cAMP levels and PKA activity. Levels of putative PKA substrate phosphoproteins were reduced in the Aβ42 fly brains. Alterations in mitochondrial transport in neurons were sufficient to disrupt PKA signaling and induce late-onset behavioral deficits, suggesting a mechanism whereby mitochondrial mislocalization contributes to Aβ42-induced neuronal dysfunction.
Zhao and colleagues (2010) studied transgenic adult flies that express wild-type or arctic form of Aβ42 for neuronal structure/function, particularly axons and presynaptic terminals. They found Aβ accumulated intracellularly and induced age-dependent changes, including depletion of presynaptic mitochondria, slowdown of bi-directional transports of axonal mitochondria, decreased synaptic vesicles, increased large vacuoles, and elevated synaptic fatigue. These structural and functional synaptic changes correlated with age-dependent deficit in motor behavior. The depletion of presynaptic mitochondria was the earliest detected phenotype and was not caused by the change in axonal transport of mitochondria (Zhao et al., 2010).
Using transgenic flies that overexpress wild-type human tau (0N3R isoform), Mudher et al. (2004) studied tau pathology and neurodegeneration. They found that overexpressed tau disrupts axonal transport causing vesicle aggregation and this is associated with loss of locomotor function. Co-expression of constitutively active glycogen-synthase kinase-3beta (GSK-3beta) enhanced impaired axonal transport, and GSK-3beta inhibitors, lithium and AR-A014418, reversed both the axon transport defects and locomotor phenotypes, suggesting that the pathological effects of tau are phosphorylation dependent. These data show that tau abnormalities significantly disrupt neuronal function, in a phosphorylation-dependent manner, before the classical pathological hallmarks are evident (Mudher et al., 2004).
Overall, these Drosophila studies suggest that overexpressed tau and Aβ42, fragment mitochondria, disrupt mitochondrial distribution and cause synaptic degeneration.
Mitochondrial dysfunction in 3xTgAD mice
Several groups investigated mitochondrial dysfunction and oxidative damage in 3xTg-AD mice, and found mitochondria dysfunctional in these mice (Yao et al., 2009; Resende et al., 2008; Sensi et al., 2008; Drago et al., 2008). Yao et al. (2009) conducted mitochondrial functional analyses of female 3xTg-AD and age-matched, nontransgenic mice. Mitochondrial dysfunction in the 3xTg-AD brain was evidenced by decreased mitochondrial respiration and decreased pyruvate dehydrogenase protein levels and activity in mice as young as 3 months of age. 3xTg-AD mice also exhibited increased oxidative stress, manifested by increased hydrogen peroxide production and lipid peroxidation. The level of mitochondrial Aβ was significantly increased in the 3xTg-AD female mice at 9 months of age. Embryonic neurons derived from the 3xTg-AD mouse hippocampus exhibited significantly decreased mitochondrial respiration and increased glycolysis. Results form these analyses indicated compromised mitochondrial function in embryonic hippocampal neurons, which continued unabated in females throughout their reproductive period and was exacerbated during reproductive senescence. Reproductive senescence in the 3xTg-AD mouse brain markedly exacerbated mitochondrial dysfunction. Collectively, the data indicated significant mitochondrial dysfunction early in AD pathogenesis, in this female model of AD.
Drago et al. (2008) investigated whether Aβ-metal complexes have detrimental effects on intraneuronal Ca2+ homeostasis and mitochondrial function in vitro. They found that, when conjugated with aluminum, Aβ perturbed neuronal Ca2+i homeostasis and inhibited mitochondrial respiration. They also found that aluminum is increased in the cortex of these mice.
Resende et al. (2008) investigated whether oxidative damage occurs early in AD development. They evaluated oxidative stress and the levels of antioxidants in the 3xTg-AD mouse model. They found levels of the antioxidants glutathione and vitamin E decreased and the level of lipid peroxidation, increased. They also observed increased activity of the antioxidant enzymes glutathione peroxidase and superoxide dismutase. These increases were evident during the Aβ oligomerization period, before the appearance of Aβ plaques and NFTs, supporting the view that oxidative stress occurs early in AD development, before Aβ plaques and NFTs are observed.
Sensi et al. (2008) evaluated ROS-driven [Zn(2+)](i) increases in neurons obtained from 3xTg-AD mice that express mutant APP, PS1, and tau. The increase in [Zn(2+)](i), triggered by prolonged exposure to the membrane-permeant oxidizing agent 2,2′-dithiodipyridine, was significantly higher in 3xTg-AD neurons than in control neurons, suggesting that neuronal expression of pro-AD factors facilitated altered Zn(2+) homeostasis.
These studies indicate that mitochondrial dysfunction and oxidative stress are present in 3xTg-AD mice, and both Aβ and tau are linked to oxidative stress early in AD progression.
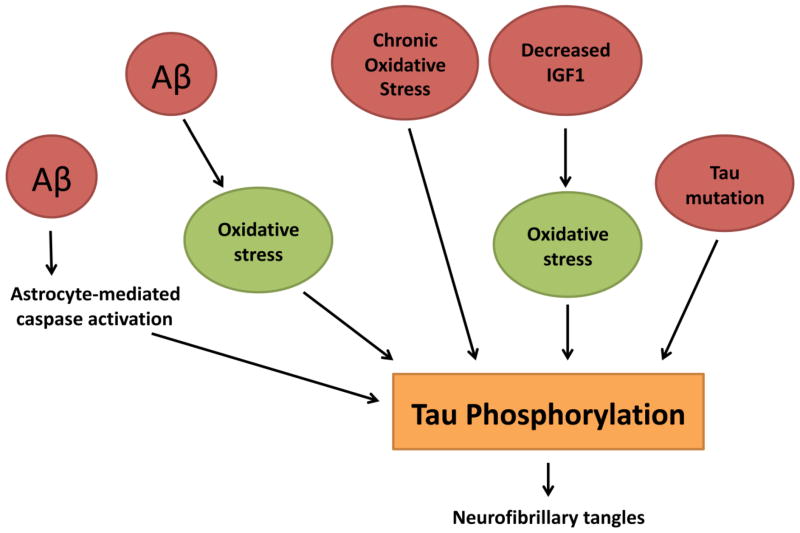
Possible factors that cause hyperphosphorylation of tau in AD brains
In AD brains, the formation of NFTs has been observed in late stage of disease progression and has been considered an important, but a secondary event. A possible reason for considering tau pathology as secondary is that mutations in the tau gene have not been found in AD patients. However, phosphorylation of tau and NFT formation have been extensively reported in the AD literature (Braak and Braak, 1991; Selkoe, 2001; Lee et al., 2001; LaFerla and Oddo, 2005). Causal factors affecting phosphorylation of tau and NFT formation are not fully understood. However, as depicted in Fig. 2, recent research has revealed several important factors in hyperphosphorylation of tau. The most convincing evidence came from research with 3xTg-AD mice.
Figure 2.
Possible factors that induce hyperphosphorylation of tau. 1. Aβ-mediated caspase activation, 2. Aβ-mediated oxidative stress, 3. Chronic oxidative stress, 4. Reduced IGF1-mediated oxidative stress and 5. Mutations in tau gene.
As described above, 3xTg-AD mice express PS1, APP, and tau mutations, and develop Aβ and pathology (at 5 months and 12–13 months, respectively). Although both mutant transgenes are expressed at same time, Aβ deposition develops before tau hyperphosphorylation and NFTs (Oddo et al., 2003), so Aβ may trigger and facilitate tau pathology.
Melov et al. (2007) determined whether increased mitochondrial oxidative stress could modulate or regulate tau phosphorylation and Aβ deposition. AD mice lacking superoxide dismutase 2 died within the first week of life and developed a complex, heterogeneous phenotype arising from mitochondrial dysfunction and oxidative stress. Treatment of these mice in utero with catalytic antioxidants increased the lifespan of newborns and rescued peripheral phenotypes and central nervous system pathology. Next, Melov and colleagues (2007) studied superoxide dismutase 2 null mice that were differentially treated with high and low doses of a catalytic antioxidant. These mice exhibited striking elevations in tau phosphorylation (at Ser-396 and other phospho-epitopes of tau) in the mice treated with low doses of antioxidants. These high levels of tau hyperphosphorylation were prevented with high doses of the antioxidants – doses that were previously reported to be sufficiently high to prevent neuropathology. These authors genetically combined a well-characterized mouse model of AD (Tg2576) with heterozygous superoxide dismutase 2 knockout mice to study the interactions between mitochondrial oxidative stress and cerebral Aβ load. They found that a deficiency in mitochondrial superoxide dismutase 2 exacerbated the amyloid burden and increased levels of Ser-396 phosphorylated tau. These findings mechanistically linked mitochondrial oxidative stress with tau and Aβ in AD.
Cheng et al. (2005) reported that reduced insulin-like growth factor 1 (IGF-I) promotes hyperphosphorylated tau in the aged brain. To investigate whether IGF-I promotes or retards brain aging, they examined signs of oxidative stress and neuropathological aging in brains from 400-d-old Igf1−/ − and WT mice. Although the accumulation of the lipofuscin pigment reflects oxidative stress and aging (Cheng et al., 2005), they found no accumulation of lipofuscin in the Igf1−/ − and WT brains. Likewise, there was no apparent difference in nitrotyrosine residues, in the Igf1−/ − and WT brains, except in the layer IV/V of the cerebral cortex, where nitrotyrosine residues were about 20% higher in the Igf1−/ − brain. Cheng and colleagues found no difference in the levels of oxidative stress-related enzymes, neuronal nitric oxide synthase, inducible nitric oxide synthase, and superoxide dismutase in the Igf1−/ − and WT brains. They found tau phosphorylation dramatically increased on two specific residues, Ser-396 and Ser-202, both glycogen synthase kinase target sites that have been implicated in neurodegeneration. These observations indicate that IGF-I may have a major role in regulating tau phosphorylation in the aging brain.
Using a mouse neuroblastoma cell line M17, Su et al. (2010) investigated whether chronic, long-term oxidative stress is involved in tau hyperphosphorylation. They established a novel, in vitro model of chronic oxidative stress by inhibiting glutathione synthesis with BSO. In examination of the response of these cells to, they found that these were under chronic, mild oxidative stress, the induction of heme oxygenase 1 without neuronal death. Chronic oxidative stress increased levels of tau phosphorylation at PHF-1 epitope (serine 399/404) in a time-dependent manner. Their data further suggest that increased activity of JNK and p38, and decreased activity of PP2A are likely involved in chronic oxidative stress-induced tau phosphorylation.
Using murine primary cultures, Garwood et al. (2011) studied the role of astrocytes in mediating Aβ-induced neurotoxicity and tau phosphorylation. Garwood and colleagues (2011) used primary rat neuronal, astrocytic, and mixed cortical cultures to investigate the contribution of astrocyte-mediated inflammatory responses during an Aβ-induced neuronal loss. They found that the presence of small numbers of astrocytes exacerbated Aβ-induced neuronal death, caspase-3 activation, and the production of caspase-3-cleaved tau. The release of soluble inflammatory factor(s) from astrocytes accompanied these events. The inhibition of astrocyte activation with the anti-inflammatory agent, minocycline, reduced astrocytic inflammatory responses and the associated neuronal loss. When the astrocytic response was subsequently attenuated with minocycline, the former Aβ-induced increases in caspase-3 activation and the production of caspase-3-truncated tau species in neurons were reduced. Astrocytes were shown to be important mediators of the neurotoxic events downstream of elevated Aβ.
Recently, using in vivo multiphoton imaging tools and living tau transgenic mice (Tg4510 line), de Calignon et al. (2010) studied caspase activation and NFTs formation. They found that caspase activation occurs first, and precedes NFT formation. After NFTs formation, the neuron remains alive and caspase activity decreased. They also introduced wild-type 4-repeat tau (tau-4R) into wild-type animals and observed caspase activation, tau truncation and tau aggregation. These authors propose that caspase activation cleaves tau first, followed by NFTs formation AD mice.
Overall, findings from these studies indicate that tau hyperphosphorylation and NFT formation are caused by multiple factors in AD progression.
Aβ and tau colocalization in AD neurons
Fein and colleagues (2008) examined the regional distribution and co-localization of Aβ and phosphorylated tau in synaptic terminals of neurons from postmortem AD brains. To analyze synaptosomes prepared from cryopreserved AD tissue, they used flow cytometry and quantitatively examined large populations of individual synaptic terminals. An average 68.4% of synaptic terminals were positive for Aβ, and 32.3% were positive for phospho-tau. Aβ and phospho-tau fluorescence was lowest in neurons from the cerebellum. In contrast to synaptic phospho-tau, which was highest in the entorhinal cortex and hippocampus, synaptic fluorescence was significantly lower in the entorhinal cortex and hippocampus, relative to the neocortical regions. Synaptic and phospho-tau fluorescence was significantly correlated, and dual-labeling experiments demonstrated that 24.1% of Aβ-positive terminals were also positive for phospho-tau, with the highest fraction of dual labeling (39.3%) in the brain region known to be the earliest area affected by AD – the entorhinal cortex.
Takahashi and colleagues (2010) investigated whether phosphorylated tau is localized to Aβ in synapses of neurons from Tg2576 mice. They found hyperphosphorylated tau co-localized with Aβ42 in dystrophic neurites surrounding Aβ plaques. They also examined alterations in tau, considered normally to be an axonal protein, in relation to Aβ42, in dystrophic neurites around Aβ plaques in Tg2576 mice at an ultrastructural level, utilizing dual-labeling immuno-EM. Hyperphosphorylated tau was mislocalized near Aβ42, on tubular-filamentous structures and in clusters associated with the microtubule network, in dendritic profiles, including postsynaptic compartments. Similar mislocalization of hyperphosphorylated tau was also found in biopsies of human AD brain tissues.
Using molecular modeling tools, Miller et al. (2011) investigated the possible interaction between Aβ and tau, particularly Aβ and tau oligomeric complexes. Based on the molecular structure of Aβ and tau, they predicted three tau complexes interacted with Aβ, and all complexes contained high β-structure propensity in their R2, R3, and R4 repeats. They showed that Aβ oligomers likely interact with the R2 domain to form a stable complex with better alignment in the turn region and the β-structure domain. Based on these results, they proposed that the R2 domain of tau interacts with soluble Aβ oligomers and promotes tau-Aβ aggregation. These predicted interactions need to be further studied at biochemical levels, using co-immunoprecipitation and immunostaining analyses, in brain tissues from AD patients, and antibodies that label reactive to Aβ- and tau.
Overlapping Aβ and tau pathologies are consistent with a model in which both synaptic loss and dysfunction are linked to Aβ and tau in AD neurons.
Abnormal tau and impaired axonal transport
Normal tau has several cellular functions: 1) stabilization of microtubules, 2) promotion of neurite outgrowth, 3) membrane interactions 4) facilitation of enzyme enchoring, and 5) facilitation axonal transport of organelles to nerve terminals (Ittner and Gotz, 2011). In a disease such as AD, tau is hyperphosphorylated, accumulates in neurons, and forms paired helical filaments. As a result, tau loses its capability to bind with microtubules, which ultimately leads to neurodegeneration (Garcia and Cleveland, 2001).
It is well established that neurons are elongated cells. To maintain neuronal function they need efficient delivery of cellular organelles (such as mitochondria, endoplasmic reticulum, lysosomes), proteins, and lipids from soma to axons, dendrites and synapses. As shown in Figure 3, the delivery of organelles is purely based on microtubules, which serves as rail tracks, motor proteins that represent engines, organelles are cargoes; and microtubule associated proteins (MAPs), including tau, and other MAP proteins - tightly bind to microtubules and deliver the organelles smoothly from soma to nerve terminals. And also transport organelles back to soma in a retrograde fashion for recycling or re-energizing the organelles for next anterograde transport. This works as railroad in an organized fashion to transport organelles. In AD neuron, the detachment of MAP proteins, particularly tau, may lead to impaired axonal transport of organelles (see Fig. 3). Overexpressed normal tau and/or hyperphosphorylated tau has been found to impair axonal transport of organelles, including mitochondria (Stamer et al., 2002; Mandelkow et al., 2003; Dubey et al., 2008).
Figure 3.
Schematic representation of axonal transport of organelles in neurons A. represents neuron from control subject and B. represents neuron from Alzheimer’s disease patient. In healthy neuron, tau protein binds tightly to microtubule and allow normal axonal transport of organelles, including mitochondria and axonal transport of organelles is impaired in AD neuron because of destabilization hyperphosphorylated tau to microtubules.
Stamer and colleagues (2002) investigated how tau affects the trafficking of vesicles and organelles in primary cortical neurons, retinal ganglion cells, and neuroblastoma cells. They found that tau inhibits kinesin-dependent transport of peroxisomes, neurofilaments, and Golgi-derived vesicles into neurites. Loss of peroxisomes makes cells vulnerable to oxidative stress and leads to cellular degeneration. In particular, tau inhibits the transport of APP into axons and dendrites, and this inhibition causes the accumulation of APP in the cell body. APP tagged with yellow fluorescent protein and transfected by adenovirus associates with vesicles moving rapidly forward in the axon and slowly back. Both movements were strongly inhibited by cotransfection with fluorescently tagged tau (cyan fluorescent protein-tau) as seen by two-color confocal microscopy.
Using several neuronal and nonneuronal cell lines, Mandelikow’s group (2003) investigated the effects of tau-impaired axonal transport of organelles on synapses. They found that: 1) tau impairs axonal transport of organelles, 2) tau impairs APP trafficking to a large extent, and 3) the depletion of organelles, including mitochondria, causes oxidative stress and reduces ATP in synapses. These activities occur long before tau detaches from microtubules in cells.
Using neuroblastoma cells, Dubey et al. (2008) studied the role of overexpressed tau in the transport and stability of axons. They transfected neuroblastoma cultures with a construct that expresses full-length, 4-repeat tau. They found that tau, when overexpressed, retracted axonal transport of organelles. However, axonal neurites of day 14 cells were more resistant to tau-mediated retraction. To test whether this resistance was derived from the additional neurofilament content, day 3 cells were co-transfected with constructs expressing the full-length, 4-repeat tau and neurofilament M. Overexpression of neurofilament M attenuated tau-mediated retraction of day 3 cells. In contrast, co-transfection with constructs expressing tau and vimentin (which increases axonal neurites length) did not attenuate tau-mediated neurite retraction. Cultures simultaneously transfected with constructs expressing neurofilament M and tau, the level of examined vesicles was maintained. Their data indicate that neurofilaments stabilize developing axonal neurites and can counteract the destabilizing force resulting from overexpression of tau.
Using primary neurons from tau knockout mice, Vossel and colleagues (2010) studied the effects of Aβ and tau on axonal transport of mitochondria and the neurotrophin receptor TrkA, cargoes that are critical for neuronal function in AD. Aβ oligomers rapidly inhibited axonal transport in WT neurons. When they reduced the level of tau, these defects did not occur. Thus, Aβ requires tau to impair axonal transport, and tau reduction protects against Aβ-induced axonal transport abnormalities.
The findings from cell culture studies indicate that: 1) overexpressed tau impairs axonal transport of organelles, including mitochondria, causing synapse degeneration and neuronal damage, and 2) tau reduction and/or overexpressed neurofilament maintains normal axonal transport in neurons.
Tau and its association with mitochondria
Several groups recently investigated the relationship between tau and mitochondria and found that N-terminal-truncated tau is localized to mitochondrial membranes (Amadoro et al., 2011; Atlante et al., 2008; Quintanilla et al., 2009) (see Fig. 4). Amadoro et al. (2011) reported that a 20–22 kDa NH2-truncated tau fragment was largely enriched in human mitochondria from cryopreserved synaptosomes of AD brains and that the amount of tau in the terminal fields correlated with pathological synaptic changes and with organelle functional impairment. This form of NH2-truncated tau was also found in other brain cells from persons with AD, not AD-tauopathies, while its presence in AD patients is linked to Aβ multimeric species and likely to pathology severity. Native, patient-derived, Aβ oligomer-enriched extracts likely impaired mitochondrial function by the in vitro production of 20–22 kDa NH2-tau fragments in mature human SY5Y and in rat hippocampal neurons. These researchers suggest that the distribution of mitochondrial NH2-derived tau peptides may exacerbate synapse degeneration in tauopathies, including AD, and may sustain the in vivo NH-2 tau cleavage inhibitors as an drug discovery strategies for AD therapy.
Figure 4.
N-terminal tau and Aβ peptide association with mitochondria and causes abnormal mitochondrial dynamics, mitochondrial dysfunction and neuronal damage.
Quintanilla et al. (2009) demonstrated that the expression of tau that they induced at Asp-421 to mimic caspase cleavage (T4C3) was toxic to immortalized cortical neurons compared with a full-length tau isoform (T4). Expression of T4C3 induced mitochondrial fragmentation and elevated oxidative stress levels in comparison with T4-expressing cells. Thapsigargin treatment of T4 or T4C3 cells, which causes an increase in intracellular calcium levels, resulted in a significant decrease in mitochondrial potential. It also resulted in the loss of mitochondrial membrane integrity in T4C3 cells when compared with cells expressing T4. Mitochondrial fragmentation and membrane damage were ameliorated in T4C3 cells when they were pretreated with cyclosporine A or FK506, implicating the calcium-dependent phosphatase calcineurin in these pathogenic events. Increased calcineurin activity has been reported in the AD brain; thus, inhibition of this phosphatase may provide a therapeutic target for AD treatment.
Atlante et al. (2008) studied the relationship between overexpressed N-terminal tau fragments (1–25 aa and 26–44 aa) and mitochondrial dysfunction. They tested both fragments for ATP synthesis, membrane potential, and activity of the adenine nucleotide translocator. They found that oxidative phosphorylation was not affected by the NH(2)-1–25 aa tau fragment, but was dramatically impaired by the NH(2)-26–44 aa tau fragment. Both cytochrome c oxidase and the adenine nucleotide translocator are targets of the NH(2)-26–44 tau fragment, but the adenine nucleotide translocator is the unique mitochondrial target responsible for impairment of oxidative phosphorylation by the NH(2)-26–44 tau fragment, which then exerts deleterious effects on cellular availability of ATP synthesized into mitochondria.
These studies suggest that the N-terminal fragment of tau may cause mitochondrial dysfunction and defects in oxidative phosphorylation, in AD neurons. However, additional research is needed to understand the truncated and/or full-length tau or phospho tau-induced mitochondrial structure changes, particularly abnormal fission and fusion.
Tau and mitochondrial proteins
Several investigators studied mRNA and protein expressions, using microarray and global proteomics approaches and tissues from tau transgenic mice and triple transgenic AD mice. Chou et al. (2010) investigated protein alterations in mitochondrial proteins, in 3xTg-AD mice. They used 2D gel electrophoresis and tandem mass spectrometry to determine quantitative differences in the mitochondrial proteome, in the cerebral cortices of 6-month-old male 3xTg-AD and non-transgenic mice. They identified 23 different proteins, the expression levels of which differed significantly between the triple transgenic and non-transgenic mitochondria. The down-regulated and up-regulated mitochondrial proteins were observed in the transgenic cortices. The proteins functioned in a wide variety of metabolic pathways, including the citric acid cycle, oxide phosphorylation, pyruvate metabolism, glycolysis, oxidative stress, fatty acid oxidation, ketone body metabolism, ion transport, apoptosis, and mitochondrial protein synthesis. These alterations occurred before the development of a significant number of Aβ deposits and NFTs, indicating that mitochondrial dysregulation is an early event in AD.
Using hippocampal tissues from mice that express mutations of tau P301L, APP(sw), and PS2(N141I), Rhein et al. (2009) studied protein changes. In 1,275 proteins that they studied, they found massive deregulation in 24 of them. One-third of the 24 were mainly related to complexes I and IV of oxidative phosphorylation. Deregulation of complex I was tau-dependent, whereas deregulation of complex IV was Aβ-dependent, both at the protein and activity levels. Synergistic effects of Aβ and tau were evident in the 8-month-old, Aβ/tau transgenic mice as they were the only age group that showed a reduction of the mitochondrial membrane potential. At the age of 12 months, the mice exhibited the strongest defects: oxidative phosphorylation, synthesis of ATP, and reactive oxygen species were exhibited, again emphasizing synergistic, age-associated effects of Aβ and tau in perishing mitochondria
These studies suggest that deregulated mitochondrial protein levels occur in AD neurons, and these imbalanced mitochondrial proteins are a compensatory response to mitochondrial dysfunction caused by Aβ and tau.
Dendritic role of Aβ and tau
Recently, Ittner and colleagues (2010) studied tau for its localization in dendrites. Missorting of tau in transgenic mice expressing truncated tau (Δtau) and the absence of tau in tau−/−mice both disrupted the kinase Fyn, a postsynaptic target. This uncoupled NR-mediated excitotoxicity and mitigated Aβ toxicity. Δtau expression and tau deficiency prevented memory deficits and improved survival in Aβ-forming APP23 mice, a model of AD. These deficits were also fully rescued with a peptide that uncouples the Fyn-mediated interaction of NR and PSD-95 in vivo. They suggest that this dendritic role of tau confers Aβ toxicity at the postsynapse with direct implications for pathogenesis and treatment of AD.
In another study, Zempel et al. (2010) investigated differentiated primary hippocampal neurons for early localized changes following exposure to Aβ oligomers. Initial events became evident by missorting of endogenous tau into the somatodendritic compartment, in contrast to axonal sorting in normal neurons. In missorted dendritic regions, there was a depletion of spines, a local increase in Ca2+, and a breakdown of microtubules. In these regions, tau showed elevated phosphorylation at certain sites diagnostic of AD-Tau and local elevation of certain kinase activities, including MARK/par-1, BRSK/SADK, p70S6K and cdk5. These local effects occur without global changes in tau, tubulin, or kinase levels. Somatodendritic missorting occured not only with tau but also with other axonal proteins (e.g., neurofilaments), and correlated with pronounced depletion of microtubules and mitochondria. The Aβ-induced effects on microtubule and mitochondria depletion, tau missorting, and loss of spines were prevented by taxol, indicating that Aβ-induced microtubule destabilization and corresponding traffic defects are key factors in incipient degeneration. In contrast, taxol could not prevent the rise in Ca2+ levels, kinase activities, and tau phosphorylation.
Hoover et al. (2010) investigated the localization of abnormal tau in dendritic spines using rTgP301L tau mice. They found that early tau-related deficits develop not from the loss of synapses or neurons, but rather as a result of synaptic abnormalities caused by the accumulation of hyperphosphorylated tau within intact dendritic spines. Mutagenesis of 14 disease-associated serine and threonine amino acid residues to create pseudohyperphosphorylated tau caused tau mislocalization while creation of phosphorylation-deficient tau blocked the mistargeting of tau to dendritic spines. They conclude that tau phosphorylation plays a critical role in mediating tau mislocalization and subsequent synaptic impairment.
Overall, findings from these studies suggest that the mislocalization of tau and Aβ oligomers cause synapse deprivation and loss, and neuronal damage in AD
Tau-deficient mouse models
To understand the normal function of tau and the role of deficient tau in neurons, several groups created tau knockout mice. Harada et al. (1994) knocked out the endogenous tau gene in mice and found that the mice showed no phenotypic changes and the brains showed normal immunohistological features. They also found that axonal elongation was not affected in the knockout mice. However, in some caliber axons, microtubule stability was decreased and microtubule organization was significantly changed. An increase in MAP 1A was observed, which may have been to compensate for the functions of tau in large-caliber axons. These results argue against the suggested role of tau in axonal elongation but confirm that it is crucial in the stabilization and organization of microtubules in a certain type of axon. In another tau knockout study, Dawson et al (2001) investigated axonal transport in primary neurons. They observed delayed and reduced axonal growth in primary neurons from the tau knockout mice, indicating that tau is critical for the growth and maintenance of axons (Dawson et al. 2001).
Further, Dawson et al. (2010) crossed tau knockout mice with APP transgenic mice in order to understand the effect of missing functional tau in AD state The overexpression of APP (swe) in tau knockout mice elicited an extensive formation of axonal spheroids. While spheroids were found associated only with Aβ plaques in mice expressing APP (swe) on an endogenous mouse tau background, APP(sw)/mTau(−/ −) mice have spheroids not only surrounding Aβ plaques but also in white matter tracks and in the neurosis. Aβ plaques associated with neuropil dystrophic neurites and spheroids are prominent features of AD, and loss of normal tau function may lead to neurodegeneration.
Overall findings from tau knockout mice studies suggest that: 1) tau is important for axonal growth, 2) tau is important for microtubule stabilization, and 3) deficient tau or lack of tau may lead to neurodegeneration in neurons from AD mice.
Tau knockout mice and mitochondrial dysfunction
Recent studies revealed that mitochondrial dysfunction and oxidative stress are related to each other, in tau knockout mice. Tau has also been observed in the nuclei of neuronal and non-neuronal cells. Sultan and colleagues (2011) demonstrated that acute oxidative stress and mild heat stress induced the accumulation of dephosphorylated tau in neuronal nuclei. Using chromatin immunoprecipitation assays, they demonstrated that the capacity of endogenous tau to interact with neuronal DNA increased following heat stress. Comet assays performed on both WT and tau-deficient neurons showed that tau fully protected the genomic DNA in the neurons against HS-induced damage. Interestingly, this heat stress-induced DNA damage was completely rescued after the overexpression of human tau targeted to the nucleus.
Overall, the results from tau knockout mice highlight a novel role for nuclear tau as a key player in early stress response.
Conclusions and future directions
There is increasing evidence to suggest that hyperphosphorylated tau and mitochondrial dysfunction play a significant role in AD development. Tau is a major microtubule associated protein and important to maintain microtubule binding, stabilization and axonal transport of organelles. Abnormal and/or overexpressed tau destabilizes microtubule binding and cause impairment in the axonal transport of organelles, including mitochondria, and ultimately leading to synaptic deprivation and neuronal damage in AD. Recent studies found that tau cause defects in mitochondria and oxidative stress. Multiple factors, including intraneuronal Aβ, chronic oxidative stress, astrocytic-mediated Aβ oligomers, and caspase activation cause tau hyperphosphorylation and NFT pathology. However, the mechanisms of intraneuronal Aβ and tau in causing synaptic damage are unclear. Experiments that focus on the import of tau into mitochondrial membranes may provide new insights in understanding the mechanisms of tau association with mitochondria. Further, future experiments that focus on tau phosphorylation may be useful for developing drug targets.
Acknowledgments
This research was supported by NIH grants AG028072, RR00163, and Alzheimer Association grant IIRG-09-92429.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alzheimer Association Report. Alzheimer’s Disease Facts and Figures. 2011. [Google Scholar]
- Amadoro G, Corsetti V, Ciotti MT, Florenzano F, Capsoni S, Amato G, Calissano P. Endogenous Aβ causes cell death via early tau hyperphosphorylation. Neurobiol Aging. 2011;32(6):969–990. doi: 10.1016/j.neurobiolaging.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Atlante A, Amadoro G, Bobba A, de Bari L, Corsetti V, Pappalardo G, Marra E, Calissano P, Passarella S. A peptide containing residues 26–44 of tau protein impairs mitochondrial oxidative phosphorylation acting at the level of the adenine nucleotide translocator. Biochim Biophys Acta. 2008;17771(10):1289–1300. doi: 10.1016/j.bbabio.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004;84(2):361–384. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Gräber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25(16):3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, Yue M, Wszolek Z, Ashe K, Knight J, Dickson D, Andorfer C, Rosenberry TL, Lewis J, Hutton M, Janus C. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci. 2007;27(14):3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingsley ML, Kincaid RL. Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J. 1997;323(Pt 3):577–591. doi: 10.1042/bj3230577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolmont T, Clavaguera F, Meyer-Luehmann M, Herzig MC, Radde R, Staufenbiel M, Lewis J, Hutton M, Tolnay M, Jucker M. Induction of tau pathology by intracerebral infusion of amyloid-beta -containing brain extract and by amyloid-beta deposition in APP x Tau transgenic mice. Am J Pathol. 2007;171(6):2012–2020. doi: 10.2353/ajpath.2007.070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda DJ, Castellani RJ, Zhu X, Nunomura A, Lee HG, Perry G, Smith MA. A Novel Perspective on Tau in Alzheimer Disease. Curr Alzheimer Res. 2011 May 23; doi: 10.2174/156720511796717131. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;(3):213–216. doi: 10.1111/j.1750-3639.1991.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Brandt R, Hundelt M, Shahani N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim Biophys Acta. 2005;1739(2–3):331–354. doi: 10.1016/j.bbadis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Casas C, Sergeant N, Itier JM, Blanchard V, Wirths O, van der Kolk N, Vingtdeux V, van de Steeg E, Ret G, Canton T, Drobecq H, Clark A, Bonici B, Delacourte A, Benavides J, Schmitz C, Tremp G, Bayer TA, Benoit P, Pradier L. Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Pathol. 2004 Oct;165(4):1289–300. doi: 10.1016/s0002-9440(10)63388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CM, Tseng V, Wang J, Wang D, Matyakhina L, Bondy CA. Tau is hyperphosphorylated in the insulin-like growth factor-I null brain. Endocrinology. 2005;146(12):5086–5091. doi: 10.1210/en.2005-0063. [DOI] [PubMed] [Google Scholar]
- Chou JL, Shenoy DV, Thomas N, Choudhary PK, Laferla FM, Goodman SR, Breen GA. Early dysregulation of the mitochondrial proteome in a mouse model of Alzheimer’s disease. J Proteomics. 2011;74(4):466–479. doi: 10.1016/j.jprot.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Coomaraswamy J, Kilger E, Wölfing H, Schäfer C, Kaeser SA, Wegenast-Braun BM, Hefendehl JK, Wolburg H, Mazzella M, Ghiso J, Goedert M, Akiyama H, Garcia-Sierra Dawson HN, Cantillana V, Jansen M, Wang H, Vitek MP, Wilcock DM, Lynch JR, Laskowitz DT. Loss of tau elicits axonal degeneration in a mouse model of Alzheimer’s disease. Neuroscience. 2010;169(1):516–531. doi: 10.1016/j.neuroscience.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson HN, Cantillana V, Jansen M, Wang H, Vitek MP, Wilcock DM, Lynch JR, Laskowitz DT. Loss of tau elicits axonal degeneration in a mouse model of Alzheimer’s disease. Neuroscience. 2010;169(1):516–531. doi: 10.1016/j.neuroscience.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci. 2011;114(Pt 6):1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, Spires-Jones TL, Hyman BT. Caspase activation precedes and leads to tangles. 2010;464(7292):1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago D, Cavaliere A, Mascetra N, Ciavardelli D, di Ilio C, Zatta P, Sensi SL. Aluminum modulates effects of beta amyloid(1–42) on neuronal calcium homeostasis and mitochondria functioning and is altered in a triple transgenic mouse model of Alzheimer’s disease. Rejuvenation Res. 2008;11(5):861–871. doi: 10.1089/rej.2008.0761. [DOI] [PubMed] [Google Scholar]
- Dubey M, Chaudhury P, Kabiru H, Shea TB. Tau inhibits anterograde axonal transport and perturbs stability in growing axonal neurites in part by displacing kinesin cargo: neurofilaments attenuate tau-mediated neurite instability. Cell Motil Cytoskeleton. 2008;65(2):89–99. doi: 10.1002/cm.20243. [DOI] [PubMed] [Google Scholar]
- Fein JA, Sokolow S, Miller CA, Vinters HV, Yang F, Cole GM, Gylys KH. Co-localization of amyloid beta and tau pathology in Alzheimer’s disease synaptosomes. Am J Pathol. 2008;172(6):1683–1692. doi: 10.2353/ajpath.2008.070829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia ML, Cleveland DW. Going new places using an old MAP: tau, microtubules and human neurodegenerative disease. Curr Opin Cell Biol. 2001;13(1):41–48. doi: 10.1016/s0955-0674(00)00172-1. [DOI] [PubMed] [Google Scholar]
- Garwood CJ, Pooler AM, Atherton J, Hanger DP, Noble W. Astrocytes are important mediators of Aβ-induced neurotoxicity and tau phosphorylation in primary culture. Cell Death Dis. 2011 Jun 2;2:e167. doi: 10.1038/cddis.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Golde TE, Janus C. Homing in on intracellular Abeta? Neuron. 2005;45(5):639–642. doi: 10.1016/j.neuron.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Gómez-Ramos P, Asunción Morán M. Ultrastructural localization of intraneuronal Abeta-peptide in Alzheimer disease brains. J Alzheimers Dis. 2007;11(1):53–59. doi: 10.3233/jad-2007-11109. [DOI] [PubMed] [Google Scholar]
- Götz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293(5534):1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Abeta42 accumulation in human brain. Am J Pathol. 2000;156(1):15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueninger F, Bohrmann B, Czech C, Ballard TM, Frey JR, Weidensteiner C, von Kienlin M, Ozmen L. Phosphorylation of Tau at S422 is enhanced by Abeta in TauPS2APP triple transgenic mice. Neurobiol Dis. 2010;37(2):294–306. doi: 10.1016/j.nbd.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Gyure KA, Durham R, Stewart WF, Smialek JE, Troncoso JC. Intraneuronal abeta-amyloid precedes development of amyloid plaques in Down syndrome. Arch Pathol Lab Med. 2001;125(4):489–492. doi: 10.5858/2001-125-0489-IAAPDO. [DOI] [PubMed] [Google Scholar]
- Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369(6480):488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- Harold D, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43(5):429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, Ashe KH, Liao D. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68(6):1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima-Ando K, Hearn SA, Shenton C, Gatt A, Zhao L, Iijima K. Mitochondrial mislocalization underlies Abeta42-induced neuronal dysfunction in a Drosophila model of Alzheimer’s disease. PLoS One. 2009;4(12):e8310. doi: 10.1371/journal.pone.0008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Alonso Adel C, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739(2–3):198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Iseki E, Yamamoto R, Murayama N, Minegishi M, Togo T, Katsuse O, Kosaka K, Akiyama H, Tsuchiya K, de Silva R, Andrew L, Arai H. Immunohistochemical investigation of neurofibrillary tangles and their tau isoforms in brains of limbic neurofibrillary tangle dementia. Neurosci Lett. 2006;405(1–2):29–33. doi: 10.1016/j.neulet.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Götz J. Amyloid-β and tau--a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12(2):65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wölfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Götz J. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142(3):387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Oddo S. Alzheimer’s disease: Abeta, tau and synaptic dysfunction. Trends Mol Med. 2005;11(4):170–176. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- LaFerla FM. Pathways linking Abeta and tau pathologies. Biochem Soc Trans. 2010;38(4):993–995. doi: 10.1042/BST0380993. [DOI] [PubMed] [Google Scholar]
- Lambert JC, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;2293(5534):1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Lord A, Kalimo H, Eckman C, Zhang XQ, Lannfelt L, Nilsson LN. The Arctic Alzheimer mutation facilitates early intraneuronal Abeta aggregation and senile plaque formation in transgenic mice. Neurobiol Aging. 2006;27(1):67–77. doi: 10.1016/j.neurobiolaging.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Stamer K, Vogel R, Thies E, Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging. 2003;24(8):1079–1085. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631– 639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Adlard PA, Morten K, Johnson F, Golden TR, Hinerfeld D, Schilling B, Mavros C, Masters CL, Volitakis I, Li QX, Laughton K, Hubbard A, Cherny RA, Gibson B, Bush AI. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS One. 2007;2(6):e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Y, Ma B, Nussinov R. Synergistic Interactions between Repeats in Tau Protein and Aβ Amyloids May Be Responsible for Accelerated Aggregation via Polymorphic States. Biochemistry. 2011;50(23):5172–5181. doi: 10.1021/bi200400u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira PI, Liu Q, Honda K, Smith MA, Santos MS, Oliveira CR. Is intraneuronal amyloid beta-peptide accumulation the trigger of Alzheimer’s disease pathophysiology? J Alzheimers Dis. 2004;6(4):433–434. doi: 10.3233/jad-2004-6411. discussion 443–449. [DOI] [PubMed] [Google Scholar]
- Mudher A, Shepherd D, Newman TA, Mildren P, Jukes JP, Squire A, Mears A, Drummond JA, Berg S, MacKay D, Asuni AA, Bhat R, Lovestone S. GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol Psychiatry. 2004;9(5):522–530. doi: 10.1038/sj.mp.4001483. [DOI] [PubMed] [Google Scholar]
- Na AC, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RL, Harris P, Kosik KS, Kurnit DM, Donlon TA. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res. 1986;387(3):271–280. doi: 10.1016/0169-328x(86)90033-1. [DOI] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26v(40):10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Pierrot N, Santos SF, Feyt C, Morel M, Brion JP, Octave JN. Calcium-mediated transient phosphorylation of tau and amyloid precursor protein followed by intraneuronal amyloid-beta accumulation. J Biol Chem. 2006;281(52):39907–39914. doi: 10.1074/jbc.M606015200. [DOI] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, Ladu M, Busciglio J, Brady S. Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid beta. Proc Natl Acad Sci U S A. 2009;106(14):5907–5912. doi: 10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Quintanilla RA, Matthews-Roberson TA, Dolan PJ, Johnson GV. Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of Alzheimer disease. J Biol Chem. 2009;284(28):18754–18766. doi: 10.1074/jbc.M808908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, Guimaraes A, Yue M, Lewis J, Carlson G, Hutton M, Ashe KH. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) J Neurosci. 2005;(46):10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeck GW, Hoe HS, Moussa CE. Beta-amyloid1–42 gene transfer model exhibits intraneuronal amyloid, gliosis, tau phosphorylation, and neuronal loss. J Biol Chem. 2010;285(10):7440–7446. doi: 10.1074/jbc.M109.083915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Manczak M, Mao P, Calkins MJ, Reddy AP, Shirendeb U. Amyloid-beta and mitochondria in aging and Alzheimer’s disease: implications for synaptic damage and cognitive decline. J Alzheimers Dis. 2010;20(Suppl 2):S499–512. doi: 10.3233/JAD-2010-100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende R, Moreira PI, Proença T, Deshpande A, Busciglio J, Pereira C, Oliveira CR. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic Biol Med. 2008;44(12):2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Dröse S, Brandt U, Savaskan E, Czech C, Götz J, Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci U S A. 2009;106(47):20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316(5825):750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Rogaeva E, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg GD, Bird TD, Wijsman EM, Orr HT, Anderson L, Nemens E, White JA, Bonnycastle L, Weber JL, Alonso ME, et al. Genetic linkage evidence for a familial Alzheimer’s disease locus on chromosome 14. Science. 1992;258:668–671. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Sensi SL, Rapposelli IG, Frazzini V, Mascetra N. Altered oxidant-mediated intraneuronal zinc mobilization in a triple transgenic mouse model of Alzheimer’s disease. Exp Gerontol. 2008;43(5):488–492. doi: 10.1016/j.exger.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Shie FS, LeBoeur RC, Jin LW. Early intraneuronal Abeta deposition in the hippocampus of APP transgenic mice. Neuroreport. 2003;14(1):123–129. doi: 10.1097/01.wnr.0000051151.87269.7d. [DOI] [PubMed] [Google Scholar]
- Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J Cell Biol. 2002;156(6):1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Wang X, Lee HG, Tabaton M, Perry G, Smith MA, Zhu X. Chronic oxidative stress causes increased tau phosphorylation in M17 neuroblastoma cells. Neurosci Lett. 2010;468(3):267–271. doi: 10.1016/j.neulet.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Sultan A, Nesslany F, Violet M, Bégard S, Loyens A, Talahari S, Mansuroglu Z, Marzin D, Sergeant N, Humez S, Colin M, Bonnefoy E, Buée L, Galas MC. Nuclear tau, a key player in neuronal DNA protection. J Biol Chem. 2011;286(6):4566–4575. doi: 10.1074/jbc.M110.199976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Capetillo-Zarate E, Lin MT, Milner TA, Gouras GK. Co-occurrence of Alzheimer’s disease β-amyloid and τ pathologies at synapses. Neurobiol Aging. 2010;31(7):1145–1152. doi: 10.1016/j.neurobiolaging.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwel D, Muyllaert D, Dewachter I, Borghgraef P, Croes S, Devijver H, Van Leuven F. Amyloid activates GSK-3beta to aggravate neuronal tauopathy in bigenic mice. Am J Pathol. 2008;172(3):786–798. doi: 10.2353/ajpath.2008.070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo T, Akiyama H, Iseki E, Uchikado H, Kondo H, Ikeda K, Tsuchiya K, de Silva R, Lees A, Kosaka K. Immunohistochemical study of tau accumulation in early stages of Alzheimer-type neurofibrillary lesions. Acta Neuropathol. 2004;107(6):504–508. doi: 10.1007/s00401-004-0842-2. [DOI] [PubMed] [Google Scholar]
- Tomiyama T, Matsuyama S, Iso H, Umeda T, Takuma H, Ohnishi K, Ishibashi K, Teraoka R, Sakama N, Yamashita T, Nishitsuji K, Ito K, Shimada H, Lambert MP, Klein WL, Mori H. A mouse model of amyloid beta oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci. 2010;30(14):4845–4856. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda T, Tomiyama T, Sakama N, Tanaka S, Lambert MP, Klein WL, Mori H. Intraneuronal amyloid β oligomers cause cell death via endoplasmic reticulum stress, endosomal/lysosomal leakage, and mitochondrial dysfunction in vivo. J Neurosci Res. 2011;89 (7):1031–1042. doi: 10.1002/jnr.22640. [DOI] [PubMed] [Google Scholar]
- Van Broeck B, Vanhoutte G, Pirici D, Van Dam D, Wils H, Cuijt I, Vennekens K, Zabielski M, Michalik A, Theuns J, De Deyn PP, Van der Linden A, Van Broeckhoven C, Kumar-Singh S. Intraneuronal amyloid beta and reduced brain volume in a novel APP T714I mouse model for Alzheimer’s disease. Neurobiol Aging. 2006 Nov 15; doi: 10.1016/j.neurobiolaging.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, Cui B, Mucke L. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330(6001):198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JZ, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol. 2008;85(2):148–175. doi: 10.1016/j.pneurobio.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Winton MJ, Lee EB, Sun E, Wong MM, Leight S, Zhang B, Trojanowski JQ, Lee VM. Intraneuronal APP, Not Free A{beta} Peptides in 3xTg-AD Mice: Implications for Tau versus A{beta}-Mediated Alzheimer Neurodegeneration. J Neurosci. 2011;31(21):7691–7699. doi: 10.1523/JNEUROSCI.6637-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wirths O, Multhaup G, Czech C, Blanchard V, Moussaoui S, Tremp G, Pradier L, Beyreuther K, Bayer TA. Intraneuronal Abeta accumulation precedes plaque formation in beta-amyloid precursor protein and presenilin-1 double-transgenic mice. Neurosci Lett. 2001;306(1–2):116–120. doi: 10.1016/s0304-3940(01)01876-6. [DOI] [PubMed] [Google Scholar]
- Yao J, Irwin R, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106(34):14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XL, Wang WA, Tan JX, Huang JK, Zhang X, Zhang BZ, Wang YH, YangCheng HY, Zhu HL, Sun XJ, Huang FD. Expression of beta-amyloid induced age-dependent presynaptic and axonal changes in Drosophila. J Neurosci. 2010;30(4):1512–1522. doi: 10.1523/JNEUROSCI.3699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempel H, Thies E, Mandelkow E, Mandelkow EM. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30(36):11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbinatti CV, Wahrle SE, Kim H, Cam JA, Bales K, Paul SM, Holtzman DM, Bu G. Apolipoprotein E and low density lipoprotein receptor-related protein facilitate intraneuronal Abeta42 accumulation in amyloid model mice. J Biol Chem. 2006;281(47):36180–36186. doi: 10.1074/jbc.M604436200. [DOI] [PubMed] [Google Scholar]