Abstract
Purpose
Many solid tumors express cell surface mesothelin making them attractive targets for antibody-based therapies of cancer. SS1P (anti-mesothelin(Fv)PE38) is a recombinant immunotoxin (RIT) that has potent cytotoxic activity on several cancer cell lines and clinical activity in mesothelioma patients. Pancreatic cancers express mesothelin and are known to be resistant to most chemotherapeutic agents. The goal of the current study is to treat pancreatic cancer with RIT by targeting mesothelin.
Experimental Design
We measured the cytotoxic activity of an anti-mesothelin immunotoxin on pancreatic cancer cells. We also measured the levels of several pro- and anti-apoptotic proteins, as well as the ability of TRAIL or the anti-TRAIL receptor 2 agonist antibody (HGS-ETR2) to kill pancreatic cells and the cytotoxic activity of the two agents together in cell culture and against tumors in mice.
Results
In two pancreatic cancer cell lines immunotoxin treatment inhibited protein synthesis, but did not produce significant cell death. The resistant lines had low levels of the pro-apoptotic protein Bak. Increasing Bak expression enhanced the sensitivity to immunotoxins, while Bak knockdown diminished it. We also found that combining immunotoxin with TRAIL or HGS-ETR2 caused synergistic cell death, and together triggered caspase-8 recruitment and activation, Bid cleavage and Bax activation. Combining SS1P with HGS-ETR2 also acted synergistically to decrease tumor burden in a mouse model.
Conclusion
Our data show that low Bak can cause cancer cells to be resistant to immunotoxin treatment, and that combining immunotoxin with TRAIL or a TRAIL agonist antibody can overcome resistance.
Keywords: Resistance, Apoptosis, Immunotoxin, TRAIL, Bak, Bax
Introduction
Since the FDA approved the first monoclonal antibody (mAb), Rituximab, in 1997 for the treatment of B cell lymphomas, many mAbs have been evaluated for their ability to cause tumor regressions (1), but only a few have been effective. To improve the therapeutic usefulness of antibodies, they are now being used with a variety of cytotoxic substances such as small molecular drugs, radioisotopes or protein toxins (2).
Our laboratory has focused on the development of recombinant immunotoxins (RITs) for cancer treatment (3).These proteins are composed of an Fv that binds to an antigen on a cancer cell fused to a 38-kD portion of Pseudomonas exotoxin A (PE38). They derive their potency from the toxin and their specificity from the antibody fragment to which they are attached. We now have several RITs in clinical trials. One of these is Moxetumomab pasudotox (also known as CAT-8015 or HA22), which targets CD22 on B cells malignancies. It has produced a very high complete remission rate in chemotherapy resistant hairy cell leukemia and is now being evaluated in other B cell malignancies (4–6). Another is SS1P that targets mesothelin, a 40-kD cell surface glycoprotein that is present on mesotheliomas and pancreatic, ovarian and lung cancers and cholangiocarcinomas (7–11). SS1P is composed of an anti-mesothelin Fv linked to PE38. It has shown significant cytotoxic activity in vitro against ovarian, mesothelioma, lung and cholangiocarcinoma cancer cells (7–11). SS1P has been evaluated in two phase I clinical trials. It was well tolerated and showed some anti-tumor activity in patients with mesothelioma (12, 13). A new trial in which SS1P is being given in combination with cisplatin and pemetrexed is ongoing (14).
In order to kill cells an immunotoxin must be internalized and the toxin portion delivered to the cytosol via the endoplasmic reticulum (Fig. 1A). In the cytosol, protein synthesis is inhibited by the ADP-ribosylation of elongation factor 2, and then the apoptosis cascade is activated and cell death ensues. The details of how the apoptosis pathway is activated after protein synthesis inhibition are still not completely understood. Our experiments with mouse embryonic fibroblast (MEF) cells showed that degradation of the anti-apoptotic protein Mcl-1 is required, and that Bak, a mediator of the intrinsic pathway of apoptosis is critical for apoptosis induced by PE (15).
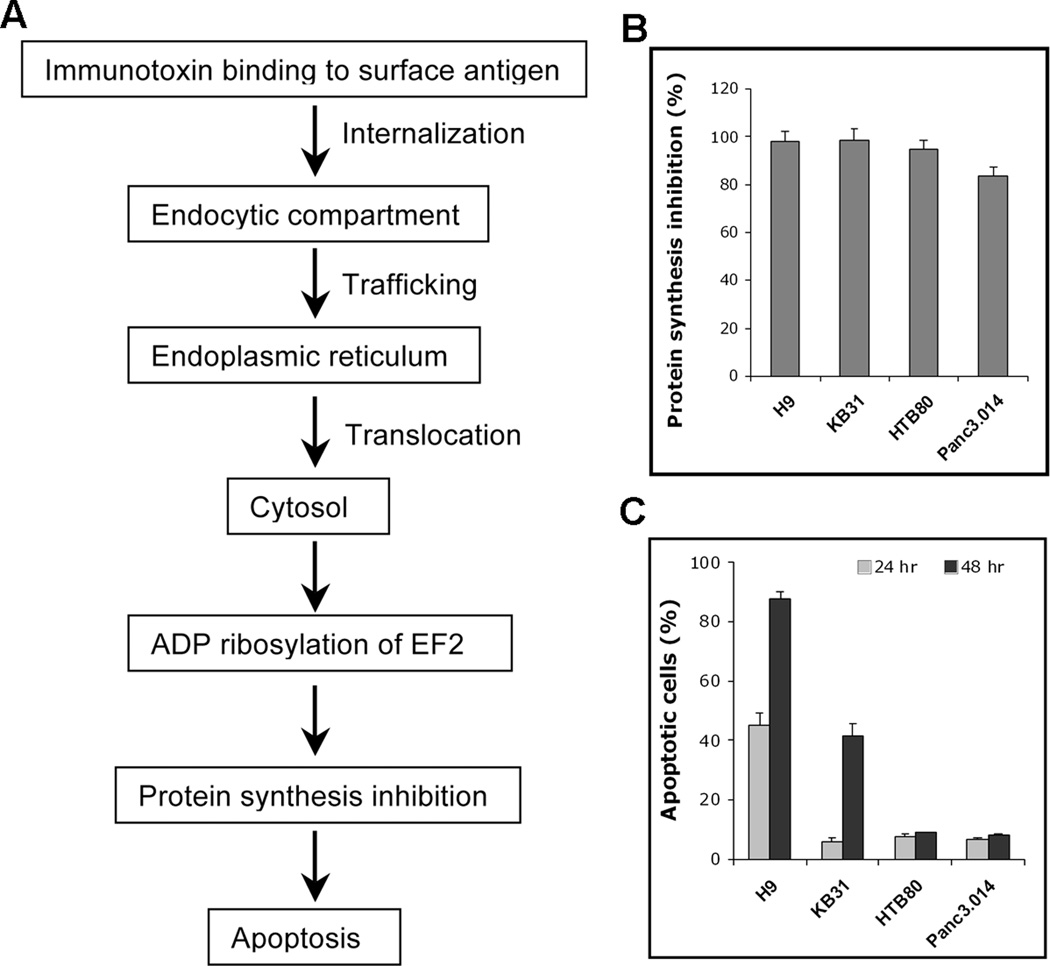
Figure 1.
Mechanism of cell killing by recombinant immunotoxin and efficient protein synthesis inhibition does not result in cell death in resistant cells. A, Schematic diagram of how PE-based recombinant immunotoxins kill cells. B, Protein synthesis inhibition by 300 ng/ml SS1P-KDEL. Results are shown as reduced H3-leucine incorporation compared to cells without immunotoxin. C, Cells were treated with 300 ng/ml SS1P-KDEL for 24 and 48 hr. Apoptotic cells are shown as Annexin V positive population (n=4).
To expand the usefulness of anti-mesothelin immunotoxin therapy, and to further understand the mechanism of immunotoxin induced apoptosis, we evaluated the activity of SS1P or SS1P-KDEL, a mutant RIT with enhanced intracellular trafficking ability (16), on pancreatic cancer cells, which are known to be resistant to conventional treatments (17, 18). Recent findings indicate that constitutively activated autophagy may contribute to pancreatic ductal adenocarcinoma pathogenesis (19, 20). We demonstrate here that Mcl-1 and Bak also regulate apoptosis in human cells, and that pancreatic cancer cells with low Bak protein are resistant to immunotoxin treatment despite efficient protein synthesis inhibition. This resistance can be overcome by combining immunotoxin with TRAIL, or an anti-TRAIL receptor 2 agonist antibody that activates the extrinsic pathway. The cell killing produced by the combination treatment is highly synergistic and mitochondrial-dependent, and is initiated by caspase-8 recruitment and activation. Synergistic anti-tumor activity was also observed in mice receiving a combination of immunotoxin and anti-TRAIL receptor 2 agonist antibody.
Material and Methods
Reagents
Recombinant human and mouse TRAIL were purchased from R&D Systems (Minneapolis, MN). HGS-ETR2 was provided by Human Genome Science (Rockville, MD). Immunotoxins SS1P, SS1P-KDEL, HB21(Fv)-PE40, TGFα-PE38 and LMB9 were produced in our lab. Human insulin was obtained from the NIH pharmacy. Puromycin was purchased from Invitrogen (Carlsbad, CA). 3H-leucine was purchased from GE Healthcare (Piscataway, NJ).
Cells
A431/H9 (mesothelin stable cell line), KB31, Hela, MEF and MEF (Bak−/−) were maintained in Dulbecco's Modified Eagle Medium with 10% fetal bovine serum (FBS). Pancreatic cell line Panc3.014 was obtained from Dr. Elizabeth Jaffee (Department of Oncology, Johns Hopkins University, Baltimore, MD) and maintained in RPMI-1640 with 20% FBS and 0.2 unit/ml human insulin. Pancreatic cell line HTB80 (Capan-2, ATCC, Rockville, MD) was maintained in RPMI-1640 with 10% FBS.
Surface TRAIL receptor and mesothelin expression, protein synthesis and apoptosis assay, immunoblot and immunoprecipitation
TRAIL receptor expression was detected with anti-human TRAIL R1 and R2 antibodies (R&D System). Mesothelin expression was detected with MORAB-009 (SS1 humanized mAb). Protein synthesis inhibition was measured with 3H-leucine incorporation. Detailed methods are described in the Supplementary Methods.
After treatment, both floating and adherent cells were collected, washed with cold DPBS twice, and solubilized in lysis buffer (50 mM Tris•HCl pH 7.5, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) with protease inhibitors and subjected to western blotting. For immunoprecipitation and Bax activation, cell lysates were prepared in 1% CHAPS lysis buffer and incubated with mouse anti-Bax (6A7). Detailed methods and antibodies for western blotting are described in the Supplementary Methods.
Bak overexpression and siRNA knockdown of Bak, Bax and Bid
A cDNA for human Bak was cloned into a lentiviral expression vector pLVX-PURO (Clontech, Mountain View, CA). Lentivirus was produced by SAIC-Frederick (Frederick, MD) and transduced into KB31. Stable clones were selected with 1.5 µg/ml puromycin. Bak expression levels were confirmed by western blot and cells were treated with 100 ng/ml SS1P for indicated times and subjected to apoptosis analysis.
siRNAs (Control, Hs-Bak1_5, Hs_Bax_9, Hs_Bid_7; Qiagen, Valencia, CA) were transfected into cells at the indicated concentrations with Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s protocol.
Xenograft tumor model
KB31 (1.7×106) with matrigel (4.0 mg/ml, 200 µl/mouse) were implanted s.c. into the thigh area of the rear leg of 5- to 6-week-old athymic nude mice (18–20 g). Tumor dimensions were determined every other day with a caliper. Tumor volume (mm3) was calculated by the formula: (a) × (b2) × 0.4, where (a) is tumor length and (b) is tumor width in millimeters.
When tumor size reached 110 mm3 (day 8), 10 mg/kg HGS-ETR2 was given i.p., and 4 hr later three doses of SS1P 0.3 mg/kg was given i.v. QOD (days 8, 10, and 12). The animal protocol was approved by the National Cancer Institute Animal Care and Use Committee. All animal experiments were stopped when tumors reached 1,000 mm3 based on animal protocols.
Statistics
All data are presented as mean ± standard deviation from at least three independent assays. Statistical analysis of synergy on tumor experiments was done by Drs. David Venzon and David Liewehr (Biostatistics and Data Management Section/Center for Cancer Research/National Cancer Institute, Bethesda, MD). Tumor volumes were logarithmically transformed before analysis to normalize their distribution and to stabilize variances. Differences between treatment groups were assessed by repeated measures analysis of variance, and two-tailed p values from Dunnett’s test are reported for the multiple comparisons within days. Synergy was defined as an interaction effect significantly greater than the sum of the SS1P and HGS-ETR2 effect.
Results
Immunotoxin does not induce cell death despite efficient protein synthesis inhibition
Pancreatic cell lines HTB80 and Panc3.014 have strong cell surface mesothelin expression (Supplementary Fig. S1), and the expression levels are in the range of two sensitive cell lines often used in our lab, KB31 and A431/H9. We then treated the four cell lines with SS1P at 300 ng/ml and found that protein synthesis was completely arrested in A431/H9 and KB31 cells but not in HTB80 and Panc3.014 (data not shown). We have previously shown that in some cell lines changing the c-terminus of an immunotoxin to KDEL, which binds more tightly to the recycling receptor, can improve protein synthesis inhibition and indeed found SS1P-KDEL at 300 ng/ml profoundly inhibited protein synthesis in the two pancreatic cell lines (Fig. 1B), and employed SS1P-KDEL in all further experiments with pancreatic cells. When examined at 24 hr, over 45% of the A431/H9 were undergoing apoptosis, which increased to over 90% at 48 hr (Fig. 1C). Significant apoptosis became evident at 48 hr in the KB31 (Fig. 1C). No significant apoptosis was detected in HTB80 or Panc3.014 at 48 hr and even 72 hr (Fig. 1C and unpublished data). These results indicate that despite profound protein synthesis inhibition, SS1P-KDEL does not cause the death of HTB80 and Panc3.014 cells, indicating that these two cell lines are highly resistant to immunotoxin, as observed in a recent study that DLD1 cells do not undergo apoptosis when protein synthesis is inhibited by immunotoxins (21).
Low expression of Bak protein in resistant cells and Bak-dependent apoptosis induced by immunotoxin
Using MEFs we have previously found that apoptosis induced by PE is Bak-dependent (15). In this study we examined Bak protein levels and found that expression levels were low in these two cell lines (Fig. 2A), compared to A431/H9 and KB31 cells. The levels of Bax, another pro-apoptotic multi-BH domain protein, were similar in all the cell lines. We found that treatment with SS1P-KDEL resulted in a large fall in Mcl-1 levels as previously shown in MEFs treated with native PE toxin (15), but this fall was not sufficient to induce apoptosis in HTB80 and Panc3.014 cells.
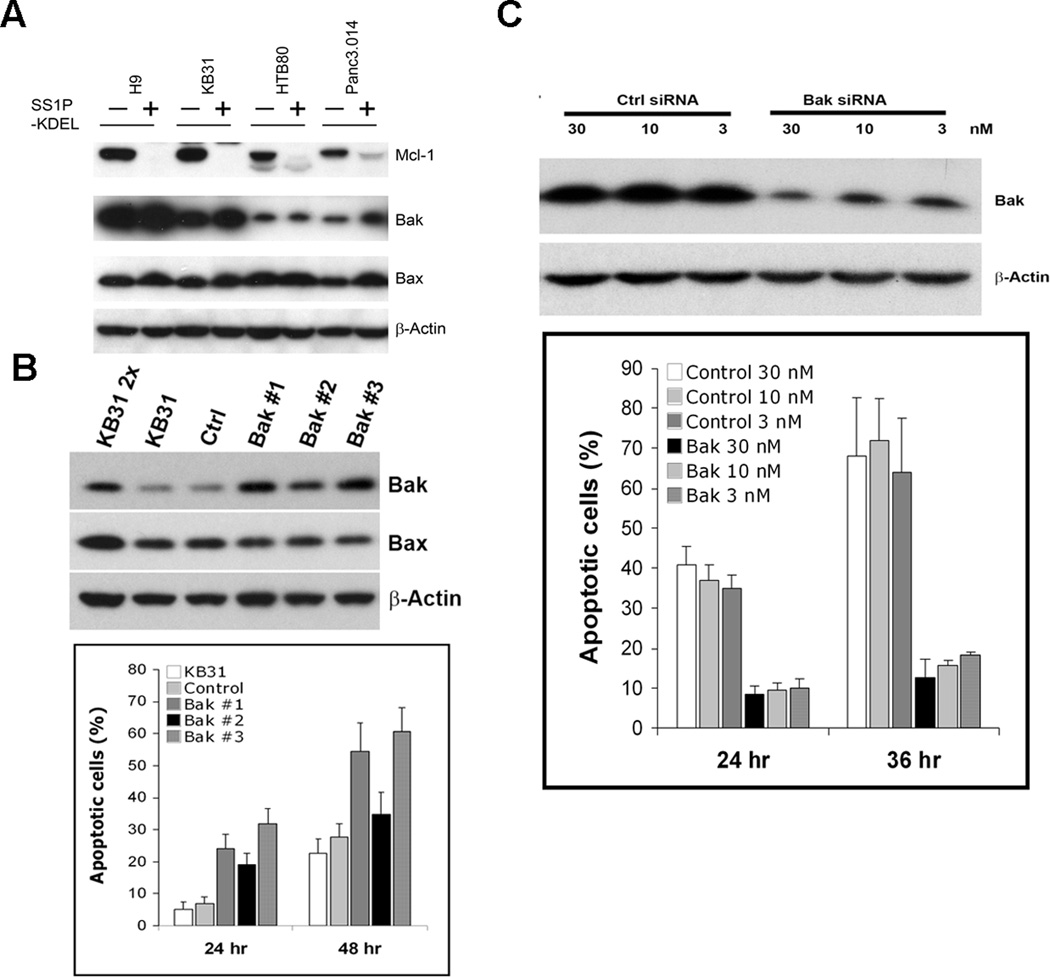
Figure 2.
Low Bak protein in resistant cells and Bak-dependent apoptosis induced by immunotoxin. A, Western blotting after SS1P-KDEL treatment for 24 hr. B, Bak and Bax expression in lentiviral infected stable Bak KB31 clones detected with western blotting, and compared to the same amount of KB31, empty lentiviral infected KB31 (control), and 2-fold amount of KB31 (KB31 2×). Cells were treated with 300 ng/ml SS1P for 24 or 48 hr. Apoptotic cells are shown as Annexin V positive population (n=3). C, A431/H9 transfected with control and Bak siRNA. Western blot was conducted 48 hr after transfection. Cells were further treated with 100 ng/ml SS1P for 24 and 36 hr. Apoptotic cells are shown as Annexin V positive population (n=3).
We reasoned that if Bak were important for apoptosis induced by immunotoxin, then increasing Bak expression would increase sensitivity to immunotoxin. We tried to make stable lines with high Bak by using HTB80 and Panc3.014, but failed due to low transfection efficiency. We were able to easily transfect KB31 cells that have lower BAK and are less sensitive to immunotoxins compared to A431/H9 cells.
We established several stable KB31 clones expressing Bak and found that each clone had about a 2-fold increase of Bak compared to parental KB31 cells. Bax protein levels were unchanged (Fig. 2B). All Bak clones showed increased sensitivity to SS1P, which induced 20–35% apoptotic cells after 24 hr, and 34% to 60% after 48 hr. These values are significantly higher than the parental KB31 cells, or KB31 cells transduced with empty virus (Fig. 2B). Clone #2 had the lowest level of Bak, and showed the lowest apoptosis. This data shows that raising Bak levels increases immunotoxin killing.
To show that a decrease in Bak can cause resistance to immunotoxin, we used siRNA to knockdown Bak in A431/H9, because it has high Bak levels and is immunotoxin sensitive (Fig. 2C). We found that apoptotic cells induced by SS1P decreased from 39% to 8% at the 24 hr time point, and from 65% to 14% at 36 hr.
To determine whether the apoptosis induced by other PE-based immunotoxins is also Bak dependent, we treated A431/H9 with HB21(Fv)-PE40 targeting the transferrin receptor, and LMB9 targeting the Lewis Y antigen (3). Bak knockdown reduced the apoptotic cells induced by SS1P, HB21(Fv)-PE40 or LMB9 (Supplementary Fig. S2). To determine whether Bak dependence exists in other cell types, we knocked down Bak in Hela cells and found substantial reduction of apoptotic cells induced by HB21(Fv)-PE40 or TGFα-PE38 (transforming growth factor α fused to PE38) targeting EGFR (3). Hela cells do not express Lewis Y, and are not responsive to LMB9 (Supplementary Fig. S2).
Immunotoxin combined with TRAIL synergistically induce cell death
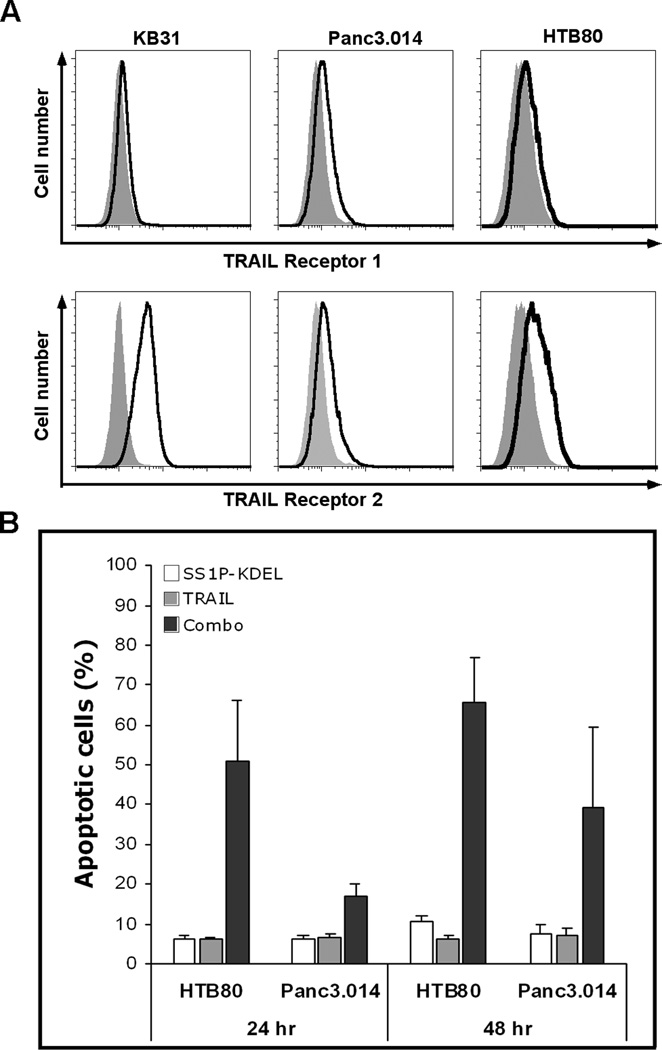
Because immunotoxins kill cells by lowering Mcl-1 levels in a Bak-dependent process, we hypothesized that activating the extrinsic apoptosis pathway would increase immunotoxin cell killing if Bak levels were low, since Bax is important for the extrinsic apoptosis pathway (22, 23). To do this we needed to use cell lines expressing TRAIL receptors. We examined the pancreatic cancer lines and KB31 cells and found they all expressed TRAIL 1 and TRAIL 2 receptors, and that the TRAIL 2 receptor was more highly expressed (Fig. 3A). Despite TRAIL receptor expression these cells were all resistant to human TRAIL at 20 ng/ml 48 hr after treatment (Fig. 3B). SS1P-KDEL alone did not cause cell death of either HTB80 or Panc3.014 cells (Fig. 3B). When SS1P-KDEL and TRAIL were combined, there was considerable cell death; 51% apoptotic cells in HTB80 after 24 hr and 66% after 48 hr, 17% in Panc3.014 after 24 hr and 40% after 48 hr.
Figure 3.
TRAIL receptor expression and synergistic cell killing by combination of immunotoxin and TRAIL. A, Trypsinized KB31, Panc3.014 and HTB80 stained with PE conjugated isotype antibody (filled region) or PE conjugated anti-TRAIL receptor 1 or 2 antibody (open region). B, HTB80 and Panc3.014 incubated with 300 ng/ml SS1P-KDEL, 20 ng/ml rh-TRAIL or combination for 24 or 48 hr. Apoptotic cells are shown as Annexin V positive population (n=4).
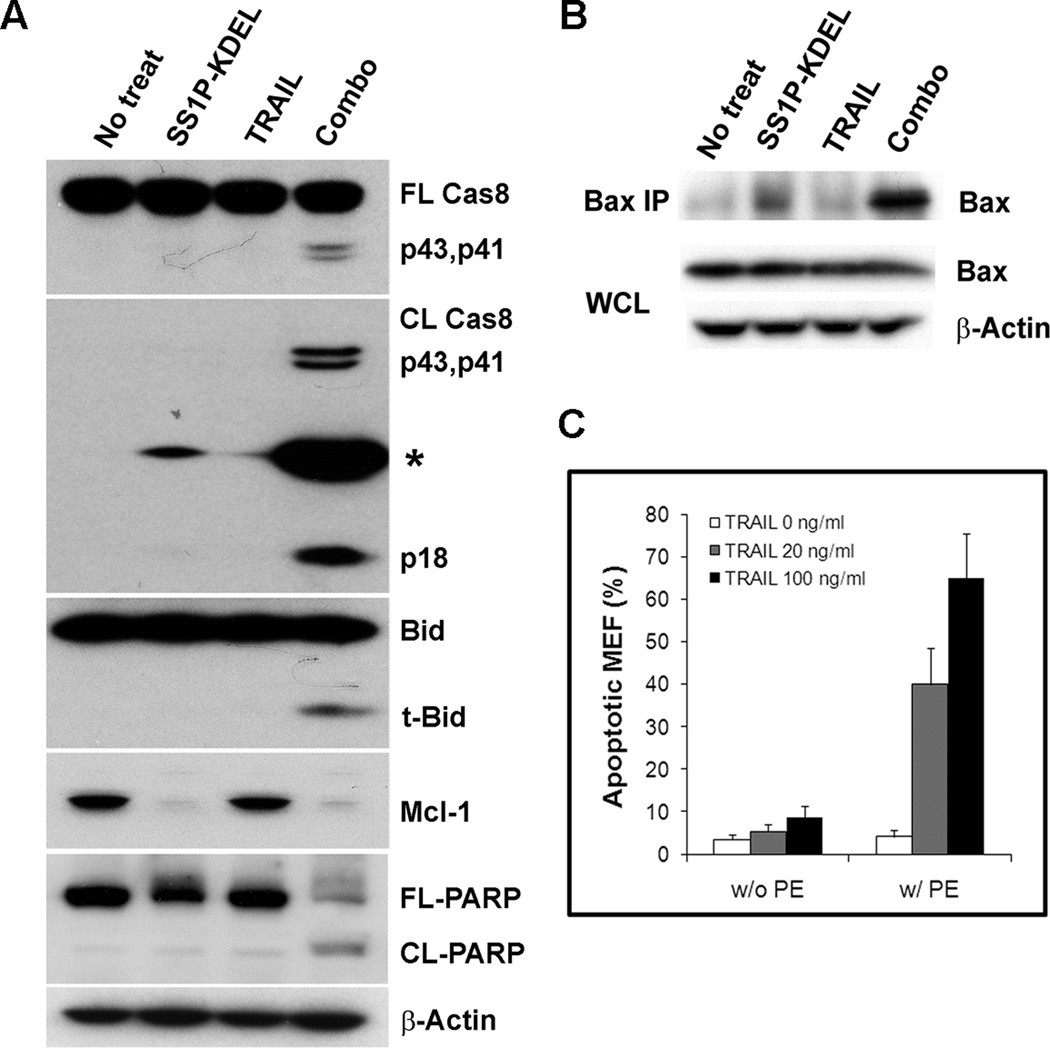
We examined the activation of cell death pathway proteins Caspase-8, Bid and PARP in cells treated with immunotoxins and TRAIL. SS1P-KDEL or TRAIL alone did not induce apoptosis in HTB80 cells, and did not activate caspase-8 or cause Bid cleavage (Fig. 4A). There was no PARP cleavage, which is consistent with the Annexin V staining assay data. Only the combination of SS1P-KDEL with TRAIL resulted in caspase-8, Bid and PARP cleavage (Fig. 4A), and activation of Bax (Fig. 4B), indicating that the combination of immunotoxin and TRAIL successfully triggered the Bax mediated apoptosis cascade by recruitment and activation of caspase-8.
Figure 4.
Immunotoxin and TRAIL combination activates Bax mediated apoptosis pathway, and Bak is dispensable for the synergism. A, HTB80 treated with 300 ng/ml SS1P-KDEL, 50 ng/ml TRAIL, or combination for 24 hr. Western blots were conducted with full-length caspase-8, cleaved caspase-8, Bid, Mcl-1, PARP, and Actin antibodies. *, unknown protein. B, HTB80 treated with 300 ng/ml SS1P-KDEL, 50 ng/ml TRAIL, or combination for 24 hr. Cell lysates were prepared with CHAPS buffer, immunoprecipitated with mouse mAb 6A7, followed by western blotting. WCL, whole cell lysate. C, Bak−/− MEFs treated with 30 ng/ml PE toxin, or 20, 100 ng/ml recombinant mouse TRAIL, or combination for 24 hr. Apoptotic cells are shown as Annexin V positive population (n=3).
To rule out the possible role of Bak in the Bax pathway, we utilized Bak knockout MEFs (Bak−/− MEF). We found that after 24 hr treatment with PE toxin alone or recombinant mouse TRAIL alone there was not measurable apoptosis. However, PE combined with TRAIL induced massive apoptotic Bak−/− MEFs (Fig. 4C), suggesting that the combination of PE toxin and TRAIL synergistically activates Bax mediated cell death, and that Bak is dispensable for this apoptosis pathway. Similar synergistic combination results were obtained when using Bak−/− HCT116 colorectal cancer cell line (Supplementary Fig. S3).
Synergistic anti-tumor activity with a combination of TRAIL receptor 2 agonist antibody HGS-ETR2 and SS1P
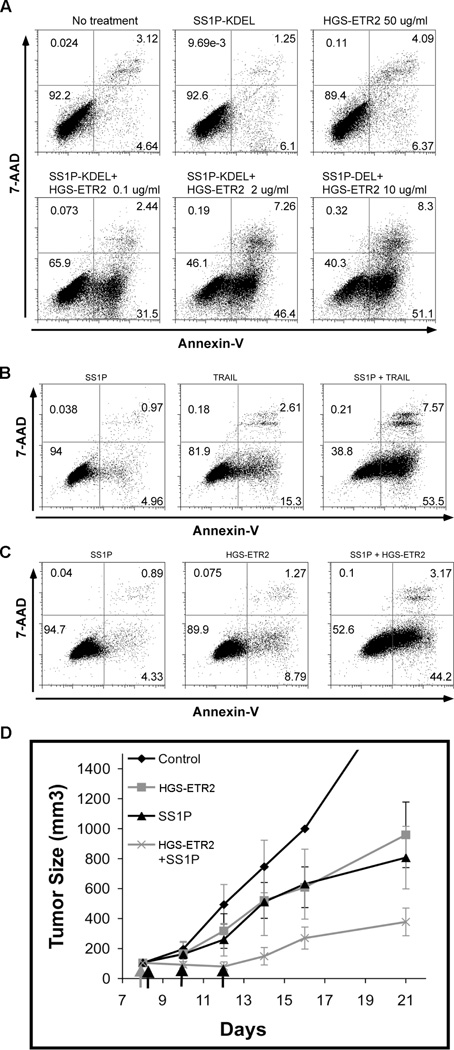
Lexatumumab (HGS-ETR2) is a fully human agonistic mAb targeting TRAIL receptor 2 that activates the extrinsic apoptosis pathway. It has preclinical anti-tumor activity and is being evaluated in clinical trials (24–27). To examine if combining immunotoxin with HGS-ETR2 could overcome the resistance of Bak deficient cancer cells, we treated HTB80 cells with HGS-ETR2. As shown in Fig. 5A, neither a very high concentration of HGS-ETR2 (50 µg/ml) nor SS1P-KDEL alone induced apoptosis. However, when SS1P-KDEL and HGS-ETR2 were combined they synergistically triggered massive cell death, and the apoptosis was HGS-ETR2 dose-dependent (Fig. 5A).
Figure 5.
Synergistic anti-tumor effect from the combination of immunotoxin and TRAIL receptor 2 agonist antibody HGS-ETR2. A, HTB80 treated with 300 ng/ml SS1P-KDEL, HGS-ETR2, or combination for 24 hr. B, KB31 treated with 100 ng/ml SS1P, 10 ng/ml rh-TRAIL, or combination for 24 hr. C, KB31 cells treated with 100 ng/ml SS1P, 0.5 µg/ml HGS-ETR2, or combination for 24 hr. Apoptosis was analyzed with Annexin V-PE and 7-AAD staining. D, KB31 cells (1.7×106) with matrigel (4.0 mg/ml, 200 µl/mouse) implanted into nude mice, five mice per group. When tumor size reached 110 mm3, 10 mg/kg HGS-ERT2 was given i.p, and 4 hr later 3 doses of SS1P 0.3 mg/kg was given QOD i.v.
To examine the effect of the two agents in an animal model we used the KB31 xenograft tumors because these cells grow well in immunodeficient mice and the pancreatic cancer cell lines do not (28, 29). Cell culture experiments showed that at 24 hr neither SS1P nor TRAIL nor HGS-ETR2 alone induced significant cell death. When SS1P was combined with either TRAIL or HGS-ETR2, substantial cell death was produced (Fig. 5B and 5C). For tumor experiments, mice bearing KB31 tumors were treated on day 8 with a single dose of HGS-ETR2, or on days 8, 10 and 12 with SS1P, or with the two agents together. We found that each agent alone had a modest anti-tumor effect, and when combined the effect was much greater than with each agent alone (Fig. 5D). On day 12 we found the combination had substantially smaller tumors than the single treatments (p<0.0001 for each). The calculated synergistic effect is significantly different from zero (p=0.013). On day 14 the combination again had substantially smaller tumors than the single treatments (p<0.0001 for each). The synergistic effect is slightly larger than on day 12 (p=0.0028).
The mitochondrial-dependent synergism between immunotoxin and HGS-ETR2 is not due to c-FLIP down-regulation or mitochondrial feedback amplification loop
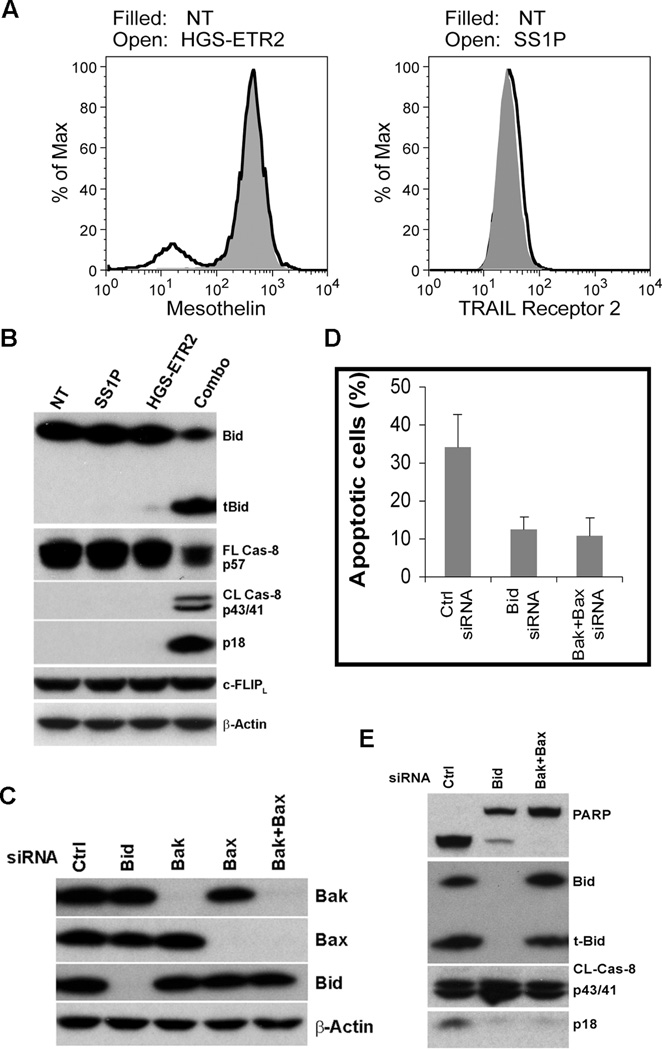
To understand the molecular mechanism behind such robust synergistic cell killing, we chose KB31 for further study because of its high transfection efficiency. We first determined whether the immunotoxin and HGS-ETR2 increases the mesothelin or TRAIL receptor 2 expression. As shown in Figure 6A, there was no change of mesothelin expression by HGS-ETR2 treatment or TRAIL receptor 2 expression by SS1P treatment. These findings rule out the possibility that increased surface receptors are the cause for the synergism of SS1P and HGS-ETR2 combination.
Figure 6.
Mitochondrial-dependent synergism between immunotoxin and HGS-ETR2 is not due to c-FLIP down-regulation or mitochondrial feedback amplification loop. A, KB31 treated with 100 ng/ml SS1P, 0.5 µg/ml of HGS-ETR2, or no treatment (NT) for 24 hr. Cell surface mesothelin and TRAIL receptor 2 were analyzed. B, Western blotting of treated cells. C, Control, Bid, Bak, Bax, Bak and Bax siRNAs (5 nM) were transfected into KB31 and knockdown efficiency was checked after 72 hr. D, siRNA transfected KB31 cells were further treated with 100 ng/ml SS1P and 0.5 µg/ml HGS-ETR2 for 20 hr. Apoptotic cells are shown as Annexin V positive population (n=3). E, Western blotting of combination treated cells.
We found that caspase-8 and Bid cleavage in KB31 were activated by the combination of SS1P and HGS-ETR2 (Fig. 6B), similar to what we observed in HTB80 (Fig. 4A). To identify the protein responsible for the synergistic activation of caspase-8, we measured the intracellular apoptosis inhibitor cellular-FLIP (c-FLIP), a regulator protein of caspase-8 activation, but we found no change of FLIP expression (Fig. 6B).
There is also the possibility that the combination of immunotoxin and TRAIL or agonist antibody could induce a mitochondrial feedback amplification loop and cause synergistic cell killing as suggested by the combination of TRAIL and etoposide (30). To this end, we knocked down Bid, Bak or Bax (Fig. 6C). We found that Bid, or Bak/Bax double knockdown reduced cell death induced by the combination of SS1P and HGS-ETR2 (Fig. 6D), suggesting that cell death by combination treatment is still mitochondrial dependent. However, the production of the active form of caspase-8, p43/41, was not affected by either Bid knockdown, or by Bak/Bax double knockdown (Fig. 6E). Bid cleavage was also not affected by Bak/Bax double knockdown (Fig. 6E). These data suggest that a mitochondrial feedback amplification loop does not exist when cells are treated with the combination of SS1P and HGS-ETR2 because the downstream events did not affect the upstream events.
Discussion
In the current study, we examined pancreatic cancer cell lines to see if an immunotoxin against mesothelin that has previously been shown to kill other mesothelin expressing cancer cell lines (7–11) can kill them. We found that two pancreatic lines, HTB80 and Panc3.014, were resistant to immuntoxin killing despite complete inhibition of protein synthesis, and demonstrated that the resistance was associated with low expression of Bak. We then carried out experiments to determine if Bak regulated the ability of immunotoxins to kill cells and found that increasing Bak makes cells more immunotoxin sensitive and that lowering Bak makes cells immunotoxin resistant. Thus it is clear that after arrest of protein synthesis, Bak, which acts through the intrinsic mitochondrial apoptosis pathway, has an important role in the ability of immunotoxins to kill cells. To our knowledge, this is the first report showing that cancer cells with low levels of endogenous Bak are resistant to some anti-cancer drugs. We did not expect the level of Bak to be so crucial that even a 2-fold increase of Bak could sensitize cells to immunotoxin.
Certain cancer cells such as DU145 and LoVo carry frame shift mutations in the Bax gene, and do not express Bax protein (31). To determine if mutations in the Bak gene are a cause of low Bak expression in HTB80 and Panc3.014, we sequenced the genomic DNA, and found no changes except silent mutations in the Bak gene (data not shown). Other cancer cells such as colon cancer cell line HCT116 also contain low Bak protein (unpublished data and ref. 32). HCT116 is resistant to HB21(Fv)-PE40 despite profound protein synthesis inhibition and growth arrest, and no significant apoptotic cells can be detected even after 48 hr treatment (Supplementary Fig. S2 and unpublished data). Thus there are cancer cells that contain much less Bak protein for unknown reasons, and are resistant to immunotoxin.
To overcome immunotoxin resistance, we simultaneously targeted both the mitochondrial (intrinsic) and death receptor (extrinsic) apoptosis pathways; this has been shown to be an effective strategy with the drugs etoposide, bortezomib and vorinostat (30, 33–35). We found that HTB80 and Panc3.014 express TRAIL receptors, yet are resistant to treatment with TRAIL. Remarkably, when SS1P-KDEL and TRAIL were combined on HTB80 and Panc3.014 cells, they synergized to produce massive cell death accompanied by caspase-8 and Bid cleavage, and Bax activation.
Antibodies to TRAIL receptors have advantages over TRAIL itself as therapeutic agents because they do not bind to decoy receptors and have a long half-life. HGS-ETR2 is a human IgG1 agonist antibody to TRAIL receptor 2 that has been evaluated in several studies for its anti-tumor activity (24–27). We found that combining SS1P-KDEL with HGS-ETR2 induced synergistic cell killing in HTB80 and that combining SS1P with HGS-ETR2 or TRAIL induced synergistic cell killing of KB31. When given to mice with KB31 tumors, SS1P combined with HGS-ETR2 had greater anti-tumor activity than either agent alone on slowing tumor growth, although the tumor regressions were not observed. Optimization of the timing of administration of immunotoxin and HGS-ETR2 may enhance tumor penetration and improve their anti-tumor effect.
Recruitment and activation of caspase-8 appears to be the mechanism for the synergism observed with the combination of immunotoxin and TRAIL or HGS-ETR2 (Fig. 4A and 6B). To investigate how it was initiated we analyzed the expression of mesothelin and TRAIL receptor 2, and found they did not change. Down-regulation of c-FLIP, a negative regulator of caspase-8 activation, has been reported to be important for the synergism of the combination of mitochondrial and death receptor apoptosis agents in some studies (33, 36, 37), but not in others (34, 38). In the current study, we found no change of c-FLIP expression after treatment with SS1P, HGS-ETR2, or the combination.
A mitochondrial feedback amplification loop was suggested to account for the synergistic cell killing by the combination of TRAIL and etoposide (30). In that scenario, extensive caspase-8 cleavage seen during TRAIL-etoposide synergy is a consequence and not a cause of the apoptotic cascade activated downstream of Bid, and Bid knockdown was shown to disrupt the mitochondrial feedback loop, and inhibit the production of p43/41, the active form of caspase-8 (30). When we combined immunotoxin and HGS-ETR2, Bid knockdown or Bak/Bax double knockdown did not inhibit caspase-8 p43/41 formation (pro-domain cleavage, Ref 39 and 40). Also, Bak/Bax double knockdown did not affect Bid cleavage. Collectively, these data indicate that the combination of immunotoxin and TRAIL or HGS-ETR2 does not induce mitochondrial feedback amplification. Although we found the combination of immunotoxin and TRAIL or HGS-ETR2 synergistically induces the cleavage and activation of caspase-8, and triggers downstream the apoptosis pathway, the mechanism of caspase-8 activation under combination treatment remains unclear. It is noteworthy that caspase-8 p18 formation was reduced by either Bid knockdown or Bak/Bax double knockdown, suggesting that the maturation of caspase-8 into p18 (inter-domain cleavage) may occur downstream of mitochondrial involvement (39, 40).
In summary, cancer cells with endogenous low Bak protein are resistant to immunotoxin treatment despite profound protein synthesis inhibition. Combining the immunotoxin with TRAIL or TRAIL agonist antibodies can induce synergistic mitochondrial-dependent cell killing by recruitment and activation of caspase-8, although the mechanism needs further study. Combining immunotoxin with activators of the death receptor pathway is an effective approach to overcome resistance due to Bak deficiency.
Translational Relevance.
New treatments for pancreatic cancer are needed because they are resistant to most chemotherapeutic agents. Many solid tumors express cell surface mesothelin, which makes them attractive targets for antibody-based cancer therapies. We assessed the activity of an anti-mesothelin immunotoxin on pancreatic cancer cells and found that they are immunotoxin resistant and that low levels of the pro-apoptotic protein Bak contribute to the resistance. We show that combining TRAIL or an anti-TRAIL receptor 2 agonist antibody, HGS-ETR2, with immunotoxin caused synergistic cell death and synergized in mice to cause a reduction in tumor size. Our results demonstrate that low Bak cancer cells are resistant to immunotoxin treatment, and that combining immunotoxin with TRAIL or TRAIL agonist antibody is an effective way to overcome such resistance.
Supplementary Material
Acknowledgements
We thank Dr. David FitzGerald for reading the manuscript and helpful comments, Dr. Raffit Hassan for providing the pancreatic cell lines and MORAB antibodies, Dawn A. Walker and other lab members for their helpful discussion, and Dr. Dan Soppet (Laboratory of Molecular Technology, NCI-SAIC) for genomic Bak sequencing. We are grateful to Drs. Richard Youle and Chunxin Wang (NINDS, NIH) for providing a human Bak gene cDNA and HCT116 cells, and for their helpful comments.
Support Statement: This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Disclosure of Potential Conflict of Interest: The authors declare no conflict. Ira Pastan is an inventor on immunotoxin patents that are owned by the National Institutes of Health.
References
- 1.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 2.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 3.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 4.Kreitman RJ, Wilson WH, Bergeron K, Raggio M, Stetler-Stevenson M, FitzGerald DJ, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med. 2001;345:241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 5.Kreitman RJ, Squires DR, Stetler-Stevenson M, Noel P, FitzGerald DJ, Wilson WH, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol. 2005;23:6719–6729. doi: 10.1200/JCO.2005.11.437. [DOI] [PubMed] [Google Scholar]
- 6.Wayne AS, Kreitman RJ, Findley HW, Lew G, Delbrook C, Steinberg SM, et al. Anti-CD22 immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-positive hematologic malignancies of childhood: preclinical studies and phase I clinical trial. Clin Cancer Res. 2010;16:1894–1903. doi: 10.1158/1078-0432.CCR-09-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 8.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Verschraegen CF, Mendoza J, Hassan R. Cytotoxic activity of the recombinant anti-mesothelin immunotoxin, SS1(dsFv)PE38, towards tumor cell lines established from ascites of patients with peritoneal mesotheliomas. Anticancer Res. 2004;24:1327–1335. [PubMed] [Google Scholar]
- 10.Ho M, Bera TK, Willingham MC, Onda M, Hassan R, FitzGerald D, et al. Mesothelin expression in human lung cancer. Clin Cancer Res. 2007;13:1571–1575. doi: 10.1158/1078-0432.CCR-06-2161. [DOI] [PubMed] [Google Scholar]
- 11.Yu L, Feng M, Kim H, Phung Y, Kleiner DE, Gores GJ, et al. Mesothelin as a potential therapeutic target in human cholangiocarcinoma. J Cancer. 2010;1:141–149. doi: 10.7150/jca.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 13.Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan R, Sharon E, Chen HX, Conlon K, Ling A, Steinberg SM, et al. Phase I clinical trial of antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of advanced pleural mesothelioma. J Clin Oncol. 2010;28:e17518. (Abstract) [Google Scholar]
- 15.Du X, Youle RJ, FitzGerald DJ, Pastan I. Pseudomonas exotoxin A-mediated apoptosis is Bak dependent and preceded by the degradation of Mcl-1. Mol Cell Biol. 2010;30:3444–3452. doi: 10.1128/MCB.00813-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreitman RJ, Pastan I. Importance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptor. Biochem J. 1995;307:29–37. doi: 10.1042/bj3070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 18.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 19.Kang R, Tang D, Schapiro NE, Livesey KM, Farkas A, Loughran P, et al. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010;17:666–676. doi: 10.1038/cdd.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traini R, Ben-Josef G, Pastrana DV, Moskatel E, Sharma AK, Antignani A, et al. ABT-737 overcomes resistance to immunotoxin-mediated apoptosis and enhances the delivery of pseudomonas exotoxin-based proteins to the cell cytosol. Mol Cancer Ther. 2010;9:2007–2015. doi: 10.1158/1535-7163.MCT-10-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han J, Goldstein LA, Gastman BR, Rabinovitz A, Wang GQ, Fang B, et al. Differential involvement of Bax and Bak in TRAIL-mediated apoptosis of leukemic T cells. Leukemia. 2004;18:1671–1680. doi: 10.1038/sj.leu.2403496. [DOI] [PubMed] [Google Scholar]
- 23.Theodorakis P, Lomonosova E, Chinnadurai G. Critical requirement of BAX for manifestation of apoptosis induced by multiple stimuli in human epithelial cancer cells. Cancer Res. 2002;62:3373–3376. [PubMed] [Google Scholar]
- 24.Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 25.Wakelee HA, Patnaik A, Sikic BI, Mita M, Fox NL, Miceli R, et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol. 2010;21:376–381. doi: 10.1093/annonc/mdp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng Y, Wu XX, Fiscella M, Shimada O, Humphreys R, Albert V, et al. Monoclonal antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) induces apoptosis in primary renal cell carcinoma cells in vitro and inhibits tumor growth in vivo. Int J Oncol. 2006;28:421–430. doi: 10.3892/ijo.28.2.421. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Zhang X, Barrisford GW, Olumi AF. Lexatumumab (TRAIL-receptor 2 mAb) induces expression of DR5 and promotes apoptosis in primary and metastatic renal cell carcinoma in a mouse orthotopic model. Cancer Lett. 2007;251:146–157. doi: 10.1016/j.canlet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Xiang L, Hassan R, Paik CH, Carrasquillo JA, Jang BS, et al. Synergistic antitumor activity of taxol and immunotoxin SS1P in tumor-bearing mice. Clin Cancer Res. 2006;12:4695–4701. doi: 10.1158/1078-0432.CCR-06-0346. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Xiang L, Hassan R, Pastan I. Immunotoxin and Taxol synergy results from a decrease in shed mesothelin levels in the extracellular space of tumors. Proc Natl Acad Sci USA. 2007;104:17099–17104. doi: 10.1073/pnas.0708101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broaddus VC, Dansen TB, Abayasiriwardana KS, Wilson SM, Finch AJ, Swigart LB, et al. Bid mediates apoptotic synergy between tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and DNA damage. J Biol Chem. 2005;280:12486–12493. doi: 10.1074/jbc.M408190200. [DOI] [PubMed] [Google Scholar]
- 31.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 32.Gillissen B, Wendt J, Richter A, Richter A, Müer A, Overkamp T, et al. Endogenous Bak inhibitors Mcl-1 and Bcl-xL: differential impact on TRAIL resistance in Bax-deficient carcinoma. J Cell Biol. 2010;188:851–862. doi: 10.1083/jcb.200912070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frew AJ, Lindemann RK, Martin BP, Clarke CJ, Sharkey J, Anthony DA, et al. Combination therapy of established cancer using a histone deacetylase inhibitor and a TRAIL receptor agonist. Proc Natl Acad Sci USA. 2008;105:11317–11322. doi: 10.1073/pnas.0801868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanker A, Brooks AD, Tristan CA, Wine JW, Elliott PJ, Yagita H, et al. Treating metastatic solid tumors with bortezomib and a tumor necrosis factor-related apoptosis-inducing ligand receptor agonist antibody. J Natl Cancer Inst. 2008;100:649–662. doi: 10.1093/jnci/djn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith MR, Jin F, Joshi I. Bortezomib sensitizes non-Hodgkin's lymphoma cells to apoptosis induced by antibodies to tumor necrosis factor related apoptosis-inducing ligand (TRAIL) receptors TRAIL-R1 and TRAIL-R2. Clin Cancer Res. 2007;13:5528s–5534s. doi: 10.1158/1078-0432.CCR-07-0982. [DOI] [PubMed] [Google Scholar]
- 36.Hietakangas V, Poukkula M, Heiskanen KM, Karvinen JT, Sistonen L, Eriksson JE. Erythroid differentiation sensitizes K562 leukemia cells to TRAIL-induced apoptosis by downregulation of c-FLIP. Mol Cell Biol. 2003;23:1278–1291. doi: 10.1128/MCB.23.4.1278-1291.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, Shen HM, Ong CN. Down-regulation of c-FLIP contributes to the sensitization effect of 3,3'-diindolylmethane on TRAIL-induced apoptosis in cancer cells. Mol Cancer Ther. 2005;4:1972–1981. doi: 10.1158/1535-7163.MCT-05-0249. [DOI] [PubMed] [Google Scholar]
- 38.Morizot A, Mérino D, Lalaoui N, Jacquemin G, Granci V, Iessi E, et al. Chemotherapy overcomes TRAIL-R4-mediated TRAIL resistance at the DISC level. Cell Death Differ. 2011;18:700–711. doi: 10.1038/cdd.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–21781. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.