Abstract
Purpose
To identify the neural correlates of cognitive improvement in mild AD subjects, following 12 weeks of donepezil treatment.
Materials and Methods
Resting-state functional connectivity MRI (R-fMRI) was used to measure the hippocampal functional connectivity (HFC) in 14 mild AD and 18 age-matched normal (CN) subjects. AD subjects were scanned at baseline and after donepezil treatment. CN subjects were scanned only at baseline as a reference to identify regions correlated or anticorrelated to the hippocampus. Before each scan, participants underwent cognitive, behavioral and functional assessments.
Results
After donepezil treatment, neural correlates of cognitive improvement measured by Mini-Mental State Examination scores were identified in the left parahippocampus, dorsolateral prefrontal cortex (DLPFC) and inferior frontal gyrus. Improvement in AD Assessment Scale-cognitive subscale scores correlated with the HFC changes in the left DLPFC and middle frontal gyrus. Stronger recovery in the network connectivity was associated with cognitive improvement.
Conclusion
R-fMRI may provide novel insight into the brain's functional responses to AD treatment in clinical pharmacological trials, and also may predict clinical response.
Keywords: Resting-state Functional MRI, Hippocampus, Alzheimer's disease, Donepezil
Introduction
In Alzheimer's disease (AD), the degree of memory impairment is associated with the degeneration of the basal forebrain cholinergic nuclei and deprivation of the cholinergic supply to the hippocampus and neocortical areas (1-3). These findings constitute the basis for the “cholinergic hypothesis” in AD, and have led to the development of cholinesterase inhibitors (ChEIs), including donepezil. The latter indirectly enhances central cholinergic neurotransmission. It improves cognitive performance, delays cognitive decline and shows neuroprotective properties in AD patients (4, 5). However, the benefits of prescribing ChEIs to all individuals with AD are debatable, because positive response to these agents is seen only in a subgroup of patients. Even in treatment responders, cognitive improvement is modest at best, and the clinical benefit observed is heterogeneous (6). The neural network and neurophysiological mechanism by which donepezil modifies brain function and produces treatment effects in a subset of AD patients is not fully understood. Further, traditionally used efficacy measures in AD clinical trials have inherent limitations. Therefore, greater emphasis is now being placed on identifying sensitive imaging markers that can be used to evaluate treatment efficacy and neuroprotective effects earlier in pharmacological studies (7).
Functional magnetic resonance imaging (fMRI) has been used to measure the pharmacodynamic changes induced by ChEIs in the brain regions associated with cognition in patients with mild cognitive impairment (MCI) and AD (8-14). After ChEI treatment, AD patients show increased medial temporal lobe and neocortical activity during cognitive tasks relative to healthy elderly people (8, 11, 12). However, fMRI task-activation studies have limitations. For instance, the neuronal response depends on the type of task, the severity of cognitive impairment, and the subject's attention and motivation (15). It is also difficult to study the functional connections between spatially isolated brain regions activated by fMRI task-specific methods. AD is increasingly being viewed as a disconnection syndrome (16) in which cognitive dysfunction is a result of alterations in the functional connections between brain regions, rather than isolated regional changes (17).
Resting-state functional connectivity MRI (R-fMRI) is a novel technique that overcomes the above limitations and shows great potential in AD research (18-25). R-fMRI measures interregional correlations between the spontaneous blood oxygenation level-dependent (BOLD) fluctuations in spatially separated, but functionally related brain regions at rest (26, 27). Interestingly, hippocampal disconnection with other functionally related cortical and subcortical regions is found in early AD (22, 23). Although R-fMRI has shown promise as a surrogate marker for AD, no studies have evaluated whether the R-fMRI method can monitor the neurophysiological mechanisms of treatment responses in patients with AD.
The study objective was to use R-fMRI to test a hypothesis that donepezil would improve hippocampal functional connectivity (HFC), and that cholinergic enhancement of the medial temporal lobe network would significantly correlate with improvement in standardized cognitive measures.
Subjects and Methods
Participants
Fourteen subjects with probable AD and 18 age-matched healthy control subjects were recruited through an academic institution's Memory Disorders Clinic and local advertising. Written informed consent was obtained from all subjects in accordance with the Institutional Review Board.
All participants underwent psychiatric assessments, neurological examinations, extensive neuropsychological testing, and MRI scanning. All subjects were healthy and did not have significant medical, neurological or psychiatric conditions, history of head trauma with loss of consciousness, current or past history of alcohol or substance abuse, or other factors that can affect the participant's cognition or hemodynamic response while in the scanner. Participants also had adequate visual and auditory acuity to allow cognitive testing, and had a reliable informant to accompany them to each clinic visit.
Mild AD was diagnosed using the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association criteria for probable AD (28) during the baseline clinical visit (14 ± 10 days prior to the baseline R-fMRI scan [day 0]). Exclusion criteria included claustrophobia, Hachinski Ischemia Scale score > 4, Mini-Mental State Examination (MMSE) score < 18/30, contraindications to MRI, major psychiatric diagnoses, including primary psychotic and affective disorders, and other types of neurodegenerative or secondary dementias, as assessed with current clinical diagnostic criteria. Two neurologists with expertise in dementia reviewed the medical, neurological, functional, behavioral and neuropsychological data, and consensus diagnoses were reached for all patients.
The cognitive and behavioral assessments performed at a baseline clinical visit and at week 12 included the MMSE, Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog), Neuropsychiatric Inventory (NPI), and Instrumental Activities of Daily Living (IADL) scale. AD subjects underwent R-fMRI scans at baseline (day 0) and end point (week 12). AD patients received donepezil 5 mg/day after the baseline scan with the dose increased to 10 mg/day after four weeks.
Control subjects were required to have a Clinical Dementia Rating (CDR) global score of 0 and an MMSE score ≥ 24 (25, 29). All control subjects underwent an R-fMRI scan.
Resting-state R-fMRI Acquisition
All scans were performed using a whole-body 3T Signa GE scanner (Waukesha, Wisconsin) with a standard quadrature transmit-receive head coil. During the resting-state acquisitions, no specific cognitive tasks were performed, and the study participants were instructed to close their eyes, relax, and stay awake. Sagittal resting-state fMRI datasets of the whole brain were obtained in 6 minutes with a single-shot gradient echo-echo planar imaging pulse sequence. The fMRI imaging parameters were: TE = 25 ms, TR = 2 s, flip angle of 90°; number of slices = 36; slice thickness = 4 mm, matrix size = 64 × 64, field of view = 24 × 24 cm. High-resolution 3D spoiled gradient-recalled echo axial images were acquired for anatomical reference. The parameters were: TE/TR/TI = 4/10/450 ms; flip angle of 12°; number of slices = 144; slice thickness = 1 mm; matrix size = 256 × 192. To make sure that cardiac and respiratory frequencies did not account for any significant artifacts in the low-frequency spectrum, a pulse oxymeter and respiratory belt were employed to measure these physiological noise sources. They were further processed to minimize the potential aliasing effects (30, 31).
Data Analysis
Data Preprocessing
R-fMRI data analysis was carried out by using Analysis of Functional NeuroImages (AFNI) software (http://afni.nimh.nih.gov/afni) and MATrix LABoratory (MATLAB) programs (The MathWorks, Inc., Natick, Massachusetts). Briefly, motion correction was performed by volume registration on the resting-state fMRI data (program 3dvolreg); then, detrending was carried out to remove Legendre polynomials (program 3dDetrend). The cardiac aliasing was minimized (AFNI command 3dretroicor -card) (30), the respiratory-volume variation was minimized, based on the respiratory belt signal (AFNI command 3dretroicor -resp) (31). The signals in white matter and cerebrospinal fluid were regressed out using averaged signals from the white matter and the ventricles, and the six-motion parameter vectors were regressed out from each voxel time series (program 3dDeconvolve) (32). In addition, global signals were regressed out from the whole brain. A band-pass filter was applied to keep only low-frequency fluctuations within the frequency range of 0.015 Hz and 0.1 Hz (33).
HFC Network
Because of the remarkable atrophy in AD subjects, the seed region in the hippocampus was not obtained from the standard space after stereotactical normalization of the data. Rather, the right and left hippocampal “seed voxels” were manually identified before and after treatment in individual subjects, respectively (32). The time course of each hippocampal voxel was cross-correlated to the time courses of all other voxels in the brain mask, and Pearson correlation coefficients (rij) were obtained, where i is a hippocampal voxel and j is a brain voxel. The cross-correlation coefficients (rij) were converted to mij-values, using Fisher's transformation, , where N is the number of subjects. The Fisher's transformation yields the variants approximately normal distribution (34). The AD subjects had a limited number of hippocampal voxels, because of atrophy, and different study subjects had a different number of hippocampal voxels, therefore, a fixed number of voxels was selected for comparison in the present study. Thirty voxel time courses were selected with the highest averaged m-values from each subject as a “seed” region for functional connectivity analysis. We defined 30 voxels as the “seed” region, because all subjects, except one AD subject (16 voxels), had a minimum of 30 hippocampal voxels. Repeated analysis indicated that with or without inclusion of this AD subject, there was no significant effect on the results and conclusion, as reported.
For those subjects with more than 30 hippocampal voxels, only 30 voxels with the highest mi-scores were selected, according to Equation [1] below:
| [1] |
where N was the number of voxels in masked brain regions, mi is the mean of absolute mij-score of hippocampus voxel i, as voxelwise functional connectivity.
For each brain voxel j, its overall connectivity strength is the mean of mij over the 30 selected voxels according to Equation [2]
| [2] |
The distribution of mj was registered to the study subject's own anatomical images and transformed to the Talairach space with 2 × 2 × 2 mm3 cubic interpolation (program adwarp), followed by applying a 6-mm full-width half-maximum Gaussian kernel filter to generate individual HFC maps. These procedures were designed to compensate for intersubject anatomical variability for cross-subject comparison.
Statistical Analysis
Comparison of the HFC Network Patterns within and between Groups of Study Subjects
For each group (AD group at baseline [ADB], AD group after treatment [ADT] and cognitively normal group of subjects [CN]), the pattern of the HFC map was generated by applying a voxelwise one-sample t-test within groups against a null hypothesis of no connectivity (p < 0.01, cluster size > 1072 mm3, corrected with AlphaSim,). For the between-group comparison, a two-sample t test was performed (p < 0.05, cluster size > 4048 mm3, corrected with AlphaSim).
Linear Regression Analysis
To determine the cognitive significance of the HFC alteration, a linear regression analysis was performed to examine the relationship between the change in voxelwise HFC strength (Δmj or simply Δm) and the change in MMSE scores (ΔMMSE) and ADAS-cog scores (ΔADAS) of all AD subjects before and after treatment. The voxelwise linear regression map was further cluster-corrected (program AlphaSim) with a cluster threshold of > 4048 mm3 at the significance level of p < 0.05 to identify the correlation maps of behavioral scores.
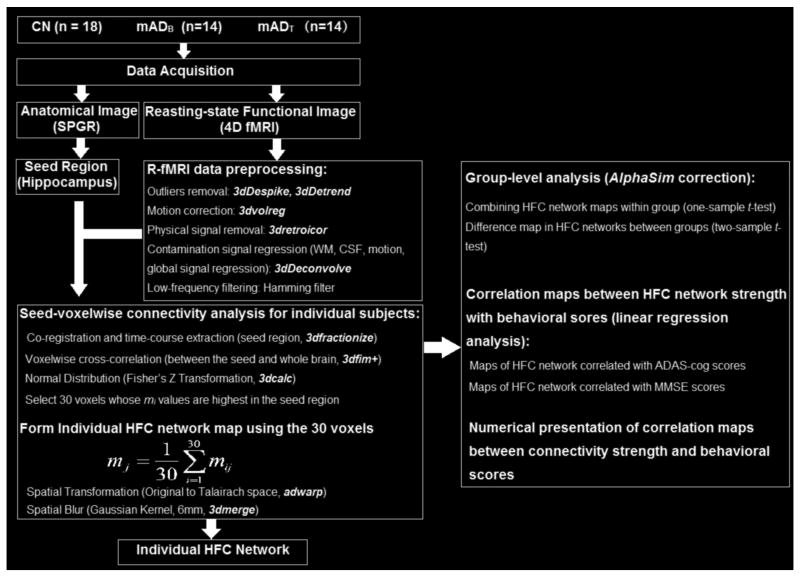
In addition to the voxelwise correlation maps of behavioral scores, a numeric presentation of the correlation can be obtained by identifying a center voxel with the highest z-score in each region of the voxelwise correlation maps. Twenty-seven voxels around the center voxel were selected and the averaged Δm value over the 27 voxels was linearly correlated with ΔMMSE and ΔADAS across AD subjects to obtain the correlation graphs. Figure 1 summarizes the imaging and data-processing procedures.
Fig 1.
A flow diagram depicts the image and data processing procedures. Left: Data acquisition and preprocessing to obtain individual HFC network map. Right: Statistical analysis for group-level comparisons and linear regression analysis. CN: Cognitively Normal; mADB: mild Alzheimer's disease, baseline group; mADT: mild Alzheimer's disease, treatment group; SPGR, Spoiled Gradient-Recalled Echo sequence; fMRI: functional Magnetic Resonance Imaging; HFC, hippocampus functional connectivity; ADAS-cog: Alzheimer's Disease Assessment Scale-cognitive subscale; MMSE: Mini-Mental State Examination.
Results
Subject Characteristics
Fourteen mild AD and 18 CN subjects were used for the final data analysis. Demographic information and clinical evaluations are shown in Table 1. No significant difference in age or gender was noted between groups. MMSE scores in AD study subjects after donepezil treatment were not significantly different from baseline. However, ADAS-cog scores were significantly improved at the 12-week study end point (p < 0.01). Table 2 provides a detailed description of the MMSE and ADAS-Cog scores for each AD subject at baseline and after treatment.
Table 1. Demographic and Clinical Information.
| Characteristic | Mild AD (n = 14) | CN (n = 18) | |
|---|---|---|---|
| Baseline | After Treatment | ||
| M ± SD | M ± SD | M ± SD | |
| Sex (female/male) | 5/9 | 5/9 | 8/10 |
| Age, years | 77.6 ± 6.6 | 77.9 ± 6.6 | 74.9 ± 6.3 |
| MMSE | 26.1 ± 1.3 | 26.1 ± 2.7 | 29.4 ± 0.9a |
| ADAS-cog | 12.0 ± 4.8 | 10.5 ± 6.1b | |
Significant difference of MMSE scores between mild AD and CN group.
Significant difference of ADAS-cog scores before and after donepezil treatment, from Student's t-test.
There were no significant gender differences between groups (X2=0.50, p=0.48). AD = Alzheimer's disease; CN = cognition normal; M = mean; SD = standard deviation; MMSE = Mini-Mental State Examination; ADAS-cog = Alzheimer's Disease Assessment Scale-cognitive subscale.
Table 2. MMSE and ADAS-Cog scores at baseline (BL) and after treatment (TM) of the mild AD patients.
| Subject Number | MMSE | ADAS-Cog | ||
|---|---|---|---|---|
| BL | TM | BL | TM | |
| 1 | 26 | 30 | 6.3 | 4.7 |
| 2 | 25 | 27 | 16.3 | 12.0 |
| 3 | 27 | 26 | 6.0 | 5.0 |
| 4 | 25 | 25 | 10.7 | 6.7 |
| 5 | 26 | 28 | 12.3 | 6.7 |
| 6 | 25 | 22 | 9.3 | 9.7 |
| 7 | 26 | 30 | 9.0 | 5.7 |
| 8 | 28 | 30 | 9.3 | 6.7 |
| 9 | 25 | 24 | 12.3 | 9.0 |
| 10 | 28 | 26 | 10.7 | 10.0 |
| 11 | 25 | 22 | 18.3 | 24.0 |
| 12 | 24 | 27 | 15.7 | 13.7 |
| 13 | 27 | 24 | 23.0 | 22.3 |
| 14 | 28 | 25 | 7.7 | 8.3 |
Neuroimaging Data
HFC Network Patterns in CN, ADB and ADT Groups
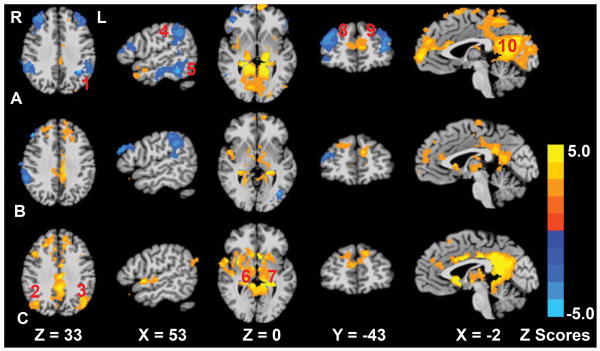
To establish a comparison of the AD subjects, we analyzed R-fMRI data from a group of CN subjects to identify regions correlated or anticorrelated to the hippocampus. Using a one-sample t-test, the HFC network in the CN (A), ADB (B), and ADT groups (C) was obtained (Figure 2). As shown in Figure 2A, there were positively correlated HFC networks (the mj value was positive) in the posterior cingulate cortex and negatively correlated or anticorrelated HFC networks (the mj value was negative) in the bilateral dorsolateral prefrontal cortex and bilateral inferior parietal cortex. The information of correlated/anticorrelated HFC networks in the CN group serves as the reference in identifying how donepezil treatment alters these networks in AD subjects.
Fig 2.
The hippocampal functional connectivity network patterns for the cognitively normal group (row A), mild Alzheimer's disease (AD) group at baseline (row B) and mild AD group after treatment (row C). The images are in radiological format, the right side image represents the left side anatomy. The numbers in red are: 1: the left inferior parietal cortex; 2: the right middle temporal gyrus; 3: the left middle temporal gyrus; 4: the right inferior parietal cortex; 5: the right fusiform gyrus; 6: the right thalamus; 7: the left thalamus; 8: the right dorsolateral prefrontal cortex; 9: the left dorsolateral prefrontal cortex; 10: the left posterior cingulate cortex. Bright color indicates positive correlation pattern, and blue color indicates negative correlation (anticorrelation) pattern. Color bar provided to indicate z-scores.
Changes in the HFC Network Connectivity after Donepezil Treatment
Before donepezil treatment, a two-sample t-test between ADB and CN groups showed that the positively correlated HFC network connectivity was markedly reduced in ADB in brain regions, including the left middle occipital gyrus, left fusiform gyrus, left posterior cingulate cortex (PCC), and left superior parietal cortex; bilateral lingual gyrus and cuneus; and right precuneus and postcentral gyrus (Table 3). Also, the anticorrelated hippocampal network strength (negative mj values) in the ADB group was significantly decreased (less negative) in the right dorsolateral prefrontal cortex (DLPFC), inferior frontal gyrus (IFG) and anterior cingulate cortex (ACC), relative to the controls. Detailed Talairach coordinates of these regions are presented in Table 3.
Table 3. Significant Differences in HFC Network Connectivity between the Mild AD Group at Baseline and the CN Group.
| Brain Region | Side | BA | Cluster Size (mm3) | Talairach Coordinates (LPI) | z-Score | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Decreased connectivity in the positive HFC network | |||||||
| MOG | L | 28816 | -33 | -73 | 8.0 | -3.93 | |
| Fusiform gyrus | L | -24 | -55 | -10 | -2.88 | ||
| PCC | L | 30 | -4 | -67 | 10 | -3.14 | |
| Lingual gyrus | L | 18 | -6 | -69 | 5 | -2.81 | |
| Lingual gyrus | R | 18 | 6 | -75 | 2 | -2.73 | |
| Cuneus | L | 30 | -4 | -67 | 9 | -3.14 | |
| Cuneus | R | 18 | 14 | -81 | 27 | -3.25 | |
| SPC | L | 7 | 6032 | -7 | -65 | 58 | -2.93 |
| Precuneus | R | 7 | 5 | -52 | 52 | -2.87 | |
| Postcentral gyrus | R | 4 | 10 | -39 | 63 | -2.68 | |
| Decreased connectivity in the negative (anticorrelation) HFC network | |||||||
| DLPFC | R | 9 | 8776 | 27 | 39 | 36 | -3.76 |
| IFG | R | 13 | 41 | 23 | 11 | -2.90 | |
| ACC | R | 32 | 11 | 24 | 36 | -2.56 | |
The results were obtained with two sample t test, p < 0.05, cluster size > 4048 mm3, the whole brain corrected.
BA = Brodmann's area; LPI = left, posterior, and inferior; R = right; L = left; x, y, z = coordinates of peak locations in the Talairach space; HFC = hippocampal functional connectivity; MOG = middle occipital gyrus; PCC = posterior cingulate cortex; SPC = superior parietal cortex; DLPFC = dorsolateral prefrontal cortex; IFG = inferior frontal gyrus; ACC = anterior cingulate cortex.
After donepezil treatment, a two-sample t-test (ADTvs. ADB) showed an increase in positively correlated HFC network connectivity. The enhanced HFC network was seen in regions of the precentral gyrus, parahippocampus, insula, lentiform nucleus, thalamus, middle frontal gyrus (MFG) and pons on the left side, and PCC on the right, as shown in Table 4. Additionally decreased anticorrelated HFC network strength in the ADT group was observed in the left inferior parietal cortex and supramarginal gyrus, left posterior MTG and right DLPFC.
Table 4. Significant Differences in HFC Network Connectivity in the Mild AD Group before and after Donepezil Treatment.
| Brain Region | Side | BA | Cluster Size (mm3) | Talairach coordinates (LPI) | z-Score | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Increased connectivity in the positive HFC network | |||||||
| Precentral gyrus | L | 5 | 19024 | -31 | -15 | 30 | 4.49 |
| Parahippocampus | L | 30 | -22 | -28 | -2 | 3.95 | |
| PCC | R | 31 | 2 | -41 | 33 | 2.42 | |
| Insula | L | 13 | -33 | 8 | -5 | 3.69 | |
| Pons | L | -11 | -21 | -21 | 2.39 | ||
| Lentiform nucleus | L | -31 | -20 | -2 | 3.09 | ||
| Thalamus | L | -10 | -27 | -3 | 3.06 | ||
| MFG | L | 6 | -50 | 2 | 43 | 3.44 | |
| Decreased connectivity in the anticorrelation HFC network | |||||||
| IPC/SMG | L | 39 | 6168 | -45 | -53 | 26 | 3.86 |
| pMTG | L | 19 | -47 | -51 | 12 | 2.91 | |
| DLPFC | R | 9 | 5408 | 39 | -5 | 46 | 3.51 |
The results were obtained with paired t test, p < 0.05, cluster size > 4048 mm3, the whole brain corrected.
BA = Brodmann's area; LPI = left, posterior, and inferior; R = right; L = left; x, y, z = coordinates of peak locations in the Talairach space; HFC = hippocampal functional connectivity; PCC = PCC = posterior cingulate cortex; MFG = middle frontal gyrus; IPC = inferior parietal cortex; SMG = supramarginal gyrus; pMTG = posterior middle temporal gyrus; DLPFC = dorsolateral prefrontal cortex.
Neural Correlates of Behavioral Changes before and after Donepezil Treatment
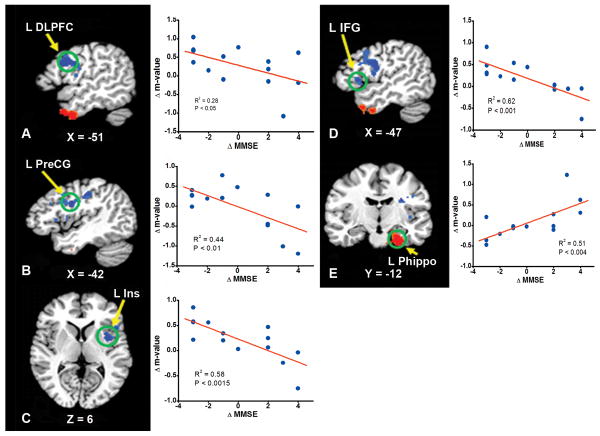
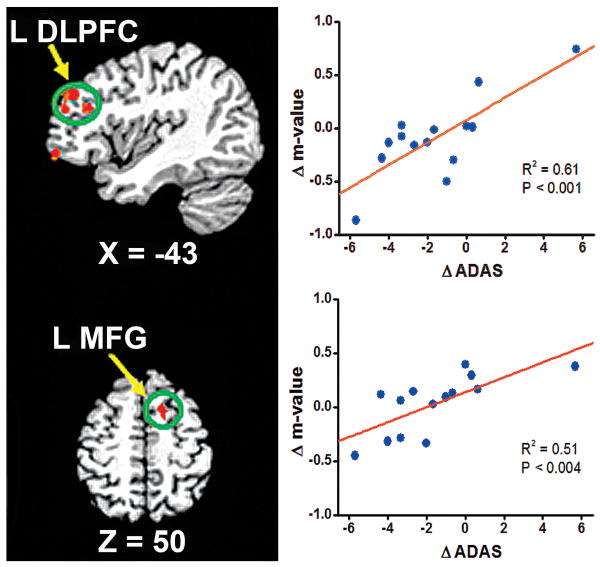
As demonstrated in Figure 3, the neural correlates of changes in MMSE scores after donepezil treatment were located in the left inferior frontal and precentral gyri, insula, parahippocampus and DLPFC. Similarly, the neural correlates of changes to ADAS-cog scores are shown in Figure 4. The improvement in ADAS-cog scores correlated with the change in HFC strength in the left DLPFC and middle frontal gyrus. The detailed Talairach coordinates of these regions are presented in Table 5.
Fig 3.
The neural correlates between changes in the hippocampal functional connectivity network activity and changes in Mini-Mental State Examination (MMSE) scores after donepezil treatment. The stronger the anticorrelation strength (Δm is more negative) in the left dorsolateral prefrontal cortex (L DLPFC), precentral gyrus(L PreCG), and inferior frontal gyrus (L IFG), the better the improvement in MMSE scores (ΔMMSE is more positive). In the left parahippocampus (L Parahippo) region, the stronger the correlation strength (the Δm is more positive), the better the MMSE improvement. For the correlation in the left insula (L Ins), please see the discussion in the text.
Fig 4.
The neural correlates between changes in hippocampal functional connectivity network activity in the left dorsolateral prefrontal cortex (L DLPFC) and the left middle frontal gyrus (L MFG) correlated to changes in AD Assessment Scale-cognitive subscale (ADAS-cog) scores after donepezil treatment. The stronger the anticorrelation strength (Δm is more negative), the better improvement in ADAS-cog scores (ΔADAS is more negative).
Table 5. Cognitive Neural Correlates between Changes in Connectivity Strength in the HFC Network with ΔMMSE Scores and ΔADAS-cog Scores in Mild AD after Donepezil Treatment.
| Brain Region | Side | BA | Cluster Size (mm3) | Talairach Coordinates (LPI) | z-Score | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| ΔMMSE scores | |||||||
| Parahippocampus | L | 28 | 6576 | -27 | -13 | -28 | 3.99 |
| IFG | L | 9 | 5968 | -49 | 11 | 26 | -4.42 |
| Precentral gyrus | L | 6 | -47 | -2 | 26 | -3.29 | |
| Insula | L | 13 | -37 | -1 | 5 | -3.54 | |
| DLPFC | L | 9 | -46 | 6 | 37 | -2.37 | |
| ΔADAS-cog scores | |||||||
| DLPFC | L | 9 | 5544 | -38 | 39 | 30 | 2.73 |
| MFG | L | 6 | -13 | 10 | 50 | 2.90 | |
The results were obtained with linear regression analysis, p < 0.05, cluster size > 4048 mm3, the whole brain corrected.
BA = Brodmann's area; LPI = left, posterior, and inferior; R = right; L = left; x, y, z = coordinates of peak locations in the Talairach space; r = regression coefficient; MMSE = Mini-Mental State Examination; IFG, inferior frontal gyrus; DLPFC, dorsolateral prefrontal cortex; ADAS-cog = Alzheimer's Disease Assessment Scale-cognitive subscale; MFG = middle frontal cortex.
Discussion
Clinical trials have demonstrated that ChEIs positively affect cognition in AD and other conditions, but few studies have examined where effects occur in the brain. The objective of this study was to identify neural correlates of cognitive improvement after three months of donepezil treatment in subjects with mild AD. Our main hypothesis was that donepezil would improve the HFC network, which would significantly correlate with cognitive improvement. Specifically, in nonhuman primates and humans, cholinergic fibers primarily originate from the cholinergic nuclei located in the basal forebrain and pons, and densely innervate the thalamus, limbic regions and neocortical regions (35). The hippocampus, in particular, is one of the limbic areas with the highest concentration of cholinergic innervation. In AD, a decrease in cortical and hippocampal cholinergic neurotransmission is observed (1, 35). In vivo visualization of donepezil binding in the brains of AD patients reveals a significant reduction in the hippocampus (36). Recent neuroimaging approaches, in particular resting-state R-fMRI studies in AD, have shown hippocampal disconnection with the limbic, thalamic, and neocortical areas (18, 22-24). In addition, the hippocampal network is involved in the default mode network, which showed alterations in the AD subjects (20, 21, 37).
Currently, very little is known regarding how donepezil affects functional brain networks in patients with mild AD. After 12 weeks of donepezil treatment, subjects demonstrated significant enhancement of the positively connected hippocampal functional networks, especially in the regions of the right PCC, and the left insula and thalamus. It is hypothesized that donepezil treatment significantly increases cholinergic activity, and improves hippocampal network activity. Recent studies demonstrated that the PCC is an important region as a part of the connectivity found in the backbones and brain module (38). The improved connectivity between the PCC and the hippocampus after the cholinergic treatment could improve the information flow, resulting in cognitive improvement.
The improved HFC network seen after donepezil treatment involves similar regions that have shown improvement in previous acute and prolonged ChEI-challenged fMRI task-activation studies reported in the literature. For example, during a working-memory task paradigm, a single dose of rivastigmine increased activation in the right prefrontal cortical regions in mild AD subjects (8). Similarly, after only five days of galantamine treatment, MCI patients demonstrated increased left hippocampal, prefrontal, anterior cingulate, and occipital cortical activation during face encoding, and right precuneus and middle frontal gyrus activity with working-memory tasks (9). MCI patients also have shown increased DLPFC, superior frontal gyrus, temporal lobe, and occipital region activity during working-memory tasks (10) after an average of 10 weeks of donepezil treatment. Other fMRI task-specific studies have focused on the effects of prolonged administration (20 weeks) of rivastigmine (13) and galantamine (12) in mild AD. During various cognitive tasks, increased activation was found in the frontal lobe, inferior parietal lobe and basal ganglia structures. In this study, using resting-state R-fMRI and a shorter duration of donepezil treatment, we found similar results and have extended the findings to include other brain regions, such as the insula, thalamus and pontine regions.
Unlike fMRI studies where the brain responses to treatment are dependent on the type of cognitive task, R-fMRI studies can identify brain networks in which changes in the network activity correlate with changes in cognitive function. In the present study, we employed the changes in the MMSE and ADAS-cog scores as independent variables to identify their neural correlates. As shown in Figure 3E, changes in the parahippocampus activity (Δm) significantly correlated with changes in MMSE scores, such that the stronger the connectivity between the parahippocampus and the hippocampus, the greater the improvement in the MMSE. It is plausible that improvement of the network connectivity in this important cognitive pathway is resultant of the cholinergic enhancement after donepezil treatment.
The regions of the left DLPFC, left IFG and left precentral gyrus in the anticorrelated HFC network showed a negative correlation with changes in MMSE scores. The negative slope represented the increase or recovery in anticorrelation activity. This is because the sign of functional connectivity in these regions is negative in these anticorrelation networks in the CN group, as shown in Figure 2. The better the recovery of this anticorrelation network activity is, the greater the cognitive improvement is in these subjects after treatment. The better recovery of anticorrelation connectivity in the DLPFC-hippocampus network may help memory reconsolidation, thereby facilitating signal processing from the hippocampus to the DLPFC (39).
Similar neural correlates related to changes in the ADAS-cog score were found in the regions of the left DLPFC and the left MFG, as shown in Figure 4. Because the DLPFC and MFG regions belonged in the anticorrelated HFC network, and the negative values of ΔADAS-cog represent improvement of cognitive function, the slopes in Figure 4 are positive. This indicates that greater cognitive improvement (represented by negative ΔADAS-cog scores) is associated with better connectivity recovery in these anticorrelated networks (represented by negative Δm values). Clearly, connectivity recovery between the temporal and frontal networks in mild AD could result in cognitive improvement, as measured by ADAS-cog scores. To our knowledge, this is the first study in which neural correlates of cognitive improvement, as evidenced by MMSE and ADAS-cog scores, are revealed and can be employed to monitor or assess therapeutic responses to AD treatment.
It is intriguing that the brain regions with altered HFC network connectivity after treatment (Table 4, ADB vs. ADT) did not overlap with those regions that had reduced HFC network connectivity (Table 3, ADB vs. CN). In other words, donepezil treatment in patients with AD did not improve the originally reduced HFC connectivity. Instead, the donepezil treatment boosted HFC connectivity in those regions (Table 4) where the HFC connectivity was normal in the CN group. It is plausible that because the oral donepezil treatment increases the cholinergic activity in the whole brain, this medication increases activity in some regions (as listed in Table 4), even though they were relatively normal before treatment. It is suggested that brain reorganization may occur after treatment. However, such increased connectivity in certain regions (Table 4) after donepezil treatment could induce some of the imbalances in the neural network, resulting in modest improvement of the cognitive performance.
Justification for Selecting the “Seed” Region
For the determination of functional connectivity, the “seed” method is an original and simple technique, which extracts the intrinsic time course from a region of interest, and then determines the temporal correlation between this extracted signal and the time courses from all other voxels (26). Although this method requires a priori definition of a seed region, it is widely employed, because this approach provides high sensitivity and easy interpretation of results. In the related research topic, a majority of previous studies averaged all voxel time courses in the hippocampus as the “seed” signal (18, 22), while others selected only five voxel time courses as the “seed” signal (23). Other studies employed the independent component analysis (ICA) method without requiring a priori specification of a seed region to calculate functional connectivity (19, 40). There is no consensus, as of yet, on how to choose the optimal number of components, or the manner by which to assign each component with specific neurophysiological meaning (27). In the present study, we selected 30 voxel time courses with the highest averaged m-values from each subject as a “seed” region because all but one AD subject (16 voxels), had a minimum of 30 hippocampal voxels. Our rationales are: 1) the number of hippocampal voxels in each individual subject is different. Some have bigger hippocampi than others; 2) the mean number of hippocampal voxels in AD subjects is significantly smaller than that of the CN subjects; 3) each subject has the same number of voxels with the best functional connectivity as the seed region. We compared results from the literature and found that the selection of different numbers of voxels in the hippocampus could affect the results of functional connectivity (19, 22, 23). In the present study, we reanalyzed the entire dataset with “averaged voxel time courses over all hippocampus voxels.” The results of the hippocampal functional connectivity differences between mild AD and control subjects are consistent with previous publications (22), but the differences in functional connectivity are less than the 30-voxel approach. It is possible that the method of averaging all voxel time courses to generate the seed signal may desensitize the difference in the functional connectivity. Although a comprehensive investigation on how the seed selection would affect the hippocampal connectivity is beyond the scope of this study, a sophisticated design, such as clustering analysis, could address this question in a future study.
This study is not without limitations. The study duration was relatively brief compared to some other pharmacological fMRI studies, although we were able to measure the responses of HFC activity to donepezil treatment in AD study subjects, and show the cognitive significance of hippocampal functional alterations in this population. Second, the sample size was relatively small, because the population was limited to mild AD subjects, who never received AD-related medication. Third, this was an open-label study instead of a scientifically ideal double-blind placebo-controlled study. As a result, there is a possibility that the observed results may represent a placebo effect. However, a randomized, placebo-controlled trial of donepezil relative to AD is no longer considered ethical, as the Food and Drug Administration approved this medication for clinical use in mild to severe AD. In practice, such a possibility is low, because previous studies showed that during the clinical trials of donepezil, there was no significant placebo effect observed in AD subjects in three months after treatment (4, 41). Fourth, another limitation in our study design is that the controls were only scanned at baseline and not at 12 weeks. Finally, while our data analysis was limited to the HFC network, the neural network changes in AD are clearly not limited to the HFC network; we will perform a global functional connectivity analysis with this cohort that will consider all pairs of anatomically defined regions of interest from the whole-brain template in Talairach space.
Acknowledgments
The authors thank Ms. Carrie O'Connor for editorial assistance, Mr. Douglas Ward for statistical analysis help, and Ms. Judi Zaferos-Pylant and Mr. Yu Liu for MRI technical support. Additional editorial support was provided by R. Daniel, Ph.D., of PAREXEL. This study was funded by Eisai Inc. and Pfizer Inc. Also, this study was supported, in part, by NIH-NIA R01 grant AD 20279.
Grant support: This work was supported by National Institutes of Health grants: R01 AG20279 (Dr. Shi-Jiang Li), General Clinical Research Center Grant M01 RR00058 (Dr. Shi-Jiang Li) the DANA Foundation, Pfizer Inc., and the Extendicare Foundation (Dr. Shi-Jiang Li). Dr. Shi-Jiang Li is currently funded by NIH grants 2R01AG020279-06A2 and 2R01DA010214-11A1 and previously received research support from Pfizer Inc.
Dr. Goveas receives research funding support from the Young Investigator Award of National Alliance for Research on Schizophrenia and Depression (NARSAD), Extendicare Foundation, the Advancing Healthier Wisconsin Endowment for Research to the Medical College of Wisconsin and the NHLBI, NIH Women's Health Initiative contract N01-WH 44221 (PI: Dr. Sally Shumaker). Dr. Xie receives support from the China Scholarship Council CSC20070320. Dr. Zhilin Wu, Wenjun Li and Jennifer Jones do not report disclosures. Drs. Antuono and Franczak receive research support from Bristol Myers Squibb, Danone, Elan, Genentech and Octapharma AG. Dr. Antuono serves on the speaker bureau of Novartis and Pfizer. Dr. Antuono also receives research support from the NIH grant # 2R01AG020279-06A2 (PI: Dr. Shi-Jiang Li). Dr. Franczak serves as a consultant/speaker for Novartis and Forest Pharmaceuticals.
Footnotes
Disclosure: All authors have made substantial intellectual contribution to this manuscript in one or more of the following areas: design or conceptualization of the study, analysis or interpretation of the data, or drafting and revising the manuscript. All authors have given final approval of this manuscript. None of the authors of this paper have any possible conflicts of interest, financial or otherwise, related directly or indirectly to this work.
References
- 1.Mesulam M. The cholinergic lesion of Alzheimer's disease: pivotal factor or side show? Learn Mem. 2004;11:43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- 2.Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 3.Coyle JT, Price DL, DeLong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 4.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Group. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson SA, Sabbagh MN. Donepezil: potential neuroprotective and disease-modifying effects. Expert Opin Drug Metab Toxicol. 2008;4:1363–1369. doi: 10.1517/17425255.4.10.1363. [DOI] [PubMed] [Google Scholar]
- 6.Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer's disease: systematic review of randomized clinical trials. BMJ. 2005;331:321–327. doi: 10.1136/bmj.331.7512.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venneri A. Imaging treatment effects in Alzheimer's disease. Magn Reson Imaging. 2007;25:953–968. doi: 10.1016/j.mri.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Rombouts SA, Barkhof F, Van Meel CS, Scheltens P. Alterations in brain activation during cholinergic enhancement with rivastigmine in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2002;73:665–671. doi: 10.1136/jnnp.73.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goekoop R, Rombouts SA, Jonker C, et al. Challenging the cholinergic system in mild cognitive impairment: a pharmacological fMRI study. Neuroimage. 2004;23:1450–1459. doi: 10.1016/j.neuroimage.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Saykin AJ, Wishart HA, Rabin LA, et al. Flashman LA, McHugh TL, Mamourian AC, Santulli RB. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain. 2004;127:1574–1583. doi: 10.1093/brain/awh177. [DOI] [PubMed] [Google Scholar]
- 11.Kircher TT, Erb M, Grodd W, Leube DT. Cortical activation during cholinesterase-inhibitor treatment in Alzheimer disease: preliminary findings from a pharmaco-fMRI study. Am J Geriatr Psychiatry. 2005;13:1006–1013. doi: 10.1176/appi.ajgp.13.11.1006. [DOI] [PubMed] [Google Scholar]
- 12.Shanks MF, McGeown WJ, Forbes-McKay KE, Waiter GD, Ries M, Venneri A. Regional brain activity after prolonged cholinergic enhancement in early Alzheimer's disease. Magn Reson Imaging. 2007;25:848–859. doi: 10.1016/j.mri.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 13.McGeown WJ, Shanks MF, Venneri A. Prolonged cholinergic enrichment influences regional cortical activation in early Alzheimer's disease. Neuropsychiatr Dis Treat. 2008;4:465–476. doi: 10.2147/ndt.s2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrella JR, Prince SE, Krishnan S, Husn H, Kelley L, Doraiswamy PM. Effects of donepezil on cortical activation in mild cognitive impairment: a pilot double-blind placebo-controlled trial using functional MR imaging. AJNR Am J Neuroradiol. 2009;30:411–416. doi: 10.3174/ajnr.A1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould RL, Brown RG, Owen AM, Bullmore ET, Williams SC, Howard RJ. Functional neuroanatomy of successful paired associate learning in Alzheimer's disease. Am J Psychiatry. 2005;162:2049–2060. doi: 10.1176/appi.ajp.162.11.2049. [DOI] [PubMed] [Google Scholar]
- 16.Lakmache Y, Lassonde M, Gauthier S, Frigon JY, Lepore F. Interhemispheric disconnection syndrome in Alzheimer's disease. Proc Natl Acad Sci U S A. 1998;95:9042–9046. doi: 10.1073/pnas.95.15.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grady CL, Furey ML, Pietrini P, Horwitz B, Rapoport SI. Altered brain functional connectivity and impaired short-term memory in Alzheimer's disease. Brain. 2001;124:739–756. doi: 10.1093/brain/124.4.739. [DOI] [PubMed] [Google Scholar]
- 18.Li SJ, Li Z, Wu G, Zhang MJ, Franczak M, Antuono PG. Alzheimer Disease: evaluation of a functional MR imaging index as a marker. Radiology. 2002;225:253–259. doi: 10.1148/radiol.2251011301. [DOI] [PubMed] [Google Scholar]
- 19.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Zang Y, He Y, et al. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage. 2006;31:496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 23.Allen G, Barnard H, McColl R, et al. Reduced hippocampal functional connectivity in Alzheimer disease. Arch Neurol. 2007;64:1482–1487. doi: 10.1001/archneur.64.10.1482. [DOI] [PubMed] [Google Scholar]
- 24.Wang K, Liang M, Wang L, et al. Altered functional connectivity in early Alzheimer's disease: a resting-state fMRI study. Hum Brain Mapp. 2007;28:967–978. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Xu G, Wu G, Antuono P, Rowe DB, Li SJ. The phase shift index for marking functional asynchrony in Alzheimer's disease patients using fMRI. Magn Reson Imaging. 2008;26:379–392. doi: 10.1016/j.mri.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 27.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 28.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 29.Xu G, Antuono PG, Jones J, et al. Perfusion fMRI detects deficits in regional CBF during memory-encoding tasks in MCI subjects. Neurology. 2007;69:1650–1656. doi: 10.1212/01.wnl.0000296941.06685.22. [DOI] [PubMed] [Google Scholar]
- 30.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–7.31. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 31.Birn RM, Murphy K, Bandettini PA. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Hum Brain Mapp. 2008;29:740–50.30. doi: 10.1002/hbm.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rombouts SA, Stam CJ, Kuijer JP, Scheltens P, Barkhof F. Identifying confounds to increase specificity during a “no task condition” evidence for hippocampal connectivity using fMRI. Neuroimage. 2003;20:1236–1245. doi: 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- 33.Orfanidis SJ. Introduction to signal processing. Upper Saddle River, NJ: Prentice Hall; 1996. p. 798. [Google Scholar]
- 34.Zar JH. Biostatistical analysis. 3rd. Upper Saddle River, NJ: Prentice-Hall; 1996. p. 662. [Google Scholar]
- 35.Mesulam MM. Central cholinergic pathways: neuroanatomy and some behavioral implications. In: Avoli M, Reader TA, Dykes RW, Gloor P, editors. Neurotransmitters and cortical function. New York: Plenum Press; 1988. pp. 237–260. [Google Scholar]
- 36.Okamura N, Funaki Y, Tashiro M, et al. In vivo visualization of donepezil binding in the brain of patients with Alzheimer's disease. Br J Clin Pharmacol. 2008;65:472–479. doi: 10.1111/j.1365-2125.2007.03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(e159):1479–1493. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Euston DR, Tatsuno M, McNaughton BL. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science. 2007;318:1147–1150. doi: 10.1126/science.1148979. [DOI] [PubMed] [Google Scholar]
- 40.Sorg C, Riedl V, Mühlau M, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seltzer B, Zolnouni P, Nunez M, et al. Donepezil “402” Study Group. Efficacy of donepezil in early-stage Alzheimer disease: a randomized placebo-controlled trial. Arch Neurol. 2004;61:1852–1856. doi: 10.1001/archneur.61.12.1852. [DOI] [PubMed] [Google Scholar]