Abstract
Substance abuse is linked to numerous mental and physical health problems, including disturbed sleep. The association between substance use and sleep appears to be bidirectional, in that substance use may directly cause sleep disturbances, and difficulty sleeping may be a risk factor for relapse to substance use. Growing evidence similarly links substance use to disturbances in circadian rhythms, although many gaps in knowledge persist, particularly regarding whether circadian disturbance leads to substance abuse or dependence. Given the integral role circadian rhythms play in regulating sleep, circadian mechanisms may account in part for sleep-substance abuse interactions. Furthermore, a burgeoning research base supports a role for the circadian system in regulating reward processing, indicating that circadian mechanisms may be directly linked to substance abuse independently of sleep pathways. More work in this area is needed, particularly in elucidating how sleep and circadian disturbance may contribute to initiation of, and/or relapse to, substance use. Sleep and circadian-based interventions could play a critical role in the prevention and treatment of substance use disorders.
Keywords: circadian rhythms, sleep, insomnia, substance abuse, substance dependence, addiction, alcohol, opiates, methamphetamine, cocaine
INTRODUCTION
Substance abuse, defined here as the maladaptive and non-medical use of psychoactive substances, is linked to numerous mental and physical health problems, including disturbed sleep. Many substances of abuse have acute deleterious consequences on sleep, consequences that may be maintained or expanded upon during chronic use, and further exacerbated during withdrawal. Likewise, sleep disturbances may well lead to substance abuse.
In parallel, a substantive literature has documented associations between substance use and disruptions of circadian rhythms, the 24-hour biological rhythms present in many if not most physiological processes, including the sleep/wake cycle. Furthermore, accumulating evidence from animal models supports strong links between appetitive processes and various circadian genes. Much of this emerging literature suggests bidirectional effects; that is, substance abuse results in circadian disturbance, and circadian disturbance leads to increased substance use and greater potential for abuse and/or dependence.
In this article, we will review what is known about how sleep and circadian rhythms are respectively linked to substance abuse, focusing most closely on those studies that have implications for how circadian rhythms and sleep may be intertwined, whether as predictors or consequences of substance abuse. We will emphasize human research, but will integrate the animal literature that supports the mechanisms underlying the human findings. Based on the current evidence, we will suggest clinical guidelines for assessment and treatment of patients who have some combination of substance abuse and sleep and/or circadian disturbance.
SUBSTANCE ABUSE AND SLEEP
There is “a convincing link between substance abuse and sleep problems” (1). For example, one study found that patients presenting to a medical clinic seeking treatment for sleep complaints were more likely to have problems with drug and/or alcohol use as compared to those without sleep complaints (2). Sleep disturbance, particularly insomnia, is associated with various comorbid conditions including substance use disorders (3). Because there are excellent reviews on the impact of substance use and its disorders on sleep (1, 4, 5), only a brief summary is included below.
The acute effects of substance use on sleep largely depend upon the substance in question. Stimulants such as cocaine and amphetamine cause light, restless, and disrupted sleep (5). Drugs from other classes, such as depressants (e.g., benzodiazepines, alcohol) and opiates (e.g., heroin) initially produce soporific effects, including increased daytime sleepiness and reduced sleep latency, but cause sleep disruptions later in the night (e.g., increased night awakenings) due to acute withdrawal effects (1, 5, 6).
Chronic use of substances can further impact sleep quality and quantity. Unlike acute effects which may be dependent on the specific substance, the effects of chronic substance use on mood and other psychiatric symptoms are similar across different substances (7). Sleep-related outcomes of chronic substance use are also broadly similar across substances and include extended sleep onset latency (SOL), reduced total sleep time (TST), more nighttime awakenings, and decreased slow-wave sleep (SWS) and rapid eye movement (REM) sleep. Substance-specific effects of chronic use have been documented as well, such as different temporal patterns of TST and REM changes during the course of withdrawal from alcohol versus stimulants (5, 8).]
Withdrawing or abstaining from substance use has its own unique impact on sleep parameters, which may be dependent on the duration of abstinence. Sleep disturbance tends to be associated with acute withdrawal from any substance, as evidenced by extended SOL, reduced TST, and reduced SWS (5, 6). In addition, REM sleep often shows a rebound effect during abstinence (6). As the period of abstinence is extended, sleep patterns tend to return to baseline. There is evidence, however, that some sleep characteristics (e.g., REM sleep disturbances) persist well into abstinence (5, 6). Contrary to subjective reports of improvements in sleep quality with sustained abstinence from cocaine, polysomnographic sleep variables in cocaine-dependent participants show continued disruptions over 3 weeks of abstinence as indicated by increased SOL and decreased TST, SWS, and REM sleep (9). A recent polysomnographic study of heavy marijuana users showed that across two weeks of abstinence, participants showed increases in wake time after sleep onset (WASO) and decreases in TST, sleep efficiency and REM sleep (10). The persistent sleep disturbances observed in these populations may be a risk factor for relapse to substance use (6, 9, 10)
Although extensive research supports the existence of a relationship between substance use and sleep disturbances, few longitudinal or prospective studies have been done to clarify the specific causal relationship(s) between the two. Preliminary evidence suggests that the pathways are bidirectional in that substance use may directly cause sleep disturbances and sleep disturbance may be a risk factor for relapse to substance use (if not for initiation of use) (1).
SLEEP AND CIRCADIAN RHYTHMS
The substance use-sleep and the substance use-circadian rhythms literatures have largely developed independently of one another, with few studies considering all three factors. This segmentation has the unfortunate effect of delaying progress on understanding the mechanisms that link substance abuse, sleep, and circadian rhythms.
Sleep and circadian rhythms are bi-directionally linked, with substantial overlap and integration of their underlying neurobiological mechanisms (11), and thus it is critical to consider both in attempts to understand the pathways leading to and from substance abuse. Sleep timing and consolidation are partially governed by the central circadian clock, located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Sleep propensity is a function of the complex interaction between two oscillators: a circadian signal for wakefulness that is tuned to the 24-hour light/dark cycle, and a homeostatic drive for sleep that increases with continued wakefulness (12, 13). Likewise, the selection of a sleep schedule, which dictates exposure to light, impacts the circadian timing of a host of processes subject to circadian regulation.
Given that the timing of sleep is dependent upon circadian regulation, it is plausible that the association between sleep disturbance and substance abuse is partly mediated by circadian pathways, or that the link between circadian disruption and substance abuse is due in part to disturbed sleep. Indeed, limited evidence supports this possibility, although the literature also points to pathways that are multifactorial and complex. Even so, keeping the intersecting regulation of sleep and circadian rhythms in mind is critical when considering the evidence reviewed below.
SUBSTANCE ABUSE AND CIRCADIAN RHYTHMS
Despite the considerable attention paid to the relationship between substance abuse and disturbed sleep, few studies have addressed whether concomitant disturbed circadian rhythms are present. This is unfortunate, given that circadian disruption is often inherent to sleep disturbance, and that the circadian clock not only regulates the sleep-wake cycle, but is also implicated in many other behavioral and physiological processes relevant to substance abuse (e.g., mood and cognition) (14, 15). The neglect of circadian factors may be due in part to considering sleep-timing issues to be primarily the consequence of lifestyle factors (e.g., erratic schedules and all-night drug binges). In at least one study, the investigators explicitly disregarded circadian timing issues, explaining that “sleeping at inappropriate times appears to be a disturbance in circadian rhythms associated with the nocturnal lifestyle of addicts in general,” implying that such a measure was less likely to be a consequence of drug use per se (16). Interestingly, the same investigators noted that their sample tended to have delayed sleep times, although they did not report the actual data. Even if it is lifestyle factors, rather than drug abuse per se, that lead to delayed timing, these forces still may be critically contributing to a vicious cycle of circadian disruption, sleep disturbance, and substance abuse.
A common assumption in the literature is that chronic drug users have disturbed circadian rhythms, although direct evidence of this remains sparse. Anecdotal reports describe irregular sleep/wake timing, often with late bedtimes or even reversed schedules due to “all-nighter” drug binges. Indeed, cross-sectional evidence across age groups bears this out. In a Finnish study of adolescents, irregular sleep schedules and daytime sleepiness together accounted for 26% of the variance in substance use in 15 year-old boys (12% in girls) (17). Likewise, a majority (63%) of adult psychiatric inpatients reported irregular sleep schedules prior to admission, and this irregularity was significantly more common in those inpatients also reporting high levels of illicit drug and alcohol abuse (18). In addition to this cross-sectional evidence, a prospective study of 63 adult recovering alcoholics (abstinent 15.7 ± 20.2 months) reported highly irregular sleep schedules, with wake time varying an average 218 minutes (SD = 84.2 minutes, range = 45-690 minutes) across a week (19). Moreover, the alcoholics in this study were more prone to sleep-onset insomnia than sleep-maintenance insomnia, suggesting a tendency towards delayed phase.
The image of drug abusers as night owls has some support. Cross-culturally, and across adolescents and adult samples, self-reported eveningness is associated with later circadian phase (23), later and more irregular sleep schedules, as well greater substance use and dependence across substance types, including alcohol, marijuana, nicotine, and caffeine (20-25). This increased substance use and sleep instability among evening-types may be a consequence of a mismatch between biological timing and societal demands (26). In contrast, among a sample of adults with bipolar disorder, those patients with a co-morbid alcohol abuse disorder were more of the morning-type than those with bipolar disorder alone, even after controlling for age, sex, education, psychotropic medication, smoking, and body mass index (27). Because individuals with bipolar disorder without co-morbid alcohol abuse tend to be evening-types (27-29), the authors hypothesized that prolonged alcohol misuse could lead to a shift towards a morningness-preference. Following this logic, the change in preference could occur through eventual acclimation to the early morning awakening that can result after alcohol intake, or through more direct effects of chronic alcohol abuse on the circadian system.
Direct evidence concerning substance abuse and circadian rhythms
By far, the largest literature concerning the relationship between circadian rhythms and substances of abuse in humans has focused on alcohol. Because substances of abuse broadly impact the same neural circuits, but also show important mechanistic differences, it is important to remember that the respective interactions of various substances with the circadian system will likely vary as well. Time-of-day effects of alcohol impact a range of sleep and circadian-related processes, although it remains unclear whether these effects are occurring at the level of the central clock in humans. Substance use can indirectly affect the clock, as most or all of the known zeitgebers (time-givers) for the central clock—the timing of light exposure, meals, exercise, and social activities (30)—are likely to be impacted during acute or chronic substance use. Furthermore, few of these studies have addressed what, if any, effects these changes have on sleep. Likewise, in contrast to reports that sleep disturbance increases the risk of relapse for substance abuse or dependence, almost no published human studies address whether persistent circadian disruption affects substance use.
Does acute substance use influence circadian rhythms? (Table 2)
Table 2.
Circadian measures during acute substance use
| Study | Substance | Sample | Methods | Key Results | Limitations |
|---|---|---|---|---|---|
| Danel et al, 2001 (41) | Alcohol | 9 healthy young adult males (age = 23.3 ± 2.9 yr; range 21–30) | All participants underwent a 26-h alcohol session and a 26-h placebo session, separated by 2-5 weeks. Alcohol administered orally during waking hours and intravenously during sleep to maintain a target BAC between 0.5 and 0.7 g/l throughout the session. Core body temperature (CBT) measured every 20 min throughout 26-h session. |
Lower CBT during afternoon (1220-1400) and higher CBT during early morning (0300-0820) relative to placebo. | Sleep/wake schedule prior to study was not controlled |
| Danel and Touitou, 2006 (40) | Alcohol | 11 healthy young adult males (age = 23.3 ± 2.9 yr; range 18-30) | All participants underwent a 26 h alcohol session and a 26 h placebo session. Alcohol administered orally during waking hours and intravenously during sleep to maintain a BAC between 0.29 and 0.78 g/l throughout the session. Blood samples collected from 1200 on day 1 until 1500 on day 2. Lux < 50 |
No differences in melatonin levels in group comparisons. Apparent phase delay in the melatonin profiles of six out of 11 participants. |
Reported statistical analysis may have missed transient suppression in melatonin levels. Poor ecological validity of alcohol administration paradigm. |
| Devaney et al, 2003 (42) | Alcohol | 8 moderate drinker adults (4 females, age = 25.1 ± 6.0 yr; range 18-40, M = 4.3 ± 2.3 standard drinks/day) reporting a pre-study average of two drinking days per week with 4.3 ± 2.3 (M ± SD) drinks per occasion | Compared alcohol consumption at 1300 and 1800 (sessions separated by > 1 week). Target peak BAC of 1.0 g/l. Core body temperature measured every minute starting 1 h before consumption and ending at 0900 the following morning. |
Effects depended on time-of-day of consumption. Both session times were associated with lower CBT throughout the sleep period (2330-0830). The 1300 session was also associated with higher CBT for 5-9 hours post-consumption. |
Sleep/wake schedule prior to study was not controlled |
| Ekman et al, 1993 (37) | Alcohol | 9 healthy medical students (5 females, aged 21-23 years). No alcohol for at least 1 week prior to study No medications or smoking during 3-week study. |
Single oral dose of 0, 0.5, or 1.0 g/kg alcohol between 1900-1945. Within-participant comparison, with doses administered at 1-week intervals. Collected plasma samples at 1800, 2000, 2200, 2400, 0100, 0200, 0400, and 0700. Collected urine from 1900-2400 and 2400-0700 for urinary MT excretion. Lights on until 2300 when participants sent to bed; < 2 lux from 2300-0700. |
Both alcohol doses associated with lower plasma melatonin levels; 41 % reduction for both at 2400, and 33 and 18% reduction at 0100 and 0200, respectively, for the 1.0 g/kg dose. No differences in urinary MT levels. |
High light levels <2300. Sleep/wake schedule prior to study was not controlled, light exposure history was unknown, and circadian phase was not assessed (unknown circadian time of administration). |
| Plenzler et al., 1996 (39) | Alcohol | 10 healthy adult males (mean age = 23.6 yr) | All participants tested on three nonconsecutive nights separated by at least 48 hours. In random order, received alcohol (0.8 g/kg dose) at either 2005 or 2305, or received placebo beverage. Saliva collected hourly from 2000-0200. Lux ≤ 100 throughout collection. |
No differences in saliva melatonin levels at either dose. | Small collection window may have missed delayed melatonin suppression. |
| Rodjmark et al, 1993 (36) | Alcohol | 7 healthy non-obese participants (4 females, age = 29 ± 1 yr) All unmedicated. |
Experiment A-C: Alcohol administered at one of two doses (0.34 g/kg in A, 0.52 g/kg in B) at 1800, 2000, and 2200. Water was administered at same time points in Experiment C. Serum samples collected every 2 hours from 1800-0800. Collected urine from 2200-0700 for urinary melatonin excretion. For Experiments A-C: Lux 280-430 until 2300 when participants sent to bed. A 25W red light was used from 2300-0700. |
Only higher alcohol dose (Exp B) was associated with lower serum melatonin levels: 20% reduction in total melatonin secretion relative to control group (Exp C). No differences in urinary melatonin levels. |
High light levels <2300; unreported lux 2300-0700 (although red light likely to exert minimal suppression). Sleep/wake schedule prior to study was not controlled and light exposure history was unknown. Small sample sizes and between-group comparison. |
| Rupp et al, 2007 (31) | Alcohol | 29 healthy young adults (20 females, age = 22.6 ± 1.2 yr; range 21-25) | All participants underwent a placebo night and an alcohol night, counterbalanced and separated by 5-7 nights. Both nights included 30-minute beverage session ending 1 h before bed. Alcohol dose of 0.54 and 0.48 g/kg for men and women, respectively. Saliva samples collected every ~30 min starting 5 h before and ending 4.5 h after habitual bedtime. Lux < 20 throughout collection. |
Alcohol consumption associated with lower salivary melatonin levels (19 and 15%, respectively, relative to placebo) at 140 and 190 minutes post-consumption. No sex effects. No effects on sleep as based on PSG measures of subset (n = 8) of sample. |
Sleep only measured via PSG on subset of sample. |
Notes: All times provided in military time (e.g., 1900 = 7 PM)
Abbreviations: CBT = core body temperature; DLMO = Dim light melatonin onset; 6-SM = 6-sulfatoxymelatonin
Investigations of acute circadian effects of alcohol administered to healthy individuals have produced mixed findings, with most reporting changes in the amplitude of melatonin and core body temperature rhythms. As noted by Rupp and colleagues (31), interpretation of many of these studies is confounded by uncertainty regarding the circadian phase of alcohol administration (in part due to a lack of controlled sleep-wake history), questionable control of light exposure during the study, small samples, and negligence of potential sex differences. Controlling for the circadian phase of administration is critical because of evidence that alcohol’s effects on other sleep-related measures (e.g., sedation) are based on time-of-day of administration (32). Similarly, controlled light exposure through the protocol is critical because even moderate indoor lighting (<200 lux) can markedly suppress melatonin levels depending on light exposure history and the wavelength of light (33, 34).
Rupp and colleagues (31) attempted to address these methodological concerns in their investigation of 29 healthy young adults, who underwent both placebo and alcohol laboratory study nights that were counter-balanced and separated by 5-7 nights on a habitual schedule at home. During a 30-minute session ending one hour prior to bedtime, and controlling for individual circadian phase, participants imbibed either a placebo or alcoholic beverage (0.54 and 0.48 g/kg for men and women respectively) with a target breath alcohol content (BrAC) of 0.05 g% (the equivalent of 2-3 standard drinks). Saliva was collected under dim light conditions (< 20 lux) in order to monitor melatonin levels and to estimate circadian phase via the dim light melatonin onset (DLMO; see Table 1 for definitions of circadian terminology). Alcohol administration did not result in any changes in circadian phase; however, there was evidence of suppressed (15-19% versus placebo) melatonin secretion 2-3 hours post-consumption in the alcohol condition, apparently during the elimination phase of alcohol metabolism. Effects were unrelated to sex, and did not appear to be linked to any acute effects on sleep, at least as based on a subset (eight participants) of the sample that underwent polysomnography (PSG) (35).
Table 1.
Circadian terminology
| Term | Definition |
|---|---|
| Circadian phase | Temporal location within the 24-hour circadian cycle |
| Constant routine protocol | Research protocol designed to unmask the endogenous rhythmicity of physiological and psychological processes in humans. Participants are kept awake in a semi-recumbent position under constant light conditions, with meals, assessments, and other stimuli delivered at equal time intervals, typically for 24-48 hours. |
| Core body temperature (CBT) | A robust physiological output of the human circadian pacemaker, albeit one masked by activity, sleep, and posture. Under normally entrained conditions, the CBT minimum occurs a few hours before waking. |
| Dim light melatonin onset (DLMO) | A commonly-used human circadian phase marker based on the abrupt rise in melatonin that normally occurs in the evening. Given that light suppresses melatonin secretion, the DLMO is so-named because it must be collected in dim light conditions (typically <30 lux). |
| Entrainment | The process of synchronization of the internal circadian clock to the external environment. Entrainment is achieved via rhythmic environmental signals known as zeitgebers. Light is the most powerful zeitgeber in humans. |
| Forced desynchrony protocol | Research protocol designed to separate the confounding effect of the sleep/wake schedule (homeostatic sleep drive) from the output of central circadian pacemaker. Participants live on enforced 20- or 28-hour sleep/wake schedules in constant dim light and deprived of time cues for multiple circadian cycles. Participants are unable to entrain to these schedules, thus uncoupling their sleep/wake timing from their circadian phase. |
| Masking | Disruption in the observed expression of circadian rhythms such as melatonin or core body temperature without directly affecting the function of the central pacemaker. For example, physical activity masks the core body temperature rhythm. |
| Melatonin | A robust physiological output of the human circadian pacemaker. Known as a “hormone of darkness”, it is secreted into the blood by the pineal gland at night under normal conditions, with peak levels in the middle of the night and low levels throughout the day. It is suppressed by light, but is otherwise less influenced by masking effects such as activity than is CBT. |
| Phase angle | The temporal interval between a circadian phase marker (e.g., DLMO) and the timing of sleep (e.g., sleep offset), or in the case of the phase angle of entrainment, between a circadian marker and the timing of light exposure. |
| Phase response curve | A graph illustrating the relationship between a zeitgeber and its influence on the circadian clock. The curve specifies the extent to which the circadian clock will advance (shift earlier) or delay (shift later) in response to a particular zeitgeber (e.g., light) at different circadian phases across the 24-hour cycle. |
Two earlier studies also reported a delayed and moderate suppression in melatonin levels after alcohol administration the previous afternoon or evening, although methodological differences between the studies obscure comparison. Rodjmark and colleagues (36) compared two doses of alcohol (0.34 and 0.52 g/kg), each administered to seven healthy adults at 18:00, 20:00, and 22:00 on separate study nights. Only a 25W red light was on between bedtime (23:00) and rise time (07:00). Neither dose affected urinary melatonin levels, but the larger dose resulted in 20% lower serum melatonin levels. Somewhat larger suppression effects were reported by Ekman and colleagues (37), who studied nine healthy young adults in a double-blind crossover design and compared alcohol doses of 0.5 and 1 g/kg to placebo. Alcohol administration between 19:00-19:45 resulted in a 41% reduction (relative to control) at midnight for both doses. Lights were lowered to less than two lux at the 23:00 bedtime. The higher dose was associated with a more sustained reduction in plasma melatonin levels, continuing to show 33 and 18% reductions at the 01:00 and 02:00 time points, respectively. Neither dose affected urinary melatonin levels, consistent with the findings of Rodjmark and colleagues (36). Taken together, these studies suggest that acute alcohol administration leads to moderate melatonin suppression, with a duration of effect potentially contingent on the dose. However, interpretation is challenged by two main methodological concerns in the earlier studies; first, neither study assessed or controlled for the circadian phase of alcohol administration. Second, neither study controlled pre-bedtime light levels, which may have lingering suppressive effects for more than 60 minutes (38), thus confounding interpretation of the apparent suppression at the midnight and 01:00 time points.
Indeed, two other studies that maintained lower light levels throughout the assessment periods did not report any direct suppressive effects of acute administration of alcohol. Plenzler and colleagues (39) probed time-of-day effects of alcohol administration on salivary melatonin levels in a randomized, double-blind crossover design. Depending on the testing day, 10 healthy adult males received either placebo or alcohol (0.8 g/kg) at 20:05 and 23:05—administration times intended to occur before and after the onset of melatonin secretion, respectively. Although alcohol at the earlier time resulted in greater sleepiness (Stanford Sleepiness Scale), neither time of administration resulted in significant changes in melatonin levels. A more recent study by Danel and Touitou (40) arguably provided the largest dose of alcohol by maintaining blood alcohol concentrations between 0.29 g and 0.78 g/l1 over a full 24 hours in a sample of 11 healthy adult males, but did not report evidence of any suppression in plasma melatonin levels relative to levels during a 24-hour control period. Interestingly, the investigators reported that six out of the 11 participants showed evidence of a phase delay in their melatonin profiles, suggesting that individual differences may be an important determinate in the effect of alcohol on circadian parameters.
Alcohol use may have acute effects on the core body temperature (CBT) rhythm that vary according to time-of-day of administration. Danel and colleagues (41) tested nine healthy male young adults in a single-blind, randomized, crossover design in which alcohol (0.5-0.7 g/l) or placebo was administered throughout a 26-hour protocol. Participants remained in bed throughout the protocol; lights were turned off and participants were permitted to sleep between 22:00 and 06:00. The alcohol condition was marked by significantly lower temperatures during the early afternoon and significantly higher temperatures during the early morning hours, relative to the placebo condition. The maximal (43%) reduction in amplitude occurred at the nadir of the CBT rhythm, with seven of nine participants showing this effect. Another study compared the effects of alcohol consumption (peak blood alcohol content of 0.10 g/100 mL) at 13:00 and 18:00 (sessions separated by > 1 week) in eight moderate drinkers (42)2. In contrast to the previous study, these discrete administration sessions allowed detection of time-of-day effects. Indeed, only the 13:00 session was associated with a hypothermic effect that lasted 5-9 hours. However, both sessions were associated with a hyperthermic effect that was observed throughout the sleep period. Of note, this study permitted participants to go home after the first 6 hours of sampling and sleep under uncontrolled conditions; a potential confound given that both sleep and activity have masking effects on core body temperature (43).
Two recent animal studies also support an effect of acute alcohol administration on circadian rhythms, although neither of these studies used melatonin or CBT as the output signal of circadian clock, precluding direct comparison with the human findings. Alcohol administration (60 mM solution) blocked the phase-shifting effects of glutamate but enhanced the phase-shifting effects of serotonin on the molecular clock in an in vitro preparation of the mouse SCN, suggesting complex effects of alcohol on both photic (light-induced) and nonphotic (e.g., activity-induced) pathways (44). In a follow-up study in hamsters, acute alcohol administration (doses ranged from 0.5-2.0 g/kg) attenuated both photic and non-photic (serotonergic) phase advances in locomotor activity rhythms, but did not affect phase delays (45). The attenuation of phase advances occurred whether alcohol was administered intraperitoneally or directly to the SCN via reverse microdialysis, indicating that alcohol is directly affecting the central clock. However, in the absence of a light stimulus, alcohol had no phase-shifting effects on its own, indicating that alcohol is interfering with photic and nonphotic pathways, but is not a phase-shifting agent per se. Complementing this evidence, mice with a mutation in the circadian gene Period2 (Per2) show attenuated phase delays in response to light, elevated glutamate levels in the brain, as well as increased alcohol intake (46, 47). Taken together, these implicate disruptions of the circadian system, and perhaps specifically the glutamatergic photic pathway, in increased alcohol abuse behavior.
To summarize, the evidence is mixed on whether a moderate dose of alcohol has any acute effect on circadian rhythms, although human studies generally favor a transient suppressive effect on the melatonin rhythm. Similarly, in studies of both “healthy” (no substance use-related diagnosis) and “moderate drinker” samples, alcohol consumption led to a hypothermic effect during the daytime while showing a hyperthermic effect during the nighttime trough of the core body temperature (CBT) rhythm. Together, these suggest a blunting effect of alcohol on the circadian amplitude of the temperature rhythm, perhaps parallel to the effects on melatonin discussed above. However, it has been suggested that the acute hypothermia is due to an alcohol-induced dysregulation of the thermoregulatory system, rather than having a chronobiological basis (48).
Other questions remain about the importance of the size and timing of dose, as well individual differences in susceptibility to any suppressive or phase-shifting effects. As suggested by Rupp and colleagues (31), repeated heavy drinking that continues into the night might lead to greater circadian (and sleep) effects; including perhaps, larger suppressions exacerbating the phase delays associated with weekends. Such delays could plausibly contribute to cycles of polysubstance abuse as individuals attempt to cope with morning fatigue and sleep-onset insomnia via stimulants and depressants, respectively. Future studies should employ larger doses, include prospective measures of sleep continuity and structure, and assess beyond the first night post-intake. Such studies will be required to determine whether circadian changes associated with chronic use and discontinuation of use, as described below, are due to dysfunction in the circadian system per se, or to changes in lifestyle sleep schedule habits.
Does chronic substance use impact circadian rhythms? (Table 3)
Table 3.
Circadian measures during chronic substance use
| Study | Substance | Sample | Methods | Key Results | Limitations |
|---|---|---|---|---|---|
| Danel et al., 2009 (49) | Alcohol | 7 alcohol-dependent patients (1 female, age = 46.3 ± 5.1 yr; range 39-52, mean 222.86 g/day) | Assessed night/day ratio of melatonin secretion by collecting urine samples fom 2000-0800 (night) and 0800-2000 (day) and comparing the urinary 6-SM levels. Collection done at home. | “Inverted” night/day melatonin ratios ranged from 0.29 to 0.96 | Participants selected for this abnormality from a sample with a high drop-out rate, thus confounding assessment of prevalence. Low temporal resolution of collection bins precludes assessment of relative phase. Collection done at home and sleep/wake and light/dark schedules were not controlled or reported. |
| Hasler et al., 2008 (52) | Polysubstance (primarily alcohol, marijuana, cocaine, and hallucinogens) | 21 sleep-disturbed adolescents (7 females, age = 16.3 ± 1.4; range 14-19) recruited from substance-abuse treatment programs | Assessed DLMO based on salivary melatonin levels, collected hourly samples from 1930-0330. Lux < 30 throughout collection. |
Greater substance use was associated with delayed DLMOs and shorter DLMO-wake time phase angles | Highly variable substance use profiles across participants. No control group. |
| Majumdar et al, 1987 (50) | Alcohol | 28 male chronic alcoholic patents of unknown age | Assessed plasma melatonin levels at 1500 | Eight of 28 patients had “detectable” melatonin concentrations during afternoon (mean +/- SEM = 13 1.6 ng/L) | Letter to the editor, not peer-reviewed. Limited data on important methodological details (e.g., light levels) |
| Murialdo et al, 1991 (51) | Alcohol | 10 alcohol-dependent men (age = 45.5 ± 2.9 years) 10 male healthy controls (age = 41.4 ± 2.5 years). Hamilton Rating Scale for Depression scores ranged between 21 and 30 for patients. |
Assessed night/day ratio of melatonin secretion by collecting urine samples from 2000-0800 (night) and 0800-2000 (day) and comparing the urinary 6-sulfatoxymelatonin (6-SM) levels. | The patients’ 24-hour urine melatonin levels were higher than in controls. Daytime urinary melatonin levels were also higher in patients than in controls, with a night/day ratio of ~1. | Low temporal resolution of collection bins precludes assessment of relative phase. Patients had significant depressive symptoms. |
Notes: All times provided in military time (e.g., 1900 = 7 PM)
Abbreviations: CBT = core body temperature; DLMO = Dim light melatonin onset; 6-SM = 6-sulfatoxymelatonin
Although a number of previous reviews have stated that chronic substance use is accompanied by circadian disturbance, empirical evidence of effects on human circadian rhythms other than the sleep/wake cycle is sparse. The limited available evidence suggests that circadian disruption is present in some portion of chronic users that parallels, and is perhaps contributing to, any present sleep disturbance.
Some studies and anecdotal reports have described an “inversion” of the melatonin rhythm during chronic alcohol use in some alcohol-dependent patients (49-51), referring to a reversed secretion pattern in which daytime melatonin levels are higher than nighttime levels. In the most recent study (49), melatonin secretion was inferred via urinary 6-sulfatoxymelatonin levels, sampled from 08:00-20:00 (daytime) and 20:00-08:00 (nighttime). The assumption of the ratio measure was that daytime melatonin levels should normally be lower than nighttime levels, resulting in a ratio of night/day 6SM > 1. In this small pilot study, seven of 12 patients were selected for having an “inversion” of melatonin secretion (ratio < 1) during chronic alcohol use at baseline. (The authors do not report the number of patients screened in order to obtain the study sample, so the prevalence of this abnormality remains unknown.) Ratios ranged from 0.29 to 0.96; at the most extreme, the ratios indicate that more than 75% of the melatonin secretion occurred during the daytime sampling period. The ratios were unrelated to duration of dependence or daily alcohol intake, but were significantly correlated to the ages at which consumption and dependence began.
Although these studies did not report any sleep parameters, the overall findings suggest that elevated daytime levels of melatonin could indicate misaligned circadian timing that would interfere with attempts to sleep at conventional times. However, interpretation of these studies is challenged by methodology that precludes more precise description of the melatonin profile, including the presence of advanced or delayed phase that could account for this apparent “inversion”. Nevertheless, the association between the extent of inversion and the ages at which consumption and dependence began suggest that the disturbed timing is either a consequence of long-time alcohol abuse, or may be an important individual difference predicting vulnerability to alcohol abuse.
In accordance with this possibility, a study in adolescents suggests a role for circadian misalignment in the pathophysiology of ongoing substance abuse. Hasler and colleagues (52) examined a sample of 21 adolescents who had recently completed substance abuse treatment programs and continued to report sleep disturbance. Given this post-treatment timing of assessment, substance use was greatly curtailed, but not eliminated, in the sample. Nonetheless, the adolescents still showed a positive association between their circadian phase (as measured by DLMO), and the degree of substance use; that is, more delayed DLMOs were associated with higher scores on a self-report measure of illicit drug and alcohol use over the past 30 days (r =0.44, p < 0.05). Notably, the alignment between the adolescents’ self-selected sleep-wake schedule and their endogenous circadian phase seemed to be even more critically linked to the level of substance use (r =0.62, p < 0.01); more delayed DLMOs relative to actigraphy-based wake time were associated with greater frequency and intensity of use. Interestingly, although DLMO was associated with sleep-onset difficulties (later DLMOs were associated with longer sleep latencies), the DLMO-wake time phase angle was unrelated to measures of sleep continuity, suggesting some other mechanism beyond sleep disturbance is responsible for the elevated substance use. While preliminary, this finding underscores the importance of considering the potential consequences of a mismatch between endogenous circadian timing and the sleep-wake schedules that are often imposed by sociocultural factors such as school or work. Of course, it is also plausible that the substance abuse and associated lifestyle may have contributed to the delayed circadian timing. Prospective follow-up studies are needed to address questions of directionality. To the best of our knowledge, there are no experimental human studies that clearly address pre-withdrawal effects of chronic substance use on circadian rhythms, or whether circadian factors maintain substance use.
Experimental studies in the animal literature provide strong evidence that chronic alcohol use disrupts circadian timing and impairs circadian clock re-setting, although the ramifications for entrainment remain unclear (53-56). At least in the case of alcohol, these consequences appear to be due to direct effects on the central clock (44, 53, 54). The studies indicate that chronic alcohol’s effects on circadian timing are dose-dependent, suggesting that the mixed results in the human literature may be partly explained by between-study variability in alcohol dosage.
A recent study by Brager and colleagues (53) provides the most conclusive demonstration of chronic alcohol consumption leading to circadian disturbance via effects on the SCN. Alcohol was provided to freely-behaving mice in their drinking water at 10 or 15% concentrations. Based on the 24-hour ethanol levels within the SCN and periphery, peak consumption and peak alcohol levels both occurred during the dark phase, particularly during times overlapping with the phase-delay portion of the light phase-response curve. Notably, the locomotor activity patterns of alcohol-consuming mice showed smaller phase delays in response to a light pulse administered within this phase-delay, suggesting that the elevated alcohol levels during this time were directly inhibiting the phase-resetting effects of light at the level of the SCN. Despite these apparent impairments in the phase-resetting mechanisms, however, the alcohol-consuming animals showed no differences in re-entraining to a 6-hour phase advance of the light/dark cycle, mirroring the lack of direct attenuation of phase advances in response to a light pulse. The authors suggest that lower SCN levels of alcohol during the advance portion of the phase-response curve, or an acute tolerance to the effects of alcohol on phase-resetting (as demonstrated in vitro, (44)), could be responsible. Interestingly, there were subtle effects on stable entrainment—the alcohol-consuming animals showed a less robust increase in activity at the start of their biological day, somewhat consistent with a lengthening in the phase angle of entrainment. These effects of chronic alcohol use on phase-resetting are mostly consistent with previous animal studies, although there are apparent species differences; hamsters show attenuated phase advances, not delays (54-56). None of these studies have investigated physiological markers such as melatonin or core body temperature, limiting comparison to the human literature. Furthermore, the model of chronic alcohol use in animals is generally approximately two weeks of forced or voluntary consumption, limiting our understanding of how long-term alcohol use (more representative of typical alcohol dependence in humans) is related to circadian disruption. Finally, with particular relevance to the focus of the current review, none of these studies assessed sleep.
Circadian disturbance during discontinuation of and subsequent abstinence from substance use (Table 4)
Table 4.
Circadian measures during discontinuation of substance use
| Study | Substance | Sample | Methods | Key Results | Limitations |
|---|---|---|---|---|---|
| Conroy et al., 2010, (58) | Alcohol | 22 alcohol-dependent patients (6 females, age = 34 ± 10.2) 14 healthy controls (10 females, age = 33 ± 11.7) |
Assessed multiple parameters of saliva melatonin profile. Collected saliva from 1930-0200. |
No group differences in any parameters of melatonin profile. Patients reported worse sleep. | Patients had been abstinent for widely varying durations (3-12 weeks). All participants adhered to single sleep-wake schedule for two weeks prior to assessment. Published abstract |
| Danel et al., 2009 (49) | Alcohol | 7 alcohol-dependent patients (1 female, age = 46.3 ± 5.1 yr; range 39-52, mean 222.86 g/day) | Assessed night/day ratio of melatonin secretion by collecting urine samples from 2000-0800 (night) and 0800-2000 (day) and comparing the urinary 6-SM levels. Assessed during initial acute withdrawal (day 1) and at 15 days post-withdrawal. All patients were being treated with benzodiazepines during acute withdrawal, but were psychotropic medication-free at day 15. |
Inverted melatonin profile normalized over time; it was present in only five out of the seven patients at day 1, and it was present in three out of the seven patients at day 15 | Low temporal resolution of collection bins precludes assessment of relative phase. |
| Fonzi et al, 1994 (59) | Alcohol | 8 male alcohol-dependent adult patients (age = 53.3 ± 7.1 yrs, range 39-64) 8 age-matched healthy male controls |
Collected plasma melatonin every 2-4 hr from 2000 to 0600 on day 1 and day 14 post-withdrawal. Light levels unreported. |
Patients had non-significantly higher melatonin levels and a loss of rhythmicity at 24 hours post-withdrawal. No differences at 14 days post-withdrawal. |
Light conditions during experiment, as well as sleep/wake and light exposure history, were all unreported. |
| Kuhlwein et al., 2003 (57) | Alcohol | 11 alcohol-dependent adults (age = 39.4 ± 5.5 years), averaging 27.1± 6.5 drinking days/ month and 15.8±16.2 drinks/day 10 healthy controls (age = 38.2 ± 7.0 years) All participants were African-American males. Patients were abstinent for more than 2 weeks prior to assessment. |
Assessed melatonin and cortisol profiles by collecting blood samples every 30 minutes between 2200-0630. Sleep was assessed via polysomnography. |
Lower melatonin levels in early part of night, and a 90 minute delay in peak secretion. Longer sleep latency was correlated with delay in peak secretion. |
All participants adhered to single sleep/wake schedule for two weeks prior to assessment. |
| Li et al., 2009 (62) | Heroin | 16 male heroin-dependent adult patients (age = 30.1 ± 4.2 years, 90 ± 59 months of dependence) 8 male healthy controls age = 29.0 ± 4 years |
Assessed 21-h profiles of hPER1, hPER2, hCLOCK, as well as cortisol, ACTH, β-endorphin, leptin and IL-2 on day 3, 10, and 30 post-withdrawal | Loss of rhythmicity in hPER1, hPER2, ACTH, β-endorphin, and IL-2 through day 30. Loss of rhythmicity in cortisol on day 3. | The hPER1 and hPER2 were sampled from periphery rather than the CNS. Sleep-wake schedules prior to and during the experiment were not reported. |
| Murialdo etal., 1991 (51) | Alcohol | 10 alcohol-dependent men (age = 45.5 ± 2.9 years) 10 male healthy controls (age = 41.4 ± 2.5 years) Hamilton Rating Scale for Depression scores ranged between 21 and 30 for patients. |
Assessed night/day ratio of melatonin secretion by collecting urine samples from 2000-0800 (night) and 0800-2000 (day) and comparing the urinary 6-SM levels. | In contrast with chronic use condition in same patients, nighttime urinary melatonin levels were higher than daytime levels in patients, similar to that of controls. | Low temporal resolution of collection bins precludes assessment of relative phase. Patients had significant depressive symptoms. |
Notes: All times provided in military time (e.g., 1900 = 7 PM)
Abbreviations: CBT = core body temperature; DLMO = Dim light melatonin onset; 6-SM = 6-sulfatoxymelatonin
Attempts to characterize circadian disturbance following discontinuation of substance use have produced mixed results. Given that other withdrawal symptoms tend to improve as the duration of abstinence increases, the mixed findings may be due to between-study differences in the timing of assessment. Only one study began assessment prior to acute discontinuation, thus precluding differentiation between the effects of chronic abuse and those directly attributable to withdrawal. On the plus side, several studies reported on concomitant sleep disturbance. Finally, nearly all of the studies looked at alcohol.
The most common reported circadian disturbance in these studies is an apparent phase shift in the melatonin rhythm. A study of 11 alcohol-dependent adults at two weeks into withdrawal reported apparent phase differences in their melatonin profiles relative to 10 healthy controls (57). Although the study’s blood sampling began too late in order to assess DLMO, the alcohol-dependent group was characterized by lower melatonin levels during the early part of the night and a 90-minute delay in the time of peak secretion, both consistent with delays in the overall melatonin profile. Cortisol was also assessed in the study, and relative to controls, patients had lower cortisol levels during the early part of the night and higher levels during the later part of the night--also consistent with a general delay in circadian timing. Notably, the patients reported a longer sleep latency, which was correlated with the time-to-peak melatonin secretion, suggesting that delayed circadian phase may account in part for the patients’ sleep-onset insomnia. In contrast, a more recent study comparing 22 alcohol-dependent adults to 14 healthy controls also reported worse sleep among the drinkers, but no group differences in any parameters of the circadian profile (e.g., volume, time of DLMO, peak secretion, and time of peak secretion) (58). However, duration of abstinence in the alcohol-dependent group—3-12 weeks—was both longer and more variable than in the previous study. Given evidence (see below) suggesting that circadian disturbance normalizes with extended abstinence, the patients may have experienced greater “normalizing” of their circadian rhythm, and/or the variability in duration of abstinence may have obscured within-group differences in melatonin profiles.
Several studies suggest that abnormalities in the melatonin profile of abstinent alcohol-dependent individuals resolve over time. The aforementioned study of “inverted” melatonin profiles (higher daytime than nighttime levels) in seven alcohol-dependent patients also reported that this inversion was present in only five out of the seven patients at day 1 of abstinence, and it was present in three out of the seven patients at day 15 (49). This timeline of normalization is consistent with two earlier studies reporting that melatonin abnormalities present during chronic use (inverted profile) or acute withdrawal (loss of rhythmicity) were absent by day 14 (51, 59). The patients in one of these studies were also depressed, raising a confound for interpretation given that affective disturbance is linked to both sleep and circadian abnormalities (60, 61). Unfortunately, only a minority of published studies on alcohol-dependent individuals report on the extent of concomitant affective disturbance (40, 51, 57)
Only one published study has examined circadian functioning following discontinuation of a substance of abuse other than alcohol. This study also found evidence of initial circadian disruption followed by some improvement during continued abstinence. A sample of 16 adult male heroin-dependent individuals were compared to eight age-matched healthy male volunteers at three times post-cessation: 35-72 hours (day 3), day 10, and day 30 (62). Relative to the healthy controls, the patients showed disruptions in the rhythms of circadian gene expression (PER1 and PER2, as measured in peripheral blood mononuclear cells, or PMBCs), cortisol, and several peptides that largely persisted through day 30. Specifically, PER1 and PER2, as well as ACTH, β-endorphin, and IL-2 all showed significant daily rhythms in the healthy controls but not in the patients at any point. The cortisol rhythm was also disrupted in the patients at day 3, but was restored by day 10, and cortisol levels were higher in the patients throughout the study, although they declined over time. The PER1 and PER2 results are of particular interest, given that these genes have been implicated in reward processing (as discussed below), suggesting that the continued disruption of their rhythmic expression may contribute to craving during withdrawal. The same authors ran a parallel study of morphine withdrawal in rats, and similarly found that a loss of rhythmicity in the expression of the rat gene homologues (Per1 and Per2), both in the hypothalamus and in PMBCs, accompanied other signs of withdrawal.
The rest of the animal literature is consistent with the human studies, showing continued circadian disturbance during acute discontinuation of chronic alcohol use, with improvements in circadian functioning as abstinence continues. The aforementioned study by Brager and colleagues (53) found similar attenuation of photic phase-resetting during chronic use and ~1 day withdrawal, suggesting that circadian abnormalities during acute discontinuation may simply reflect pre-withdrawal disturbance rather than any new exacerbation. A previous study in hamsters followed discontinuation through day 3, and found an actual enhancement in light-induced phase advances relative to controls on days 2 and 3 (but not on day 1) (54). The authors suggested that this rebound in circadian functioning may be due to the disinhibition of glutamatergic signaling following alcohol discontinuation.
Taken together, despite their limitations, these findings provide a potential context for the variability of findings. Circadian disturbance may be most severe immediately post-cessation, and less apparent several more weeks out, highlighting the first few weeks as a key time for intervention via chronobiologically-informed treatment. However, it is unclear if sleep disturbance follows the same trajectory (which would suggest shared mechanisms), or to what extent methodological factors (e.g., imposing a stable sleep/wake schedule) obscure the findings.
Circadian disturbance and relapse for substance use and abuse
Other than previously-noted evidence that sleep disturbance is a risk factor for relapse to alcohol abuse, no direct human evidence links chronic circadian disturbance to changes in substance use, let alone substance abuse relapse. However, such a pathway is plausible based on the persistence of circadian and sleep disturbances for weeks into abstinence, and reports from alcoholics that coping with sleep disturbance is a significant motivation for heavy drinking (19). Furthermore, limited cross-sectional data suggests that chronic exposure to circadian challenges is linked to increased alcohol use. This is found in studies of nurses on rotating or night shifts (63) and travelers subjected to frequent jet lag (64). The nurses working night shifts also reported more smoking. However, neither of these studies directly assessed the extent of circadian disturbance, nor correlated it with the drinking behavior, and neither study addresses relapse in previously substance-dependent individuals. In addition, neither study linked these outcomes to any measures of sleep disruption.
The animal literature offers mixed findings regarding the effects of shift work and jetlag-like circadian challenges on substance use. One study reported that a single 8-h light/dark phase advance or delay resulted in a transient increase in alcohol consumption (3 or 1 days, respectively), while a more chronic circadian challenge—repeated phase shifts over 28 days—resulted in sustained increases in consumption (65). In contrast, two recent studies reported that alcohol consumption declines during periods of persistent circadian challenge (66, 67). The studies examined the effect of repeated 6-h phase advances of the light/dark cycle on three rat strains with different genetic backgrounds associated with a range of innate preference for alcohol (although none were alcohol-avoiding). Interestingly, despite between-strain differences in speed of re-entrainment to the shifting light/dark schedules, the researchers found either no difference or reductions in alcohol consumption relative to controls across all three strains. If circadian disruption is conceptualized as a non-specific stressor, this is consistent with other evidence that stress can lead to increased or decreased voluntary alcohol consumption depending on the circumstances (68). None of these studies addressed the question of whether prior substance dependence moderates the effect of circadian disruption on substance use.
Determining whether circadian disturbance leads to increased substance use, accelerates the onset of substance dependence, or leads to increased risk of relapse during initial discontinuation or subsequent abstinence, are critically important questions deserving further study. Prospective observational and experimental research in this area could potentially elucidate some of the mechanisms linking circadian and sleep disturbance with substance abuse, and would also inform both prevention and intervention efforts. Actigraphy could have a role in expanding substance abuse research to measure both sleep and circadian rhythms as it is a convenient and minimally invasive method of measuring circadian functioning (69).
Circadian-based treatment during substance discontinuation
Although several studies have examined the effect of treating sleep disturbance in withdrawing or abstinent substance users (as reviewed in (1)), none of the studies assessed circadian parameters. Because most behavioral sleep treatments aim to improve the regularity of the sleep/wake schedule, it is likely that circadian functioning may be impacted over the course of treatment. The effects of sleep- (or circadian-focused) treatment on relapse is a key area for further inquiry which could lead to supplementing current substance abuse treatments with focused sleep and circadian interventions.
A single open trial suggests that the use of bright-light therapy may simultaneously impact circadian functioning and improve sleep in alcoholics during withdrawal (70, 71). In this study of 10 alcohol-dependent adults, bright-light treatment on day 3 of withdrawal was associated with both a reduction (compared to day 2) in serum melatonin levels and improvement in PSG-assessed sleep, as measured by reductions in sleep latency, more rapid onset of slow-wave sleep, increase in REM sleep, and increase in subjective sleep quality. Blood samples were collected hourly from 20:00 to 08:00 on days 2, 3, and 4 post-withdrawal. The study is limited by the lack of a control intervention and the lack of a pre-withdrawal control group. These two limitations are problematic given the well-documented large inter-individual variability present in the melatonin levels of healthy individuals. Furthermore, the bright-light treatment (4500 lux) was administered during both the morning (07:00-09:00) and evening (17:00-21:00), a combination which may “cancel out” any phase-shifting effects, and the study did not report sleep timing or circadian phase measures to assess such effects. The treatment periods also overlapped with the sampling period and thus likely directly contributed to suppressing the melatonin levels. Finally, the authors did not report whether light levels (other than the bright light treatment) were controlled during the study, raising the possibility of uncontrolled light suppression effects.
Another study compared melatonin to placebo for treating benzodiazepine withdrawal-associated sleep disturbances in adult patients undergoing methadone maintenance treatment who had been abusing benzodiazepines (72). Under double-blind conditions, the melatonin treatment (5 mg at approximately 20:00-21:00, (E. Peles, personal communication, June 10, 2010)) was associated with improved self-reported sleep quality and a longer time to relapse to benzodiazepine use. This may indicate that the melatonin was stabilizing disrupted circadian rhythms in the patients, and that by doing so, reduced the vulnerability to relapse. However, the lack of circadian assessment precludes any firm conclusions.
Circadian genes, reward, and substance abuse
In addition to the role circadian rhythms may play in the pathway linking sleep disturbance and substance abuse, the circadian system may be directly linked to addictive processes. Over the past decade, the concept of a supreme central clock in the SCN as the solitary source of rhythmicity has been supplanted by a model wherein an orchestra of peripheral clocks throughout the tissues of the body and brain are all inherently rhythmic, but require signals from the SCN in order to remain appropriately synchronized (73). This paradigm shift has come with the identification of the molecular machinery underlying rhythmicity in both the SCN and peripheral clocks, which is comprised of a negative-positive autofeedback loop of transcription and translation (73). The continued characterization of the circadian genes responsible for these rhythms has led to accumulating evidence that the bidirectional links between circadian processes and substance abuse are partly mediated at the cellular and genetic level (74). Most intriguingly, as reviewed below, circadian genes are active in brain areas within the mesolimbic reward circuit and have been directly linked to drug abuse-related behaviors.
Animal studies indicate that a range of drugs of abuse can serve as zeitgebers for these peripheral clocks in non-SCN brain areas. This is evidenced by the robust entrainment of locomotor activity rhythms to the timing of drug or alcohol administration (75). For example, methamphetamine administration desynchronizes the locomotor rhythm from the light/dark cycle, leading to a pseudo-free-running rhythm with a period of >24 hours (76). This is not a true free-run, because the rhythm shows signs of relative coordination, which is assumed to be due to the influence of the SCN. While influenced by the SCN, this methamphetamine-induced rhythm is also expressed in drinking, feeding, body temperature, and plasma corticosterone in SCN-lesioned rats, and can persist for up to 14 cycles post-withdrawal, indicating that it is a true oscillatory process being generated outside the SCN. Recent evidence suggests that it may not depend on the same molecular machinery, as even mice made arrhythmic by mutations in the involved circadian genes have their rhythms restored by methamphetamine administration (77). On the other hand, the influence of the methamphetamine-induced rhythm on the SCN is less clear. No direct effects of the methamphetamine oscillator on the SCN are evident, but one study showed that methamphetamine-treated rats with access to a running wheel did show an altered SCN period (76). Given that wheel-running is inherently rewarding to rodents (78), this may indicate a pathway via the reward system to influence circadian functioning.
Assuming these SCN-independent pacemakers behave similarly in humans, these findings have intriguing implications for understanding the consequences of substance abuse on sleep. For example, the possibility for entrainment of these peripheral clocks to a different phase than that of the central pacemaker implies that the timing of these drug-induced activity rhythms could conflict with the timing of the sleep/wake cycle, leading to difficulty in falling or maintaining sleep.
Furthermore, evidence suggests that reward-related behaviors follow circadian rhythms; rhythms that could potentially be dissociated from the timing of the central pacemaker and/or sleep/wake cycle. In rats, alcohol consumption parallels the circadian pattern of wheel-running, which is also an innately rewarding activity as mentioned above, thus suggesting a common source of rhythmicity (67). Indeed, both drug-seeking behavior and responsiveness to drugs of abuse show diurnal variation, implying circadian rhythmicity in appetitive motivation and response to reward (79, 80). This rhythmicity is paralleled by the expression of the dopamine transporter and tyrosine hydroxylase (the rate-limiting enzyme in dopamine synthesis), as well as in the expression of several circadian genes in multiple reward-related brain areas associated with the mesolimbic dopamine system, such as the striatum and ventral tegmental area (80, 81). Some of these rhythms may be regulated by the SCN, as lesioning the SCN leads to a dampening of the diurnal variation in the dopamine transporter and tyrosine hydroxylase expression (81). Another series of studies demonstrated that melatonin is not only required for mouse Per1 rhythmicity in the striatum, but for concomitant diurnal rhythms in locomotor and reward measures of cocaine sensitization (82-84). Other evidence suggests that these rhythms can be hijacked away from SCN control, as methamphetamine administration shifts the expression of mouse Per1 and Per2 genes in the striatum but not the SCN (85), paralleling the aforementioned methamphetamine shifts in activity rhythms. This again suggests that the timing of drug-related behaviors could fall into conflict with the timing of other SCN-regulated processes, including the sleep/wake cycle.
Transgenic mouse models support a role for the circadian genes in these reward-related brain areas in the regulation of appetitive behavior, although it is not yet clear from these studies if the genes are serving a circadian or pleiotropic role. For example, mice with a mutation of the Clock gene show an increased sensitization to cocaine paralleled by increased dopaminergic activity in the ventral tegmental area (86). Other mouse studies have demonstrated that the lack of a functional Per1 gene is associated with hyposensitivity to cocaine reward, while, conversely, lacking a functional Per2 gene is associated with hypersensitivity to cocaine exposure (87). This apparent role of Period2 in the reward value of substances of abuse may extend to humans, as preliminary evidence suggests that the human PER2 gene is not directly involved in alcohol dependence (88), but may have a role in the regulation of alcohol consumption as based on studies in adult inpatient (47) and adolescent (89) samples. Interestingly, PER1 (protein) levels are higher and PER2 levels are lower for at least 30 days into withdrawal from heroin (62). Based on the previous studies, this pattern would be consistent with hyper-responsivity to reward, and thus may be contributing to craving during heroin withdrawal. Others have argued that a hyper-responsivity to the reward value of the drug during withdrawal is the primary contributor to relapse (90). In contrast to the alcohol and heroin findings, another human study found no relationship between polymorphisms in PER1 or PER2 and cocaine dependence (91). In general, further research is needed to disentangle potentially distinct interactions between the circadian system and different substance classes, particularly with regard to sedating substances, such as alcohol and marijuana, versus stimulants.
Appetitive processes may be subject to circadian regulation in humans as well. Across healthy and mood-disordered samples, subjective mood is partly a product of circadian phase and duration of wakefulness, and circadian modulation may be specific to positive affect (15, 92, 93). Demonstrating endogenous circadian modulation requires specialized chronobiological protocols designed to control for environmental contributors to diurnal patterning (constant routine) or to parse out the effects of circadian timing versus homeostatic sleep drive (forced desynchrony, see Table 1). A recent forced desynchrony study reported that both subjective (positive affect) and a psychophysiological measure of reward motivation are influenced by both circadian and homeostatic processes (15). Positive affect and reward motivation decline with time spent awake but recover during sleep; based on the circadian component, positive affect and reward motivation are at their lowest in the early morning and highest during the evening. Notably, this temporal pattern parallels that of alcohol consumption among the general U.S. population, which troughs in the early morning and peaks in the early evening (94). As with the regulation of sleep propensity, proper alignment of the circadian and homeostatic components results in stable levels of appetitive motivation throughout the 24-hour light/dark cycle, albeit with apparently relative lower levels during the dark phase (14, 15). These findings are highly relevant to addiction, as they suggest that misalignment of sleep/wake timing with that of the central pacemaker might result in unstable or more variable appetitive motivation. For example, such misalignment could lead to windows of hypersensitivity or hyposensitivity to reward that increases vulnerability to substance abuse, particularly at the beginning and end of the day. These changes are likely to interact with other relevant processes, such as the effect of decreasing blood concentration of the relevant substance following use, social influences (including sociocultural rhythms), and other affective processes (e.g., negative emotionality such as fear and anxiety). Thus, both circadian misalignment and decreasing overnight blood alcohol levels may independently contribute to the shift in peak craving to the morning hours that is reported for the majority of alcohol-dependent adults (95).
Lastly, proper alignment of sleep with the central clock also consolidates cognitive performance so that performance is better during the biological day and worse during the biological night (14). Some of these aspects of cognitive performance shown to be influenced by both circadian and homeostatic processes, such as attention and executive control, have also been implicated in emotion regulation and impulse control (96, 97). Thus, this provides another overlapping pathway by which circadian and sleep disturbance are linked to substance abuse.
In summary, this emerging literature linking the circadian system to appetitive motivation, as well as behavioral indicators and the neural circuitry of reward, offers the potential for specific mechanistic hypotheses about how drug abuse and circadian misalignment might fall into a vicious cycle.
Integrations and hypothetical mechanisms
Although many gaps remain, current human and animal evidence collectively points to substantial, and perhaps bidirectional, interactions between the circadian system and substance abuse. Less evidence directly speaks to substance dependence or addiction per se, with relatively greater support for effects on substance intake and craving. Likewise, too few of the reviewed studies simultaneously addressed sleep disturbance as an outcome or mediator of the observed associations. However, given the well-established circadian regulation of sleep/wake timing, as well as circadian influences on sleep structure and continuity, it is plausible that sleep disturbance will often accompany any substance use-associated circadian disruption. Likewise, the sleep disturbance associated with acute use, chronic use, and discontinuation of use, may lead to circadian changes as well, thus compounding the negative effects. For example, the increases in sleep latency associated with chronic use and withdrawal may encourage later bedtimes, thus exposing the individual to more light before bed and thus shifting his or her circadian timing later, further exacerbating the sleep onset difficulties.
In part because of the current gaps in the evidence base, particularly the lack of studies addressing chronic use, any integration of the above findings into mechanistic models must remain speculative. A number of intriguing and non-mutually exclusive pathways come to mind. Substance abuse has apparent direct consequences on sleep and circadian rhythms, and may also impact them indirectly via associated affective disturbance and stress. Addiction, regardless of the specific substance, has negative emotional consequences that serve to maintain the addiction (7) and these consequences may also serve to disrupt sleep and circadian rhythms directly or via behavorial changes that influence the timing of photic and/or non-photic zeitgebers. Bidirectional effects are plausible, thus risking a vicious cycle. Perhaps the most simple circadian-addiction model, as suggested by others in the context of alcohol, is that circadian (and sleep) disturbance acts as a non-specific chronic stressor leading to a vulnerability for excessive substance use (98). More specifically, circadian (and sleep disturbance) may increase substance use due to attempts to cope with the accompanying increased sleepiness and decreased alertness. For example, users may employ stimulants earlier in the day to stay awake, and rely on depressants at night to help fall asleep. Persistent usage of these substances could lead to tolerance and eventually dependence, as well as to continued sleep and circadian disturbance.
Taking a more explicitly circadian perspective, the impact of substance use on circadian phase-resetting could lead to a misalignment between the central clock and the light/dark cycle or sleep/wake cycle. The consistent association between substance abuse and eveningness preference, which is associated with chronic misalignment and irregular sleep schedules due to societal school and work demands that are more geared to morning-types, adds credence to this model. Such misalignment could thus lead to sleep disruption calling for compensatory drug use. Misalignment could also impact any or all of a range of systems ostensibly under circadian regulation, thus leading to impaired cognitive control, a dysregulated reward system, and increased drug seeking.
Figure 1 shows a conceptual model of how substance abuse may be both a cause and consequence of circadian misalignment, operating via pathways including sleep disruption, direct circadian effects, and reward processing (which includes both cognitive and motivational components). This model is informed by evidence that sleep, reward motivation, and cognition are all modulated by interacting circadian and homeostatic processes, as consistent with the two-process model of sleep regulation (12, 14, 15). Thus, in the case of reward processing, a misalignment between circadian and homeostatic processes could lead to windows of hypersensitivity and/or hyposensitivity to reward that increases vulnerability to substance abuse and/or dependence (windows that are likely to occur at beginning and end of day).
Figure 1.
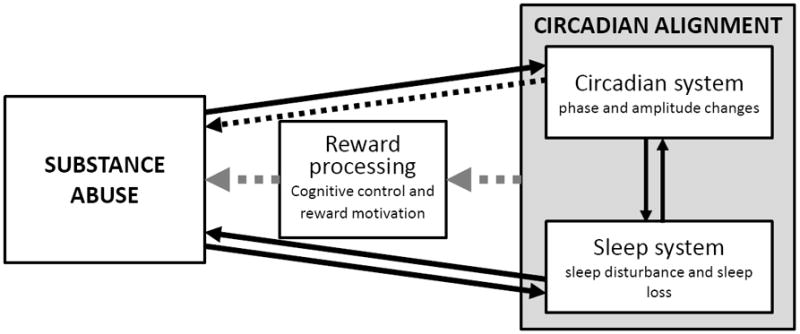
Conceptual model of circadian and sleep pathways to substance abuse. The solid arrows reflect the rich literature supporting bidirectional effects between sleep and substance abuse, and the cumulative human and animal evidence supporting the acute effects of substance use on circadian parameters. The hatched arrows reflect for hypothetical pathways awaiting more research attention, including direct circadian effects on substance abuse, and the reward system-mediated effect of circadian misalignment on substance abuse.
Taking a developmental perspective, this model may have particular relevance during adolescence, a qualitatively distinct period with regard to substance use and other risk-taking behavior, reward processing, and emotion regulation (99). Notably, this period is also marked by changes in sleep and circadian timing, in which sleep times and circadian phase are trending later while school start times are moving earlier (100). The resulting misalignment and instability may impair reward processing and emotion regulation, perhaps in part due to effects on executive control, increasing the risk for early onset of substance use, and eventual abuse and/or dependence. The onset of substance use during adolescence may in turn exacerbate these changes in sleep, circadian, reward, and emotion regulation. The limited relevant circadian research in adolescent samples precludes any conclusions regarding the validity of this model. This is a critical area of the literature to strengthen, given that a better understanding of these mechanisms during adolescence could lead to prevention efforts to decrease substance use during a particularly vulnerable time, and thus shift the trajectory away from the eventual development of substance abuse and/or dependence.
Evidence that drugs themselves can become zeitgebers for the central clock or SCN-independent pacemakers add another level of complexity to the potential pathways. A disturbed, and thus weakened, circadian system may increase susceptibility to drugs of abuse becoming zeitgebers that entrain activity (and thus sleep) to a time at odds with the preferred sleep and work (or school) schedules. The identification of an SCN-independent methamphetamine-induced pacemaker raises another intriguing possibility. Methamphetamine use could lead to a conflict between the methamphetamine-specific pacemaker and the central pacemaker in the SCN, resulting in a conflict between both the external behaviors and internal processes respectively regulated by these two pacemakers. In addition to sleep disturbance, such desynchrony may also contribute to other pathophysiological effects observed with substance abuse.
Conclusion
A substantial literature now links perturbations in the circadian system with the processes of addiction. Although gaps remain, evidence suggests that circadian mechanisms may partially account for the well-documented association between sleep disturbance and substance abuse. Furthermore, exciting new research concerning circadian regulation of the reward system suggests a direct link between circadian disturbance and substance abuse behaviors. Further work in each of these areas is critical for the enhancement of current substance abuse prevention and intervention methods, as well as the development of new approaches. The range of existing non-pharmacological sleep (e.g., stimulus control and sleep restriction) and circadian-based interventions (e.g., light therapy) may prove particularly useful in patients exhibiting problematic substance use.
PRACTICE POINTS.
Assessment of substance use should be routine in all sleep-disturbed patients
In the assessment and treatment of substance use disorders, consideration of sleep (and perhaps circadian factors) may improve outcomes
When physiological measures of circadian functioning are not feasible, use of actigraphy may be useful for assessing both sleep and circadian disturbance in clinics
Disturbance in sleep and circadian rhythms may be at their worst during early withdrawal, highlighting the need for appropriate non-pharmacological intervention during this time.
Further research is needed on the effects associated with different substances of abuse. Alcohol, opiates, and stimulants have all been linked to circadian disturbance
The timing of acute alcohol consumption is instrumental in determining the
RESEARCH AGENDA.
Further research in this area should focus on:
Prospective or longitudinal human studies that assess both sleep and circadian rhythms during acute and chronic substance use, as well as after discontinuation of use, and including chronobiological paradigms such as constant routine and forced desynchrony to separate circadian effects from those of sleep and the environment
Developmental factors in circadian-substance abuse interactions, particularly whether circadian changes during adolescence contribute to increased risk for substance abuse
The use of comparison samples with well-characterized substance use patterns
Dose-dependent effects of acute alcohol administration, and more studies on the acute effects of other substances of abuse
Evaluating whether sleep and circadian disturbance during withdrawal contributes to the risk of relapse, particularly in substances other than alcohol
Probing whether sleep and circadian-focused interventions during chronic use or withdrawal lead to better addiction-relevant outcomes
Differentiating the effects of substance abuse from concomitant affective disturbance on sleep and circadian rhythms
Investigating genetic, behavioral, psychological, and neural-based individual differences in associations between substance abuse and sleep and/or circadian disturbance
Mechanistic research to determine the biobehavioral pathways by which sleep and circadian disruption lead to increased substance use, and vice versa
Circadian regulation of reward processing in humans, particularly with regard to its neurobehavioral underpinnings, and dysregulation in these pathways may contribute to increased substance abuse
Acknowledgments
Support for the first author (BPH) was provided by a postdoctoral Kirschstein-NRSA (T32HL082610) from the National Heart Lung Blood Institute (NHLBI).
LIST OF ABBREVIATIONS
- 6-SM
6-sulfatoxymelatonin
- BrAC
Breath alcohol content
- CBT
Core body temperature
- DLMO
Dim light melatonin onset
- PSG
Polysomnography
- PMBCs
Peripheral blood mononuclear cells
- REM sleep
Rapid eye movement sleep
- SCN
Suprachiasmatic nucleus
Footnotes
Doses measured in g/l are roughly equivalent to doses in g/kg
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- *1.Roth T. Does effective management of sleep disorders reduce substance dependence? Drugs. 2009;69(Suppl 2):65–75. doi: 10.2165/11531120-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Teplin D, Raz B, Daiter J, Varenbut M, Tyrrell M. Screening for substance use patterns among patients referred for a variety of sleep complaints. American Journal of Drug and Alcohol Abuse. 2006;32(1):111–20. doi: 10.1080/00952990500328695. [DOI] [PubMed] [Google Scholar]
- 3.Passarella S, Duong MT. Diagnosis and treatment of insomnia. American Journal of Health-System Pharmacy. 2008 May 15;65(10):927–34. doi: 10.2146/ajhp060640. [DOI] [PubMed] [Google Scholar]
- 4.Gillin JC, Drummond SPA, Clark CP, Moore P. Medication and substance abuse. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. fourth edition. Philadelphia: Elsevier Saunders; 2006. pp. 1345–58. [Google Scholar]
- 5.Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of illicit recreational drugs upon sleep: cocaine, ecstasy and marijuana. Sleep Med Rev. 2008 Oct;12(5):381–9. doi: 10.1016/j.smrv.2007.12.004. [DOI] [PubMed] [Google Scholar]
- *6.Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Research & Health. 2001;25(2):110–25. [PMC free article] [PubMed] [Google Scholar]
- 7.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004 Jan;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 8.Thompson PM, Gillin JC, Golshan S, Irwin M. Polygraphic sleep measures differentiate alcoholics and stimulant abusers during short-term abstinence. Biological Psychiatry. 1995 Dec 15;38(12):831–6. doi: 10.1016/0006-3223(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 9.Morgan PT, Pace-Schott E, Pittman B, Stickgold R, Malison RT. Normalizing effects of modafinil on sleep in chronic cocaine users. American Journal of Psychiatry. 2010 Mar;167(3):331–40. doi: 10.1176/appi.ajp.2009.09050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Wang NY, Funderburk FR, et al. Polysomnogram changes in marijuana users who report sleep disturbances during prior abstinence. Sleep Med. 2010 Oct;11(9):882–9. doi: 10.1016/j.sleep.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenwasser AM. Functional neuroanatomy of sleep and circadian rhythms. Brain Research Reviews. 2009 Oct;61(2):281–306. doi: 10.1016/j.brainresrev.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- 13.Dijk DJ, Lockley SW. Integration of human sleep-wake regulation and circadian rhythmicity. Journal of Applied Physiology. 2002 Feb;92(2):852–62. doi: 10.1152/japplphysiol.00924.2001. [DOI] [PubMed] [Google Scholar]
- 14.Dijk DJ, von Schantz M. Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J Biol Rhythms. 2005 Aug;20(4):279–90. doi: 10.1177/0748730405278292. [DOI] [PubMed] [Google Scholar]
- 15.Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, et al. Nature’s clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009 Oct;9(5):705–16. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- 16.Oyefeso A, Sedgwick P, Ghodse H. Subjective sleep-wake parameters in treatment-seeking opiate addicts. Drug and Alcohol Dependence. 1997 Oct 25;48(1):9–16. doi: 10.1016/s0376-8716(97)00097-5. [DOI] [PubMed] [Google Scholar]
- 17.Tynjala J, Kannas L, Levalahti E. Perceived tiredness among adolescents and its association with sleep habits and use of psychoactive substances. Journal of Sleep Research. 1997 Sep;6(3):189–98. doi: 10.1046/j.1365-2869.1997.00048.x. [DOI] [PubMed] [Google Scholar]
- 18.McCloud A, Barnaby B, Omu N, Drummond C, Aboud A. Relationship between alcohol use disorders and suicidality in a psychiatric population: in-patient prevalence study. British Journal of Psychiatry. 2004 May;184:439–45. doi: 10.1192/bjp.184.5.439. [DOI] [PubMed] [Google Scholar]
- 19.Currie SR, Clark S, Rimac S, Malhotra S. Comprehensive assessment of insomnia in recovering alcoholics using daily sleep diaries and ambulatory monitoring. Alcoholism, Clinical and Experimental Research. 2003 Aug;27(8):1262–9. doi: 10.1097/01.ALC.0000081622.03973.57. [DOI] [PubMed] [Google Scholar]
- 20.Broms U, Kaprio J, Hublin C, Partinen M, Madden PA, Koskenvuo M. Evening types are more often current smokers and nicotine-dependent-a study of Finnish adult twins. Addiction. 2010 Sep 30; doi: 10.1111/j.1360-0443.2010.03112.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gau SS, Shang CY, Merikangas KR, Chiu YN, Soong WT, Cheng AT. Association between morningness-eveningness and behavioral/emotional problems among adolescents. Journal of Biological Rhythms. 2007 Jun;22(3):268–74. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- 22.Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. Journal of Sleep Research. 2002;11(3):191–9. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- 23.Negriff S, Dorn LD, Pabst SR, Susman EJ. Morningness/eveningness, pubertal timing, and substance use in adolescent girls. Psychiatry Research. Jul 29; doi: 10.1016/j.psychres.2010.07.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pieters S, Van Der Vorst H, Burk WJ, Wiers RW, Engels RC. Puberty-dependent sleep regulation and alcohol use in early adolescents. Alcoholism, Clinical and Experimental Research. 2010 Sep 1;34(9):1512–8. doi: 10.1111/j.1530-0277.2010.01235.x. [DOI] [PubMed] [Google Scholar]
- 25.Randler C. Evening types among german university students score higher on sense of humor after controlling for big five personality factors. Psychology Reports. 2008 Oct;103(2):361–70. doi: 10.2466/pr0.103.2.361-370. [DOI] [PubMed] [Google Scholar]
- 26.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: Misalignment of biological and social time. Chronobiology International. 2006;23(1&2):497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 27.Hatonen T, Forsblom S, Kieseppa T, Lonnqvist J, Partonen T. Circadian Phenotype in Patients with the Co-Morbid Alcohol Use and Bipolar Disorders. Alcohol Alcohol 2008. 2008 September 1;43(5):564–8. doi: 10.1093/alcalc/agn057. [DOI] [PubMed] [Google Scholar]
- 28.Ahn YM, Chang J, Joo YH, Kim SC, Lee KY, Kim YS. Chronotype distribution in bipolar I disorder and schizophrenia in a Korean sample. Bipolar Disorders. 2008;10(2):271–5. doi: 10.1111/j.1399-5618.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 29.Mansour HA, Wood J, Chowdari KV, Dayal M, Thase ME, Kupfer DJ, et al. Circadian phase variation in Bipolar I disorder. Chronobiology International. 2005;22(3):571–84. doi: 10.1081/CBI-200062413. [DOI] [PubMed] [Google Scholar]
- 30.Mistlberger RE, Skene DJ. Nonphotic entrainment in humans? Journal of Biological Rhythms. 2005 Aug;20(4):339–52. doi: 10.1177/0748730405277982. [DOI] [PubMed] [Google Scholar]
- *31.Rupp TL, Acebo C, Carskadon MA. Evening alcohol suppresses salivary melatonin in young adults. Chronobiology International. 2007;24(3):463–70. doi: 10.1080/07420520701420675. [DOI] [PubMed] [Google Scholar]
- 32.Roehrs T, Zwyghuizen-Doorenbos A, Knox M, Moskowitz H, Roth T. Sedating effects of ethanol and time of drinking. Alcoholism, Clinical and Experimental Research. 1992 Jun;16(3):553–7. doi: 10.1111/j.1530-0277.1992.tb01416.x. [DOI] [PubMed] [Google Scholar]
- 33.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. Journal of Neuroscience. 2001 Aug 15;21(16):6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. Journal of Pineal Research. 2002 Nov;33(4):198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Reen E, Arantes HB, Rupp TL, Maxwell JL, Acebo C, Carskadon MA. Residual effects of a moderate dose of alcohol on sleep stages of young men. Sleep. 2006;29(Abstract Supplement):A46. [Google Scholar]
- 36.Rojdmark S, Wikner J, Adner N, Andersson DE, Wetterberg L. Inhibition of melatonin secretion by ethanol in man. Metabolism. 1993 Aug;42(8):1047–51. doi: 10.1016/0026-0495(93)90021-f. [DOI] [PubMed] [Google Scholar]
- 37.Ekman AC, Leppaluoto J, Huttunen P, Aranko K, Vakkuri O. Ethanol inhibits melatonin secretion in healthy volunteers in a dose-dependent randomized double blind cross-over study. Journal of Clinical Endocrinology & Metabolism. 1993 Sep;77(3):780–3. doi: 10.1210/jcem.77.3.8370699. [DOI] [PubMed] [Google Scholar]
- 38.Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. New England Journal of Medicine. 1995 Jan 5;332(1):6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 39.Plenzler SC, Badia P, Myers BL, Wright KP., Jr Effects of alcohol and time of administration on melatonin levels. Sleep Res. 1996;25:568. [Google Scholar]
- 40.Danel T, Touitou Y. Alcohol consumption does not affect melatonin circadian synchronization in healthy men. Alcohol & Alcoholism. 2006 Jul-Aug;41(4):386–90. doi: 10.1093/alcalc/agl036. [DOI] [PubMed] [Google Scholar]
- 41.Danel T, Libersa C, Touitou Y. The effect of alcohol consumption on the circadian control of human core body temperature is time dependent. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2001 Jul;281(1):R52–5. doi: 10.1152/ajpregu.2001.281.1.R52. [DOI] [PubMed] [Google Scholar]
- 42.Devaney M, Graham D, Greeley J. Circadian variation of the acute and delayed response to alcohol: investigation of core body temperature variations in humans. Pharmacology Biochemistry & Behavior. 2003 Jul;75(4):881–7. doi: 10.1016/s0091-3057(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 43.Czeisler CA, Buxton OM. The human circadian timing system and sleep–wake regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5. Elsevier Saunders; 2011. [Google Scholar]
- 44.Prosser RA, Mangrum CA, Glass JD. Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro. Neuroscience. 2008 Mar 27;152(3):837–48. doi: 10.1016/j.neuroscience.2007.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2009 Feb;296(2):R411–8. doi: 10.1152/ajpregu.90782.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. MPer1 and mper2 are essential for normal resetting of the circadian clock. Journal of Biological Rhythms. 2001 Apr;16(2):100–4. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]
- 47.Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nature Medicine. 2005 Jan;11(1):35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- 48.Wasielewski JA, Holloway F. Alcohol’s Interactions with Circadian Rhythms. Alcohol Research & Health. 2001;25(2):94–100. [PMC free article] [PubMed] [Google Scholar]
- 49.Danel T, Cottencin O, Tisserand L, Touitou Y. Inversion of melatonin circadian rhythm in chronic alcoholic patients during withdrawal: preliminary study on seven patients. Alcohol & Alcoholism. 2009 Jan-Feb;44(1):42–5. doi: 10.1093/alcalc/agn091. [DOI] [PubMed] [Google Scholar]
- 50.Majumdar SK, Miles A. Disturbed melatonin secretion in chronic alcoholism and withdrawal. Clinical Chemistry. 1987 Jul;33(7):1291. [PubMed] [Google Scholar]
- 51.Murialdo G, Filippi U, Costelli P, Fonzi S, Bo P, Polleri A, et al. Urine melatonin in alcoholic patients: a marker of alcohol abuse? Journal of Endocrinological Investigation. 1991 Jun;14(6):503–7. doi: 10.1007/BF03346853. [DOI] [PubMed] [Google Scholar]
- 52.Hasler BP, Bootzin RR, Cousins JC, Fridel K, Wenk GL. Circadian phase in sleepdisturbed adolescents with a history of substance abuse: a pilot study. Behavioral Sleep Medicine. 2008;6(1):55–73. doi: 10.1080/15402000701796049. [DOI] [PubMed] [Google Scholar]
- *53.Brager AJ, Ruby CL, Prosser RA, Glass JD. Chronic ethanol disrupts circadian photic entrainment and daily locomotor activity in the mouse. Alcoholism, Clinical and Experimental Research. 2010 Jul;34(7):1266–73. doi: 10.1111/j.1530-0277.2010.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. Am J Physiol Regul Integr Comp Physiol. 2009 Sep;297(3):R729–37. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seggio JA, Fixaris MC, Reed JD, Logan RW, Rosenwasser AM. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. Journal of Biological Rhythms. 2009 Aug;24(4):304–12. doi: 10.1177/0748730409338449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacology Biochemistry & Behavior. 2007 Aug-Sep;87(3):297–305. doi: 10.1016/j.pbb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuhlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biological Psychiatry. 2003;54:1437–43. doi: 10.1016/s0006-3223(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 58.Conroy DA, Hairston IS, Arnedt J, Armitage R, Brower K. Alcohol dependent patients in recovery show distinct dim light melatonin onset profile compared to controls. Sleep. 2010;33(Abstract Supplement):A239. [Google Scholar]
- 59.Fonzi S, Solinas GP, Costelli P, Parodi C, Murialdo G, Bo P, et al. Melatonin and cortisol circadian secretion during ethanol withdrawal in chronic alcoholics. Chronobiologia. 1994 Jan-Jun;21(1-2):109–12. [PubMed] [Google Scholar]
- 60.Benca RM, Okawa M, Uchiyama M, Ozaki S, Nakajima T, Shibui K, et al. Sleep and mood disorders. Sleep Med Rev. 1997 Nov;1(1):45–56. doi: 10.1016/s1087-0792(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 61.Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Human Psychopharmacology. 2008 Oct;23(7):571–85. doi: 10.1002/hup.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li SX, Shi J, Epstein DH, Wang X, Zhang XL, Bao YP, et al. Circadian alteration in neurobiology during 30 days of abstinence in heroin users. Biological Psychiatry. 2009 May 15;65(10):905–12. doi: 10.1016/j.biopsych.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 63.Trinkoff AM, Storr CL. Work schedule characteristics and substance use in nurses. American Journal of Industrial Medicine. 1998 Sep;34(3):266–71. doi: 10.1002/(sici)1097-0274(199809)34:3<266::aid-ajim9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 64.Rogers HL, Reilly SM. A survey of the health experiences of international business travelers. Part One--Physiological aspects. AAOHN Journal. 2002 Oct;50(10):449–59. [PubMed] [Google Scholar]
- 65.Gauvin DV, Baird TJ, Vanecek SA, Briscoe RJ, Vallett M, Holloway FA. Effects of time-of-day and photoperiod phase shifts on voluntary ethanol consumption in rats. Alcoholism, Clinical and Experimental Research. 1997 Aug;21(5):817–25. [PubMed] [Google Scholar]
- 66.Clark JW, Fixaris MC, Belanger GV, Rosenwasser AM. Repeated light-dark phase shifts modulate voluntary ethanol intake in male and female high alcohol-drinking (HAD1) rats. Alcoholism, Clinical and Experimental Research. 2007 Oct;31(10):1699–706. doi: 10.1111/j.1530-0277.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- 67.Rosenwasser AM, Clark JW, Fixaris MC, Belanger GV, Foster JA. Effects of repeated light-dark phase shifts on voluntary ethanol and water intake in male and female Fischer and Lewis rats. Alcohol. May 19; doi: 10.1016/j.alcohol.2010.03.002. In press. [DOI] [PubMed] [Google Scholar]
- 68.Sillaber I, Henniger MS. Stress and alcohol drinking. Annals of Medicine. 2004;36(8):596–605. doi: 10.1080/07853890410018862. [DOI] [PubMed] [Google Scholar]
- 69.Ancoli-Israel S, Cole R, Alessi C. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 70.Schmitz M, Frey R, Pichler P, Ropke H, Anderer P, Saletu B, et al. Sleep quality during alcohol withdrawal with bright light therapy. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1997 Aug;21(6):965–77. doi: 10.1016/s0278-5846(97)00092-4. [DOI] [PubMed] [Google Scholar]
- 71.Schmitz MM, Sepandj A, Pichler PM, Rudas S. Disrupted melatonin-secretion during alcohol withdrawal. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1996 Aug;20(6):983–95. doi: 10.1016/0278-5846(96)00078-4. [DOI] [PubMed] [Google Scholar]
- 72.Peles E, Hetzroni T, Bar-Hamburger R, Adelson M, Schreiber S. Melatonin for perceived sleep disturbances associated with benzodiazepine withdrawal among patients in methadone maintenance treatment: a double-blind randomized clinical trial. Addiction. 2007 Dec;102(12):1947–53. doi: 10.1111/j.1360-0443.2007.02007.x. [DOI] [PubMed] [Google Scholar]
- 73.Cuninkova L, Brown SA. Peripheral circadian oscillators: interesting mechanisms and powerful tools. Annals of the New York Academy of Sciences. 2008;1129:358–70. doi: 10.1196/annals.1417.005. [DOI] [PubMed] [Google Scholar]
- 74.Falcon E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology. 2009;566(Suppl 1):91. doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kosobud A, Gillman AG, Leffel JK, Pecoraro NC, Rebec GV, Timberlake W. Drugs of abuse can entrain circadian rhythms. The Scientific World Journal. 2007;7:203–12. doi: 10.1100/tsw.2007.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Honma K, Honma S. The SCN-independent clocks, methamphetamine and food restriction. European Journal of Neuroscience. 2009 Nov;30(9):1707–17. doi: 10.1111/j.1460-9568.2009.06976.x. [DOI] [PubMed] [Google Scholar]
- *77.Mohawk JA, Baer ML, Menaker M. The methamphetamine-sensitive circadian oscillator does not employ canonical clock genes. Proceedings of the National Academy of Science U S A. 2009 Mar 3;106(9):3519–24. doi: 10.1073/pnas.0813366106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sherwin CM. Voluntary wheel running: a review and novel interpretation. Animal Behaviour. 1998 Jul;56(1):11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- 79.Sleipness EP, Sorg BA, Jansen HT. Time of day alters long-term sensitization to cocaine in rats. Brain Res. 2005 Dec 14;1065(1-2):132–7. doi: 10.1016/j.brainres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 80.Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. Journal of Biological Rhythms. 2009 Dec;24(6):465–76. doi: 10.1177/0748730409346657. [DOI] [PubMed] [Google Scholar]
- 81.Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Research. 2007 Jan 19;1129(1):34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 82.Akhisaroglu M, Ahmed R, Kurtuncu M, Manev H, Uz T. Diurnal rhythms in cocaine sensitization and in Period1 levels are common across rodent species. Pharmacology Biochemistry & Behavior. 2004 Sep;79(1):37–42. doi: 10.1016/j.pbb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 83.Kurtuncu M, Arslan AD, Akhisaroglu M, Manev H, Uz T. Involvement of the pineal gland in diurnal cocaine reward in mice. European Journal of Pharmacology. 2004 Apr 12;489(3):203–5. doi: 10.1016/j.ejphar.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 84.Uz T, Akhisaroglu M, Ahmed R, Manev H. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003 Dec;28(12):2117–23. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- 85.Iijima M, Nikaido T, Akiyama M, Moriya T, Shibata S. Methamphetamine-induced, suprachiasmatic nucleus-independent circadian rhythms of activity and mPer gene expression in the striatum of the mouse. European Journal of Neuroscience. 2002 Sep;16(5):921–9. doi: 10.1046/j.1460-9568.2002.02140.x. [DOI] [PubMed] [Google Scholar]
- 86.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005 Jun 28;102(26):9377–81. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *87.Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proceedings of the National Academy of Science U S A. 2002 Jun 25;99(13):9026–30. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kovanen L, Saarikoski ST, Haukka J, Pirkola S, Aromaa A, Lonnqvist J, et al. Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol and Alcoholism. Jul-Aug;45(4):303–11. doi: 10.1093/alcalc/agq035. [DOI] [PubMed] [Google Scholar]
- 89.Comasco E, Nordquist N, Gokturk C, Aslund C, Hallman J, Oreland L, et al. The clock gene PER2 and sleep problems: association with alcohol consumption among Swedish adolescents. Upsala Journal of Medical Sciences. Feb;115(1):41–8. doi: 10.3109/03009731003597127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nature Neuroscience. 2001 Sep;4(9):943–7. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- 91.Malison RT, Kranzler HR, Yang BZ, Gelernter J. Human clock, PER1 and PER2 polymorphisms: lack of association with cocaine dependence susceptibility and cocaine-induced paranoia. Psychiatric Genetics. 2006 Dec;16(6):245–9. doi: 10.1097/01.ypg.0000242198.59020.ca. [DOI] [PubMed] [Google Scholar]
- 92.Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Archives of General Psychiatry. 1997;54(2):145–52. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- 93.Koorengevel KM, Beersma DG, den Boer JA, van den Hoofdakker RH. Mood regulation in seasonal affective disorder patients and healthy controls studied in forced desynchrony. Psychiatry Research. 2003 Jan 25;117(1):57–74. doi: 10.1016/s0165-1781(02)00305-0. [DOI] [PubMed] [Google Scholar]
- 94.Arfken CL. Temporal pattern of alcohol consumption in the United States. Alcoholism: Clinical and Experimental Research. 1988 Feb;12(1):137–42. doi: 10.1111/j.1530-0277.1988.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 95.Danel T, Jeanson R, Touitou Y. Temporal pattern in consumption of the first drink of the day in alcohol-dependent persons. Chronobiol International. 2003 Nov;20(6):1093–102. doi: 10.1081/cbi-120025533. [DOI] [PubMed] [Google Scholar]
- 96.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005 Nov;8(11):1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 97.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005 May;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- *98.Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol consumption and the body’s biological clock. Alcoholism, Clinical and Experimental Research. 2005 Aug;29(8):1550–7. doi: 10.1097/01.alc.0000175074.70807.fd. [DOI] [PubMed] [Google Scholar]
- 99.Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcoholism, Clinical and Experimental Research. 2008 Mar;32(3):375–85. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 100.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Medicine. 2007;Sep8(6):602–12. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]


