Abstract
Objective
Based on promising in vitro and in vivo activity of several histone deacetylase inhibitors (HDACi’s) in HL (Hodgkin lymphoma), we investigated SNDX-275, an oral class 1 isoform–selective HDACi in HL-derived cell lines.
Materials and Methods
Proliferation and cell death were examined by MTS assay, Annexin-V/propidium iodide, and FACS analysis. Gene and protein expression were measured by RT-PCR, Western blotting, and immunohistochemical analysis. A multiplex assay was used to determine cytokines and chemokines.
Results
SNDX-275 induced cell death in a dose- and time-dependent manner with an IC50 at the sub- and lower micromolar range at 72 hours. At the molecular level, SNDX-275 increased histone H3 acetylation, up-regulated p21 expression, and activated the intrinsic apoptosis pathway by down-regulating the X-linked inhibitor of apoptosis protein (XIAP). SNDX-275 down-regulated the expression of antiapoptotic Bcl-2 and Bcl-xL proteins without altering Mcl-1 or Bax levels. Combination studies demonstrated that two Bcl-2 inhibitors (ABT-737 and obatoclax) significantly enhanced the effect of SNDX-275. SNDX-275 modulated the level of several cytokines and chemokines, including IL-12 p40-70, IP-10, RANTES, IL-13, IL-4, and TARC, and variably induced the cancer/testis antigen expression of MAGE-A4 and survivin in HL cell lines.
Conclusions
SNDX-275 has antiproliferative activity in HL cell lines, involving several mechanisms: induction of apoptosis, regulation of cytokines and chemokines, and alteration of CTAs. Clinical investigation of SNDX-275 alone or in combination with Bcl-2 inhibitors is warranted in patients with HL. Phase 2 studies with SNDX-275 in HL are ongoing, and future clinical studies should investigate combinations with SNDX-275.
Keywords: Hodgkin lymphoma, Histone deacetylase inhibitor (HDACi), SNDX-275
Introduction
Classic Hodgkin lymphoma (HL) is one of the most curable human cancers. However, treatment of patients with relapsed and refractory disease, especially those who relapse after autologous stem cell transplantation, remains challenging: the median survival for these patients is typically less than 3 years. Several new targeted therapy agents, currently being investigated in a clinical setting, are showing promise [1].
Posttranscriptional modification of DNA and histones can be performed by methylation, acetylation, phosphorylation, ubiquitination, and sumoylation [2]. Histone acetylation and deacetylation, important epigenetic processes, are highly regulated by various enzymes, including histone acetyltransferases and histone deacetylases (HDACs) [3]. The complex of DNA and histones tends to shut down or not be expressed when deacetylated, whereas inhibition of deacetylation results in a more open chromatin, which is functional or expressed. This epigenetic modification plays an important role in regulating the expression of genes that are responsible for cell proliferation, survival, angiogenesis, and immunity [4, 5], thus HDACs have become attractive targets for cancer therapy.
To date, 18 HDACs are known; they are either zinc-dependent or nicotinamide adenine dinucleotide (NAD)-dependent and are grouped into one of four classes: class I (HDACs 1, 2, 3, and 8), class II (HDACs 4, 5, 6, 7, 9, and 10), class III (SIRT1- SIRT7), and class IV (HDAC 11) [6, 7]. Currently, two HDAC inhibitors (HDACi’s)—vorinostat and romidepsin—have been approved by the Food and Drug Administration for the treatment of patients with relapsed cutaneous T-cell lymphoma [8, 9]. Specifically, vorinostat and romidepsin are referred to as pan-DAC inhibitors, in contrast to mocetinostat (MGCD0103) and entinostat (SNDX-275), which inhibit only certain HDAC groups [10].
SNDX-275 (formerly MS-275) is an oral class 1 isoform–selective HDACi, a synthetic benzamide derivative. It inhibits cancer cell growth with a half maximal inhibitory concentration (IC50) in the submicromolar range. Inhibition of cell growth is accompanied by cell cycle arrest and induction of the cyclin-dependent kinase (CDK) inhibitor p21waf1, one of the most commonly induced genes by HDACi’s [11]. SNDX-275 has shown promising activity, both in vitro and in vivo, against various cancer types, including colorectal, lung, ovarian, and pancreatic cancers [12], pediatric solid tumors [13], leukemia [12, 14–16], prostate cancer [17], and breast cancer [18–20].
Class I selective inhibitor MGCD0103 has shown promising clinical activity, but its adverse effects are significant [21, 22]; thus, SNDX-275 may be a more tolerable alternative for patients. In the current study, we investigated the in vitro activity and molecular mechanisms of SNDX-275 in HL-derived cell lines.
Materials and Methods
Cell lines, cell culture, and reagents
Human HL-derived cell lines (HD-LM2, L-428, KM-H2), anaplastic large cell lymphoma (ALCL) cell lines (KARPAS 299, SUP-M2, SUP-DHL-1), and mantle cell lymphoma (MCL) cell lines (Mino, Jeko-1, SP53) were obtained from the German Collection of Microorganisms and Cell Cultures, Department of Human and Animal Cell Cultures, Braunschweig. The phenotypes and genotypes of these cell lines have been previously published (HD-LM2 of T-cell origin, L-428 and KM-H2 of B-cell origin) [23]. Cell lines were cultured in RPMI 1640 medium supplemented with 10% or 20% heat-inactivated fetal bovine serum (Gibco BRL, Gaithersburg, MD), 1% L-glutamine, and penicillin/streptomycin in a humid environment of 5% CO2 at 37°C.
The HDACi SNDX-275 was obtained from Syndax (Waltham, MA). Gemzar (gemcitabine) was purchased from Lilly Pharmaceuticals (Indianapolis, IN). The proteasome inhibitor, bortezomib (PS-341) was provided by Millennium Pharmaceuticals, Inc. (Cambridge, MA). Bcl-2 inhibitor obatoclax (OBT, GX-15070) was obtained from Gemin X (Malvern, PA); Bcl-2 inhibitor ABT-737 was Dr Michael Andreeff (M. D. Anderson Cancer Center). Antibodies to acetylated histone H3; caspases 3, 8, 9; Bcl-2; Bax; HDAC-1; PARP; and X-linked inhibitor of apoptosis protein (XIAP) were purchased from Cell Signaling Technology (Beverly, MA). Antibodies for p21, Bcl-xL, and Mcl-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody to β-actin was from Sigma Chemicals Co. (St. Louis, MO). The Human Thirty-Plex Antibody bead kit was purchased from Invitrogen Corporation (Camrillo, CA).
In vitro proliferation assay
Cells were cultured in 6-, 12-, and 24-well plates at a concentration of 0.5 × 106 cells/mL. Cell viability was assessed with the nonradioactive cell proliferation MTS assay by using CellTiter96AQueous One Solution Reagent (Promega, Madison, WI), as previously described [24]. Briefly, 80 μL of cell suspension with 20 μL of CellTiter96AQueous One Solution Reagent were incubated in 96-well plates for 1 hour at 37°C, 5% CO2; formazan absorbance was measured at 490 nm on a μQuant plate reader equipped with KC4 software (Biotek Instruments, Winooski, VT). Each measurement was made in triplicate, and the mean value was determined.
Flow cytometry
Cell surface expression was determined by fluorescence-activated cell sorter (FACS), as previously described. Apoptosis was determined by Annexin-V-FLUOS and propidium iodide (PI) double staining (Roche Molecular Biochemicals, Indianapolis, IN), according to manufacturer’s instructions and our previous publications [24, 25]. Data were collected on a FACSCalibur flow cytometer by using Flowjo software (Tree Star Inc, Ashland, OR), as described previously [24, 25]. Data were analyzed with use of FlowJo software (Treestar Inc.), and results were shown as the mean of three independent experiments.
Enzyme-linked immunosorbent assay (ELISA)
HL cell lines were incubated with increasing doses of SNDX-275 (0.1 to 2 μM) and DMSO (0.1%) for 24 to 72 hours, before supernatants were collected and examined for IL-12 p40-70, IP-10, RANTES, IL-13, IL-4, and TARC levels by Human Thirty-Plex Antibody bead kit (Invitrogen Corporation) according to the manufacturer’s instructions. Each experiment was performed in triplicate, and results represent mean values from three different experiments.
Western blot analysis
Total cellular proteins were extracted by incubation in lysis buffer (Cell Signaling Technology) for 30 minutes on ice and then centrifuged to remove cellular debris. The protein in the resulting supernatant was quantified by the BCA method (Pierce Chemical Co., Rockford, IL) according to the manufacturer’s instructions. Next, protein was diluted 1:2 in protein SDS loading buffer (Cell Signaling Technology) and heated to 95°C for 5 minutes. A total of 30 μg of protein was loaded onto 12% Tris-HCl SDS polyacrylamide electrophoresis Ready Gels (Bio-Rad, Hercules, CA), transferred to a nitrocellulose transfer membrane (Bio-Rad), and detected by using SuperSignalWest Dura Extended Duration Substrate (Pierce Chemical Co.), as previously described [24, 25].
Reverse transcriptase–polymerase chain reaction
Cancer/testis antigen (CTA) expression was analyzed by reverse transcriptase–polymerase chain reaction (RT-PCR) by using an RNeasy mini kit (Qiagen, Valencia, CA) and a reverse transcriptase system (Promega). RNA, isolated from HL-derived cell lines by using Qiagen RNeasy Micro Kits (Qiagen), was used as a template for RT-PCR by using the OneStep RT-PCR Kit (Qiagen) with gene-specific primers to MAGE-A4, PRAME, SSX2, NY-ESO-1, and survivin (purchased from SABiosciences, Frederick, MA) and normalized to expression of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (SABiosciences). To measure CTA gene expression, cells were cultured with 0.1% DMSO, 0.5 μM or 1.0 μM SNDX-275, and vorinostat. At 72 hours after exposure, cells were harvested and analyzed for CTA transcripts with use of the OneStep RT-PCR with the above gene-specific primers and at conditions specified by the manufacturer. In some experiments, the hypomethylating agent, 5-aza-20-deoxycytidine (Decitabine; Sigma-Aldrich), was used as a positive control. PCR bands were visualized on 1% agarose gels with ethidium bromide (Sigma).
Immunohistochemical analysis
Cell lines were cultured in the presence of 0.1% DMSO, 0.5 μM or 1.0 μM SNDX-275, and vorinostat for 72 hours. In some experiments, the hypomethylating agent, 5-aza-20-deoxycytidine (Decitabine; Sigma-Aldrich), was used as a positive control After incubation, cells were harvested and resuspended in PBS (Sigma-Aldrich), mounted onto glass slides (Shandon Cytospin 4 Cytocentrifuge; Thermo Scientific, Waltham, MA), and then fixed with 4% paraformaldehyde (BD Cytofix; BD Biosciences, Sparks, MD). Cells were subsequently permeabilized by using 0.3% Triton-X-100 (Gibco) for 5 minutes followed by incubation with Digest ALL1 (Zymed, San Francisco, CA) for 10 minutes at 37°C. To block endogenous peroxidase activity, cells were treated with 3% hydrogen peroxide (Sigma). We used PowerVision kits (ImmunoVision Technologies, Daly City, CA) according to the manufacturer’s recommendation for immunohistochemical analysis. Briefly, slides were first incubated in pre-block/diluent for 30 minutes followed by incubation with MAGE-A4, PRAME, SSX2, NY-ESO-1, and survivin antibody (AbCam, Cambridge, MA), and diluted 1:50 in dilutant for 1 hour at room temperature. We used anti-mouse/anti-rabbit horseradish peroxidase to detect positive cells, followed by enzymatic conversion using the chromogenic substrate 3,3′-diaminobenzidine (DAB). A control picture of the slide was taken without the use of antibodies.
Statistical methods, isobologram, and combination index calculation
The effectiveness of the drugs used in the present study, and of combinations of these drugs, was analyzed with use of Calcusyn Software (Biosoft, Ferguson, MO). The combination index and isobologram plot were calculated according to the Chou-Talalay method [24, 25]. A Combination Index (CI) value of 1 indicates an additive effect between two drugs. Combination Index values <1 indicate synergy, and the lower the value, the stronger the synergy. In contrast, Combination Index values >1 indicate antagonism. Effects of certain conditions on cell proliferation, apoptosis, and cytokine production were performed in three independent experiments in triplicate. The two-tailed Student t test was used to estimate statistical significance of the differences in results from the three experiments. The level of significance was 0.05 if marked with * and was 0.005 if marked with **.
Results
SNDX-275 shows antiproliferative activity in a dose- and time-dependent manner and increases histone H3 acetylation and p21 expression
We investigated the in vitro effect of SNDX-275 in HL-derived cell lines (HD-LM2, L-428, KM-H2), ALCL cell lines (KARPAS 299, SUP-M2, SUP-DHL-1), and MCL cell lines (Mino, Jeko-1, SP53) to determine its antiproliferative activity. These cell lines were cultured with DMSO (0.1%) or SNDX-275 (0.1–2 μM) for 24 to 72 hours; cell viability was determined by MTS assay, which revealed antiproliferative activity in a dose- and time-dependent manner. Among HL-derived cell lines, HD-LM2 and L-428 were more sensitive. Among ALCL cell lines, KARPAS 299 showed remarkable sensitivity, whereas MCL cell lines were less sensitive (Fig. 1A). The more sensitive cell lines (HL: HD-LM2, L-428; ALCL: KARPAS 299) had IC50 values in the submicromolar range, whereas the rest had values in the micromolar range (Fig. 1B). HL-derived cell lines were co-cultured with either DMSO (0.1%) or SNDX-275 (0.1–2 μM) for 48 hours, and Western-blot analysis was performed.
Figure 1.
Antiproliferative activity of SNDX-275 in Hodgkin lymphoma (HL). Anaplastic large cell lymphoma (ALCL) and mantle cell lymphoma (MCL) cell lines. (A) SNDX-275 exerted its antiproliferative effect in a dose- and time-dependent manner. All cell lines were incubated with DMSO (0.1%) or increasing doses of SNDX-275 (0.1–2 μM) for 24 to 72 hours, and cell viability was determined with use of the MTS assay. Values represent a mean of at least 3 experiments ±SEM. (B) IC50 values of 9 cell lines, incubated with SNDX-275 for 72 hours. (C) Molecular effects of SNDX-275 treatment in HL cell lines. HD-LM2, L-428, and KM-H2 cells were incubated with DMSO (0.1%) or SNDX-275 (0.1 to 2 μM) for 48 hours, and intracellular proteins were determined by Western blotting. SNDX-275 increased histone H3 acetylation and up-regulated p21 protein expression from 0.1 μM concentration. HDAC1 was used as a positive control. Protein loading was verified by β-actin.
A common feature of HDACi’s is their ability to acetylate histones, resulting in the restoration of the expression of tumor suppressor genes, such as p21 [11]. We therefore first examined the effect of SNDX-275 on histone H3 acetylation and p21 expression. Acetylation of histone H3 was first seen at a 0.1 μM concentration at 48 hours, which was associated with p21 expression. Expression of HDAC1 was used as a positive control (Fig. 1C).
SNDX-275 induces apoptosis through the intrinsic apoptosis pathway by down-regulating XIAP
To determine whether antiproliferative activity of SNDX-275 works through apoptosis, Annexin-V/PI staining and FACS analysis was performed with or without DMSO (0.1%) or SNDX-275 (1 μM) for 72 hours. A representative example of three independent experiments is shown (Fig. 2A). The average percentages of Annexin-V/PI–positive cells with SNDX-275 (HD-LM2: 67.52%; L-428: 57.08%; KM-H2: 54.31%) were significantly higher than were percentages of Annexin-V/PI–positive cells with or without DMSO (HD-LM2: 7.49%, 7.67%; L-428: 7.34%, 8.23%; KM-H2: 3.83%, 4.33%) (**p < 0.005) (Fig. 2B).
Figure 2.
SNDX-275 induces apoptosis in Hodgkin lymphoma (HL) cells lines HD-LM2, L-428, and KM-H2. (A) Cells were incubated with DMSO (0.1%) or SNDX-275 (1 μM) for 72 hours, and apoptotic cells were determined by Annexin-V-FLUOS/propidium iodide (PI) staining and FACS analysis. Viable cells are shown in the lower left quadrant. Percentages in the upper right quadrant indicate Annexin-V/PI–positive cells. A representative example of three independent experiments is shown. (B) A summary of 3 independent experiments ±SEM demonstrating ratio of Annexin-V/PI–positive cells versus DMSO or RPMI. Differences shown are significant (**p < 0.005). (C) Cells from HL cell lines HD-LM2, L-428, and KM-H2 were incubated with DMSO (0.1%) or SNDX-275 (0.1 to 2 μM) for 48 hours, and intracellular proteins were determined by Western blotting. The intrinsic apoptosis pathway was activated by down-regulating the antiapoptotic X-linked inhibitor or apoptosis (XIAP) protein, which was associated with activation of caspases 9 and 3. Protein loading was verified by β-actin.
We also analyzed several components of the apoptosis pathway. Caspase 9 cleavage, observed at a 0.1–1.0 μM concentration, was associated with the down-regulation of XIAP and caspase 3. However, the caspase 8 level remained unchanged, suggesting that the intrinsic apoptosis pathway is responsible for PARP cleavage and hence apoptosis. These data are in accordance with the MTS results showing that HD-LM2 and L-428 are more sensitive cell lines and that KM-H2 is the less sensitive (Fig. 2C).
SNDX-275 decreases antiapoptotic Bcl-2 protein family expression, Bcl-2 inhibitors enhances synergistically the effect of SNDX-275
Induction of apoptosis by HDACi’s has been linked to alterations in gene expression, resulting in down-regulation of antiapoptotic and up-regulation of proapoptotic proteins [26]. Similarly, our data demonstrated that levels of antiapoptotic Bcl-2 and Bcl-xL were decreased at a concentration of 2 μM SNDX-275 after 48 to 72 hours of incubation. Interestingly, Mcl-1 and proapoptotic Bax remained unchanged (Fig. 3A). The overexpression of Bcl-2 in hematological malignancies can be the reason for poor therapeutic outcome [27], which provides the rationale for combining Bcl-2 inhibitors with SNDX-275. Hence, combination studies were performed with the MTS assay using DMSO (0.1%), SNDX-275 (0.1–2 μM), and either Bcl-2 inhibitor ABT-737 (0.01–0.2 μM) or obatoclax (0.1–2 μM); furthermore, either gemcitabine (0.001–0.02 μM) or bortezomib (0.001–0.02 μM) were used for 72 hours.
Figure 3.
Effect of SNDX-275 on Bcl-2 family proteins as a single agent and in combinations. (A) Cells from HL cell lines HD-LM2, L-428, and KM-H2 were incubated with DMSO (0.1%) or SNDX-275 (2 μM) for 48 to 72 hours, and intracellular proteins were determined by Western blotting. SNDX-275 down-regulated the expression of antiapoptotic Bcl-2 and Bcl-xL, without altering the level of Mcl-1 or Bax. Protein loading was verified by β-actin. (B) Molecular effects of incubating HL cells with combinations of SNDX-275 (2 μM) and obatoclax (2 μM) or ABT-737 (0.2 μM) for 72 hours; intracellular proteins were determined by Western blotting. The intrinsic apoptosis pathway is involved similarly to SNDX-275 alone. Caspases 9 and 3, and PARP cleavage can be seen; however, cleavage is weaker with obatoclax alone and in combination with SNDX-275 than with ABT-737. Protein loading was verified by β-actin.
All three cell lines at every investigated concentration level were found to be synergistic with ABT-737, thus showing that HDACi’s and Bcl-2 inhibitors make a reasonable combination. Similarly, obatoclax showed synergism at every investigated concentration level in KM-H2, at two conditions in HD-LM2 (SNDX-275 1 μM + obatoclax 1 μM and SNDX-275 2 μM + obatoclax 2 μM), and at one condition in L-428 (SNDX-275 2 μM + obatoclax 2 μM). With gemcitabine and with bortezomib, much poorer synergism was seen. Only at one condition in L-428 (SNDX-275 0.5 μM + gemcitabine 0.005 μM) and at all conditions in KM-H2. With bortezomib, synergy could be seen at one condition in HD-LM2 (SNDX-275 2.0 μM + bortezomib 0.02 μM) and at two conditions in KM-H2 (SNDX-275 0.5 μM + bortezomib 0.005 μM and SNDX-275 1.0 μM + bortezomib 0.01 μM). Combinations are considered synergistic when Combination Index (CI) values are less than 1 (Table 1).
Table 1.
SNDX-275 in combinations. SNDX-275 had synergistic effects when combined with Bcl-2 inhibitors ABT-737 and obatoclax in Hodgkin lymphoma (HL) cells. Less synergy can be seen with gemcitabine and the proteasome inhibitor bortezomib. HL cells were incubated with DMSO (0.1%), SNDX-275 (0.1 to 2 μM), obatoclax (0.1 to 2 μM), ABT-737 (0.01 to 0.2 μM), bortezomib (0.001–0.02 μM), gemcitabine (0.001–0.02 μM) or the combination of SNDX-275 and ABT-737, obatoclax, bortezomib or gemcitabine, respectively, and cell viability was determined at 72 hours with the MTS assay. Values represent a mean of at least 3 independent experiments ±SEM each in triplicates. Combinations are considered synergistic if CI values are less than 1 (shown bold in the table).
| SNDX-275 (μM) | ABT-737 (μM) | CI | SNDX-275 (μM) | Obatoclax (μM) | CI | SNDX-275 (μM) | Gemcitabine (μM) | CI | SNDX-275 (μM) | Bortezomib (μM) | CI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HD-LM2 | 0.1 | 0.01 | 0.955 | 0.1 | 0.1 | 1.801 | 0.1 | 0.001 | 2.103 | 0.1 | 0.001 | 1.983 |
| 0.5 | 0.05 | 0.687 | 0.5 | 0.5 | 1.229 | 0.5 | 0.005 | 1.315 | 0.5 | 0.005 | 1.248 | |
| 1 | 0.1 | 0.818 | 1 | 1 | 0.968 | 1 | 0.01 | 1.198 | 1 | 0.01 | 1.064 | |
| 2 | 0.2 | 0.845 | 2 | 2 | 0.405 | 2 | 0.02 | 1.136 | 2 | 0.02 | 0.862 | |
| L-428 | 0.1 | 0.01 | 0.256 | 0.1 | 0.1 | 2.158 | 0.1 | 0.001 | 1.447 | 0.1 | 0.001 | 1.181 |
| 0.5 | 0.05 | 0.798 | 0.5 | 0.5 | 1.06 | 0.5 | 0.005 | 0.822 | 0.5 | 0.005 | 1.069 | |
| 1 | 0.1 | 0.879 | 1 | 1 | 1.008 | 1 | 0.01 | 1.024 | 1 | 0.01 | 1.119 | |
| 2 | 0.2 | 0.866 | 2 | 2 | 0.881 | 2 | 0.02 | 1.062 | 2 | 0.02 | 1.167 | |
| KM-H2 | 0.1 | 0.01 | 0.1 | 0.1 | 0.1 | 0.186 | 0.1 | 0.001 | 0.098 | 0.1 | 0.001 | 3.761 |
| 0.5 | 0.05 | 0.494 | 0.5 | 0.5 | 0.126 | 0.5 | 0.005 | 0.014 | 0.5 | 0.005 | 0.996 | |
| 1 | 0.1 | 0.669 | 1 | 1 | 0.131 | 1 | 0.01 | 0.424 | 1 | 0.01 | 0.892 | |
| 2 | 0.2 | 0.288 | 2 | 2 | 0.23 | 2 | 0.02 | 0.176 | 2 | 0.02 | 1.65 |
We wanted to further demonstrate that combinations with Bcl-2 inhibitors work the same way as SNDX-275 alone. Hence, we used the end-concentrations of the MTS assay studies (SNDX-275: 2 μM; obatoclax: 2 μM; ABT-737: 0.2 μM) and performed Western-blot analysis. Similar to results seen with use of SNDX-275 alone, caspases 9 and 3, PARP cleavage was finally observed, thus confirming our hypothesis, although weaker cleavage could be seen both in the case of obatoclax alone and in combination with SNDX-275 than with ABT-737 (Fig. 3B).
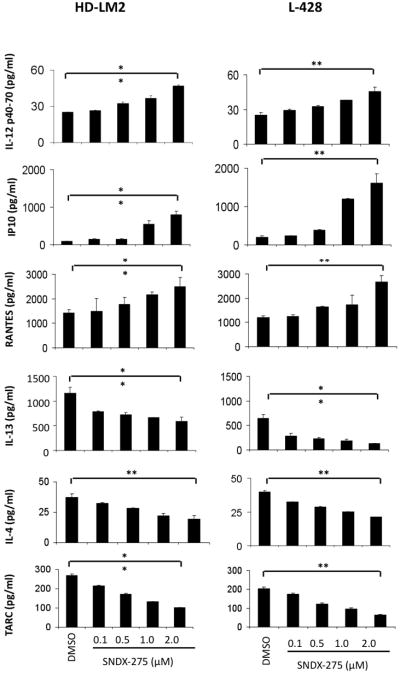
SNDX-275 has an impact on cytokines/chemokines, which leads to a more favorable Th1 response
It is well-known that HL has a dysregulated cytokine background, which helps to maintain an environment in which effective immune response against Hodgkin Reed- Sternberg (HRS) cells cannot be achieved [28]. Hence, to examine whether SNDX-275 can affect this cytokine production, we measured the level of several major cytokines/chemokines with a Human Thirty-Plex Antibody bead kit in the presence of DMSO (0.1%) or SNDX-275 (0.1–2 μM) for 48 hours. The level of cytokine IL-12 p40-70, which promotes Th1 cell differentiation, increased significantly. Also, levels of chemokines IP-10 and RANTES, which are responsible for Th1 recruitment, increased significantly. IP-10 is a ligand for CXCR3, whereas RANTES is a ligand for both CCR3 and CCR5. IL-4, which is required for Th2 cell differentiation, decreased significantly, as did IL-13, the key growth factor of HRS cells. The Th2 chemokine TARC also decreased significantly (**p < 0.005). Collectively, these data demonstrated that all investigated cytokines and chemokines responsible for Th1 immune response increased, whereas those required for Th2 response decreased (Fig. 4).
Figure 4.
Effect of SNDX-275 on cytokine/chemokine production. By using a Human Thirty-Plex Bead Kit, cytokine/chemokine production was analyzed for the effect of DMSO (0.1%) or SNDX-275 (0.1 to 2 μM). SNDX-275 increased levels of IL-12 p40-70, IP-10, and RANTES, and decreased levels of IL-13, IL-4, and TARC, thus favoring Th1-type cytokines/chemokines. Changes in the level of cytokines/chemokines are significant (**p < 0.005).
Effect of SNDX-275 is limited to the expression of CTAs
Previous studies demonstrated that epigenetic modulatory agents, including hypomethylating drugs and HDAC inhibitors, can induce the expression of CTAs (also called cancer germ-line antigens) in a variety of tumors, but little is known of the ability of HDAC inhibitors to promote CTA expression in HL.[29] [30]. We therefore investigated the effect of SNDX-275 on the expression of common CTAs. SNDX-275 slightly induced the expression of MAGE-A4 in L-428 and of survivin in HD-LM2 (Fig. 5A), but had no significant effect on SSX2 or NY-ESO-1 expression (Supplemental figure 1). In contrast, the pan HDAC inhibitors vorinostat and panobinostat variably induced the expression of these CTAs (Supplemental figure 1). Immunohistochemical analysis showed that neither a 0.5 μM (data not shown) nor a 1.0 μM concentration of SNDX-275 or vorinostat induced the expression of the investigated proteins MAGE-A4, PRAME, or survivin (Fig. 5B). However, vorinostat promoted the expression of NY-ESO-1 in the HD-LM2 cells (Supplemental figure 1). When the hypomethylating agent decitibine was used as a control, it was more potent in inducing SSX2 and NY-ESO-1 in the HD-LM2 cells (Supplemental figure 1).
Figure 5.
Expression of cancer/testis antigens (CTAs) in Hodgkin lymphoma (HL) cell lines with histone deacetylase inhibitors (HDACi’s) SNDX-275 and vorinostat. HL cell lines HD-LM2, L-428, and KM-H2 were cultured with HDACi’s SNDX-275 and vorinostat at a concentration of 0.5 μM and 1.0 μM for 72 hours. CTA expression was analyzed by reverse transcriptase–polymerase chain reaction and immunohistochemically. (A) HDACi’s induced the expression of MAGE-A4 in L-428 and survivin in HD-LM2 at the RNA level. (B) At the protein level, immunohistochemical analysis showed that none of the investigated CTAs were changed.
Discussion
Because HDACs regulate a variety of cell functions that are involved in cell survival, cell cycle progression, angiogenesis, and immunity, they are considered to be promising targets for cancer therapy, including lymphoma [31–41]. In the current study, we provided pre-clinical rationale for evaluating the HDAC inhibitor SNDX-275 (entinostat) in patients with relapsed HL
SNDX-275 showed a potent antiproliferative activity in a time- and dose-dependent manner, which is comparable to our recent experience with MGCD0103 [42]. SNDX-275 antiproliferative activity was primarily mediated by activating the intrinsic caspase pathway and induction of apoptosis. A similar effect was recently observed with other HDAC inhibitors, including vorinostat [43] and MGCD0103 [44]. This effect was augmented by downregulating the inhibitor of apoptosis protein, XIAP.
Targeting the Bcl-2 pathway remains to be a major interest, since the overexpression of these proteins can be the reason of resistance of several hematological malignancies [27, 46]. Therefore we combined SNDX-275 with Bcl-2 family inhibitor ABT-737, which is reported to bind to Bcl-2, Bcl-xL and Bcl-w in various preclinical models. [47–49] Interestingly, SNDX-275 decreased the expression level of Bcl-2 and Bcl-xL, but had no significant effect on Mcl-1 or Bax. These favorable effects, coupled with the reported mechanisms of ABT-737 and obatoclax, resulted in an enhanced caspase 3 and PARP cleavage and synergistic antiproliferative effects. In contrast, the synergistic interaction between SNDX-275 and gemcitibine or bortezomib was not as obvious, perhaps due to their potent single agent efficacy against HL cell lines.
In addition to its direct antiproliferative activity, our results demonstrate that SNDX-275 may also influence tumor growth and survival by altering the balance of cytokines and chemokines in the HL microenvironment, and by enhancing tumor antigens that may promote a favorable antitumor immune response [28, 50]. SNDX-275 increased the production of interleukin (IL)-12, which is responsible for Th1 cell differentiation, and decreased the production of IL-13 and IL-4, which promote Th2 differentiation. Furthermore, SNDX-275 altered the balance between chemokines that are involved in attracting Th2 cells to HL microenvironment, including, IP-10 and RANTES, and TARC. These changes in the microenvironment were associated with upregulation of a variety of CTAs, although to a less degree when compared with other HDAC inhibitors.
Collectively, our results indicate that SNDX-275 has a favorable antiproliferative activity by a direct induction of cell death in HL cells, in addition to an indirect effect on the microenvironment and immunity. Based on these favorable pre-clinical activities, we initiated a phase II study of SNDX-275 to determine its safety and efficacy in patients with relapsed classical HL.
Supplementary Material
A) In vitro effect of HDAC inhibitors or decitibine on the expression of SSX2 and NY-ESO-1 in HL cell lines as determined by the RT-PCR method. B) The effect of HDAC inhibitors and decitibine on NY-ESO-1 protein expression was determined by immunohistochemistry in HL cell lines.
Acknowledgments
Financial support: This research is supported in part by the National Institutes of Health (NIH) through MD Anderson’s Cancer Center Support Grant CA016672, NCI Lymphoma SPORE grant 1P50CA136411-01A1, The Living Legend Fund, and the Prince Fund for Lymphoma (Anas Younes), by NIH grant P50 CA126752, the Gillson Longenbaugh Foundation, and the M. D. Anderson Foundation (Catherine M. Bollard), and by The Hungarian American Enterprise Scholarship Fund (Ádám Jóna).
Footnotes
Conflict of Interest: none of the authors have relevant conflict of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jona A, Younes A. Novel treatment strategies for patients with relapsed classical Hodgkin lymphoma. Blood Rev. 2010;24:233–238. doi: 10.1016/j.blre.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 4.Heider U, Kaiser M, Sterz J, et al. Histone deacetylase inhibitors reduce VEGF production and induce growth suppression and apoptosis in human mantle cell lymphoma. Eur J Haematol. 2006;76:42–50. doi: 10.1111/j.1600-0609.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 5.Brogdon JL, Xu Y, Szabo SJ, et al. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. 2007;109:1123–1130. doi: 10.1182/blood-2006-04-019711. [DOI] [PubMed] [Google Scholar]
- 6.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 7.Botrugno OA, Santoro F, Minucci S. Histone deacetylase inhibitors as a new weapon in the arsenal of differentiation therapies of cancer. Cancer Lett. 2009;280:134–144. doi: 10.1016/j.canlet.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piekarz RL, Frye R, Turner M, et al. Phase II Multi-Institutional Trial of the Histone Deacetylase Inhibitor Romidepsin As Monotherapy for Patients With Cutaneous T-Cell Lymphoma. J Clin Oncol. 2009;27:5410–5417. doi: 10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan N, Jeffers M, Kumar S, et al. Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem J. 2008;409:581–589. doi: 10.1042/BJ20070779. [DOI] [PubMed] [Google Scholar]
- 11.Gui C-Y, Ngo L, Xu WS, Richon VM, Marks PA. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. PNAS. 2004;101:1241–1246. doi: 10.1073/pnas.0307708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito A, Yamashita T, Mariko Y, et al. A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. PNAS. 1999;96:4592–4597. doi: 10.1073/pnas.96.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaboin J, Wild J, Hamidi H, et al. MS-27-275, an Inhibitor of Histone Deacetylase, Has Marked in Vitro and in Vivo Antitumor Activity against Pediatric Solid Tumors. Cancer Res. 2002;62:6108–6115. [PubMed] [Google Scholar]
- 14.Gojo I, Jiemjit A, Trepel JB, et al. Phase 1 and pharmacologic study of MS-275, a histone deacetylase inhibitor, in adults with refractory and relapsed acute leukemias. Blood. 2007;109:2781–2790. doi: 10.1182/blood-2006-05-021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas DM, Davis ME, Parthun MR, et al. The histone deacetylase inhibitor MS-275 induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia cells. Leukemia. 2004;18:1207–1214. doi: 10.1038/sj.leu.2403388. [DOI] [PubMed] [Google Scholar]
- 16.Rosato RR, Almenara JA, Grant S. The Histone Deacetylase Inhibitor MS-275 Promotes Differentiation or Apoptosis in Human Leukemia Cells through a Process Regulated by Generation of Reactive Oxygen Species and Induction of p21CIP1/WAF1 1. Cancer Res. 2003;63:3637–3645. [PubMed] [Google Scholar]
- 17.Yang J, Wezeman M, Zhang X, et al. Human C-reactive protein binds activating Fcgamma receptors and protects myeloma tumor cells from apoptosis. Cancer Cell. 2007;12:252–265. doi: 10.1016/j.ccr.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Huang X, Gao L, Wang S, Lee C-K, Ordentlich P, Liu B. HDAC Inhibitor SNDX-275 Induces Apoptosis in erbB2-Overexpressing Breast Cancer Cells via Down-regulation of erbB3 Expression. Cancer Res. 2009;69:8403–8411. doi: 10.1158/0008-5472.CAN-09-2146. [DOI] [PubMed] [Google Scholar]
- 19.Singh TR, Shankar S, Srivastava RK. HDAC inhibitors enhance the apoptosis-inducing potential of TRAIL in breast carcinoma. Oncogene. 2005;24:4609–4623. doi: 10.1038/sj.onc.1208585. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Zhou J-Y, Wei W-Z, Philipsen S, Wu GS. Sp1-Mediated TRAIL Induction in Chemosensitization. Cancer Res. 2008;68:6718–6726. doi: 10.1158/0008-5472.CAN-08-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fournel M, Bonfils C, Hou Y, et al. MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther. 2008;7:759–768. doi: 10.1158/1535-7163.MCT-07-2026. [DOI] [PubMed] [Google Scholar]
- 22.Martell RE, Garcia-Manero G, Younes A, et al. Clinical Development of MGCD0103, An Isotype-Selective HDAC Inhibitor: Pericarditis/Pericardial Effusion in the Context of Overall Safety and Efficacy. Blood (ASH Annual Meeting Abstracts) 2009;114:4756. [Google Scholar]
- 23.Drexler HG. Recent results on the biology of Hodgkin and Reed-Sternberg cells. II. Continuous cell lines. Leuk Lymphoma. 1993;9:1–25. doi: 10.3109/10428199309148499. [DOI] [PubMed] [Google Scholar]
- 24.Georgakis GV, Li Y, Rassidakis GZ, Martinez-Valdez H, Medeiros LJ, Younes A. Inhibition of heat shock protein 90 function by 17-allylamino-17-demethoxy-geldanamycin in Hodgkin’s lymphoma cells down-regulates Akt kinase, dephosphorylates extracellular signal-regulated kinase, and induces cell cycle arrest and cell death. Clin Cancer Res. 2006;12:584–590. doi: 10.1158/1078-0432.CCR-05-1194. [DOI] [PubMed] [Google Scholar]
- 25.Zheng BFP, Li YV. MEK/ERK pathway is aberrantly active in Hodgkin disease: a signaling pathway shared by CD30, CD40, and RANK that regulates cell proliferation and survival. Blood. 2003;102:1019–1027. doi: 10.1182/blood-2002-11-3507. [DOI] [PubMed] [Google Scholar]
- 26.Rasheed W, Bishton M, Johnstone R, Prince H. Histone deacetylase inhibitors in lymphoma and solid malignancies. Expert Rev Anticancer Ther. 2008;8:413–432. doi: 10.1586/14737140.8.3.413. [DOI] [PubMed] [Google Scholar]
- 27.Chanan-Khan A. Bcl-2 antisense therapy in hematologic malignancies. Curr Opin Oncol. 2004;16:581–585. doi: 10.1097/01.cco.0000142074.67968.eb. [DOI] [PubMed] [Google Scholar]
- 28.Maggio E, van den Berg A, Diepstra A, Kluiver J, Visser L, Poppema S. Chemokines, cytokines and their receptors in Hodgkin’s lymphoma cell lines and tissues. Ann Onc. 2002;13:52–56. doi: 10.1093/annonc/13.s1.52. [DOI] [PubMed] [Google Scholar]
- 29.Karpf AR. A potential role for epigenetic modulatory drugs in the enhancement of cancer/germ-line antigen vaccine efficacy. Epigenetics. 2006;1:116–120. doi: 10.4161/epi.1.3.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattei V, Fonsatti E, Sigalotti L, et al. Epigenetic immunomodulation of hematopoietic malignancies. Semin Oncol. 2005;32:503–510. doi: 10.1053/j.seminoncol.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Marks PA, Xu WS. Histone deacetylase inhibitors: Potential in cancer therapy. J Cell Biochem. 2009;107:600–608. doi: 10.1002/jcb.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27:5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 33.Khaskhely NM, Buglio D, Shafer J, Bollard CM, Younes A. The Histone Deacetylase (HDAC) Inhibitor Entinostat (SNDX-275) Targets Hodgkin Lymphoma through a Dual Mechanism of Immune Modulation and Apoptosis Induction. Blood (ASH Annual Meeting Abstracts) 2009;114:1562. [Google Scholar]
- 34.Hartlapp I, Pallasch C, Weibert G, Kemkers A, Hummel M, Re D. Depsipeptide induces cell death in Hodgkin lymphoma-derived cell lines. Leuk Res. 2009;33:929–936. doi: 10.1016/j.leukres.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Gupta M, Ansell SM, Novak AJ, Kumar S, Kaufmann SH, Witzig TE. Inhibition of histone deacetylase overcomes rapamycin-mediated resistance in diffuse large B-cell lymphoma by inhibiting Akt signaling through mTORC2. Blood. 2009;114:2926–2935. doi: 10.1182/blood-2009-05-220889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buglio D, Mamidipudi V, Khaskhely NM, et al. The Histone Deacetylase Inhibitor MGCD0103 Down Regulates CD30, Activates NF-Kb, and Synergizes with Proteasome Inhibitors by HDAC6 Independent Mechanism in Hodgkin Lymphoma. Blood (ASH Annual Meeting Abstracts) 2009;114:3735. [Google Scholar]
- 37.Buglio D, Georgiakis GV, Hanabuchi S, et al. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood. 2008 doi: 10.1182/blood-2008-01-133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindemann RK, Newbold A, Whitecross KF, et al. Analysis of the apoptotic and therapeutic activities of histone deacetylase inhibitors by using a mouse model of B cell lymphoma. PNAS. 2007;104:8071–8076. doi: 10.1073/pnas.0702294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawamata N, Chen J, Koeffler HP. SAHA (vorinostat) suppresses translation of cyclin D1 in mantle cell lymphoma cells. Blood. 2007 doi: 10.1182/blood-2005-11-026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heider U, Kaiser M, Sterz J, et al. Histone deacetylase inhibitors reduce VEGF production and induce growth suppression and apoptosis in human mantle cell lymphoma. Eur J Haematol. 2006;76:42–50. doi: 10.1111/j.1600-0609.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 41.Duan H, Heckman CA, Boxer LM. Histone deacetylase inhibitors down-regulate bcl-2 expression and induce apoptosis in t(14;18) lymphomas. Mol Cell Biol. 2005;25:1608–1619. doi: 10.1128/MCB.25.5.1608-1619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buglio D, Mamidipudi V, Khaskhely NM, et al. The class-I HDAC inhibitor MGCD0103 induces apoptosis in Hodgkin lymphoma cell lines and synergizes with proteasome inhibitors by an HDAC6-independent mechanism. Br J Haematol. 151:387–396. doi: 10.1111/j.1365-2141.2010.08342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buglio D, Georgakis GV, Hanabuchi S, et al. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood. 2008;112:1424–1433. doi: 10.1182/blood-2008-01-133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buglio D, Mamidipudi V, Khaskhely NM, et al. The Histone Deacetylase Inhibitor MGCD0103 Down Regulates CD30, Activates NF-Kb, and Synergizes with Proteasome Inhibitors by HDAC6 Independent Mechanism in Hodgkin Lymphoma. Blood (ASH Annual Meeting Abstracts) 2009;114:3735. [Google Scholar]
- 45.Honscheid A, Rink L, Haase H. T-lymphocytes: a target for stimulatory and inhibitory effects of zinc ions. Endocr Metab Immune Disord Drug Targets. 2009;9:132–144. doi: 10.2174/187153009788452390. [DOI] [PubMed] [Google Scholar]
- 46.Patel MP, Masood A, Patel PS, Chanan-Khan AA. Targeting the Bcl-2. Curr Opin Oncol. 2009;21:516–523. doi: 10.1097/CCO.0b013e328331a7a4. [DOI] [PubMed] [Google Scholar]
- 47.Paoluzzi L, Gonen M, Bhagat G, et al. The BH3-only mimetic ABT-737 synergizes the antineoplastic activity of proteasome inhibitors in lymphoid malignancies. Blood. 2008;112:2906–2916. doi: 10.1182/blood-2007-12-130781. [DOI] [PubMed] [Google Scholar]
- 48.Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood. 2008;111:2300–2309. doi: 10.1182/blood-2007-06-098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitecross KF, Alsop AE, Cluse LA, et al. Defining the target specificity of ABT-737 and synergistic antitumor activities in combination with histone deacetylase inhibitors. Blood. 2009;113:1982–1991. doi: 10.1182/blood-2008-05-156851. [DOI] [PubMed] [Google Scholar]
- 50.Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99:4283–4297. doi: 10.1182/blood-2002-01-0099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) In vitro effect of HDAC inhibitors or decitibine on the expression of SSX2 and NY-ESO-1 in HL cell lines as determined by the RT-PCR method. B) The effect of HDAC inhibitors and decitibine on NY-ESO-1 protein expression was determined by immunohistochemistry in HL cell lines.