On April 28-29, 2011 a conference entitled, “Late Effects after Pediatric Hematopoietic Cell Transplantation: State of the Science, Future Directions” was held in Arlington, VA with a goal of bringing together thought leaders in the field of pediatric transplant survivorship in order to review the current state of knowledge and define gaps in the field, develop consensus on critical areas for future research, and determine the best study designs to effectively address these questions. Over the course of the next several months, six summary articles covering the major topics discussed at the conference will be published in this journal which will define and set forth recommendations for a research agenda to move this field forward over the next decade.
Study of late effects after pediatric hematopoietic cell transplantation (HCT) offers unique opportunities and challenges. The opportunities include an ability to discern the effects of treatment modalities on normal childhood growth and development. In addition, these individuals have decades of life ahead of them, with potentially new issues arising as they age. The challenges, however, are daunting. The numbers of transplants for any given condition are few, with even the largest centers performing only a handful of transplants for a given condition each year. This issue is magnified by the fact that children going through each developmental stage (infant, toddler, child pre-adolescent, and young adult) have different sensitivities to therapies, resulting in different complications (i.e. infants and toddlers are susceptible to neurocognitive damage with radiation and adolescents are at high risk of joint/bone issues with steroid therapy). Furthermore, ability to self-report symptoms changes throughout the continuum of transplant survivorship, both across different patients and even within a patient. Thus to be most effective, the study of pediatric late effects after HCT in children should ideally occur in a multi-institutional setting to maximize accrual. To date, such efforts have been limited.
Over the last few years, cooperative groups in North America involving pediatric HCT have strengthened and assembled the expertise and infrastructure to start to address these issues. The NCI/NHLBI Bone Marrow Transplant Clinical Trials Network (BMT CTN) has successfully executed combined adult/pediatric and pediatric only therapeutic trials, and has an active Pediatric Diseases committee. The Stem Cell Committee of the Children’s Oncology Group (COG) has formed a cooperative agreement with the Pediatric Blood and Marrow Transplant Consortium (PBMTC), initiating several phase II and III trials. The PBMTC has further allied itself with the clinical trials arm of the Center of International Blood and Marrow Transplant Research (CIBMTR) in order to run pilot trials for COG or the BMT CTN. Cooperative efforts between these groups have resulted in three phase III trials, three phase II, and one large minimal residual disease (MRD) biomarker trial currently underway. All of these studies are good clinical practice compliant, and the infrastructure, expertise, and personnel are increasingly ready to tackle challenging late effects trials.
Given these issues and opportunities, a consensus conference was organized with the aim of defining the most critical questions in this understudied field. The goals of this conference were threefold: 1) define methodological challenges in studying long-term outcomes after HCT including the impact of pre-transplant therapies/complications and post HCT events on late effects in survivors who underwent HCT as children; 2) discuss methodologies that incorporate the identification of biomarkers of adverse outcomes and also how the role of genetic predisposition to certain adverse events and/or late effects can be associated with exposure to specific chemotherapeutic agents or radiation; and (3) review selected high risk organ systems, persistent immunodeficiency, issues of tolerance, neurocognitive outcomes, and health-related quality of life (HR-QOL) outcomes in survivors after HCT during childhood in order to determine high-impact questions for multi-institutional trials.
Significance: The Lifetime Impact of Post-Transplant Late Effects
Expansion of the number of indications for transplantation and improvements in the availability of appropriate alternative donor stem cell sources to patients with rare HLA types through the use of cord blood and haploidentical approaches has resulted in increased numbers of HCT performed in children annually. In conjunction with this, a reduction in the mortality secondary to relapse, infections, graft vs. host disease (GVHD), and other acute transplant related complications,1 is leading to improved survival rates and thus an ever-increasing population of HCT survivors.
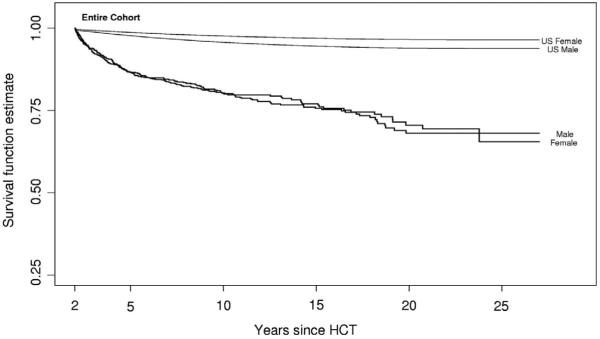
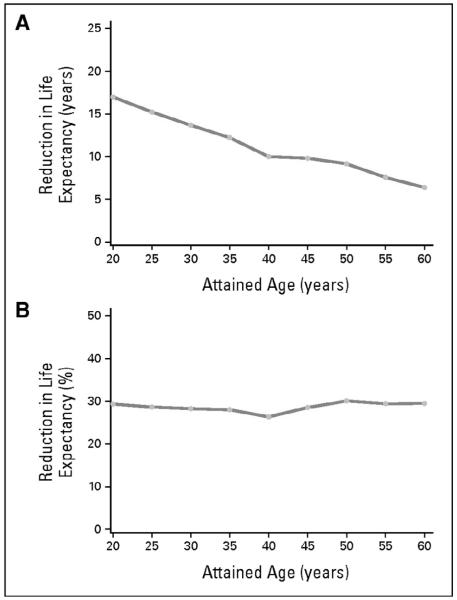
As we continue to follow HCT patients long-term, however, we are finding that in both the autologous as well as the allogeneic transplant setting, HCT survivors experience mortality rates higher than the general population.2, 3 One of the largest and most comprehensive studies of HCT survivors to date, the Bone Marrow Transplant Survivor Study (BMT-SS), examined patients treated with HCT who were alive at least 2 years post-transplant and found that allogeneic HCT survivors were at a 9.9 fold-increased risk of premature death (Figure 1). Even 15 years after transplant these patients continued to have mortality rates twice that of the general population (standardized mortality ratio = 2.2). While relapse of the primary disease and chronic graft vs. host disease (cGHVD) were the leading causes of death, late mortality was attributed to treatment-related causes in 25% of deaths including second malignancies, late infections, cardiac, and pulmonary causes. Similar findings were recently published from the Seattle group where mortality rates in patients who survived for more than 5 years after HCT were 4 to 9-fold higher than the general population for at least 30 years after transplantation.4 In this analysis, second malignancies, late infections, cardiovascular or other vascular causes, and pulmonary complications were again the most frequent causes of mortality. This resulted in an absolute decrease in estimated residual life expectancy of 17.0 years for survivors at 20 years of age to 6.4 years for survivors at 60 years of age (Figure 2A), and a proportionate reduction in life expectancy of approximately 30% regardless of attained age (Figure 2B). In both of these studies, it was difficult to discern the relative contribution of pre-transplant therapy to the risk of cardiovascular related death, as similar data has been reported in survivors of childhood cancer who have not undergone HCT as part of their therapy where the risk of non-relapse death in patients surviving for 5 years or more was 8-fold higher than the US population.5
Figure 1.
All-cause mortality in a cohort of 1479 2+-year survivors after allogeneic HCT*
* This research was originally published in Blood. Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007; 110(10):3784-92. © the American Society of Hematology
Figure 2.
Projected reduction in life expectancy in the study cohort relative to US population data as a function of attained age. (A) Absolute reduction in years; (B) percentage reduction.*
* This research was originally published in Martin PJ, Counts GW, Jr., Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol 2010; 28(6): 1011-6
While the issue of premature mortality is of obvious concern, the overall and cumulative impact of late effects in HCT survivors is also alarming. Large, comprehensive studies have shown in detail the burden of late effects in childhood cancer survivors. In two seminal studies, with a median follow-up of 25 and 17 years, the cumulative incidence of late effects after childhood cancer was 73% and 75% respectively.6, 7 In both studies, over 40% of survivors had severe, disabling and/or life- threatening late effects or died due to an adverse effect of cancer treatment. One of these studies was a multi-institutional effort by the Childhood Cancer Survivor Study (CCSS). This and many other efforts of the CCSS have created significant expertise regarding these issues in the pediatric cancer research community; however, the CCSS contains only a very small number of HCT survivors and was not designed to look specifically at HCT related issues.
A handful of single-center studies describe the cumulative incidence and severity of late effects in survivors of childhood HCT.8-12 Most of these studies focused on survivors with a particular disease, age and/or conditioning regimen (e.g. including TBI) and described the cumulative incidence of specific late effects separately. Only one study assessed comprehensively the burden of late effects according to Common Terminology Criteria for Adverse Events (CTCAE vs. 3.0). Pediatric HCT survivors had a higher cumulative incidence of late effects compared to the studies of cancer survivors who did not receive HCT as part of their treatment, with 93% of survivors having at least one late effect with a median follow-up of only 7 years. In contrast, only 25% of pediatric HCT survivors had severe or disabling/life-threatening late effects,12 but the follow up was 1-2 decades less than the childhood cancer studies.
Much remains to be learned by multi-center studies with longer follow up, however, the experience gained through studies in pediatric cancer survivors, along with experience gained at centers studying late effects in adult HCT recipients, has shaped a group of researchers capable of performing these studies. With expertise and infrastructure now available to allow this field to move into high-quality multi-center studies, the next step is obvious: we must define the best questions, approach them with the most innovative and informative methodologies, and come to a consensus about how to prioritize the work and move forward.
Post- Conference Proceedings
Over the course of the next several months (and starting with this current issue of the BBMT) a series of manuscripts will be published on topics considered to be the most critical for future research efforts. These include:
Etiology and Pathogenesis of Late Effects after HSCT performed in Childhood – Methodological Challenges
Allogeneic Immune Reconstitution and Tolerance in Children After HCT
Organ Toxicities & Metabolism
Quality of Life, Functional and Neurocognitive Outcomes
Endocrine Challenges: Thyroid Dysfunction, Growth Impairment, Bone Health and Reproductive Risks
Consensus Guidelines for Long-Term Follow-Up after Pediatric HCT
Each of these manuscripts will describe the current state of knowledge as well as what the gaps are in this current knowledge. In addition, there will be a description of what preliminary or emerging new data (clinical or pre-clinical, animal models, etc) and what new research is being done (which may or may not be focused directly on HCT populations) that sets the stage or direction for where research in that particular area needs to move in the future. Where relevant, there will be a discussion on what would be appropriate screening and management recommendations from a clinical standpoint for HCT survivors. Finally, the authors will summarize, based on their presentations and conference discussions, what the recommendations are for future research (i.e. what are the highest priority questions to be answered and how should studies be designed to answer these questions?),
Our hope is that these publications will stimulate further interest and discussion surrounding each of these very important topics and that investigators from a variety of disciplines will come together to formulate study questions, grant submissions, and protocols that will begin to provide clinically useful knowledge that can be applied to the long-term follow-up care of pediatric HCT survivors. The ultimate goal of future studies will be to modify HCT approaches, systematize post-HCT late effects screening, and improve management of late effects in a manner that reduces long-term morbidity and improves the quality and length of pediatric HCT survivors’ lives.
Acknowledgements
Funding for this work was made possible in part by grant 1R13CA159788-01 from the National Cancer Institute and the National Heart, Lung and Blood Institute. The views expressed in this manuscript do not reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. Government. Further support was provided by a generous grant from the St. Baldrick’s Foundation, and the Lance Armstrong Foundation, as well as the following pharmaceutical companies: Genzyme, Otsuka America Pharmaceutical, Inc., and Sigma-Tau Pharmaceuticals, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of those that provided funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–92. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia S, Robison LL, Francisco L, Carter A, Liu Y, Grant M, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105(11):4215–22. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin PJ, Counts GW, Jr., Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28(6):1011–6. doi: 10.1200/JCO.2009.25.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–79. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geenen MM, Cardous-Ubbink MC, Kremer LC, van den Bos C, van der Pal HJ, Heinen RC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297(24):2705–15. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 7.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 8.Ferry C, Gemayel G, Rocha V, Labopin M, Esperou H, Robin M, et al. Long-term outcomes after allogeneic stem cell transplantation for children with hematological malignancies. Bone Marrow Transplant. 2007;40(3):219–24. doi: 10.1038/sj.bmt.1705710. [DOI] [PubMed] [Google Scholar]
- 9.Perkins JL, Kunin-Batson AS, Youngren NM, Ness KK, Ulrich KJ, Hansen MJ, et al. Long-term follow-up of children who underwent hematopoeitic cell transplant (HCT) for AML or ALL at less than 3 years of age. Pediatr Blood Cancer. 2007;49(7):958–63. doi: 10.1002/pbc.21207. [DOI] [PubMed] [Google Scholar]
- 10.Faraci M, Barra S, Cohen A, Lanino E, Grisolia F, Miano M, et al. Very late nonfatal consequences of fractionated TBI in children undergoing bone marrow transplant. Int J Radiat Oncol Biol Phys. 2005;63(5):1568–75. doi: 10.1016/j.ijrobp.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Leahey AM, Teunissen H, Friedman DL, Moshang T, Lange BJ, Meadows AT. Late effects of chemotherapy compared to bone marrow transplantation in the treatment of pediatric acute myeloid leukemia and myelodysplasia. Med Pediatr Oncol. 1999;32(3):163–9. doi: 10.1002/(sici)1096-911x(199903)32:3<163::aid-mpo1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Bresters D, van Gils IC, Kollen WJ, Ball LM, Oostdijk W, van der Bom JG, et al. High burden of late effects after haematopoietic stem cell transplantation in childhood: a single-centre study. Bone Marrow Transplant. 45(1):79–85. doi: 10.1038/bmt.2009.92. [DOI] [PubMed] [Google Scholar]