Abstract
Na+ concentrations in endolymph must be controlled to maintain hair cell function since the transduction channels of hair cells are cation-permeable, but not K+-selective. Flooding or fluctuations of the hair cell cytosol with Na+ would be expected to lead to cellular dysfunction, hearing loss and vertigo. This review briefly describes cellular mechanisms known to be responsible for Na+homeostasis in each compartment of the inner ear, including the cochlea, saccule, semicircular canals and endolymphatic sac. The influx of Na+into endolymph of each of the organs is likely via passive diffusion, but these pathways have not yet been identified or characterized. Na+ absorption is controlled by gate -keeper channels in the apical (endolymphatic) membrane of the transporting cells. Highly Na+-selective epithelial sodium channels (ENaC) control absorption by Reissner’s membrane, saccular extramacular epithelium, semicircular canal duct epithelium and endolymphatic sac. ENaC activity is controlled by a number of signal pathways, but most notably by genomic regulation of channel numbers in the membrane via glucocorticoid signaling. Nonselective cation channels in the apical membrane of outer sulcus epithelial cells and vestibular transitional cells mediate Na+ and parasensory K+ absorption. The K+-mediated transduction current in hair cells is also accompanied by a Na+ flux since the transduction channels are nonselective cation channels. Cation absorption by all of these cells is regulated by extracellular ATP via apical nonselective cation channels (P2X receptors). The heterogeneous population of epithelial cells in the endolymphatic sac is thought to have multiple absorptive pathways for Na+ with regulatory pathways that include glucocorticoids and purinergic agonists.
Keywords: inner ear, sodium homeostasis, epithelial sodium channel, Meniere’s disease
1. Introduction
The luminal compartment (endolymphatic space) of the inner ear is separated from the abluminal compartment (perilymphatic space) by highly specialized epithelial cells with tight junctions. The endolymphatic space contains luminal fluid with high potassium concentration ([K+]) and low sodium concentration ([Na+]), which provides the ionic milieu needed to sustain transduction of sound and head acceleration into nerve impulses necessary for normal hearing and balance (Couloigner et al., 2006; Marcus and Wangemann, 2010). Although K+ is the primary current-carrying ion species for transduction, Na+ concentrations in endolymph must be maintained for control of hair cell function since the transduction channels of hair cells are cation-permeable, but not K+-selective (Jorgensen and Kroese, 1994). Flooding or fluctuations of the hair cell cytosol with Na+ would be expected to lead to cellular dysfunction, hearing loss and vertigo (Shi et al., 2005).
Disregulation of Na+homeostasis has been implicated in several inner ear conditions. For example, a)endolymphatic [Na+] has been observed to markedly increase during ischemic anoxia (a cause of sudden hearing loss) (Konishi, 1979; Lazarini and Camargo, 2006; Sellick and Johnstone, 1972), b) nonsyndromic autosomal recessive deafness (DFNA8/10) has been associated with mutations of a Na+ transport regulatory gene (Guipponi et al., 2002)and c) change in endolymphatic [Na+] has been proposed as a mechanism of premenstrual exacerbation of Meniere’s disease(Andrews and Honrubia, 2010) .
Although the molecular basis of K+ cycling in the inner ear has been widely reviewed (Couloigner et al., 2006; Hibino et al., 2010; Marcus and Wangemann, 2010; Wangemann, 2006; Zdebik et al., 2009), the transport systems and sites involved in Na+ homeostasis in the inner ear have largely been determined in the last decade and have received much less attention. The present review briefly describes Na+ homeostasis of the inner ear by the non-sensory epithelial cells of each compartment of the inner ear and its physiological significance. A striking homology in Na+ transport mechanisms is noted among Reissner’s membrane, saccule extramacular epithelium and semicircular canal duct epithelium and between outer sulcus epithelium and vestibular transitional cells.
2. Cochlea
Normal Na+ flux in the cochlea is only about 1% of normal K + flux (Konishi et al., 1978), indicative of the need for less active transport machinery for Na+ absorption than for K+ secretion. This is consistent with the observation of dense vascularization of the stria vascularis (seat of K+ secretion) compared with the avascular Reissners membrane and single-vessel metabolic supply of the outer sulcus. This apparently ‘low’ transport rate of Na+ actually reflects the unusually high transport of K+ transport and does not imply that Na+ movements are physiologically unimportant (see Section 6).
2.1. Distribution of Na+ transport-related channels and transporters
A number of Na+ transport-related channels and transporters have been identified in the cochlea, including the epithelial sodium channel (ENaC), non-selective cation channels, Na+H , +-exchanger (NHE-3), the Na+ pump ( Na+,K+-ATPase) and a Na+,K+,Cl−-cotransporter (NKCC1). In situhybridization and immunohistochemical studies demonstrated the expression of α-, β-and γ-subunits of ENaC in Reissner’s membrane (Couloigner et al., 2001; Zhong and Liu, 2004), striavascularis, spiral ligament, organ of Corti and spiral limbus (Couloigner et al., 2001; Grunder et al., 2001; Zhong and Liu, 2004). NHE-3 was localized in the apical surface of strial marginal cells(Bond et al., 1998; Goto et al., 1999) . Both αand β subtypes of Na +,K+-ATPase were found in stria vascularis, spiral limbusand Reissner’s membrane (Erichsen et al., 1996; Marcus et al., 2011; McGuirt and Schulte, 1994). Among the two isoforms of NKCC (NKCC1 and NKCC2), only NKCC1 was found at the basolateral membrane of strial marginal cells (Crouch et al., 1997).
Among the various tissues found to express Na+ transport genes in the cochlea, the only functionally identified active Na+ absorption sites in the cochlea are Reissner’s membrane (Kim et al., 2009a; Lee and Marcus, 2003), outer sulcus cells (Chiba and Marcus, 2000; Chiba and Marcus, 2001; Lee et al., 2001; Marcus and Chiba, 1999; Yamazaki et al., 2011)and hair cells (Lumpkin et al., 1997)(Fig 1A). Na+ transport in non-sensory epithelial cells in the cochlea is mainly mediated by apically located ENaCof Reissner’s membrane and nonselective cation channels of outer sulcus cells coupled with basolaterally located Na+,K+-ATPase (Fig 1D and E). Common to both absorption schemes, Na+,K+-ATPase in the basolateral membrane of epithelial cells provides the energy for Na+ absorption and Na+ entry pathways in the apical membrane provide a gating function for Na+entry from endolymph into the cytosol.
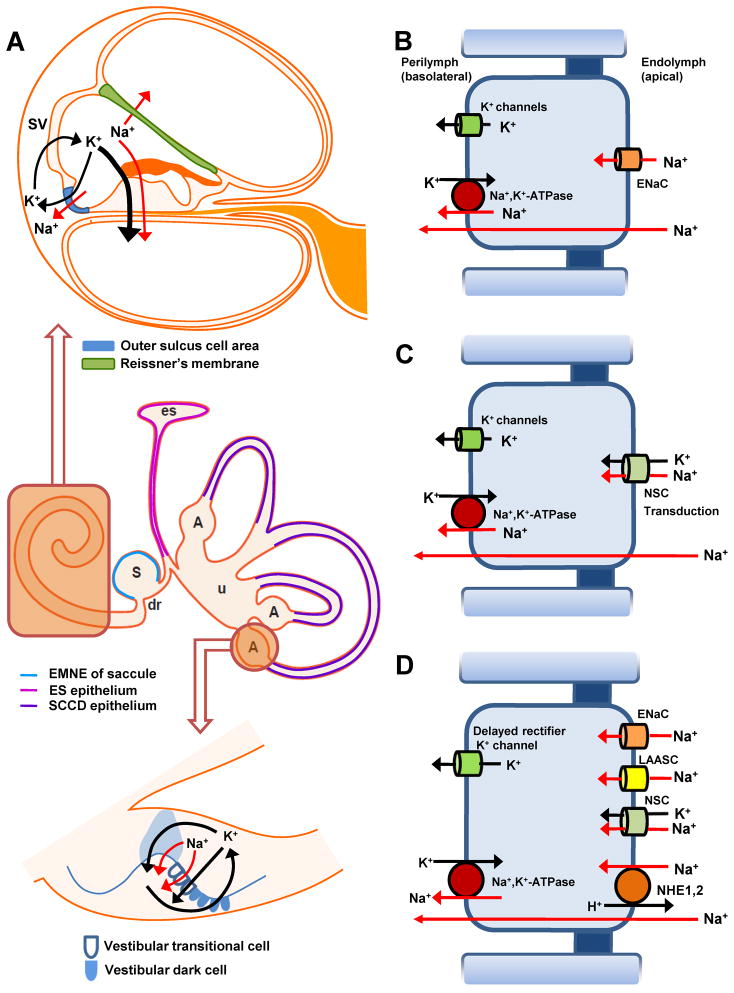
Figure 1. Schematic drawing of transepithelial Na+ transport in the inner ear.
A) Na+ ions in the endolymphatic space are absorbed (red arrows) by cochlear, vestibular and sac epithelial cells. Cochlear cross section (upper panel), vestibular system with endolymphatic sac (middle panel) and ampulla (bottom panel). Absorptive cells are rendered in colors identified in the legends.
B) Transport model for epithelial sodium channel (ENaC)-mediated Na+ absorption (Reissner’s membrane, saccule and semicircular canal duct). Na+ absorption by these cells is regulated by glucocorticoid and purinergic signaling pathways (see text).
C) Na+ absorption mediated by nonselective cation (NSC) channels (outer sulcus cells and vestibular transitional cells; transduction channels of cochlear and vestibular hair cells). Na+ absorption by all of these cells is regulated by purinergic signaling pathways (see text).
D) Na+ absorption by the endolymphatic sac mediated by cationchannels of different ion permeabilities (ENaC, NSC and low amiloride-affinity sodium channel (LAASC)) and electroneutral Na+H , +exchange (NHE). The depicted transport processes are modeled here in one cell but may occur in multiple cell types. Na+ absorption by these cells is thought to be regulated by glucocorticoid and purinergic signaling pathways (see text).
All cell models extrude Na+ from the cell cytosol into the basolateral fluid, perilymph, via the Na+-pump (Na+,K+-ATPase) in parallel with a K+ conductance. S, saccule; es, endolymphatic sac; u, utricle; dr, ductus reuniens; A, ampulla of semicircular canal duct.
2.2. Reissner’s membrane
Among the cochlear epithelial cells, Reissner’s membrane constitutes most of the luminal surface and is thought to be primarily responsible for Na+ homeostasis of cochlear endolymph. Na+ ions in the endolymphatic space are absorbed through apical ENaC and pumped out to the perilymphatic space through basolaterally located Na+,K+-ATPase (Fig 1A and D; red arrows in D). The net electrochemical driving force for Na+ across the apical membrane is thought to be directed from endolymph into the cytosol. The voltage across the apical membrane is likely greater than -100 mV (> 80 mV from the endocochlear potential and > −20 mV from the basolateral membrane voltage of the epithelial cells, both referenced to the potential of perilymph). The membrane voltage of the cells is likely controlled primarily by a large Cl− conductance (Kim and Marcus, 2010), which usually results in a membrane voltage less than that observed in cells that are dominated by K+ conductance. The electrical equivalent of the Na+ concentration is likely about 60 mV, outward directed (cytoplasmic [ Na+] ~10 mM, endolymphatic [Na+] ~1 mM). The inward-directed electrical potential difference (−100 mV) would therefore strongly overcome the outward-directed Na+ gradient (60 mV), leading to Na+ absorption across the apical membrane. Cytoplasmic K+ ions, which are exchanged with Na +ions through Na +,K+-ATPase are recycled to the perilymphatic space through and apparently low density of basolaterally located K+ channels (Kim et al., 2009a; Yamazaki et al., 2011) (Fig 1D). K+ recycling occurs through the voltage-activated K+ channel (Kv1.5) in gerbil(Lee and Marcus, 2003) whereas K + recycling occurs through multiple K+ channels (KCNJ10, KCNQ1, KCNQ3, KCNE2, KCNB1, KCNK1, KCNK2 and KCNK5) in mouse (Kim et al., 2009a). This Na+ transport scheme was determined by pharmacological responses of electrophysiological recordings of transepithelial short circuit current and epithelial cell membrane currents, employing vibrating probe and patch clamp of mouse and gerbil Reissner’s membrane (Kim et al., 2009a; Lee and Marcus, 2003; Yamazaki et al., 2011). Na+absorption through Reissner’s membrane is regulated by glucocorticoids and by purinergic agonists (see Section 5).
2.3. Outer sulcus cells
The outer sulcus epithelium is located in the lateral cochlear wall, between the K+-secreting stria vascularis and the K+-absorbing sensory hair cells in the organ of Corti. Although the outer sulcus is capable of absorbing Na+, this relatively small epithelial domain (compared to Reissner’s membrane) also provides a parasensory shunt for K+ efflux (Fig 1A) (Lee et al., 2001).
Na+(and K +) are driven from endolymph across the apical membrane of outer sulcus epithelial cells (Fig 1C) by the electrochemical gradients. The chemical gradient for Na+ is likely similar to that described above in Reissner’s membrane, but the electrical potential difference between cytosol and endolymph is likely even greater. Substantial transport homology between outer sulcus cells and vestibular transitional cells has been established (see section 3.4), and the transitional cells have a measured voltage of nearly -90 mV (Wangemann and Marcus, 1989). Support for the homology of this voltage in outer sulcus cells was provided by the measurement of a dominant basolateral K+ conductance (Chiba and Marcus, 2001). The inward driving force from endolymph is therefore the sum of the cellular basolateral voltage and the endocochlear potential, or greater than about 160 mV. It is expected that K+ would be absorbed even more strongly than Na+, since there is presumably very little concentration difference between the cytosol and endolymph and the 150-fold greater concentration would lead to greater conduction of K+ than Na+. The driving force for K+ would therefore be the entire apical membrane voltage difference, unopposed by a chemical driving force. The same considerations are applicable to the hair cells, which also have nonselective cation channels in the apical membrane although the basolateral membrane potential is not as large as in the outer sulcus cells (Fig. 1C).
The ability of the outer sulcus epithelium to absorb Na+, K+ (and Ca2+) was demonstrated in ion substitution experiments employing the vibrating probe. Removal of either Na+ or K+ from the bath solution led to significant reductions in transepithelial current (Marcus and Chiba, 1999). The identity of the apical cation entry pathway(s) was determined by patch clamp recordings from excised apical membrane. The primary conductance was ascribed to nonselective cation channels that would be active at physiological membrane voltage and Ca2+ concentrations. Large-conductance K+ channels were also observed frequently, but they would only be expected to be active at strongly-depolarized membrane potentials and/or elevated cytosolic Ca2+ levels (Chiba and Marcus, 2000).
The nonselective cation channels were blocked by relatively low concentrations of Gd3+ (IC50 of the open probability = 0.6 μM); the transepithelial current was also blocked by Gd3+ (Chiba and Marcus, 2000; Marcus and Chiba, 1999). Amiloride (10 μM–1 mM) and flufenamic acid also decreased both the transepithelial current and single-channel nonselective cation channel activity (Chiba and Marcus, 2000).
Exit of Na+ and K+ from the cytosol into perilymph is via the b asolateral Na+,K+-ATPase and basolateral K+ channels, respectively (Fig 1C). The basolateral K +channels were not identified molecularly but it was demonstrated by functional methods that they were not the Ca2+-dependent maxi (—big )-K +channel or —small -K +channels or A TP-sensitive K+ channels (Chiba and Marcus, 2001). Absorption of both cations is regulated by purinergic agonists in endolymph (see section 5.2).
3. Vestibular system
3.1. Distribution of Na+ transport-related channels and transporters
A number of Na+ transport-related channels and transporters have been identified in the vestibular system, including ENaC, non-selective cation channels, Na+, H +-exchanger (NHE-1), the Na+ pump (Na+,K+-ATPase) and a Na+,K+,Cl−-cotransporter (NKCC1). ENaC was identified in the semicircular canal duct (Pondugula et al., 2006) and non-sensorycells of the extramacular epithelium of the saccul e (Kim and Marcus, 2009). Na+,K+-ATPase was found in the basolateral surface of semicircular canal duct epithelial cells (α1-, α3-, β1- and β3-isoforms) (Pondugula et al., 2006; Pondugula et al., 2004), extramacular epithelium of the saccule (Kim and Marcus, 2009), vestibular dark cells (α1-and β 2-isoforms) (McGuirt and Schulte, 1994), and vestibular transitional cells (α1-and β 1-isoforms) (McGuirt and Schulte, 1994). NKCC1 was found in the basolateral membrane of vestibular dark cells and cellular membrane of transitional cells (Young et al., 2005). NHE-1 was expressed on the basolateral surface of dark cells and transitional cells (Wangemann et al., 1996; Wangemann et al., 1993).
Although various Na+-transporting ion channels and transporters were identified by molecular studies, functionally identified epithelial cells involved in transepithelial Na+ transport are vestibular transitional cells, semicircular canal duct epithelial cells and extramacular epithelium of the saccule. Na+ absorption occurs through apically located ENaC and nonselective cation channels and pumped out via basolaterally located Na+,K+-ATPase, which is similar to transepithelial transport in the cochlea. Regulation of Na+absorption by the vestibular system is reviewed below (Section 5).
3.2. Semicircular canal duct epithelium
Transepithelial short circuit current of cultured primary canal duct epithelial cells was observed to be mostly ENaC-dependent (Fig 1A and 1B) since most of the current was inhibited by amiloride analogs (benzamil > amiloride ≫ EIPA) (Pondugula et al., 2004). This Na+ absorption is under control of glucocorticoids (Section 5). The canal epithelium is quite complex in that it also secretes Cl−under control of beta2-adrenergic receptors and intracellular cAMP (Milhaud et al., 2002)and absorbs Ca2+via a TRPV5/TRPV6 pathway that is likely dependent on regulation by vitamin D (Yamauchi et al., 2010).
Na+ that enters the cell from endolymph is pumped out into perilymph through basolateral Na +,K+-ATPase. K+ that is pumped into the cell on the Na+,K+-ATPase is recycled to perilymph via basolaterally located Ba2+-sensitive K+ channels (Fig 1A and 1B), These transport molecules were pharmacologically identified by the sensitivity of the transepithelial current to ouabain and to low concentrations of Ba2+ (IC50:210 μM) (Pondugula et al., 2006; Pondugula et al., 2004). Candidates for the Ba+-sensitive K+ channels in the semicircular canal duct epithelial cells were found to be Kir2.1, Kir2.2, Kir2.3, Kir2.4, Kir3.1, Kir3.3, Kir4.1, Kir4.2, Kir5.1 and Kir7.1by RT -PCR (Pondugula et al., 2006).
3.3. Non-sensory extramacular epithelium of the saccule
The Na+ absorptive mechanism of extramacular epithelium of the mouse saccule is highly similar to that of mouse Reissner’s membrane (Fig 1A and 1B). Transepithelial Na + transport in extramacular epithelium of the saccule is mediated by apically located ENaC and secreted into the perilymph through basolaterally located Na+,K+-ATPase (Fig 1B and D). As before, transporters were identified by electrophysiologic and pharmacologic experiments using a vibrating probe (Kim and Marcus, 2009). Recycling of K+ at the basolateral membrane is mediated by multiple K+ channels (Fig 1B); however, the candidate K+ channels are different from Reissner’s membrane. These channels are thought to be Kv channels (including KCNQs) and Kir channels. However, the experimental observations argue against the participation of KATP, KCa and some acid-sensitive K2P channels such as KCNK1, KCNK3, KCNK5, KCNK9 and KCNK17.
3.4. Vestibular transitional cells
The vestibular transitional cells are located between the K+-secreting dark cells and the K+-absorbing sensory hair cells in the ampullae and utricle, homologous to the outer sulcus cells in the cochlea (section 2.3). There are no dark cells in the saccule. Although the transitional cells in the ampulla are capable of absorbing Na+, they are thought to primarily provide a parasensory shunt for K+ efflux (Fig 1A) (Lee et al., 2001). Ion transport by the transitional cells in the utricle and saccule have not been studied.
The cell model for transepthelial Na+ transport in the vestibular transitional cells is similar to that of cochlear outer sulcus cells (Fig 1A and C). The cations K+ and Na+ are absorbed through nonselective cation channels in the apical membrane of vestibular transitional cells. Transepithelial current at the apical surface of vestibular transitional cells towards the endolymphatic face of the epithelium was reduced by the application of Gd3+ and flufenamic acid, as for nonselective cation channels of outer sulcus epithelium (Lee et al., 2001). Measurements of intracellular voltage are consistent with the exit of Na+ across the basolateral membrane into perilymph via the Na+ pump and K + through selective basolateral channels. The cell membrane potential was reduced by oubain, an inhibitor of Na+,K+-ATPase, and by the K+ channel blockers Ba 2+, quinidine, quinine, Rb+, Cs+, NH4+ and Tl +(Wangemann and Marcus, 1989). The vestibular transitional cells are thought to provide a parasensory shunt for K + efflux in the same manner as the outer sulcus cells of the cochlea(Fig 1A) (Lee et al., 2001).
4. Endolymphatic sac
The epithelial cells of the endolymphatic sac consist primarily of two types: mitochondria-rich cells and ribosome-rich cells (Peters et al., 2002). Little is known about the ion transport properties of each cell type, since most experimental protocols used do not identify the specific cell type under study. However, at least one study of Na+ movements identified the most active cells as rich in mitochondria by staining (Miyashita et al., 2007). Further, a cooperative model of Na+ absorption involving both major cell types has been proposed (Kim and Wangemann, 2010). Many ion channels and transporters potentially involved in Na+ transport in the endolymphatic sac epithelial cells were reported, including a nonselective cation channel (Miyashita et al., 2001; Wu and Mori, 1999), low-amiloride-affinity Na+ channel (LAASC) (Mori and Wu, 1996), α-,β-and γ-subunits of ENaC (Kim et al., 2009b), NHE at the apical membrane (Son et al., 2009), NKCC1 and 2 (Akiyama et al., 2007), and Na+,K+-ATPase at the basolateral membrane (Mizukoshi et al., 1988).
Low amiloride-affinity Na+ channels, nonselective cation channels, ENaC, NHE, Na+,K+-ATPase and delayed rectifier K+ channels were identified to be involved in Na+ absorption by functional studies (Fig 1A and D). Low amiloride-affinity Na+ channels were identified in a patch clamp study and the permeability ratio of Na+ over K+ was approximately 5:1 (Mori and Wu, 1996). A nonselective cation channel was also identified in an excised patch clamp study and the relative ion permeability was in the order K+ = Na+> Ca2+≫Cl− (Miyashita et al., 2001). The nonselective cation channel was sensitive to cytosolic [Ca2+] and was voltage-dependent (Miyashita et al., 2001). ENaC and NHE were identified in cultured human endolymphatic epithelial cells by RT-PCR and by measurement of transepithelial current and its sensitivity to NHE inhibitors in confluent monlayers (Kim et al., 2009b; Son et al., 2009). Among the three isoforms of NHEs identified by RT-PCR (NHE1,2,3) in cultured human endolymphatic sac epithelial cells, NHE2 are the most likely to regulate pH and Na+ concentration of luminal fluid of endolymphatic sac, although evidence for the involvement of NHE1 (a basolateral NHE isoform in other epithelia) was also found (Son et al., 2009). Na+,K+-ATPase is thought to provide the driving force for Na+ absorption in endolymphatic sac epithelium. Immunostaining of Na+,K+-ATPase was especially prominent in the mitochondria-rich cells, which were identified by strong mitochondrial staining (Miyashita et al., 2007). This finding is correlated with the assumption that Na+,K+-ATPase activity, a major energy consuming process, requires a large number of mitochondria to supply the metabolic fuel. K+ ions in the cytosol exchanged with Na+ ions by Na+,K+-ATPase would be recycled via delayed-rectifier K+ channels, demonstrated in the guinea pig endolymphatic sac epithelial cells in a patch clamp study (Wu and Mori, 1996).
5. Regulation of Na+ absorption in the inner ear
Glucocorticoid and ATP or UTP have been observed to regulate Na+ transport in the inner ear by molecular and functional studies. Glucocorticoid increased Na+ absorption via ENaC in Reissner’s membrane (Kim et al., 2009a), semicircular canal duct epithelial cells (Pondugula et al., 2006; Pondugula et al., 2010; Pondugula et al., 2004)and extramacular epithelium of saccule (Kim and Marcus, 2009). ATP increased Na+ absorption via a ligand-gated nonselective cation channel (P2X2 receptor) in the outer sulcus cells, vestibular transitional cells (Lee et al., 2001) and endolymphatic sac epithelium (Wu and Mori, 1999) and UTP decreased Na+ absorption via the P2Y4 receptor acting on ENaC in Reissner’s membrane (Kim et al., 2010).
5.1. Glucocorticoid
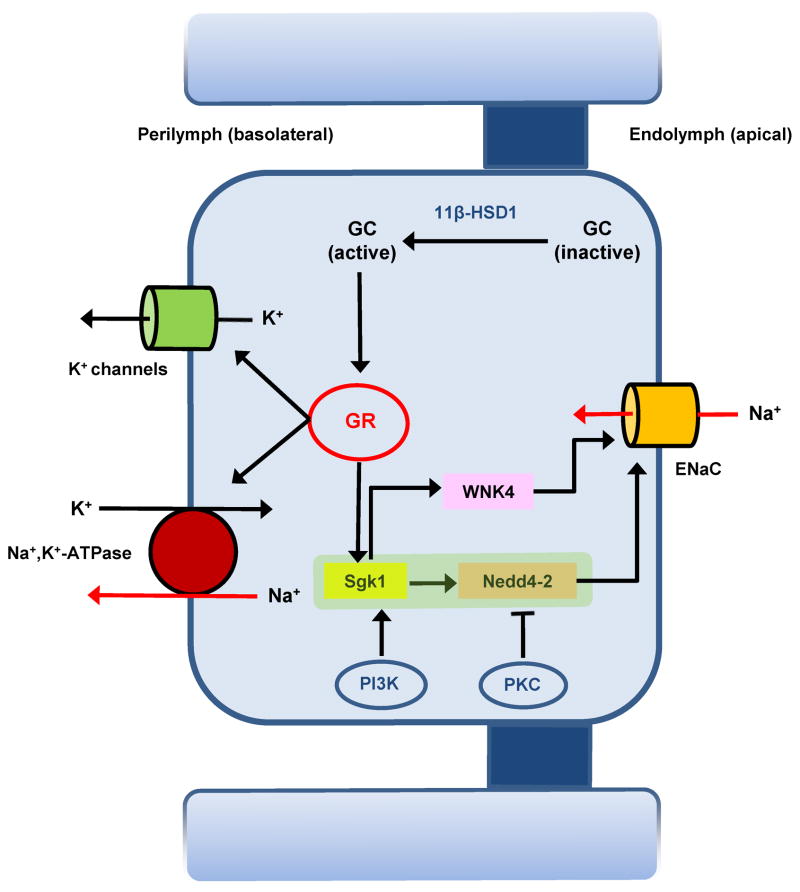
Functional and molecular studies demonstrated that the synthetic glucocorticoid dexamethasone increased transepithelial Na+ transport in Reissner’s membrane, saccule extramacular epithelium and primary cultures of semicircular canal duct epithelium via the glucocorticoid receptor (GR) pathway (Kim et al., 2009a; Kim and Marcus, 2009; Pondugula et al., 2006). The inactive forms of native glucocorticoid are converted in vivo to the active form by 11β-hydroxysteroid dehydrogenase and the active forms of glucocorticoid binds to GR (Fig 2). Activated GR then increases ENaC expression at the cell surface via the SGK1–Nedd4-2 regulatory pathway. The number of ENaC channels at the apical membrane is controlled by highly-active exocytotic and endocytotic membrane trafficking. The removal of ENaC from the apical membrane is controlled by the key regulatory proteins SGK1 (serum- and glucocorticoid-regulated kinase 1) and Nedd4-2 (neural precursor cell-expressed developmentally downregulated 4-2). Nedd4-2 is an ubiquitin protein ligase that binds to PY motifs in the C-terminal of α-, β- and γ-ENaC subunits, which reduces ENaC expression in the cell surface by ubiquitination of ENaC and its subsequent endocytosis. Activation of SGK1 by glucocorticoid leads to binding and phosphorylation of SGK1 to Nedd4-2, which decreases the binding of Nedd4-2 to ENaC and the subsequent increase in ENaC expression at the cell surface (Snyder et al., 2004; Stockand, 2002).
Figure 2.
Schematic drawing of glucocorticoid-regulated Na+ absorption in the mouse Reissner’s membrane and rat semicircular canal duct epithelial cells. Inactive forms of glucocorticoid are activated by 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1), which then increases ENaC expression via the glucocorticoid receptor (GR) – serum glucocorticoid-regulated kinase 1 (SGK1) - Neural precursor cell-expressed developmentally downregulated 4-2 (Nedd4-2) pathway. WNK4 was also suggested to be involved in the glucocorticoid regulated-Na+ transport pathway in Reissner’s membrane. Glucocorticoid-stimulated Na+ absorption is positively regulated by phosphatidylinositol 3-kinase (PI3K) and negatively regulated by protein kinase C (PKC) in canal epithelial cells. The rate of Na+ absorption is stimulated by glucocorticoid activation of GR in mouse saccule; however, the mechanism of glucocorticoid-enhanced Na+ absorption via ENaC was not defined molecularly.
Activity of Na+,K+-ATPase (Reissner’s membrane and semicircular canal) and K+ channels (semicircular canal) involved in Na+ absorption were also observed to be increased by dexamethsone. WNK, a protein kinase, was reported to increase Na+ transport if phosphorylated by SGK1 in some cell types, and four isoforms of WNK are present in Reissner’s membrane, with WNK4 upregulated by exposure to dexamethasone (Kim et al., 2009a) (Fig 2). In semicircular canal duct epithelium, glucocorticoid-stimulated Na+ absorption was reported to be positively regulated by phosphatidylinositol 3-kinase and negatively regulated by protein kinase C (Fig 2) (Pondugula et al., 2010). Although the mechanism of glucocorticoid-enhanced Na+ absorption via ENaC was not defined molecularly, dexamethasone also increased ENaC dependent Na+ absorption via genomic action of glucocorticoid receptors in the saccular extramacular epithelium (Kim and Marcus, 2009).
Channelopathies of inner ear epithelial cells have been suggested as one important etiology of endolymphatic hydrops (Gates, 2005). Synthetic glucocorticoids, such as dexamethasone and prednisolone, have been used to treat Meniere’s disease, and have been effective in controlling vertigo in ~52% to 76% of cases (Barrs et al., 2001). Therefore, it is tempting to speculate that some cases of Meniere’s disease result from increased endolymphatic [Na+] and that effective treatment by glucocorticoids is a result of increased Na+absorption by Reissner’s membrane, the saccule and the semicircular canals.
5.2. Purinergic receptors
Some mechanisms and pathways for purinergic signaling in the control of K+ secretion and cation absorption in the inner ear were recently reviewed (Lee and Marcus, 2008). Extracellular ATP exerts its effects through P2 purinergic receptors, which are divided into two families of ligand-gated ion channels and G protein-coupled receptors termed P2X and P2Y receptors, respectively (Lee and Marcus, 2008). Na+absorption from endolymph is modulated by at least two purinergic receptors: ionotropic P2X2 receptors and metabotropic P2Y4 receptors.
Purinergic receptors are expressed and regulate transport in Reissner’s membrane, outer sulcus cells, vestibular transitional cells, semicircular canal duct and endolymphatic sac epithelia. In addition, functional P2X receptors permeable to Na+ have been demonstrated at the apical aspect of hair cells (Housley et al., 1998). Further, transepithelial ion currents across Reissner’s membrane are controlled by P2Y4 receptors (Kim et al., 2010) (Fig 1). The P2X2 receptor channels are nonselective cation channels that open upon binding with purinergic agonists and are permeable to Na+, K+ and Ca2+(North, 2002) . ATP was reported to increase in endolymph during noise exposure, which would lead to an increased parasensory K+ flux via P2X2 receptors (Lee and Marcus, 2008). This has been proposed in the cochlea to serve as a protective mechanism to reduce the flux through the sensory pathway during intense stimulation and is likely also operant in the vestibular system (Lee and Marcus, 2008).
Extracellular UTP decreased ENaC-dependent transepithelial current via P2Y4 receptors in Reissner’s membrane, although the membrane location of the receptor was not determined (Kim et al., 2010). Signaling was thought to be mediated by a decrease of phosphatidylinositol 4,5-bisphosphate in the plasma membrane through phospholipase C activation. It is not yet known if the effect is directly on proteins in the Na+ transport pathway or if the effect is indirect, such as through a membrane potential change.
Extracellular ATP increased transepithelial current via P2X2 receptors in outer sulcus cells and vestibular transitional cells (Fig 1) (Lee et al., 2001). These ligand-gated channels must be in the apical membrane in order to account for the direction of current changes. This stimulated current is in addition to nonselective cation channel-mediated current in the absence of exogenous purinergic agonists (Sections 2.3 and 3.4). It is not yet resolved whether these two currents are mediated by separate types of channels or whether the P2X2 receptors in these cells mediate the currents observed in both the presence and absence of exogenous agonist. It is conceivable that there may be constitutive autocrine ATP/UTP release into the unstirred layer adjacent to the apical membrane that partially activated the receptors in the absence of exogenous purines (Corriden and Insel, 2010).
Extracellular ATP caused a biphasic change to the transepithelial voltage and resistance of semicircular canal duct epithelials cells in primary culture when added to the basolateral side but not when added to the apical side (Pondugula et al., 2010). These effects were consistent with regulation of Cl− secretion and paracellular permeability but not Na+ absorption.
The endolymphatic sac expresses several P2Y receptors (Mori et al., 2009) and ATP-stimulated membrane currents (Kd12 μM) were observed in one or more of the epithelial cells types (Wu and Mori, 1999). The functional significance for sac ion absorption and/or secretion is not yet known.
6. Etiology of elevated endolymphatic [Na+]
Elevated endolymphatic [Na+] can lead to hearing loss through alterations of the mechanical properties of the tectorial membrane (Kronester-Frei, 1979)and function of the inner hair cells, which can form blebs of their apical membrane in response to Na+ loading (Shi et al., 2005). Changes in net transport rate of Na+ (as well as other solutes) leads to volume changes of endolymph, since net solute flux is coupled to water movements. Hearing loss and/or vertigo associated with clinical conditions identified as Meniere’s syndrome and Scheibe’s deformity usually include swelling and shrinking of the inner ear lumen, respectively.
Endolymphatic [Na+] rises markedly within minutes during anoxia in the cochlea (Konishi, 1979; Sellick and Johnstone, 1972)(ca. 10 -fold in 30 minutes) and vestibular labyrinth (Sellick et al., 1972). Reductions in cochlear blood flow (ischemic anoxia) has been implicated in a large number of idiopathic hearing loss patients (Lazarini and Camargo, 2006). TNF-alpha has been proposed as a cellular mediator between infection, autoimmune disorders and systemic inflammatory responses and vasoconstriction (ischemia) (Scherer et al., 2010). If a patient with hearing loss due to ischemia and the accompanying elevated endolymphatic [Na+] is to recover, it is reasonable to speculate that part of that process would involve stimulated Na+ reabsorption.
Other pathologic conditions may also lead to elevated endolymphatic [Na+]. Increases in endolymphatic [Na+] likely occur during genetic or acute conditions that down-regulate the activity of the K+ channel KCNJ10 (Kir4.1), the channel expressed in the plasma membrane of strial intermediate cells that constitutes the site of EP generation (Marcus et al., 2002). A large, graded reduction in [K+] of cochlear endolymph was observed in heterozygous and homozygous KCNJ10 mice compared to wild type mice (Marcus et al., 2002). It is assumed that large decreases in endolymphatic [K+] are accompanied by increases in [ Na+] to maintain approximate osmotic balance. However, small decreases in endolymphatic [K+] have been observed during cisplatin administration that were not accompanied by a measureable increase in [Na+](Laurell et al., 1995) . Oxidative and nitrative stress in the stria vascularis occurs under a number of conditions and it has been shown that sufficient stress leads to a downregulation of KCNJ10 (Singh and Wangemann, 2007). It remains to be demonstrated directly whether [Na+] is elevated under these conditions. It should be noted that an often-used model of endolymphatic hydrops created by obliteration or obstruction of the endolymphatic sac and/or duct does not result in changes to the cochlear endolymphatic [Na+] (e.g., (Ikeda and Morizono, 1991; Konishi et al., 1981)).
Nonsyndromic autosomal recessive deafness (DFNA8/10) has been associated with mutations of the transmembrane serine protease TMPRSS3 (Guipponi et al., 2002). TMPRSS3 activates the epithelial Na+ channel (ENaC), a key pathway for Na+absorption by several inner ear epithelia (vide infra). Thus, it is possible that inner ear ENaC is a substrate of TMPRSS3 and that a dysfunction of ENaC is involved in the pathogenesis of DFNA8/10. Other isoforms of TMPRSS are also implicated in hearing loss, including TMPRSS1, 2, 5 and 10 (Guipponi et al., 2008), although their capacity to activate ENaC has not been reported.
The Na+ transport mechanisms described here were determined in mature healthy animal models. Constellations of expressed transport genes are known to vary during development in many systems due to markedly evolving needs of the developing organism, during changed ionic and hormonal status of fluids and in the presence of gene mutations. For example, recent studies have shown that mutations of a deafness gene (Slc26a4) in one cell type can lead to pathological changes in transport genes in other cells (Singh and Wangemann, 2007; Wangemann et al., 2004). Further, important transport functions such as cell-cell communication can be altered during embryonic times and lead to developmental problems in hearing (Kim and Wangemann, 2011). Epithelial Na+ transport genes in particular are known to undergo postpartum changes in activity and expression with critical functional significance in the lung (Eaton et al., 2009). Genetic and disruptions to ion homeostasis, including that of Na+, have been proposed as important factors in the etiology of Meniere’s syndrome by way of making the ear more fragile and susceptible to instability and dysfunction of hearing and balance function (Rauch, 2010).
Summary
This review has focused on the epithelial domains in the inner ear that have been shown to absorb Na+ from endolymph. The transport pathways responsible and the known regulatory pathways were described. Dysfunction of Na+ transport in the inner ear can be one of the causes of endolymphatic hydrops or sensory hair cell dysfunction. However, there are likely yet-to-be-determined Na+ transport pathways in inner ear epithelial cells whose discovery will enable our understanding and eventual treatment of inner ear disorders. Potentially important aspects of the topic that have not been addressed include embryonic development of Na+ transport, mutations of Na+ transport-related genes that lead to hearing loss and vertigo, and Na+ transport genes whose specific function in the inner ear have not yet been clearly established.
Acknowledgments
This work was supported by a grant from NIH R01-DC00212 to DCM.
Abbreviations
- ATP
adenosine triphosphate
- cAMP
cyclic adenosine monophosphate
- EIPA
ethylisopropylamiloride
- ENaC
epithelial sodium channel
- LAASC
low-amiloride-affinity Na+ channel
- Nedd
neural precursor cell-expressed developmentally downregulated
- NHE
Na+, H +-exchanger
- NKCC
Na+,K+,Cl− cotransporter
- NSC
non-selective cation
- PI3K
phosphatidylinositol 3-kinase
- RT-PCR
reverse transcriptase polymerase chain reaction
- SGK
serum- and glucocorticoid-regulated kinase
- UTP
uridine triphosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama K, Miyashita T, Mori T, Mori N. Expression of the Na+-K+-2Cl- cotransporter in the rat endolymphatic sac. Biochem Biophys Res Commun. 2007;364:913–917. doi: 10.1016/j.bbrc.2007.10.107. [DOI] [PubMed] [Google Scholar]
- Andrews JC, Honrubia V. Premenstrual exacerbation of Meniere's disease revisited. Otolaryngol Clin North Am. 2010;43:1029–1040. doi: 10.1016/j.otc.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Barrs DM, Keyser JS, Stallworth C, McElveen JT., Jr Intratympanic steroid injections for intractable Meniere's disease. Laryngoscope. 2001;111:2100–2104. doi: 10.1097/00005537-200112000-00003. [DOI] [PubMed] [Google Scholar]
- Bond BR, Ng LL, Schulte BA. Identification of mRNA transcripts and immunohistochemical localization of Na/H exchanger isoforms in gerbil inner ear. Hear Res. 1998;123:1–9. doi: 10.1016/s0378-5955(98)00089-6. [DOI] [PubMed] [Google Scholar]
- Chiba T, Marcus DC. Nonselective cation and BK channels in apical membrane of outer sulcus epithelial cells. J Membr Biol. 2000;174:167–179. doi: 10.1007/s002320001041. [DOI] [PubMed] [Google Scholar]
- Chiba T, Marcus DC. Basolateral K+ conductance establishes driving force for cation absorption by outer sulcus epithelial cells. J Membr Biol. 2001;184:101–112. doi: 10.1007/s00232-001-0079-0. [DOI] [PubMed] [Google Scholar]
- Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal. 2010;3:re1. doi: 10.1126/scisignal.3104re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couloigner V, Fay M, Djelidi S, Farman N, Escoubet B, Runembert I, Sterkers O, Friedlander G, Ferrary E. Location and function of the epithelial Na channel in the cochlea. Am J Physiol Renal Physiol. 2001;280:F214–F222. doi: 10.1152/ajprenal.2001.280.2.F214. [DOI] [PubMed] [Google Scholar]
- Couloigner V, Sterkers O, Ferrary E. What's new in ion transports in the cochlea? Pflugers Arch. 2006;453:11–22. doi: 10.1007/s00424-006-0103-4. [DOI] [PubMed] [Google Scholar]
- Crouch JJ, Sakaguchi N, Lytle C, Schulte BA. Immunohistochemical localization of the Na-K-Cl co-transporter (NKCC1) in the gerbil inner ear. J Histochem Cytochem. 1997;45:773–778. doi: 10.1177/002215549704500601. [DOI] [PubMed] [Google Scholar]
- Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol. 2009;71:403–423. doi: 10.1146/annurev.physiol.010908.163250. [DOI] [PubMed] [Google Scholar]
- Erichsen S, Zuo J, Curtis L, Rarey KE, Hultcrantz M. Na,K-ATPase α- and β-isoforms in the developing cochlea of the mouse. Hear Res. 1996;100:143–149. doi: 10.1016/0378-5955(96)00105-0. [DOI] [PubMed] [Google Scholar]
- Gates P. Hypothesis: could Meniere's disease be a channelopathy? Intern Med J. 2005;35:488–489. doi: 10.1111/j.1445-5994.2005.00891.x. [DOI] [PubMed] [Google Scholar]
- Goto S, Oshima T, Ikeda K, Takasaka T. Expression and localization of the Na+-H+ exchanger in the guinea pig cochlea. Hear Res. 1999;128:89–96. doi: 10.1016/s0378-5955(98)00191-9. [DOI] [PubMed] [Google Scholar]
- Grunder S, Muller A, Ruppersberg JP. Developmental and cellular expression pattern of epithelial sodium channel alpha, beta and gamma subunits in the inner ear of the rat. Eur J Neurosci. 2001;13:641–648. doi: 10.1046/j.1460-9568.2001.01426.x. [DOI] [PubMed] [Google Scholar]
- Guipponi M, Toh MY, Tan J, Park D, Hanson K, Ballana E, Kwong D, Cannon PZ, Wu Q, Gout A, Delorenzi M, Speed TP, Smith RJ, Dahl HH, Petersen M, Teasdale RD, Estivill X, Park WJ, Scott HS. An integrated genetic and functional analysis of the role of type II transmembrane serine proteases (TMPRSSs) in hearing loss. Hum Mutat. 2008;29:130–141. doi: 10.1002/humu.20617. [DOI] [PubMed] [Google Scholar]
- Guipponi M, Vuagniaux G, Wattenhofer M, Shibuya K, Vazquez M, Dougherty L, Scamuffa N, Guida E, Okui M, Rossier C, Hancock M, Buchet K, Reymond A, Hummler E, Marzella PL, Kudoh J, Shimizu N, Scott HS, Antonarakis SE, Rossier BC. The transmembrane serine protease (TMPRSS3) mutated in deafness DFNB8/10 activates the epithelial sodium channel (ENaC) in vitro. Hum Mol Genet. 2002;11:2829–2836. doi: 10.1093/hmg/11.23.2829. [DOI] [PubMed] [Google Scholar]
- Hibino H, Nin F, Tsuzuki C, Kurachi Y. How is the highly positive endocochlear potential formed? The specific architecture of the stria vascularis and the roles of the ion-transport apparatus. Pflugers Arch. 2010;459:521–533. doi: 10.1007/s00424-009-0754-z. [DOI] [PubMed] [Google Scholar]
- Housley GD, Raybould NP, Thorne PR. Fluorescence imaging of Na+ influx via P2X receptors in cochlear hair cells. Hear Res. 1998;119:1–13. doi: 10.1016/s0378-5955(97)00206-2. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Morizono T. Ionic activities of the inner ear fluid and ionic permeabilities of the cochlear duct in endolymphatic hydrops of the guinea pig. Hear Res. 1991;51:185–192. doi: 10.1016/0378-5955(91)90035-8. [DOI] [PubMed] [Google Scholar]
- Jorgensen F, Kroese AB. Ionic selectivity of the mechano-electrical transduction channels in the hair cells of the frog sacculus. Acta Physiol Scand. 1994;151:7–16. doi: 10.1111/j.1748-1716.1994.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Kim CH, Kim HY, Lee HS, Chang SO, Oh SH, Lee JH. P2Y4-mediated regulation of Na+ absorption in the Reissner's membrane of the cochlea. J Neurosci. 2010;30:3762–3769. doi: 10.1523/JNEUROSCI.3300-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Wangemann P. Failure of fluid absorption in the endolymphatic sac initiates cochlear enlargement that leads to deafness in mice lacking pendrin expression. PLoS One. 2010;5:e14041. doi: 10.1371/journal.pone.0014041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Wangemann P. Epithelial cell stretching and luminal acidification lead to a retarded development of stria vascularis and deafness in mice lacking pendrin. PLoS One. 2011;6:e17949. doi: 10.1371/journal.pone.0017949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KX, Marcus DC. Inward-rectifier chloride currents in Reissner's membrane epithelial cells. Biochem Biophys Res Commun. 2010;394:434–438. doi: 10.1016/j.bbrc.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim KX, Raveendran NN, Wu T, Pondugula SR, Marcus DC. Regulation of ENaC-mediated sodium transport by glucocorticoids in Reissner's membrane epithelium. Am J Physiol, Cell Physiol. 2009a;296:C544–C557. doi: 10.1152/ajpcell.00338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Marcus DC. Endolymphatic sodium homeostasis by extramacular epithelium of the saccule. J Neurosci. 2009;29:15851–15858. doi: 10.1523/JNEUROSCI.3044-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Park HY, Choi HS, Chung HP, Choi JY. Functional and molecular expression of epithelial sodium channels in cultured human endolymphatic sac epithelial cells. Otol Neurotol. 2009b;30:529–534. doi: 10.1097/MAO.0b013e31819a8e0e. [DOI] [PubMed] [Google Scholar]
- Konishi T. Some observations on negative endocochlear potential during anoxia. Acta Otolaryngol. 1979;87:506–516. doi: 10.3109/00016487909126459. [DOI] [PubMed] [Google Scholar]
- Konishi T, Hamrick PE, Walsh PJ. Ion transport in guinea pig cochlea. I. Potassium and sodium transport. Acta Otolaryngol. 1978;86:22–34. doi: 10.3109/00016487809124717. [DOI] [PubMed] [Google Scholar]
- Konishi T, Salt AN, Kimura RS. Electrophysiological studies of experimentally induced endolymphatic hydrops in guinea pigs. 1981:47–58. [Google Scholar]
- Kronester-Frei A. The effect of changes in endolymphatic ion concentrations on the tectorial membrane. Hear Res. 1979;1:81–94. doi: 10.1016/0378-5955(79)90019-4. [DOI] [PubMed] [Google Scholar]
- Laurell G, Teixeira M, Sterkers O, Ferrary E. Effect of cisplatin administration on the electrochemical composition of endolymph in the rat cochlea. Hear Res. 1995;87:16–20. doi: 10.1016/0378-5955(95)00074-e. [DOI] [PubMed] [Google Scholar]
- Lazarini PR, Camargo AC. Idiopathic sudden sensorineural hearing loss: etiopathogenic aspects. Braz J Otorhinolaryngol. 2006;72:554–561. doi: 10.1016/S1808-8694(15)31004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Chiba T, Marcus DC. P2X2 receptor mediates stimulation of parasensory cation absorption by cochlear outer sulcus cells and vestibular transitional cells. J Neurosci. 2001;21:9168–9174. doi: 10.1523/JNEUROSCI.21-23-09168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Marcus DC. Endolymphatic sodium homeostasis by Reissner's membrane. Neuroscience. 2003;119:3–8. doi: 10.1016/s0306-4522(03)00104-0. [DOI] [PubMed] [Google Scholar]
- Lee JH, Marcus DC. Purinergic signaling in the inner ear. Hear Res. 2008 doi: 10.1016/j.heares.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Marquis RE, Hudspeth AJ. The selectivity of the hair cell's mechanoelectrical-transduction channel promotes Ca2+ flux at low Ca2+ concentrations. Proc Natl Acad Sci USA. 1997;94:10997–11002. doi: 10.1073/pnas.94.20.10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DC, Chiba T. K+ and Na+ absorption by outer sulcus epithelial cells. Hear Res. 1999;134:48–56. doi: 10.1016/s0378-5955(99)00074-x. [DOI] [PubMed] [Google Scholar]
- Marcus, Raveendran, Wu Reissner's membrane, mouse, GEO GSE6196. 2011 http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE6196.
- Marcus DC, Wangemann P. Inner ear fluid homeostasis. 2010. pp. 213–230. [Google Scholar]
- Marcus DC, Wu T, Wangemann P, Kofuji P. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol, Cell Physiol. 2002;282:C403–C407. doi: 10.1152/ajpcell.00312.2001. [DOI] [PubMed] [Google Scholar]
- McGuirt JP, Schulte BA. Distribution of immunoreactive alpha- and beta-subunit isoforms of Na,K-ATPase in the gerbil inner ear. J Histochem Cytochem. 1994;42:843–853. doi: 10.1177/42.7.8014467. [DOI] [PubMed] [Google Scholar]
- Milhaud PG, Pondugula SR, Lee JH, Herzog M, Lehouelleur J, Wangemann P, Sans A, Marcus DC. Chloride secretion by semicircular canal duct epithelium is stimulated via β2-adrenergic receptors. Am J Physiol, Cell Physiol. 2002;283:C1752–C1760. doi: 10.1152/ajpcell.00283.2002. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Tatsumi H, Furuta H, Mori N, Sokabe M. Calcium-sensitive nonselective cation channel identified in the epithelial cells isolated from the endolymphatic sac of guinea pigs. J Membr Biol. 2001;182:113–122. doi: 10.1007/s00232-001-0035-z. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Tatsumi H, Hayakawa K, Mori N, Sokabe M. Large Na(+) influx and high Na(+), K (+)-ATPase activity in mitochondria-rich epithelial cells of the inner ear endolymphatic sac. Pflugers Arch. 2007;453:905–913. doi: 10.1007/s00424-006-0166-2. [DOI] [PubMed] [Google Scholar]
- Mizukoshi F, Bagger-Sjoback D, Rask-Andersen H, Wersall J. Cytochemical localization of Na-K ATPase in the guinea pig endolymphatic sac. Acta Otolaryngol. 1988;105:202–208. doi: 10.3109/00016488809096999. [DOI] [PubMed] [Google Scholar]
- Mori N, Wu DZ. Low-amiloride-affinity Na+ channel in the epithelial cells isolated from the endolymphatic sac of guinea-pigs. Pflugers Arch. 1996;433:58–64. doi: 10.1007/s004240050248. [DOI] [PubMed] [Google Scholar]
- Mori T, Miyashita T, Akiyama K, Inamoto R, Mori N. The expression of P2Y1, 2, 4, and 6 receptors in rat endolymphatic sac epithelia. Neuroreport. 2009;20:419–423. doi: 10.1097/WNR.0b013e328325a926. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Peters TA, Tonnaer EL, Kuijpers W, Cremers CW, Curfs JH. Differences in endolymphatic sac mitochondria-rich cells indicate specific functions. Laryngoscope. 2002;112:534–541. doi: 10.1097/00005537-200203000-00023. [DOI] [PubMed] [Google Scholar]
- Pondugula SR, Raveendran NN, Ergonul Z, Deng Y, Chen J, Sanneman JD, Palmer LG, Marcus DC. Glucocorticoid Regulation of Genes in the Amiloride-Sensitive Sodium Transport Pathway by Semicircular Canal Duct Epithelium of Neonatal Rat. Physiol Genomics. 2006;24:114–123. doi: 10.1152/physiolgenomics.00006.2005. [DOI] [PubMed] [Google Scholar]
- Pondugula SR, Raveendran NN, Marcus DC. Ion transport regulation by P2Y receptors, protein kinase C and phosphatidylinositol 3-kinase within the semicircular canal duct epithelium. BMC Res Notes. 2010;3:100. doi: 10.1186/1756-0500-3-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondugula SR, Sanneman JD, Wangemann P, Milhaud PG, Marcus DC. Glucocorticoids stimulate cation absorption by semicircular canal duct epithelium via epithelial sodium channel. Am J Physiol Renal Physiol. 2004;286:F1127–F1135. doi: 10.1152/ajprenal.00387.2003. [DOI] [PubMed] [Google Scholar]
- Rauch SD. Clinical hints and precipitating factors in patients suffering from Meniere's disease. Otolaryngol Clin North Am. 2010;43:1011–1017. doi: 10.1016/j.otc.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Scherer EQ, Yang J, Canis M, Reimann K, Ivanov K, Diehl CD, Backx PH, Wier WG, Strieth S, Wangemann P, Voigtlaender-Bolz J, Lidington D, Bolz SS. Tumor necrosis factor-alpha enhances microvascular tone and reduces blood flow in the cochlea via enhanced sphingosine-1-phosphate signaling. Stroke. 2010;41:2618–2624. doi: 10.1161/STROKEAHA.110.593327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellick PM, Johnstone BM. Changes in cochlear endolymph Na+ concentration measured with Na+ specific microelectrodes. Pflugers Arch. 1972;336:11–20. doi: 10.1007/BF00589137. [DOI] [PubMed] [Google Scholar]
- Sellick PM, Johnstone JR, Johnstone BM. The electrophysiology of the utricle. Pflugers Arch. 1972;336:21–27. doi: 10.1007/BF00589138. [DOI] [PubMed] [Google Scholar]
- Shi X, Gillespie PG, Nuttall AL. Na+ influx triggers bleb formation on inner hair cells. Am J Physiol Cell Physiol. 2005;288:C1332–C1341. doi: 10.1152/ajpcell.00522.2004. [DOI] [PubMed] [Google Scholar]
- Singh R, Wangemann P. Free radical stress mediated loss of Kcnj10 protein expression in stria vascularis contributes to deafness in Pendred syndrome mouse model. Am J Physiol Renal Physiol. 2007 doi: 10.1152/ajprenal.00433.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PM, Olson DR, Kabra R, Zhou R, Steines JC. cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na(+) channel through convergent phosphorylation of Nedd4-2. J Biol Chem. 2004;279:45753–45758. doi: 10.1074/jbc.M407858200. [DOI] [PubMed] [Google Scholar]
- Son EJ, Moon IS, Kim SH, Kim SJ, Choi JY. Interferon-gamma suppresses Na+ -H+ exchanger in cultured human endolymphatic sac epithelial cells. J Cell Biochem. 2009;107:965–972. doi: 10.1002/jcb.22201. [DOI] [PubMed] [Google Scholar]
- Stockand JD. New ideas about aldosterone signaling in epithelia. Am J Physiol Renal Physiol. 2002;282:F559–F576. doi: 10.1152/ajprenal.00320.2001. [DOI] [PubMed] [Google Scholar]
- Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol (Lond) 2006;576:11–21. doi: 10.1113/jphysiol.2006.112888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P, Itza EM, Albrecht B, Wu T, Jabba SV, Maganti RJ, Lee JH, Everett LA, Wall SM, Royaux IE, Green ED, Marcus DC. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med. 2004;2:30. doi: 10.1186/1741-7015-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P, Liu J, Shiga N. Vestibular dark cells contain the Na+/H+ exchanger NHE-1 in the basolateral membrane. Hear Res. 1996;94:94–106. doi: 10.1016/0378-5955(96)00008-1. [DOI] [PubMed] [Google Scholar]
- Wangemann P, Marcus DC. Membrane potential measurements of transitional cells from the crista ampullaris of the gerbil. Effects of barium, quinidine, quinine, tetraethylammonium, cesium, ammonium, thallium and ouabain. Pflugers Arch. 1989;414:656–662. doi: 10.1007/BF00582132. [DOI] [PubMed] [Google Scholar]
- Wangemann P, Shiga N, Marcus DC. The Na+/H+ exchanger in transitional cells of the inner ear. Hear Res. 1993;69:107–114. doi: 10.1016/0378-5955(93)90098-l. [DOI] [PubMed] [Google Scholar]
- Wu D, Mori N. Outward K+ current in epithelial cells isolated from intermediate portion of endolymphatic sac of guinea pigs. Am J Physiol. 1996;271:C1765–C1773. doi: 10.1152/ajpcell.1996.271.5.C1765. [DOI] [PubMed] [Google Scholar]
- Wu DZ, Mori N. Extracellular ATP-induced inward current in isolated epithelial cells of the endolymphatic sac. Biochim Biophys Acta Bio-Membr. 1999;1419:33–42. doi: 10.1016/s0005-2736(99)00053-x. [DOI] [PubMed] [Google Scholar]
- Yamauchi D, Nakaya K, Raveendran NN, Harbidge DG, Singh R, Wangemann P, Marcus DC. Expression of epithelial calcium transport system in rat cochlea and vestibular labyrinth. BMC Physiol. 2010;10:1. doi: 10.1186/1472-6793-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Kim KX, Marcus DC. Sodium selectivity of Reissner's membrane epithelial cells. BMC Physiol. 2011;11:4. doi: 10.1186/1472-6793-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CJ, Ho JS, Namkung W, Lee JH, Jin SE, Wook SJ, Park HY, Sang LW, Kim HN. Vestibular malformation in mice lacking Na-K-2Cl cotransporter 1 and expression of Na-K-2Cl cotransporter 1 in human vestibular end organs. Acta Otolaryngol. 2005;125:1252–1257. doi: 10.1080/00016480510012309. [DOI] [PubMed] [Google Scholar]
- Zdebik AA, Wangemann P, Jentsch TJ. Potassium ion movement in the inner ear: insights from genetic disease and mouse models. Physiology (Bethesda) 2009;24:307–316. doi: 10.1152/physiol.00018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong SX, Liu ZH. Immunohistochemical localization of the epithelial sodium channel in the rat inner ear. Hear Res. 2004;193:1–8. doi: 10.1016/j.heares.2004.03.001. [DOI] [PubMed] [Google Scholar]