Abstract
Background
Cardiac output (CO) is a useful measure of myocardial performance. CO monitoring is frequently performed in critically ill adults in order to guide physicians’ treatment strategies. However, standard methods of determining CO in children are not without risk and can be problematic secondary to their invasive nature and other technical problems. COstatus® system (Transonic Systems Inc, NY, USA), which is based on ultrasound dilution technology, works off in situ catheters and uses an innocuous indicator to allow for routine measurements of cardiac output and blood volumes in pediatric patients. The purpose of this study was to validate CO measured by COstatus® with those obtained by the clinical standard technique of pulmonary artery (PAC) thermodilution.
Methods
This was a prospective evaluation performed at a single institution. Any child with a structurally normal heart undergoing hemodynamic evaluation in the cardiac catheterization laboratory was included. A prograde right heart catheterization was performed, and CO was first determined by using the PAC thermodilution technique. Thermodilution results were then compared with CO measurements obtained using the COstatus system. The results were analyzed by standard correlation, Bland-Altman, and Crichtley and Critchley analyses.
Results
Twenty-eight patients were evaluated with a median age of 8 yrs and a median weight of 31 kg. The mean thermodilution cardiac index = 3.18 L/min (+/− 1.35 L/min), and the mean COstatus® cardiac index = 3.17 L/min (+/− 1.31 L/min). Standard Pearson correlation tests revealed an excellent correlation coefficient of 0.95 (p<0.0001). Bland-Altman analysis revealed good clinical agreement with a mean difference of −0.004 L/min with a precision of 0.8 L/min/ at 2 SD. A percentage error of 25.4% was noticed in this study which is less than the clinically acceptable limit.
Conclusion
The ultrasound dilution technique of determining CO using the COstatus® system provides a less invasive method than the traditional pulmonary artery thermodilution for accurately determining cardiac output in children. This is the first validation of the COstatus® system in pediatric patients. Further studies are required to establish its accuracy in pediatric patients with cardiac shunts and other hemodynamically unstable conditions.
Introduction
Accurate measurement of cardiac output (CO) is an important part of advanced hemodynamic monitoring to better manage the care of the patients in the pediatric intensive care unit [1–4]. It provides valuable information about the cardiac function, helps guide titration of therapies, and also identifies low CO states that compromise vital organs which impacts patient mortality in certain disease conditions [1, 5–7].
Thermodilution using a pulmonary artery catheter (PAC) is regarded as a “clinical standard” and is routinely used to measure CO in adult patients. However, its use in pediatrics is limited [7–9] due to its invasive nature and associated complications [10]. Although less invasive methods such as femoral artery thermodilution, Doppler, bioimpedance and pulse contour methods are currently available, none of them are routinely used at the bedside with pediatric patients [1, 11–12], especially in low weight and new born patients [13]. Additionally, clinical estimation of CO using surrogate markers such as heart rate, blood pressure, capillary refill, and end organ function has been inconsistent at best and has been found to show a poor correlation with the measured CO values [14–15].
COstatus® (Transonic Systems Inc, NY, USA), a new minimally invasive system based on ultrasound dilution technology, was recently introduced as a means to measure CO in children. This system works off in situ standard arterial and central venous catheters and uses isotonic saline as an indicator [16]. The theory of ultrasound dilution is based on changes in blood ultrasound velocity. The baseline blood ultrasound velocity (~1560–1585 m/sec) is a function of the total blood protein concentration (sum of proteins in the plasma and red cells), temperature, and average ion concentration in the plasma. Injection of body temperature isotonic saline (ultrasound velocity of ~1533 m/sec) reduces the blood ultrasound velocity detected at the arterial sensor producing a dilution curve. CO is then calculated using the Stewart-Hamilton principle (Fig 2). The theory and in vitro validation of this technique has been reported in previous literature [16], and this technique was recently validated in various animal models against the “gold standard” ultrasound flow probes [17–18] and in adult humans against the PAC thermodilution [19–20] and transpulmonary thermodilution [21] techniques. However, this technique has not been validated in pediatric patients. The purpose of this study was to validate COstatus® measured CO with PAC thermodilution measured CO in the pediatric population.
Figure 2.
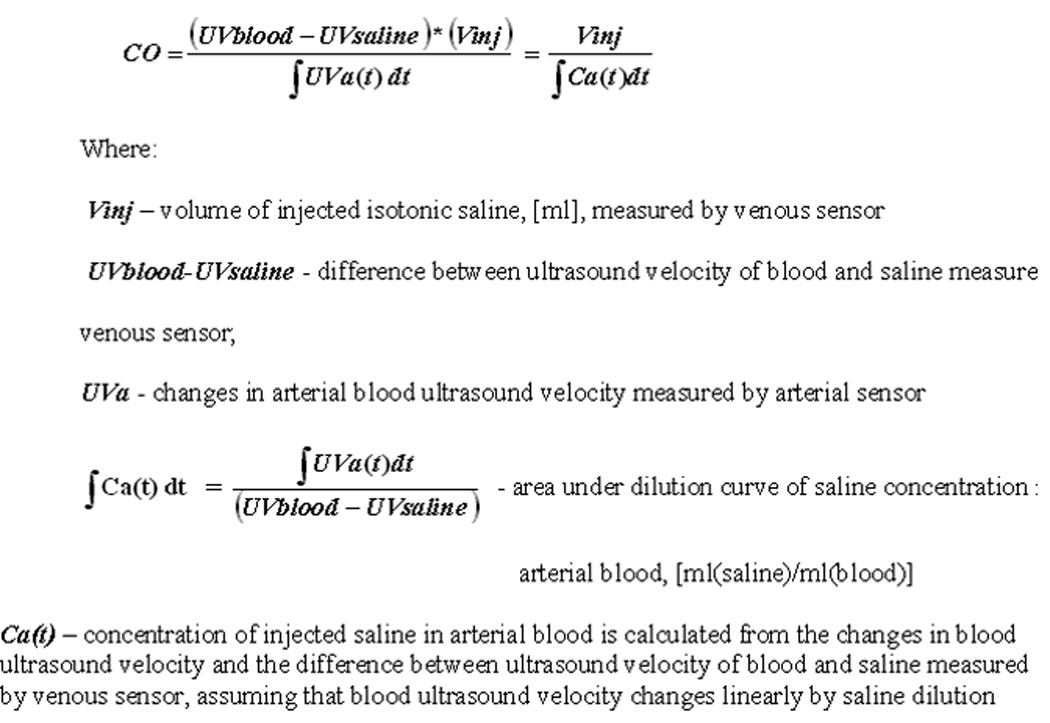
Stewart-Hamilton principle. Reproduced with permission from Krivitski et al [16].
Methods
We performed a prospective, single center evaluation, which was approved by the institutional review board. Any child with a structurally normal heart, undergoing hemodynamic assessment in the cardiac catheterization laboratory from May 2008 to June 2010 was offered the opportunity to participate in the study. Formal written consent was obtained in all cases. Of the 28 patients studied, 27 were patients who underwent cardiac transplantation and 1 patient was diagnosed with pulmonary hypertension. Children with known intra-cardiac or extra-cardiac shunting as well as those with significant atrioventricular valve regurgitation were excluded.
Thermodilution
For this study, each patient underwent a prograde right heart catheterization. The cardiac index (CI) was determined via placement of a pulmonary arterial catheter and standard thermodilution technique using room temperature saline. All patients were under general anesthesia during the study. Cardiorespiratory status was continuously monitored. Thermodilution measurements were performed in triplicates, and the results were averaged. The thermodilution catheter was then removed.
Ultrasound dilution
COstatus® measurements were then performed using an extracorporeal arterial-venous loop (AV loop with priming volume ~5ml) primed with heparinized saline connected between the indwelling femoral artery catheter and the side-arm of the central venous catheter (Fig 1). Two ultrasound flow-dilution sensors were placed on the arterial and venous ends of the AV loop. The sensor on the venous side measures the amount of injected volume and checks the quality of injection. The sensor on the arterial side is used to measure the dilution curve. The dilution curve is formed the change in the concentration of the isotonic saline indicator after it travels through the patient’s cardiopulmonary system. A roller pump is used to circulate blood through this closed AV loop from the artery to the vien at a slow rate of 10–12 ml/min for the duration of measurements, which is about 5–8 minutes including the time for 2–3 injections. Body temperature isotonic saline in the order of 0.5–1 ml/kg up to a maximum of 30ml is injected into the venous side of the AV loop to obtained CO by COstatus®. At the end of the measurement cycle (5–8 minutes of pump operation), the AV loop is flushed and the blood from the extracorporeal system is returned back into the venous side [16].
Figure 1.
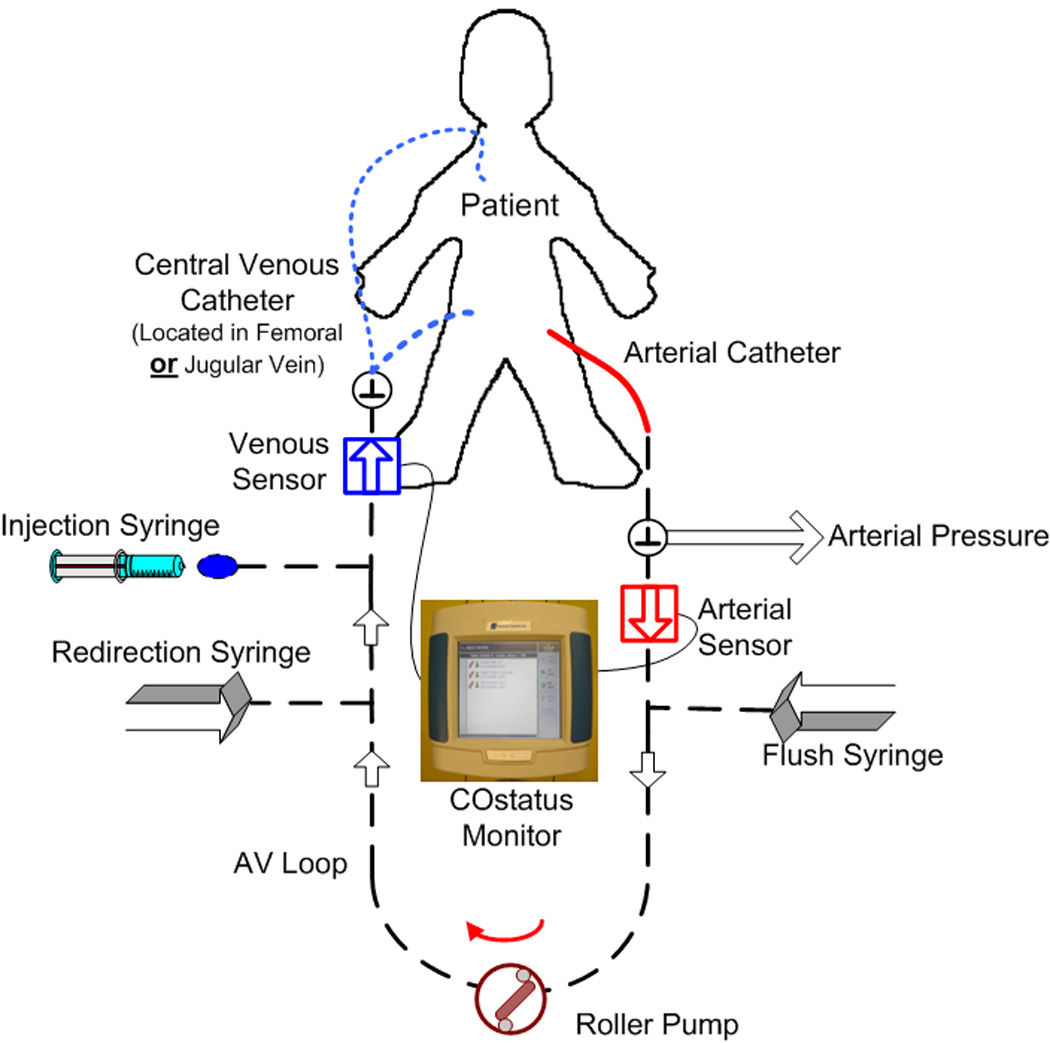
Schematic of Arterio-venous loop connected to in-situ arterial and central venous catheters in the patient.
Triplicate injections were performed using body temperature isotonic saline for ultrasound dilution measurements. An additional injection was done if an alert “Repeat” message came up on the results screen. These results were then compared to the results obtained via the thermodilution technique. Statistical analysis was performed on the cohort using standard correlation techniques, linear regression, percentage error and Bland-Altman analysis.
In collaboration with Transonic Systems Inc, NY, USA, the COstatus® ultrasound dilution algorithm was adapted for use in the pediatric population when a long venous sheath was used. This modification consisted of the insertion of a correction factor to account for the residual injected volume remaining in the long sheath and thus not entering the patient’s circulation for measurement.
Results
Twenty-eight patients (14 female / 14 male) were included in the study, and a total of 84 comparisons were performed. The median patient age was 8 yrs (range 1–17 yrs), and the median patient weight was 31.1 kg (range 9.4–73.8 kg). Ten children weighed < 20 kg and ten children were aged 5 years or less at the time of study. Of the 28 patients studied, 23 of them had the venous catheter placed in the femoral vein while 5 of them had it placed in their jugular vein. All patients had their arterial catheter placed in the femoral artery. Requirement for catheters, location, and size of the catheters was independent of this study. All patients studied had no residual shunts or valvular insufficiency of hemodynamic significance.
All patients had at least three paired measurements. The maximum number of injections performed on a single patient was 5 for PAC thermodilution. For COstatus®, the maximum number of injections performed was 4. The additional injections in the COstatus® were performed secondary to a “Repeat” message displayed on the results screen. Excluding such measurements, the remaining were averaged for comparison.
The mean CO obtained by PAC thermodilution technique was 3.18 +/−1.35 L/min (range 1.26–6.10 L/min with a median = 2.78 L/min (IQR; 2.14–4.54 L/min). The median CI was 3.02 (IQR; 2.74–3.4 L/min/m2). The mean CO obtained by the COstatus® ultrasound dilution technique was 3.17+/−1.32 L/min (range 1.51–5.90 L/min) with a median of 2.92 L/min (IQR; 2.01–4.25 L/min). The median CI was 3.05 L/min/m2 (IQR; 2.81–3.45 L/min/m2). Linear regression analysis and standard Pearson correlation technique revealed excellent correlation between the two methods with a coefficient of 0.95 (p<0.0001) (Fig 3). Bland-Altman analysis [22] revealed good clinical agreement between the two methods with a small bias of −0.004 L/min, and a precision at 2 standard deviations of 0.8 L/min/m2. The plot differences between the absolute CO values obtained by PAC thermodilution and COstatus® ultrasound dilution methods, vs. their respective means showed a good agreement with all the data points lying between the limits of agreement, which were between − 0.81 L/min and + 0.80 L/min (Fig 4). A percentage error (2SD/mean of ref method) of 25.4% was found in this study, which is less than the clinically acceptable limit of 30%, per Critchley and Critchely’s criteria [23]. Hence COstatus® CO measurements could be considered equivalent and interchangeable with thermodilution CO, in this study population. No adverse events or complications were attributed to this study.
Figure 3.
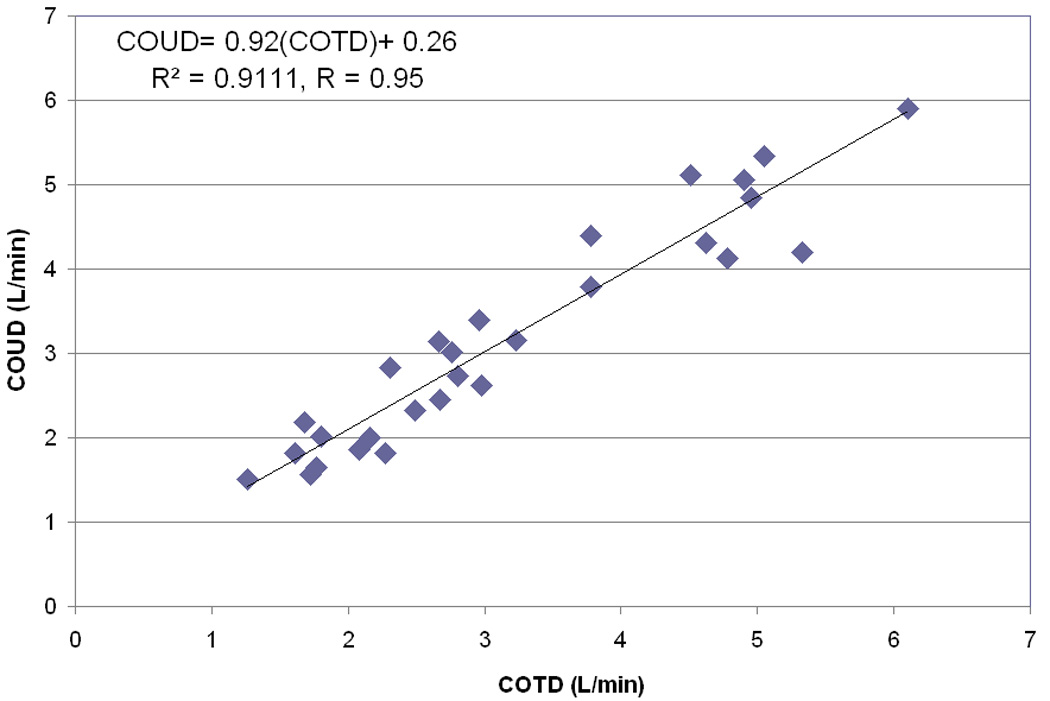
Correlation plot for the entire data set COstatus =0.92(Thermodilution) + 0.26 L/min/m2 (r = 0.95; p < 0.0001).
Figure 4.
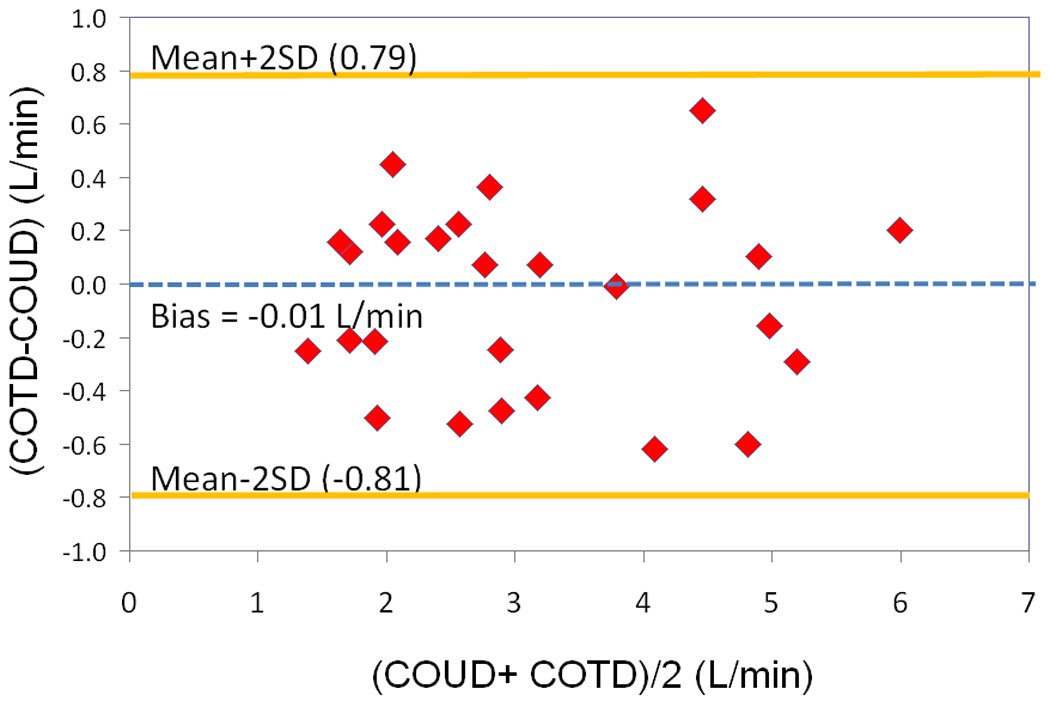
Bland- Altman analysis for the entire data set. Bias = −0.0041 L/min. Precision at 2 SD = 0.8 L/min.
Discussion
Measurement of CO is important for hemodynamic management of the critically ill in pediatric patients. CO provides an objective means by which to guide the initiation of medical or mechanical based therapies, identify low CO states, and follow a patient’s response to instituted therapies [1–4]. Though several techniques have been developed to measure CO in the last decade, some of which are in part applicable to pediatrics, none of them emerged as an ideal method for routine CO measurement in infants and children [1, 17]. As a consequence, a reliable means of monitoring cardiac output in pediatrics has been eagerly sought after.
In this study, CO measurement with the COstatus® system, which is based on ultrasound dilution technology, was compared with the PAC thermodilution method. PAC thermodilution method could be considered the current clinical standard and is commonly used to validate new CO measurement methods [4, 19–20, 24]. We chose the pediatric post heart transplant patient population undergoing hemodynamic evaluation in our catheterization laboratory primarily because all these patients have a PAC catheter and a central venous and arterial catheter placed irrespective of this study. These catheters allowed us to connect the extracorporeal AV loop between in situ catheters for COstatus® system and obtain CO measurements from both methods. To our knowledge, this is the first clinical validation study in pediatric patients.
When comparing CO measurements obtained by two different techniques, the bias and percentage error are crucial to the evaluation of clinical agreement between the new method and the reference method [1]. Prior studies in adult patients [19–21] comparing COstatus® CO with thermodilution measurements showed a good agreement with a small bias and percentage error of 20–23%. In this study in children presenting to the cardiac catheterization laboratory, we also found a small bias of −0.004 l/min with a percentage error of 25.4%, suggesting the results are comparable with those obtained in adult studies. Critchley and Critchley [23] suggest that if the percentage error between the two methods is ≤ ± 30% then the two methods are interchangeable. They [23] suggest using percentages rather than absolute values, unless the mean cardiac output approximates 5 L/min. Despite a lower mean CO in this study, we found the percentage error to be well within the accepted limits at 25.4%. Also, although the value of correlation in validation studies can be debated, some studies [3, 25, 26] suggest that a correlation coefficient of ≥ 0.8 indicates a strong positive relationship. In this study we found a correlation of 0.95 between the two methods suggesting a strong positive relationship between the two methods. This correlation is also comparable with those noticed in adult validation studies [19–21].
COstatus Advantages
The major advantages of this system include: use of existing standard arterial and venous catheters, use of a physiological indicator (isotonic saline), and the absence of blood loss. These make the system ideal for use with pediatric ICU patients. The applicability of this technique in neonatal [29] and infant [30, 31] patient populations has also been studied by different groups. Additionally, COstatus® also provides® blood volume measurements which could reflect the hemodynamic volumetric status of the patients [16, 21]. Hence, the COstatus system could potentially be used routinely with neonatal and pediatric ICU patients.
COstatus Limitations
The observed limitations of this system are that it does not provide continuous CO measurements, can be only used with patients that have both arterial and venous catheters, and it requires injection of 0.5–1ml/kg (upto a maximum of 30ml) saline for each measurement. Thus, repeat injections during multiple sessions could constitute a significant volume load for those small patients who have marginal hemodynamics. Also, the COstatus® system has not been extensively validated when intra/extra cardiac shunts are present. Currently, this precludes its use in many pre/post surgical pediatric patients with underlying congenital heart disease, although, recent animal studies suggest that the accuracy of the COstatus® system may be maintained even when intra/extra cardiac shunts are present (27).
Study Limitations
There are also some inherent limitations of this study. First, comparisons were only made to the pulmonary arterial thermodilution technique and not to the Fick estimations of CO. Pulmonary arterial thermodilution is frequently accepted as the “clinical standard” but whether it can be regarded as the “gold standard” remains a debated topic (28). Also, this study was performed in a controlled setting with all patients under general anesthesia, thus simulating a steady hemodynamic state. Critically ill patients often have considerable fluctuations in their physiology as medical/mechanical therapies are altered, and this study did not interrogate validity when more extreme hemodynamic alterations are present. Further randomized studies are warranted to ensure that the accuracy is maintained in such patients.
Conclusions
COstatus® system, which is based on ultrasound dilution technology, offers a minimally invasive approach for accurately and routinely measuring cardiac output in children. The system works off standard in situ catheters and uses isotonic saline as an indicator. This first validation study in pediatrics shows that the bias and percentage error were clinically acceptable in this population. Hence, we believe that the COstatus system could be considered interchangeable with current standard methods of CO measurement. Further studies are required to establish its accuracy in pediatric patients with cardiac shunts and other hemodynamically unstable conditions.
Acknowledgement
This study was supported by SBIR grant # R44HL061994 from National Institutes of Health, Bethesda, MD. We would like to thank NIH for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have not disclosed any potential conflicts of interest
References
- 1.Nusmeier A, Hoeven J, Lemson J. Cardiac output monitoring in pediatric patients. Expert Rev. Med. Devices. 2010;7:503–517. doi: 10.1586/erd.10.19. [DOI] [PubMed] [Google Scholar]
- 2.Tibby SM, Murdoch IA. Monitoring cardiac function in intensive care. Arch Dis Child. 2003;88:46–52. doi: 10.1136/adc.88.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calamandrei M, Lorenzo M, Muschetta S, et al. Assessment of cardiac output in children: A comparison between the pressure recording analystical method and Doppler echocardiography. Pediatr Crit Care Med. 2008;9:310–312. doi: 10.1097/PCC.0b013e31816c7151. [DOI] [PubMed] [Google Scholar]
- 4.Kim JJ, Dreyer WJ, Chang AC, et al. Arterial pulse wave analysis: An accurate means of determining cardiac output in children. Pediatr Crit Care Med. 2006;7:532–535. doi: 10.1097/01.PCC.0000243723.47105.A2. [DOI] [PubMed] [Google Scholar]
- 5.Ceneviva G, Paschall J, Maffei F, et al. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics. 1998;102:e19. doi: 10.1542/peds.102.2.e19. [DOI] [PubMed] [Google Scholar]
- 6.Masse L, Antonacci M. Low cardiac output syndrome: Identification and management. Crit Care Nurs Clin N Am. 2005;17:375–383. doi: 10.1016/j.ccell.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Mohan U, Britto J, Habibi P, et al. Noninvasive measurement of cardiac output in critically ill children. Pediatr Cardiol. 2002;23:58–61. doi: 10.1007/s00246-001-0014-2. [DOI] [PubMed] [Google Scholar]
- 8.Maruschak G, Potter A, Schauble J, et al. Overestimation of pediatric cardiac output by thermal indicator loss. Circulation. 1982;65:380–383. doi: 10.1161/01.cir.65.2.380. [DOI] [PubMed] [Google Scholar]
- 9.Singer M, Bennett D. Pitfalls of pulmonary artery catheterization highlighted by Doppler ultrasound. Crit Care Med. 1989;17:1060–1061. doi: 10.1097/00003246-198910000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Elliott CG, Zimmerman GA, Clemmer TP. Complications of pulmonary artery catheterization in the care of critically ill patients: a prospective study. Chest. 1979;76:647–652. doi: 10.1378/chest.76.6.647. [DOI] [PubMed] [Google Scholar]
- 11.Egan J, Festa M, Cole A, et al. Clinical assessment of cardiac performance in infants and children following cardiac surgery. Intens Care Med. 2005;31:568–573. doi: 10.1007/s00134-005-2569-5. [DOI] [PubMed] [Google Scholar]
- 12.Mahajan A, Shabanie A, Turner J, et al. Pulse contour analysis of cardiac output monitoring in cardiac surgery for congenital heart disease. Anesth Analg. 2003;97:1283–1288. doi: 10.1213/01.ANE.0000081797.61469.12. [DOI] [PubMed] [Google Scholar]
- 13.Soleymani S, Borzage M, Seri I. Hemodynamic monitoring in neonates: advances and challenges. J of Perinatol. 2010;30 doi: 10.1038/jp.2010.101. S38-S. [DOI] [PubMed] [Google Scholar]
- 14.Lemson J, de Boode W, Hopman J, et al. Validation of transpulmonary thermodiltuion cardiac output measurement in pediatric animal model. Pediatr Crit Care. 2008;9:313–319. doi: 10.1097/PCC.0b013e31816c6fa1. [DOI] [PubMed] [Google Scholar]
- 15.Tibby S, Hatherill M, Marsh M, et al. Clinicians’ abilities to estimate cardiac index in ventilated children and infants. Archives of Disease in Childhood. 1997;77:516–518. doi: 10.1136/adc.77.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krivitski N, Kislukhin V, Thuramalla N. Theory and in-vitro validation of a new extracorporeal approach for hemodynamic assessment in pediatric and neonatal ICU patients. Pediatr Crit Care. 2008;9:423–428. doi: 10.1097/01.PCC.0b013e31816c71bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Boode W, van Heijst A, Hopman J, et al. Cardiac output measurement using an ultrasound dilution method: A validation study in ventilated piglets. Pediatr Crit Care Med. 2009;11:103–108. doi: 10.1097/PCC.0b013e3181b064ea. [DOI] [PubMed] [Google Scholar]
- 18.Gleed R, Smith T, Callahan M, et al. Validation of novel ultrasound dilution cardiac output method for pediatric and neonatal patients. Intensive Care Med. 2006;32:S172. [Google Scholar]
- 19.Tsutsui M, Matsuoka N, Ikeda T, et al. Comparison of a New Cardiac Output Ultrasound Dilution Method With Thermodilution Technique in Adult Patients Under General Anesthesia. J Cardiothorac Vasc Anesth. 2009;23:835–840. doi: 10.1053/j.jvca.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Eremenko A, Safarov P. Flow-regulated extracorporeal arteriovenous tubing loop for cardiac output measurements by ultrasound velocity dilution: Validation in post-cardiac surgery intensive care unit patients. ASAIO J. doi: 10.1097/MAT.0b013e3181effdf8. [DOI] [PubMed] [Google Scholar]
- 21.Galstyan G, Bychynin M, Alexanyan M, et al. Comparison of cardiac output and blood volumes in intrathoracic compartments measured by ultrasound dilution and transpulmonary thermodilution methods. Intensive Care Med. doi: 10.1007/s00134-010-2003-5. [DOI] [PubMed] [Google Scholar]
- 22.Bland J, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 23.Critchley L, Critchley A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999;15:85–91. doi: 10.1023/a:1009982611386. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez J, Delafosse C, Fartoukh M, et al. Comparison of bedside measurement of cardiac output with the thermodilution method and the Fick method in mechanically ventilated patients. Crit Care. 2003;7:171–178. doi: 10.1186/cc1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou K, Tuncali K, Silverman S. Correlation and simple linear regression. Radiology. 2003;227:617–622. doi: 10.1148/radiol.2273011499. [DOI] [PubMed] [Google Scholar]
- 26.Levy R, Chiavacci R, Nicolson S, et al. An evaluation of a noninvasive cardiac output measurement using partial carbon dioxide rebreathing in children. Anesth Analg. 2004;99:1642–1647. doi: 10.1213/01.ANE.0000136952.85278.99. [DOI] [PubMed] [Google Scholar]
- 27.Vrancken S, De Boode W, Hopman J, et al. Cardiac output measurement is feasible in the presence of left to right shunt with ultrasound dilution method: a validation study in lambs. Critical care. 2010;14:97. [Google Scholar]
- 28.Stetz C, Miller R, Kelly G, et al. Reliability of thermodilution method in the determination of cardiac output in clinical practice. Am Rev Respir Dis. 1982;126:1001–1004. doi: 10.1164/arrd.1982.126.6.1001. [DOI] [PubMed] [Google Scholar]
- 29.Marr B. Novel System for Measurement of Cardiac Output and Blood Volumes in Neonatal ICU Patients. Crit Care Med. 2010;38:219. [Google Scholar]
- 30.Boehne M, Baustert M, Happel CM, et al. Novel Ultrasound Dilution Technique Detects Left-to-Right Shunts with High Accuracy in Children. Pediatric Research. 2010;117:224. [Google Scholar]
- Perez De Sa V, Johansson S, Olsson AK, et al. Accuracy and Precision of a New Method for Hemodynamic Assessment in Children Undergoing Cardiac Surgery Based on Ultrasound Dilution Method. Pediatric Research. 2010;118:226. [Google Scholar]


