Abstract
Brain-derived neurotrophic factor (BDNF) is a growth factor implicated in neuronal survival. Studies have reported altered BDNF serum concentrations in patients with Alzheimer’s disease (AD). However, these studies have been inconsistent. Few studies have investigated BDNF concentrations across multiple neurodegenerative diseases, and no studies have investigated BDNF concentrations in patients with frontotemporal dementia. To examine BDNF concentrations in different neurodegenerative diseases, we measured serum concentrations of BDNF using enzyme-linked immunoassay in subjects with behavioral-variant frontotemporal dementia (bvFTD, n=20), semantic dementia (SemD, n=16), AD (n=34), and mild cognitive impairment (MCI, n=30), as well as healthy older subjects (HS, n=38). BDNF serum concentrations were compared across diagnoses and correlated with cognitive tests and patterns of brain atrophy using voxel-based morphometry. We found small negative correlations between BDNF serum concentrations and some of the cognitive tests assessing learning, information processing speed and cognitive control in complex situationshowever, BDNF did not predict disease group membership despite adequate power. These findings suggest that BDNF serum concentration may not be a reliable diagnostic biomarker to distinguish among neurodegenerative diseases.
Keywords: Alzheimer’s disease, BDNF, frontotemporal dementia, mild cognitive impairment, neurotrophin, VBM
Introduction
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of growth factors [1, 2]. The protein promotes neuronal survival [3],modulates synaptic changes associated with learning and memory [4], and crosses the human blood-brain barrier in an intact form via a saturable transport system [5]. Moreover, plasma BDNF levels do not appear to change after blood-brain barrier damage [6].Several studies have suggested that altered BDNF serum concentrations are associated with various neurodegenerative diseases including Alzheimer’s disease (AD) (e.g., Yasutake 2005, Lee 2009, Angelucci 2010), Huntington’s disease [7], and Parkinson’s disease [8], however, results have been variable, and it remains unclear whether BDNF plays a causal role in neurodegeneration.
Investigations of BDNF serum concentrations in patients with AD have produced inconsistent results. In a study of 60 patients with severe AD (MMSE mean ± SD, 6.88 ± 6.78), patients with AD had lower average BDNF serum concentrations compared to 60 patients with vascular dementia and 33 healthy subjects [9]. Similarly, a study comparing 47 patients with moderate AD (MMSE: 13.7 ± 5.0) to 44 healthy subjects found decreased BDNF serum concentrations in patients [10]. Furthermore, in a separate study, 99 patients with amnestic MCI (MMSE: 27.00 ± 1.1) had lower BDNF concentrations compared to 99 healthy subjects and there was a positive association between serum BDNF concentrations and scores on delayed recall in the Auditory Verbal Learning Test, a test of auditory-verbal memory [11]. Nevertheless, in a study of patients with severe AD (n=35, MMSE: 6.3 ± 4.7), mild AD (n=54, MMSE: 22.3 ± 2.8), MCI (n=54, MMSE: 27.6 ± 1.8), and healthy subjects (n=27), BDNF serum concentrations were higher in patients with AD and MCI compared to healthy subjects [12]. A study of 89 patients with moderate AD (MMSE: 20.5 ± 4.8) and 89 healthy subjects reported no differences in BDNF serum concentrations [13]. Similarly, in a study of 30 patients with moderate AD (MMSE: 19.2 ± 7.0) and 10 healthy subjects, there were no differences in BDNF concentration between AD and healthy subjects. This study did, however, find increased BDNF serum concentrations in 15 patients with mild AD (MMSE ≥ 21) compared to 10 healthy subjects and 15 patients with late-stage AD [14]. Thus, the relationships among BDNF, AD and disease severity remain unclear.
Some authors have suggested that differences in medication use may account for thesediscrepant studies. Leyhe et al. found decreased BDNF serum concentrations in 19 patients with AD compared to 20 healthy subjects before donepezil treatment, but this difference disappeared after treatment [15]. Moreover, in a randomized, single-blinded, placebo-controlled study in 27 patients with AD, lithium increased BDNF serum concentrations compared to placebo [16]. AD patients also sometimes exhibit depressive symptoms [17], and a variety of antidepressant medications have been shown to increase BDNF serum concentrations in patients with depression [18]. Existing studies measuring BDNF serum concentrations in patients with AD often included patients taking different medications, which could also explain their divergent findings.
While BDNF concentrations have been measured in other neurodegenerative disease groups, we are not aware of any studies of BDNF in patients with behavioral variant frontotemporal dementia (bvFTD, a neurodegenerative disease typically characterized by bilateral frontal degeneration and progressive behavioral changes), or semantic dementia (SemD, a neurodegenerative disease typically involving more temporal and orbitofrontal atrophy and semantic loss) [19, 20]. Also, inclusion of multiple diseases in a single study allows specific BDNF-neurodegeneration relationships to be investigated. Therefore, we quantified BDNF serum concentrations in a large cohort of patients diagnosed with bvFTD, SemD, AD, and MCI as well as healthy subjects. In order to investigate possible medication effects on BDNF serum concentrations, we compared BDNF serum concentrations of patients taking medications known to affect BDNF concentrations. BDNF serum concentrations were then correlated with brain volume and a variety of cognitive tests to discover any brain volume or functional associations.
Methods
Subjects and Clinical Assessment
A total of 138 patients and healthy control subjects were recruited from the University of California, San Francisco, Memory and Aging Center, a tertiary care dementia clinic and research program. Clinical diagnosis in both patients and healthy subjects was determined after a detailed clinical history, neurologic examination, a 1-hour neuropsychological battery, laboratory screening, and 1.5 T brain MRI. We investigated 20 patients diagnosed with bvFTD, 16 with SemD, 34 with AD, 30 with MCI, and 38 healthy subjects (Table 1). Patients diagnosed with bvFTD and SemD met Neary criteria [19, 20], patients with Alzheimer disease (AD) met AD probable National Institute of Neurological and Communication Disorders/Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., 1984), and patients with MCI met the American Academy of Neurology criteria (Petersen et al., 1999). Healthy control subjects were recruited through advertisements in local newspapers and recruitment talks at local senior community centers. Healthy subjects must have had a normal neurological examination, a Clinical Dementia Rating (CDR) score of 0, and an MMSE score equal to or greater than 28/30. All subjects and, when applicable, their caregivers signed an institutional review board-approved research consent form to participate in this study. Patients seen at the clinic represented a broad sample of the population in terms of ethnicity, gender, education and socioeconomic status. An attempt was made to recruit all consecutively available patients for this study.
Table 1.
Demographic and clinical parameters of patients with neurodegenerative diseases and healthy subjects.
| Variables | bvFTD n = 20 |
SemD n = 16 |
AD n = 34 |
MCI n = 30 |
HS n = 38 |
Between groups p-value |
|---|---|---|---|---|---|---|
| Male/Female | 13/7 | 9/7 | 18/16 | 17/13 | 15/23 | ns |
|
Age
Mean ± SD |
60.9 ± 10.8* | 65.1 ± 7.5 | 66.1 ± 11.3 | 71.3 ± 11.5 | 69.2 ± 9.8 | 0.009 |
|
Education
Mean ± SD |
15.5 ±2.7* | 15.0 ± 2.6* | 15.2 ± 3.4* | 17.3 ± 2.3 | 17.9 ± 1.9 | <0.001 |
|
MMSE
Mean ± SD |
20.1 ± 9.8* | 19.5 ± 10.2* | 19.6 ± 8.2* | 28.3 ± 1.6 | 29.5 ± 0.7 | <0.001 |
| % with Medication Info | 95.0* | 100.0* | 94.1* | 70.0 | 60.5 | <0.001 |
| % onAchEIs | 21.1 | 25.0 | 83.9* | 31.8* | 4.3 | <0.001 |
| % on SSRIs and/or SNRIs | 36.8* | 31.3* | 3.2 | 13.6 | 4.4 | 0.010 |
Significant difference from healthy subjects
Blood Processing and Assay Methods
Blood was collected from patients and serum was obtained and stored at −80° C until assay. Serum was assayed for BDNF in duplicate, using a commercial BDNF ELISA assay kit (R&D Systems, Minneapolis, MN, USA). To evaluate inter-assay variability, an internal control consisting of serum obtained from a single individual, frozen in multiple aliquots, was run on each plate processed. BDNF concentrations of this control sample were measured on several different days and multiple 96-well plates. The R&D Systems Human BDNF Quantikine ELISA Kit was found to have an interassay variability of 8-14%. Intra-assay CV of duplicate samples was <10%, or samples were re-assayed.
Neuropsychological testing
Participants underwent detailed, standardized cognitive and behavioral testing as part of their assessment. The Clinical Dementia Rating Scale (CDR) was completed for each patient based on an interview with their primary caregiver-informant. Face-to-face neuropsychological testing included the Mini-Mental State Examination (MMSE); an abbreviated form of the Boston Naming Test that included 15 of the 60 items (a format which has been verified to be psychometrically valid equivalent to the full form) [21]; a modified version of the Trail Making Test, Color-Word Interference Test, and FAS Verbal Fluency Test from the Delis-Kaplan Executive Function Scale (DKEFS) [22]; the Geriatric Depression Scale (GDS) [23, 24, 25, 26]; the Immediate Recall Test and 10 Minute Delay Recall Test from the Modified Rey-Osterrieth Complex Figure test (MROCF) [27, 28]; the Number Location test from the Visual Space and Object Perception Battery (VSOP) [29]; a Design Fluency test (generate designs on an array of dots); an abstraction test (similarities and proverb interpretation); and repetition.
MRI scanning
Within 30 days of their blood draw, participants received MRI scans on a 1.5 T Magnetom VISION system (Siemens, Iselin, NJ) equipped with a standard quadrature head coil. Structural MRI sequences included a volumetric magnetization prepared rapid gradient echo MRI (repetition time/echotime/inversion time = 10/4/300 milliseconds) to obtain T1-weighted images of the entire brain, 15° flip angle, coronal orientation perpendicular to the double spin echo sequence, 1.0 × 1.0 mm2 in-plane resolution, and 1.5-mm slab thickness.
VBM
This is a technique for the detection of regional brain atrophy by voxel-wise comparison of gray and white matter volumes between groups of subjects. The technique comprises an image processing step (spatial normalization, segmentation, modulation, and smoothing) followed by statistical analysis. Both stages were implemented in the SPM5 software package (www.fil.ion.ucl.ac.uk/spm) using standard procedures. Spatially normalized, segmented, and modulated gray matter images were spatially smoothed with a 12-mm fill width at half maximum isotropic Gaussian kernel to allow inter-subject anatomic comparison. Age, gender, and total intracranial volume were entered into the design to control for any confounding effects. Regionally specific differences in gray matter volumes were assessed using the general linear model and the significance of each effect was determined by using the theory of Gaussian fields. The Anatomical Automatic Labeling atlas was used to identify regions with atrophy at a concentration of significance p<0.05 family-wise error (FWE) corrected or p<0.001 uncorrected.
Statistical Analysis
A priori power analysis (assuming 0.05 alpha, two-tailed t-test) based on the population effect sizes and SDs of previous studies reporting a significant difference in BDNF serum concentrations in patients with AD compared to healthy subjects was conducted. We had >0.8 power (1-βvalue) to detect differences in BDNF concentrations in each neurodegenerative disease group when comparing against healthy subjects assuming the predetermined effect sizes and SDs in the AD literature reported by Yasutake et al. (2006; Cohen’s d = −0.35), Lee et al. (2008; Cohen’s d = −0.38) , and Laske et al. (2006b; Cohen’s d = 0.42) respectively(AD: 1-βvalue for each study= 0.879, 0.941, 0.999; bvFTD: 0.872, 0.889, 0.970; SemD: 0.833, 0.836, 0.953; MCI: 0.923, 0.955, 0.988).Also, we had a power of >0.999 when comparing late stage AD and early stage AD when assuming the effect sizes and SDs reported by Laske et al. (2006b;Cohen’s d =-1.83). Thus, we had more than adequate power to detect the differences in BDNF concentrations between groups that have been reported in the literature.
To assess the impact of potential confounds on BDNF concentrations, general linear models were used to identify any independent relationship between BDNF and age, gender, and the cognitive tests. Next, to investigate whether disease severity could predict BDNF serum concentrations regardless of diagnostic group, a general linear model was constructed with MMSE (representing disease severity) as the primary predictor, age and gender as confounds, and BDNF concentrations as the dependent variable. To reveal any hidden effects in which membership in a single diagnostic group might predict BDNF concentrations even without a significant overall effect of diagnosis, we performed a second stage of this analysis in which each of the four diagnostic groups was parameterized (0=no, 1=yes) and entered as separate predictors. Then, a general linear model was performed to determine if diagnostic group membership predicted BDNF concentrations controlling for age and gender. Post-hoc Dunnett-Hsu tests were performed to identify whether BDNF concentrations in any diagnostic group differed from healthy subjects. Use of (1) acetylcholine esterase inhibitors (AChEIs) or (2) selective serotonin reuptake inhibitors (SSRIs) and/or serotonin & norepinephrine reuptake inhibitors (SNRIs) was analyzed in relation to BDNF concentrations. We used a mixed model to directly examine the interaction effect of medication and diagnostic group membership, although we had reduced power due to small sample sizes in some diagnosis-by-medication groups (e.g. few patients with MCI were taking AchEIs). All statistical analyses were carried out using the SAS® statistical analysis software package.
To examine the voxel-wise relationship between brain volume on BDNF serum concentrationsin VBM, a [1] and [−1] t-contrast was used assuming that lower BDNF concentrations would be associated with either lower or higher brain volumes respectively.
Results
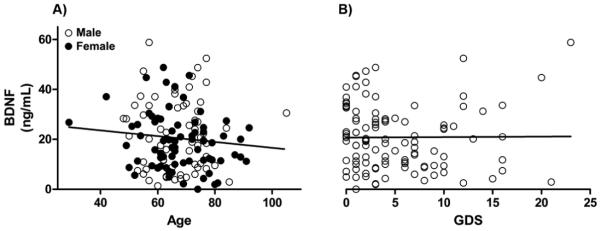
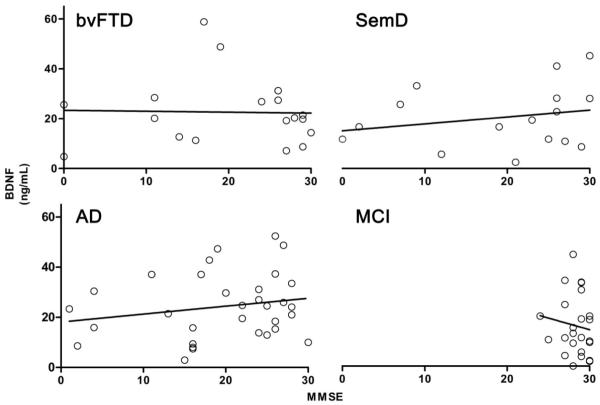
Demographic and clinical features of all subjects can be found in Table 1. There was no independent relationship of BDNF serum concentrations with age (F(1,136) = 1.381, p = 0.242), gender (F(1,135)=1.517, p = 0.220) (Fig. 1A), or depression as measured by the Geriatric Depression Scale (F(1, 99) = 1.237, p = 0.269) (Fig. 1B).There was a modest but significant negative relationship of BDNF serum concentration with some of the cognitive tests that assessed learning, information processing speed and cognitive control in complex situations including a) the filled dots switching test from the Design Fluency test (F(4, 74) = 4.40, p = 0.040),the Verbal Fluency test from the FAS (F(4, 76) = 8.99, p = 0.004), the immediate recall test from the MROCF (F(4, 115) = 4.16, p = 0.043), and the delayed 10 minute recall test from the MROCF (F(4, 112) = 8.67, p = 0.004). Partial correlations controlling for age and sex ranged from −0.18 to −0.34, suggesting small effect sizes. Disease severity, measured by MMSE, did not significantly predict BDNF serum concentrations (β = −0.005, t(119) = −0.06, p=0.954; Fig. 2). This was true within diagnostic groups as well (Fig. 3). Diagnostic group membership had no effect on BDNF concentrations (F(4,133) = 1.51, p = 0.2031; Fig. 4), even after further analyses to parameterize diagnosis and model BDNF independently in each diagnostic group.
Figure 1.
BDNF serum concentrations in relation to A) age & gender, & B) depression (while controlling for age and gender) as measured by the GDS using general linear models. Concentrations did not show significant relationships with age (F(1,136) = 1.381, p = 0.242), gender (F(1,135)=1.517, p = 0.220), or depression as measured by the Geriatric Depression Scale (F(1, 99) = 1.237, p = 0.269).
Figure 2.
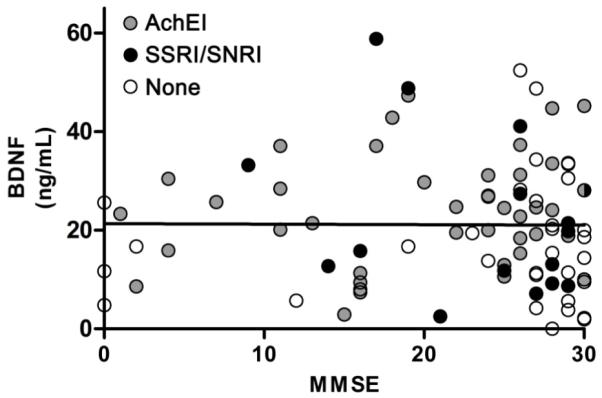
BDNF serum concentrations and disease severity, as measured by the MMSE, and medication status among all patients using general linear models controlling for age and gender. MMSE scores did not significantly predict BDNF serum concentrations (β = −0.005, t(119) = −0.06, p=0.954). Moreover, a separate model comparing patients taking AchEIs or SSRI/SNRIs compared to patients not on medications showed no significant relationships (AchEIs: F(1,86) = 1.10, p = 0.298; SSRIs/SNRIs: F(1,86) < 0.001, p = 0.999).
Figure 3.
BDNF serum concentrations & disease severity, as measured by MMSE, separated by diagnostic group. A) bvFTD, B) SemD, C) AD, D) MCI. The four diagnostic groups were parameterized and entered as separate predictors into a general linear model to determine if diagnostic group membership predicted BDNF concentrations controlling for age and gender. Disease severity did not significantly predict BDNF serum concentrations within any diagnostic group.
Figure 4.
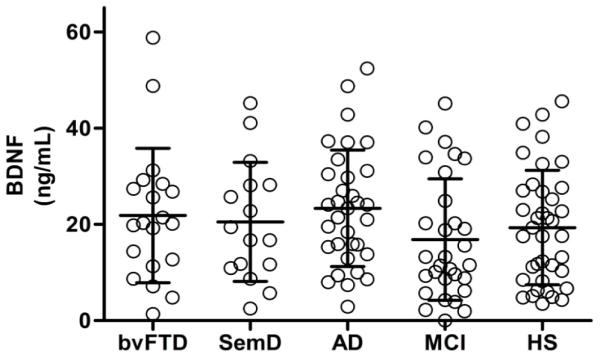
BDNF serum concentrations in relation to diagnostic group (Mean ± SD). A general linear model revealed that diagnostic group membership had no effect on BDNF concentrations (F(4,133) = 1.51, p = 0.2031) even after diagnoses were parameterized and modeled independently in each diagnostic group.
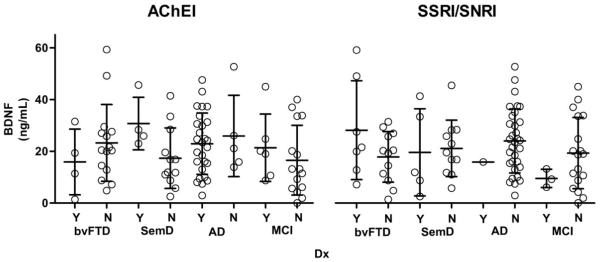
Medication status was available for a subset of subjects (Table 1). Pairwise analysis of medication status on BDNF concentrations within diagnostic groups was performed using mixed modeling of the complex interaction effect. BDNF serum concentrations did not significantly differ between participants who did not take any medications compared to participants taking acetylcholinesterase inhibitors (AchEIs) (F(1,86) = 1.10, p = 0.298), or SSRIs/SNRIs (F(1,86) < 0.001, p = 0.999; Fig. 2). No participants took a tricyclic antidepressant. Also, only one patient with bvFTD and one patient with AD took lithium, and this was not enough to perform a meaningful analysis. Diagnostic group pairwise comparisons from the least squares means showed no significant difference between BDNF concentrations and medication usage within each neurodegenerative group for AchEIs and SSRIs/SNRIs (Fig. 5).
Figure 5.
Effects of medication status on BDNF serum concentrations separated by diagnosis (Mean ± SD). Patients taking a type of medication are marked with a “Y” for yes, & patients not taking that medication are marked with an “N” for no. Least squares means (controlling for age and gender) pair-wise comparisons showed no difference between BDNF concentrations and medication usage within each neurodegenerative group.
VBM analysis of BDNF serum concentrations demonstrated no significant correlations with grey or white matter volume in any region in the brain at the more rigorous threshold of pFWE< 0.05, corrected for gender, age and total intracranial volume. Further exploration revealed no significant correlations even at an uncorrected (p < 0.001) concentration.
Discussion
In our study, we found no effect of neurodegenerative disease on BDNF serum concentrations. Studying multiple neurodegenerative diseases that affect different brain regions at once allowed us to investigate the specificity of BDNF-neurodegeneration relationships and survey a wide array of brain areas, but no pattern of brain atrophy corresponded with BDNF concentrations even at a very permissive statistical threshold. Our results suggest that BDNF serum concentrations may not serve as a reliable biomarker in detecting any of several types of neurodegenerative disease or in differentiating among these diseases. To our knowledge, this was the first study analyzing BDNF serum concentrations in patients with bvFTD or SemD. The present study is also the first to correlate BDNF serum concentrations with brain volume and cognitive impairment in patients with various neurodegenerative diseases.
The literature on BDNF concentrations in AD is mixed. Thus our results replicate some previous findings and challenge others. We did not find significant differences in BDNF serum concentrations in patients with AD compared to healthy subjects as reported by some studies [9, 30, 10] despite having adequate power to do so. Moreover, we failed to find a positive correlation between BDNF serum concentrations and MMSE or memory scores in AD as reported by previous studies [14, 10, 11]. Nevertheless, our results are consistent with those of O’Bryant et al. (2009) who used large sample sizes (98 patients with AD and 98 healthy subjects) and showed no differences in BDNF serum concentrations. Additionally, our results are consistent with three studies showing no significant correlation between BDNF serum concentrations and disease severity in AD alone [13, 12, 31]. However, our data show small negative associations of BDNF serum concentrations and learning, information processing speed and cognitive control suggesting that BDNF concentrations may still be related to subtle cognitive differences. Elevated central BDNF concentrations have been associated with poor working memory tasks in aged animals [32, 33]. Moreover, one study reported that patients with Parkinson’s disease with lower rates of central BDNF secretion performed better on a cognitive test assessing cognitive control in planning than patients with higher rates [34].
The source of the inconsistent results in the literature remains uncertain; however, we suspect that some unmeasured factors may be causing some of the discrepancies. For example, Val homozygote carriers of the BDNF gene have lower BDNF serum concentrations than the BDNF met-carrier subjects [35] which is particularly relevant for our study since the Val allele is more frequent in patients with AD compared to other neurodegenerative groups and healthy subjects [36]. Similarly, smoking [37, 38], body weight (both obesity and excessive thinness as seen in anorexia nervosa) [39], diet [40] and exercise [41, 42, 43] all influence BDNF concentrations and could be acting as confounds in studies of BDNF serum concentrations in neurodegenerative disease. Furthermore, differences in patient selection and characteristics (such as divergent pathological entities underlying a single clinical syndrome) may explain discrepancies in the literature.
Conclusion
We found no significant differences in BDNF serum concentrations of patients with bvFTD or SemD compared to healthy subjects or patients with AD or MCI. One limitation of the present study is that it examined peripheral BDNF concentrations in the blood. It remains possible that BDNF concentrations within the central nervous system may be variably affected in different neurodegenerative diseases [44], potentially warranting further study of BDNF concentrations in cerebrospinal fluid. Moreover, if a significant difference in BDNF serum concentrations exists between patients with bvFTD, SemD, MCI and healthy controls, the effect sizes may be smaller than in patients with AD. As a result, larger sample sizes in all groups may reveal differences. Lastly, low BDNF concentrations may trigger neurodegenerative diseases or fluctuate throughout the course of a disease. Measuring concentrations at multiple time points may thus be informative. With these caveats, however, our results suggest that BDNF serum concentrations may not be a useful biomarker to distinguish among neurodegenerative diseases.
Acknowledgments
We would like to thank the personnel of the Program Project Grant (PPG), Alzheimer’s Disease Research Center (ADRC), and General Clinical Research Center (GCRC), and Clinical and Translational Science Institute (CTSI) for their contributions in time and resources to this article. This research was supported in part by the National Institute on Aging (NIA) grants 5-P01 AG19724 and P50 AG023501, the State of California, Alzheimer’s Disease Research Center of California (ARCC) grant 03-7527.
Contributor Information
Joshua D. Woolley, University of California San Francisco, Langley Porter, Department of Psychiatry, 401 Parnassus Avenue, Room 159, San Francisco, CA 94143.
Eric V. Strobl, University of California San Francisco, Memory and Aging Center, Department of Neurology, 350 Parnassus Avenue, San Francisco, CA 94143.
Wendy B. Shelly, University of California San Francisco, Department of Obstetrics, Gynecology, and Reproductive Sciences, 2356 Sutter St., San Francisco, CA 94143.
Anna M. Karydas, University of California San Francisco, Memory and Aging Center, Department of Neurology, 350 Parnassus Avenue, San Francisco, CA 94143.
Robin Ketelle, University of California San Francisco, Memory and Aging Center, Department of Neurology, 350 Parnassus Avenue, San Francisco, CA 94143.
Owen M Wolkowitz, University of California San Francisco, Langley Porter, Department of Psychiatry, 401 Parnassus Avenue, Room 159, San Francisco, CA 94143.
Bruce L. Miller, University of California San Francisco, Memory and Aging Center, Department of Neurology, 350 Parnassus Avenue, San Francisco, CA 94143
Katherine P. Rankin, University of California San Francisco, Memory and Aging Center, Department of Neurology, 350 Parnassus Avenue, San Francisco, CA 94143
References
- [1].Arancio O, Chao MV. Neurotrophins, synaptic plasticity, dementia. Curr Opin Neurobiol. 2007;17:325–30. doi: 10.1016/j.conb.2007.03.013. [DOI] [PubMed] [Google Scholar]
- [2].Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–31. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- [4].Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–37. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–61. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- [6].Di Lazzaro V, Profice P, Pilato F, et al. BDNF plasma levels in acute stroke. Neurosci Lett. 2007;422:128–30. doi: 10.1016/j.neulet.2007.06.001. [DOI] [PubMed] [Google Scholar]
- [7].Ciammola A, Sassone J, Cannella M, et al. Low brain-derived neurotrophic factor (BDNF) levels in serum of Huntington’s disease patients. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:574–7. doi: 10.1002/ajmg.b.30501. [DOI] [PubMed] [Google Scholar]
- [8].Scalzo P, Kümmer A, Bretas TL, Cardoso F, Teixeira AL. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J Neurol. 2010;257:540–5. doi: 10.1007/s00415-009-5357-2. [DOI] [PubMed] [Google Scholar]
- [9].Yasutake C, Yanagawa T, Okamura T, Yoneda H. Serum BDNF, TNF-alpha, IL-1beta levels in dementia patients. World J Biol Psychiatry. 2005;256:402–6. doi: 10.1007/s00406-006-0652-8. [DOI] [PubMed] [Google Scholar]
- [10].Lee JG, Shin BS, You YS, et al. Decreased serum brain-derived neurotrophic factor levels in elderly korean with dementia. Psychiatry Investig. 2009;6:299–305. doi: 10.4306/pi.2009.6.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yu H, Zhang Z, Shi Y, et al. Association study of the decreased serum BDNF concentrations in amnestic mild cognitive impairment and the Val66Met polymorphism in Chinese Han. J Clin Psychiatry. 2008;69:1104–11. doi: 10.4088/jcp.v69n0710. [DOI] [PubMed] [Google Scholar]
- [12].Angelucci F, Spalletta G, di Iulio F, et al. Alzheimer’s disease (AD) and mild cognitive impairment (MCI) patients are characterized by increased BDNF serum levels. Current Alzheimer Research. 2010;7:15–20. doi: 10.2174/156720510790274473. [DOI] [PubMed] [Google Scholar]
- [13].O’Bryant SE, Hobson V, Hall JR, et al. Brain-derived neurotrophic factor levels in Alzheimer’s disease. J Alzheimers Dis. 2009;17:337–41. doi: 10.3233/JAD-2009-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Laske C, Stransky E, Leyhe T, et al. Stage-dependent BDNF serum concentrations in Alzheimer’s disease. J Neural Transm. 2006b;113:1217–24. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- [15].Leyhe T, Stransky E, Eschweiler GW, Buchkremer G, Laske C. Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 2008;258:124–8. doi: 10.1007/s00406-007-0764-9. [DOI] [PubMed] [Google Scholar]
- [16].Leyhe T, Eschweiler GW, Stransky E, et al. Increase of BDNF serum concentration in lithium treated patients with early Alzheimer’s disease. J Alzheimers Dis. 2009;16:649–56. doi: 10.3233/JAD-2009-1004. [DOI] [PubMed] [Google Scholar]
- [17].Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer disease. Arch Gen Psychiatry. 2008;65:439–45. doi: 10.1001/archpsyc.65.4.439. [DOI] [PubMed] [Google Scholar]
- [18].Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–32. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- [20].Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol. 2007;114:31–8. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- [21].Mack WJ, Freed DM, Williams BW, Henderson VW. Boston Naming Test: Shortened versions for use with Alzheimer’s disease. Journal of Gerontology. 1992;47:154–8. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- [22].Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) The Psychological Corporation; San Antonio, TX: 2001a. [Google Scholar]
- [23].Brink TL, Yesavage JA, Lum O, Heersema P, Adey MB, Rose TL. Screening tests for geriatric depression. Clinical Gerontologist. 1982;1:37–44. [Google Scholar]
- [24].Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey MB, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- [25].Sheikh JI, Yesavage JA. Clinical Gerontology : A Guide to Assessment and Intervention. The Haworth Press; NY: 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version; pp. 165–73. [Google Scholar]
- [26].Sheikh JI, Yesavage JA, Brooks JO, III, Friedman LF, Gratzinger P, Hill RD, Zadeik A, Crook T. Proposed factor structure of the Geriatric Depression Scale. International Psychogeriatrics. 1991;3:23–8. doi: 10.1017/s1041610291000480. [DOI] [PubMed] [Google Scholar]
- [27].Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique.(Les problems.) Archives de Psychologie. 1941;28:215–85. [Google Scholar]
- [28].Osterrieth PA. Filetest de copie d’une figure complex: Contribution a l’etude de la perception et de la memoire. Archives de Psychologie. 1944;30:286–356. [Google Scholar]
- [29].Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. Oxford University Press; Oxford: 2004. [Google Scholar]
- [30].Laske C, Stransky E, Leyhe T, et al. Decreased brain-derived neurotrophic factor (BDNF)- and β-thromboglobulin (β-TG)- blood levels in Alzheimer’s disease. Thromb Haemost. 2006a;96:102–3. doi: 10.1160/TH06-03-0173. [DOI] [PubMed] [Google Scholar]
- [31].Laske C, Stransky E, Leyhe T, et al. BDNF serum and CSF concentrations in Alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387–94. doi: 10.1016/j.jpsychires.2006.01.014. [DOI] [PubMed] [Google Scholar]
- [32].Bimonte HA, Hunter CL, Nelson ME, Granholm AC. Frontal cortex BDNF levels correlate with working memory in an animal model of Down Syndrome. Behavioural Brain Research. 2003a;139:47–57. doi: 10.1016/s0166-4328(02)00082-7. [DOI] [PubMed] [Google Scholar]
- [33].Bimonte HA, Nelson ME, Granholm AC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiology of Aging. 2003b;24:37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- [34].Foltynie T, Cheeran B, Williams-Gray CH, Edwards MJ, Schneider SA, Weinberger D, Rothwell JC, Barker RA, Bhatia KP. BDNF val66met influences time to onset of levodopa induced dyskinesia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2005;80:141–4. doi: 10.1136/jnnp.2008.154294. [DOI] [PubMed] [Google Scholar]
- [35].Ozan E, Okur H, Eker C, Eker OD, Gönül AS, Akarsu N. The effect of depression, BDNF gene val66met polymorphism and gender on serum BDNF levels. Brain Res Bull. 2010;81:61–5. doi: 10.1016/j.brainresbull.2009.06.022. [DOI] [PubMed] [Google Scholar]
- [36].Fehér A, Juhász A, Rimanóczy A, Kálmán J, Janka Z. Association between BDNF Val66Met polymorphism and Alzheimer disease, dementia with Lewy bodies, and Pick disease. Alzheimer Dis Assoc Disord. 2009;23:224–8. doi: 10.1097/WAD.0b013e318199dd7d. [DOI] [PubMed] [Google Scholar]
- [37].Kim TS, Kim DJ, Lee H, Kim YK. Increased plasma brain-derived neurotrophic factor levels in chronic smokers following unaided smoking cessation. Neurosci Lett. 2007;423:53–7. doi: 10.1016/j.neulet.2007.05.064. [DOI] [PubMed] [Google Scholar]
- [38].Bhang SY, Choi SW, Ahn JH. Changes in plasma brain-derived neurotrophic factor levels in smokers after smoking cessation. Neurosci Lett. 2010;468:7–11. doi: 10.1016/j.neulet.2009.10.046. [DOI] [PubMed] [Google Scholar]
- [39].Nakazato M, Hashimoto K, Shimizu E, et al. Decreased levels of serum brain-derived neurotrophic factor in female patients with eating disorders. Biol Psychiatry. 2003;54:485–90. doi: 10.1016/s0006-3223(02)01746-8. [DOI] [PubMed] [Google Scholar]
- [40].Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neural plasticity, and learning. Neuroscience. 2002;112:803–14. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- [41].Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gómez-Pinilla F. Exercise reverses the hamful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–40. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- [42].Soya H, Nakamura T, Deocaris CC, et al. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007;358:961–7. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- [43].Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–61. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- [44].Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–22. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]