Abstract
Chronic treatment with fetal bovine serum (FBS) causes contractility reduction, morphological alteration and DNA synthesis in organ-cultured vascular tissues. Here, we tested the hypothesis that chronic inhibition of ROCK has a protective effect on FBS-induced alterations in small arteries. Rabbit mesenteric arterial rings were cultured in FBS-supplemented culture medium with or without Y-27632, a reversible ROCK inhibitor. Chronic Y-27632 treatment prevented FBS-induced gradual arterial constriction, wall thickening, reduced contractility, and increased ROCK-specific MYPT1 Thr853 phosphorylation. Treatment with Y-27632 also prevented decreased eNOS mRNA expression, and reduced acetylcholine-induced relaxation. Sudden application of Y-27632 to pre-cultured rings reduced MYPT1 phosphorylation and re-widened the constricted rings. Chronic treatment with Y-27632, however, rather augmented than reduced the FBS-induced RhoA over-expression, also increased ROCK1 and MYPT1 expression and averted the FBS-induced reduction of MLC expression, suggesting a compensation of inhibited RhoA/ROCK activity. Sudden removal of Y-27632 caused a rebound in MYPT1 phosphorylation and vasoconstriction in rabbit mesenteric artery. To test which ROCK isoform has greater involvement in FBS-induced contraction, haploinsufficient Rock1+/− and Rock2+/− mouse mesenteric arterial rings were subjected to organ-culture. FBS-induced contraction and RhoA over-expression in either heterozygous animal was not different from wild-type animals. These results suggest that FBS-induced contraction is mediated by up-regulation of RhoA and subsequent activation of ROCK. In conclusion, chronic ROCK inhibition produces some effects that protect against FBS-stimulated vasoconstriction and remodeling. There are also negative effects that a sudden withdrawal of ROCK inhibitor might cause a stronger vasoconstriction than before it was used.
Keywords: Organ culture, Artery, Fetal serum, RhoA, ROCK
Introduction
Sustained vasoconstriction and vascular remodeling play a crucial role in the pathogenesis of a variety of vascular diseases including hypertension, vasospasm and atherosclerosis (Schwartz 1997; Noma et al. 2006). Although recent advances have led to a better understanding of the pathophysiological mechanism involved in the vascular dysfunction, further studies are needed on the mechanism in the pathogenesis of the cardiovascular disease, which remains a leading cause of death in the developed countries.
RhoA, a member of the Rho subfamily of the Rasrelated, small GTPase superfamily, acts as a molecular switch to regulate diverse cellular processes including actin cytoskeletal organization, cell proliferation, gene expression, ion channel activity, vascular permeability, reactive oxygen species production, and smooth muscle contraction (Brown et al. 2006). The Rho-associated kinase (Rho-kinase, ROCK) is the most critical RhoA effector that contributes to various physiological functions in a number of hollow organs such as blood vessels (Noma et al. 2006). Deletion of either ROCK1 or 2 causes, respectively, embryonic and postnatal death in mouse models (Shimizu et al. 2005; Thumkeo et al. 2003), indicating that both isoforms play a crucial role in development. In contrast, up-regulation of the RhoA/ROCK pathway plays a major role in the pathogenesis of various vascular dysfunctions including reduced eNOS activity and expression in endothelial cells and augmented Ca2+ sensitivity in smooth muscle contraction (Schwartz 1997; Félétou and Vanhoutte 2006), suggesting that ROCK is a therapeutic target in cardiovascular disease (Noma et al. 2006; Shimokawa and Takeshita 2005; Loirand et al. 2006).
Several compounds have been developed to inhibit ROCK, including hydroxyfasudil, Y-27632 and H-1152 (Uehata et al. 1997; Shimokawa and Rashid 2007), which target the catalytic domain and inhibit both ROCK1 and ROCK2 with equal potency. Beneficial effects of these inhibitors on the pathological roles of ROCK have been reported in animal models with various cardiovascular diseases such as hypertension, coronary and cerebral vasospasm and pulmonary arterial hypertension (Uehata et al. 1997; Shimokawa and Rashid 2007). Although in vivo models have proved useful for establishing relationships between vascular disease and therapeutic target molecules, they have certain limitations for identifying underlying mechanisms under uncontrolled conditions. In contrast, in vitro cell culture models have been used extensively to elucidate the molecular mechanism of cell-active compounds. However, results obtained with cell culture models do not always apply to physiological function, cell–cell and cell–matrix interaction, protein composition and contractile filaments. Moreover, smooth muscle cells under culture conditions also pose problems, because they rapidly lose their contractile phenotype and change to a synthetic phenotype (Owens et al. 2004; Woodsome et al. 2006; Gerthoffer 2007).
In this study, we used an organ-culture model to determine whether the RhoA/ROCK signaling mediates the direct effect of the growth stimulant FBS on the structure and function of differentiated but not proliferative smooth muscle and endothelial cells in arteries, which preserve many principal properties of native blood vessels. Organ-culture serves as a tool to investigate mechanisms of chronic effects of substances and treatments in arterial tissues (Merrilees and Scott 1982; Lindqvist et al. 1999; Ozaki and Karaki 2002; Thorne and Paul 2003). Here, we report that the ROCK inhibitor Y-27632 preserved blood vessels from FBS-induced constriction, depression of contractile activity, down-regulation of eNOS and endothelium-induced relaxation. Our results strongly support the hypothesis that RhoA/ROCK signaling is crucial for maintaining both the structural and functional phenotypes of vasculature and is a promising therapeutic target in cardiovascular diseases. We also identified potential rebound effects associated with such a treatment.
Materials and methods
Animals, tissue preparation and organ culture procedure
All animal procedures for isolation of arteries from rabbits and mice were approved by the Animal Care and Use Committees of the Boston Biomedical Research Institute. New Zealand white rabbits (2–3 kg) were obtained from Charles River Laboratories (Wilmington, MA). Heterozygous Rock1+/− and Rock2+/− mice were generated on a C57BL/6 background as described previously (Noma et al. 2008). Rabbits were euthanized with an overdose of inhalant halothane, and mice with inhalation of CO2. After thoracotomy, the mesenteric arteries were isolated. Following dissection of fat and fluffy connective tissue, branches of the mesenteric artery were cut into rings (0.5–0.6 mm in outer diameter and 0.75 mm in length for rabbits, and 0.25–0.3 mm in diameter and 0.5 mm in length for mice). The arterial rings were then placed in a silicone elastomer dish containing 4 ml Dulbecco’s Modified Eagles Medium (DMEM) supplemented with 50 U penicillin and 50 µg/ml streptomycin and 10% FBS (Sigma–Aldrich, F4135, heat-inactivated) with or without 10 µM Y-27632. The arterial rings were maintained at 37°C with 5% CO2 for 7 days for rabbit and 6 days for mouse arterial rings. The culture medium was changed every 2 days. In some preliminary experiments, arterial rings were stretched to 1.2–1.3 times slack length using two supporting stainless steel pins to mimic blood pressure in vivo. Within a few days, some smooth muscle cells started to migrate out of the rings at both cut ends along the pins. Several days later, a large number of cells from the stretched rings migrated out onto the dish. Therefore, during culture each ring was loosely laid and placed on the silicone elastomer secured by a pin at the center of the lumen without stretch (see Fig. 1). Once or twice a day during culture, the medium was gently shaken, and the rings were moved around the supporting pin to prevent the attachment to the silicone dish and subsequent cell migration.
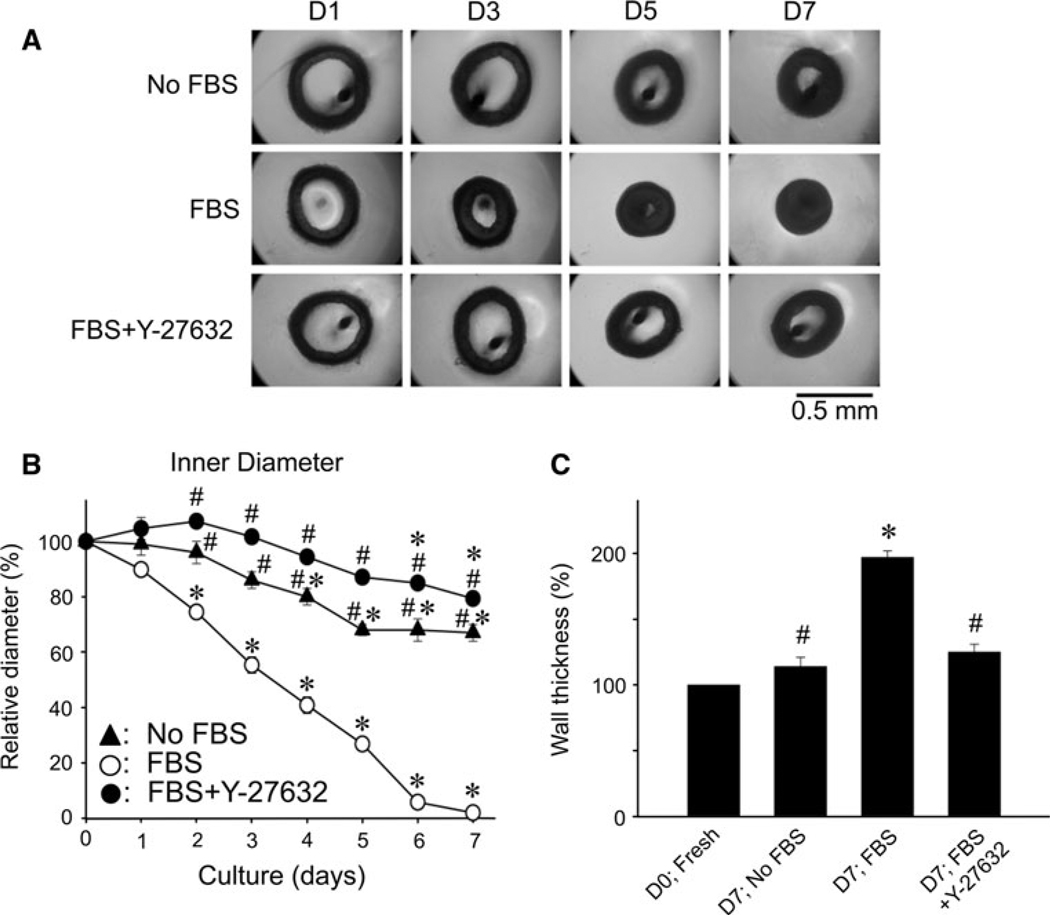
Fig. 1.
Effects of 10 µM Y-27632 on morphological changes in rabbit mesenteric arterial rings during 10% FBS-supplemented organ-culture for 7 days. A Representative arterial ring images from day 1 (D1) to day 7 (D7) in FBS-free (top panel), FBS-supplemented DMEM in the absence (middle) and presence of Y-27632 (bottom). The dark spot in the lumen is the image of a supporting pin (for details, see the Materials and methods). B Average changes in inner diameters with time. C Average change in wall thickness after 7 days organ-culture. Results are expressed as a mean ± S.E.M. (n = 4–6). * represents a significant (P < 0.05) difference from that of fresh artery on D0, and # from that of FBS-supplemented organ-cultured artery in the absence of Y-27632
Force measurement
Two tungsten needle tips were inserted into the lumen of the mesenteric artery ring. One of the needles was connected to a force transducer (AM801, SensoNor, Horten, Norway) and the other to a micromanipulator to adjust the muscle tone. Fresh or organ-cultured arterial rings were stretched to about 1.2 mN of passive tension followed by a spontaneous decrease to a lower steady level. Before the experiments, each ring was washed with a Y-27632-free modified Krebs solution at 30°C and repeatedly stimulated by high (124 mM) K+ for 5 min with an interval of 15 min rest until a steady maximum contraction was obtained. The length between two needles was step-wisely increased or decreased for readjustment of the muscle cell length to produce a maximum contraction in response to high K+. After measuring the maximum contraction, 10 µM phenylephrine (PE), 0.3 µM endothelin-1 (ET-1) or 1 µM phorbol 12,13-dibutyrate (PDBu) was applied to measure the amplitude and the time course of contraction in freshly isolated or organ-cultured arterial rings. To monitor endothelial cell-dependent and independent relaxation, 100 µM acetylcholine (ACh) and 10 µM sodium nitroprusside (SNP), respectively, was applied after 50 µM PE-induced contraction reached a plateau.
Rabbit mesenteric artery rings cultured in FBS-supplemented media for 7 days had significantly thickened vessel walls (see Fig. 1C). To precisely compare the amplitude of contraction before and after 7 days organ-culture, all samples used for force measurements were fixed and double-stained with anti α-actin antibody and 4′,6-diamidino-2-phenylindole (DAPI), and the total cell number in the whole cross-sectional area of tunica media was counted to normalize the force level.
Solutions used for force measurement
Normal and depolarizing modified Krebs solutions were prepared as described previously (Kitazawa et al. 2009). The normal external solution contained the following (mM): 150 NaCl, 4 KCl, 2 calcium methanesulfonate, 2 magnesium methanesulfonate, 5.6 glucose and 5 Hepes buffer. The high K+ depolarizing external solution had 124 mM potassium methanesulfonate substituted equally for NaCl with other chemicals at the same concentrations. The pH of both solutions was adjusted to pH 7.4 with Tris.
Immunoblotting
Immunoblotting experiments were performed as previously described (Kitazawa et al. 2003). Briefly, the arterial rings under fresh or organ-cultured conditions were quickly frozen in liquid nitrogen and kept in 10% trichloroacetic acid (TCA)-acetone at −80°C overnight. After TCA treatment, the tissues were gradually warmed and washed in acetone at room temperature, and allowed to dry. The small dried rings were homogenized in Laemmli sample buffer (LSB; with final concentrations 62.5 mM Tris, 1% SDS, 15% glycerol, 30 mM dithiothreitol and 0.005% Bromophenol Blue) using a glass-glass mini-homogenizer. The homogenates were then centrifuged and the supernatants collected. Total protein concentration was measured using a Coomassie Plus Protein Assay Reagent Kit (Pierce) and adjusted to 2 mg/ml with LSB. For actin, the samples were diluted ten-fold with LSB. Proteins were separated on a 4–20% polyacrylamide gradient gel and then transferred to nitrocellulose membranes using a wet transfer method. The membranes were blocked in Tris-buffered saline (TBS) solution containing 0.05% Tween-20 and 5% non-fat milk for 1 h at room temperature. After treatment with the primary antibody solution, the membranes were incubated with alkaline phosphatase-conjugated secondary antibodies, and the bands were developed with alkaline phosphatase substrate (Sigma). The bands were scanned and analyzed using IPLabGel image analyzing system (Signal Analytics, Vienna, VA).
MYPT1 phosphorylation was determined by immunoblotting method with 4–20% gradient SDS polyacrylamide gels using phospho-specific antibodies as described previously (Kitazawa et al. 2003). Briefly, the arterial rings in culture dishes at 37°C were quickly frozen with liquid nitrogen-cooled propane and then placed atop frozen acetone containing 10% TCA and kept at −80°C overnight. After gradual warming to room temperature, the rings were washed, dried and homogenized as described above. An equal amount of each extract was loaded into two wells of the same gels. The separated proteins were transferred to the same nitrocellulose membranes. The membranes were treated with the blocking buffer and cut into two pieces; one for phospho-MYPT1 and the other for pan-MYPT1 primary antibodies followed by secondary antibodies as described above. We compared the intensity ratio of phosphorylated MYPT1 from one set of western blots with the total amount of MYPT1 from the paired set of western blots. The relative phosphorylation levels of organ-cultured arterial rings were then compared with the phosphorylation in freshly isolated artery at Day 0 (D0) in culture.
Measurement of eNOS mRNA expression
Total RNA was isolated from fresh or organ-cultured arterial rings using the acid guanidinium thiocyanate-phenol–chloroform method with TRIzol™ reagent. The concentration of total RNA was adjusted to 0.1 µg/µl with RNase-free distilled water. RT–PCR was performed using SuperScript™ III First-Strand Synthesis System (Invitrogen). Briefly, 0.4 µg of total RNA from each preparation was reverse transcribed for the synthesis of the first strand of cDNA as per the manufacturer’s protocol. The PCR amplification of reverse transcribed cDNA was conducted using synthetic gene specific primers for eNOS and GAP-DH as described below. After initial denaturation, 25 cycles (for GAPDH) or 35 cycles (for eNOS) of amplification were performed with a thermal cycler (Bio-Rad). The oligo-nucleotide forward primers and reverse primers for eNOS and GAPDH were designed as follows: AAC CAC ATC AAG TAT GCC AC (sense for eNOS), TCT CCG TGC TCA TGT ACC AG (antisense for eNOS), GAG CTG AAC GGG AAA CTC AC (sense for GAPDH), and GGT TTG AGG GCT CTT ACT CC (antisense for GAPDH). PCR products (10 µl) were electrophoretically separated on a 1% agarose gel containing 0.1% ethidium bromide. Fluorescence bands were visualized using an ultraviolet transilluminator. The densitometric intensity of 446 base pairs for eNOS and of 359 base pairs for GAPDH was analyzed using the NIH ImageJ program and the results were shown as the ratio of the optical density of eNOS to that of GAPDH (see Fig. 2D).
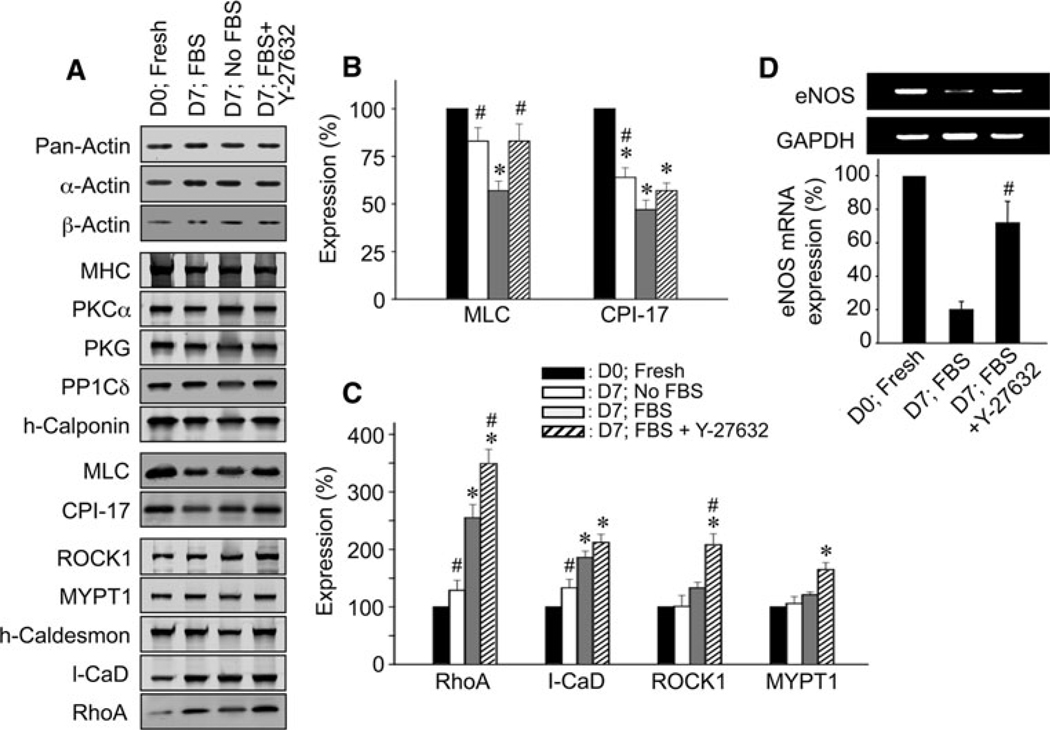
Fig. 2.
Changes in protein expression after 7 days of organ-culture and the effect of 10 µM Y-27632 in rabbit mesenteric artery. A Representative immunoblot images of various proteins from freshly isolated (D0; Fresh) and 7 days cultured arteries in FBS-free medium (D7; No FBS), and FBS-supplemented medium in the absence (D7; FBS) and presence of Y-27632 (D7; FBS + Y-27632). Total actin (pan-actin) contents under different conditions were matched. For actins, protein extracts diluted ten-fold were loaded. B and C Average expression levels of contractile/regulatory proteins (n = 4–6). D Representative RT–PCR images (upper) and a quantitative summary of eNOS mRNA expression (lower, n = 4). The symbols for statistical significance are defined as described in the legend of Fig. 1
Drugs, and chemical reagents and antibodies
Y-27632 was a gift from Yoshitomi Pharmaceutical (Mitsubishi Pharma) Co. Dulbecco’s Modified Eagles Medium was from Mediatech Inc. Endothelin-1 and PDBu from BioMol (Enzo Life Sciences) Inc. Cycloheximide, phenylephrine, acetylcholine, sodium nitroprusside, and fetal bovine serum from Sigma–Aldrich. The following primary antibodies were used in this study: monoclonal anti-myosin heavy chain (Sigma, 1:2,000); monoclonal anti-MLC (Sigma, 1:1,000); polyclonal anti-MYPT1 (BabCO, 1:5,000); anti-CPI-17 IgY (1:5,000); polyclonal anti-caldesmon (from Dr. A. Wang of BBRI, 1:10,000); polyclonal anti-h-calponin (from Dr. E. Mabuchi of BBRI, 1:10,000); polyclonal anti-PKCα (Sigma, 1:1000); polyclonal anti-PP1Cδ (from Dr. Eto of Thomas Jefferson University, 1:5,000); polyclonal anti-ROCK1 (Sigma, 1:2,000); polyclonal anti-ROCK2 (Santa Cruz Biotechnology, 1:1,000); monoclonal anti-RhoA (Santa Cruz Biotechnology, 1:1,000); polyclonal anti-PKG-Iα (Sigma, 1:5,000); polyclonal anti pMYPT1853 (Upstate, 1:5,000; the specificity of the site- and phospho-specific MYPT1 Thr853 antibody was demonstrated (Kitazawa et al. 2003; Dimopoulos et al. 2007)); polyclonal anti pan-actin (Sigma, 1:1,000); monoclonal anti α-actin (Sigma, 1:5,000); monoclonal anti β-actin (Sigma, 1:5,000). Secondary antibody against chicken IgY was from Promega (1:5,000). Anti-mouse and anti-rabbit IgG secondary antibodies (1:5,000) were from Chemicon.
Statistics
Results are expressed as the mean ± S.E.M. of n experiments. Statistical significance was evaluated with one-way ANOVA; P < 0.05 was considered statistically significant.
Results
Effect of Y-27632 on FBS-induced contraction of organ-cultured artery
To examine the morphological changes in arterial rings during FBS-supplemented organ-culture, we took an image of each ring every 24 h (Fig. 1A) to analyze the time course of changes in outer and inner diameters (i.e. thickness) of the arterial wall compared to those at day 0 (D0; Fig. 1). Although FBS (10–20%) has been reported to produce an acute and large contraction with associated increase in [Ca2+]i and dialyzed FBS has no such contractile effect (Lindqvist et al. 1999; De Mey et al. 1989), our 10% FBS (F4135, Sigma-Aldrich) in DMEM did not acutely induce any noticeable contraction in the arterial rings even for 1 day (D1; Fig. 1B). The control rings cultured in the 10% FBS medium in the absence of Y-27632, however, slowly shrank with a half time of 3.4 ± 0.2 days (n = 4) in inner diameter and the lumen was completely obstructed on day 6 (D6; Fig. 1B). In addition, the outer diameter was also reduced, and the wall thickness at day 7 was increased (Fig. 1C). These morphological changes were FBS concentration-dependent: In the absence of FBS, the rings did not significantly shrink until D3 (Fig. 1B) and the wall thickness on D7 was not increased (Fig. 1C). In a series of separate experiments, when FBS concentration was reduced to 0, 1.5, 5 and 10%, the inner diameter at D5 was 80 ± 5, 69 ± 8, 55 ± 7 and 28 ± 5% (n = 3) of that on D0, respectively. The presence of 10 µM Y-27632 markedly reduced all morphological alterations even in the presence of 10% FBS: no significant changes in wall thickness (Fig. 1C) were observed compared to those of D0 fresh and no FBS organ-cultured arteries. The inner diameter of arteries organ-cultured in the presence of FBS + Y-27632 was only slightly reduced compared to that of fresh rings on D7, while there was no significant difference from that in the absence of FBS each day of culture (Fig. 1B). Y-27632 in the absence of FBS almost completely prevented the shrinkage of the ring inner diameter at D7 (to 100 ± 8%; n = 6). Another ROCK-specific inhibitor H-1152 (Sasaki et al. 2002), and a 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase inhibitor simvastatin had similar protective effect although the latter was weaker than the former (Table 1). Together, these results suggest that the RhoA/ROCK signaling pathway plays a major role in the FBS-induced arterial contraction during organ-culture.
Table 1.
Effect of various RhoA/ROCK signaling inhibitors on relative inner diameter of organ-cultured arterial rings at D5 compared to D0 fresh artery
| Conditions | Relative inner diameter (%) | n |
|---|---|---|
| No FBS | 68 ± 6 | 6 |
| FBS | 27 ± 4 | 6 |
| FBS + 10 µM Y-27632 | 87 ± 3 | 6 |
| FBS + 3 µM H-1152 | 84 ± 2 | 6 |
| FBS + 10 µM Simvastatin | 64 ± 7 | 7 |
Results are expressed as the mean ± SEM of n experiments
Effect of Y-27632 on protein expression during organ-culture
To investigate the molecular mechanism responsible for the inhibition of FBS-induced contraction by chronic treatment with Y-27632, we examined expression levels of several regulatory/contractile proteins in 7-day organ-cultured arterial rings with and without Y-27632. Total actin content in no FBS and 10% FBS-supplemented organ-culture without and with 10 µM Y-27632 at D7 was significantly reduced to 78 ± 5%, 66 ± 10% and 74 ± 9% (n = 4; not significantly different among three conditions), respectively, of that in freshly isolated artery when the total protein contents were matched among samples. When the expression level of total actin was matched using pan-actin antibody to equalize the total content of cellular actin regardless of isoform (pan-actin in Fig. 2A), the average expression levels of α-actin and β-actin were maintained at levels similar to that of fresh artery during the 7 days organ-culture regardless whether or not FBS or Y-27632 was present (Fig. 2A), suggesting no isoform switch in actin during 7-day FBS-supplemented organ-culture. MHC, PKCα, PKG (protein kinase G), protein phosphatase type 1C δ-isoform (PP1Cδ) and h-calponin were also maintained (Fig. 2A). CPI-17 (smooth muscle-specific PKC-potentiated myosin phosphatase inhibitor protein-17 kDa) was significantly decreased in the FBS-free organ-culture for 7 days compared to that of D0 fresh artery and further decreased to 47 ± 5% in the presence of FBS (Fig. 2B). MLC expression was also decreased to 57 ± 5% in the presence but not absence of FBS (Fig. 2B). In contrast, the RhoA and l-caldesmon amounts were increased by FBS of that in fresh artery (Fig. 2C). These results suggest the phenotypic change mediated by FBS in organ-cultured artery is partly similar to the difference between fresh aorta tissues and primary aortic culture cells (Woodsome et al. 2006). Y-27632 significantly prevented down-regulation of MLC but not CPI-17 (Fig. 2B) whereas it further augmented RhoA expression to 349 ± 25% of fresh artery (Fig. 2C) but had no additional effects on l-caldesmon. Expression of ROCK1, MYPT1 and h-cal-desmon was also enhanced by Y-27632. However, ROCK2 isoform expression was not significantly changed during organ-culture regardless of the presence of Y-27632 (83 ± 9% for FBS and 118 ± 10% for FBS + Y-27632; n = 4). Since the number of intimal cells was 8 ± 3% (n = 13) of the total number of cells in the artery, the involvement of endothelial cells in measured expression level of regulatory/contractile proteins appeared to be low even if the observed expression level of these proteins was similar in both types of cells.
Figure 2D shows that the eNOS expression relative to the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression in organ-cultured artery was significantly decreased as compared to that in fresh artery, while chronic Y-27632 treatment significantly prevented the down-regulation of eNOS.
Effect of pretreatment with Y-27632 on agonist-induced contraction and acetylcholine (ACh)-induced relaxation of FBS-treated arteries
To assess the chronic effect of Y-27632 treatment during FBS-supplemented organ-culture on smooth muscle contractility, we organ-cultured arterial rings for 7 days in the presence and absence of Y-27632 and then examined isometric contraction in response to various stimulants in Y-27632-free, FBS-free modified Krebs solution. Figure 3A illustrates a comparison of the steady peak amplitude of contractions in response to high K+, PE, and ET-1 in the arteries pretreated under various conditions. The 7-day organ-culture without FBS (D7; No FBS) significantly but modestly attenuated the force stimulated with agonists except high K+ and the addition of 10% FBS (D7; FBS) markedly reduced the peak contraction. The presence of Y-27632 during organ culture (D7; FBS + Y-27632) significantly prevented the contractile reduction in the FBS-supplemented organ-culture. Contraction induced by 1 µM phorbol 12,13-dibutylate (PDBu), a direct PKC activator, was similarly reduced in the 7-day organ-culture to 23 ± 8% (n = 3) of that on D0, while the pretreatment with Y-27632 significantly averted the reduction and maintained the contractility at 70 ± 7%.
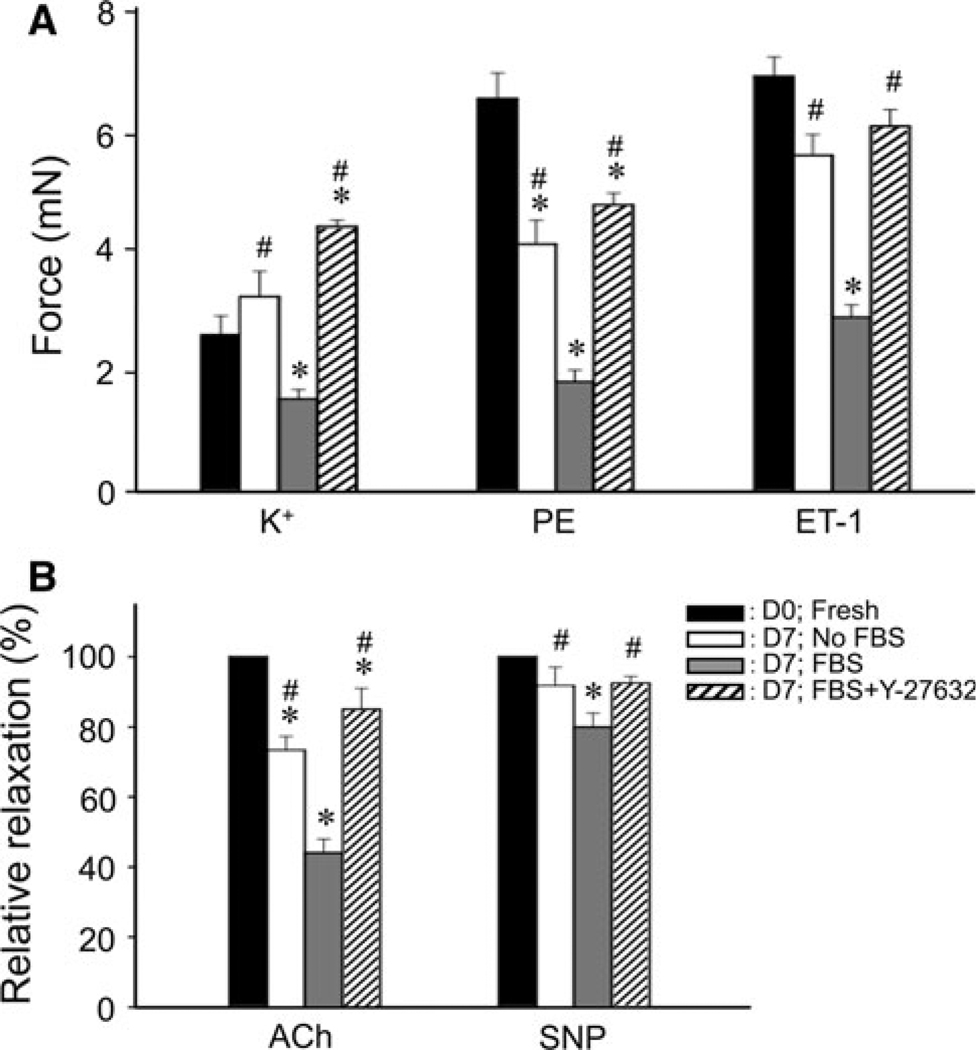
Fig. 3.
Effect of 10 µM Y-27632 pretreatment during FBS-supplemented organ-culture for 7 days on agonist-induced contractions (A) and ACh-induced relaxation (B) in rabbit mesenteric arterial rings. A Comparison of steady tonic level of contractions induced by various stimulants (124 mM K+, 30 µM PE and 0.3 µM ET-1) in arteries pretreated under various organ culture conditions (n = 9). In response to 124 mM K+, fresh arteries produced a transient contraction followed by a reduction to a low level while monotonic contraction was seen at D7 in organ-cultured arteries irrespective of pretreatment with FBS and Y-27632. B Summary of relative 100 µM ACh and 10 µM sodium nitroprusside (SNP)-induced relaxation at steady state (n = 6–9). Relative relaxation was normalized with that of the D0;Fresh. The symbols for statistical significance are defined as described in the legend of Fig. 1
We compared ACh- and SNP-induced relaxation of PE-induced contractions in FBS-supplemented organ-culture arteries with Y-27632 to that of fresh artery. Organ culturing with FBS markedly inhibited the ACh-induced relaxation while had a small effect on SNP-induced relaxation (Fig. 3B). Y-27632 in the presence of FBS markedly reduced FBS-induced inhibition of ACh-induced maximum relaxation. These changes in ACh-induced relaxation are consistent with results seen in the eNOS expression (Fig. 2D). Together, these results suggest that the FBS-supplemented organ-culture reduces more strongly the endothelium-dependent than the endothelium-independent relaxation.
Effect of Y-27632 on contracted arterial rings and increased MYPT1 phosphorylation during organ-culture
To test whether Y-27632 could reverse arteries contracted by FBS-supplemented organ-culture, the arterial rings were initially organ-cultured in a control medium containing 10% FBS for 7 days followed by the same medium containing 10 µM Y-27632 for an additional day. Figure 4A (upper panel) illustrates the representative images of arterial rings cultured on D0 and D7 in the control medium and on D8 in the Y-27632-supplemented medium for 1 day. The extra day of culture in the presence of Y-27632 markedly widened contracted arterial rings (Fig. 4B), suggesting that Y-27632 relaxes arteries from chronic FBS-induced contraction.
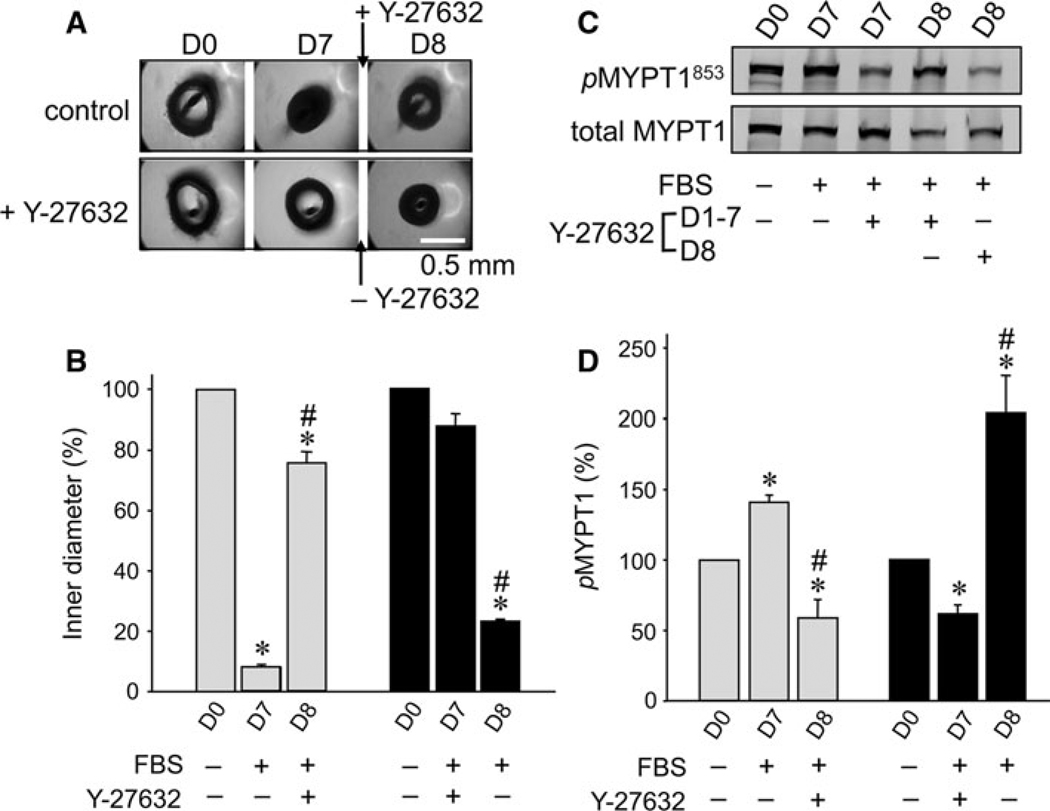
Fig. 4.
Effect of addition of 10 µM Y-27632 to pre-constricted arterial rings in organ-culture in the absence of Y-27632 for 7 days, and effect of removal of Y-27632 on arterial rings organ-cultured in the presence of Y-27632 for 7 days. A Upper panel shows arterial ring images at D0 and D7 without Y-27632 and for one additional day (D8) with 10 µM Y-27632. Lower panel shows arterial ring images at D0 and D7 with Y-27632 and D8 without Y-27632. B A quantitative summary of changes in ring inner diameter (n = 3). C Representative immunoblot images of phosphorylated (pMYPT1853, upper panel) and total MYPT1 (lower). D A quantitative summary of pMYPT1 levels (n = 3). The symbols for statistical significance are defined as described in the legend of Fig. 1
We further tested whether the removal of Y-27632 from chronic Y-27632-supplemented culture medium impaired the arterial function. The arterial rings were organ-cultured in a medium containing 10 µM Y-27632 for 7 days followed by a Y-27632-free medium for 1 day (lower panel of Fig. 4A). On D8, the arterial rings were markedly contracted within 1 day (Fig. 4B). This contraction was much faster compared with the control in the medium without Y-27632 (corresponding to the shrinkage on D5 in Fig. 1), suggesting that ROCK activity on D8 after the removal of Y-27632 was much higher than that during organ-culture in the Y-27632-free medium.
To estimate the ROCK activity in situ, we examined whether MYPT1 phosphorylation at the ROCK-specific site Thr853 (Velasco et al. 2002; Kitazawa et al. 2003) increased when rings were contracted. With 7-day culture in the absence and presence of 10 µM Y-27632, MYPT1 Thr853 phosphorylation increased and decreased, respectively, compared to that of D0 (Fig. 4C and D). Addition of 10 µM Y-27632 after 7-days culture in the absence of Y-27632 significantly reduced MYPT1 phosphorylation (Fig. 4D), whereas removal of Y-27632 for 1 day after 7-days culture in the presence of Y-27632 markedly augmented the phosphorylation (Fig. 4D).
Effect of Y-27632 on contraction and RhoA/ROCK expression during FBS-supplemented organ-culture in Rock1+/− and Rock2+/− mouse mesenteric arterial rings
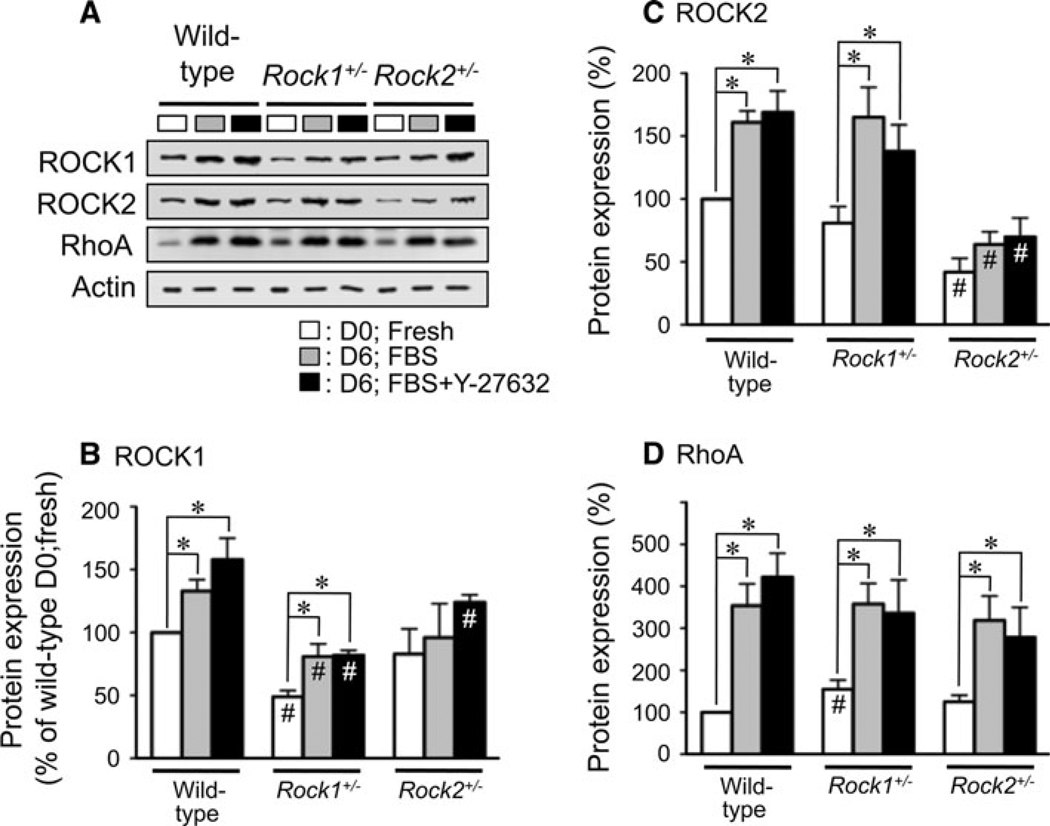
To examine which ROCK isoform is more predominantly involved and whether reduced expression of either isoform has a beneficial effect on the FBS-induced contraction, haploinsufficient Rock1+/− and Rock2+/− mouse mesenteric arterial rings were subjected to FBS-supplemented organ-culture in the presence and absence of Y-27632 (Fig. 5). In fresh mesenteric arteries, ROCK1 expression in Rock1+/− mice was significantly lower than that of D0 wild-type (49 ± 5% of D0 wild-type; n = 3; Fig. 5B), while ROCK1 in the Rock2+/− tissues was not significantly different (83 ± 20%) from that of the wild-type. ROCK2 expression in fresh arteries from Rock2+/− mice was significantly lower compared to that of the wild-type (42 ± 11%; n = 3; D0; Fig. 5C), while ROCK2 of Rock1+/− tissues was not significantly different (81 ± 7%) from that of wild-type. These changes were consistent with the previous results (Noma et al. 2008). RhoA expression in fresh arterial rings from the Rock1+/− mice was significantly higher than that of the wild-type (155 ± 22%; Fig. 5D), while RhoA of Rock2+/− mice was not (125 ± 16%).
Fig. 5.
Changes in protein expression after 6 days organ-culture and effect of 10 µM Y-27632 in wild-type, Rock1+/− and Rock2+/− mouse mesenteric arteries. A immunoblot images of ROCK1, ROCK2 and RhoA in D0 fresh (open fill), D6 organ-cultured arteries without (gray shaded) and with Y-27632 (closed fill). Total actin (pan-actin) contents under different conditions were matched. For actins, protein extracts diluted by ten-fold were loaded. B–D average expression levels of ROCK1, ROCK2 and RhoA, respectively, in fresh and organ-cultured (±Y-27632) wild-type, Rock1+/− and Rock2+/− mouse mesenteric arteries compared to that of each protein in D0 fresh wild-type artery (n = 3). * represents a significant (P < 0.05) difference from that of D0 fresh artery within each mouse type, and # from that of wild-type mice under the same conditions
After 6 days of FBS-supplemented organ-culture, RhoA expression in either wild-type, Rock1+/− or Rock2+/− mouse mesenteric artery rings was markedly up-regulated regardless of the presence of Y-27632 as compared to that of D0 (gray and black vs. white columns; Fig. 5D). ROCK1 expression on D6 was significantly elevated in the wild-type and Rock1+/− tissues regardless of Y-27632 but not in the Rock2+/− tissue as compared to that on D0 (Fig. 5B). ROCK1 expression in organ-cultured Rock1+/− arteries regardless of Y-27632 presence was smaller than that of wild-type under each condition (Fig. 5B). Unlike rabbit mesenteric artery (Fig. 2), ROCK2 expression in mice was also significantly increased in wild-type and Rock1+/− but not Rock2+/− mice after 6 days of organ-culture (Fig. 5C). ROCK2 expression in organ-cultured Rock2+/− arteries was smaller compared to that of wild-type under each condition (Fig. 5C).
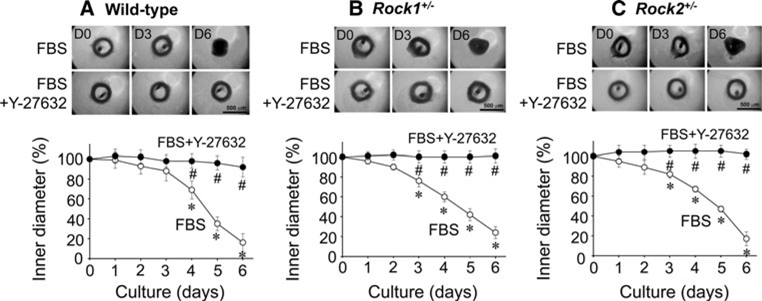
As shown in Fig. 6, FBS-supplemented organ-culture induced a gradual contraction of either Rock1+/− (Fig. 6B) or Rock2+/− mouse mesenteric artery rings (Fig. 6C). The time course of contraction in both ROCK-mutant arterial rings was similar to that of the wide-type (n = 4–5; Fig. 6A). In mesenteric arteries regardless of the mouse genotype, Y-27632 almost completely prevented the contraction and thickening of the arterial wall (filled symbols of Fig. 6).
Fig. 6.
Effects of 10 µM Y-27632 on FBS-induced morphological changes in mouse mesenteric arterial rings during 6 days organ-culture. This figure represents mouse arterial ring images (upper two panels) of D0, D3 and D6 during FBS-supplemented organ-culture with and without Y-27632, and the average time course of change in inner diameter during organ-culture in wild-type (A), Rock1+/− (B) and Rock2+/− (C) arterial rings, respectively (n = 4–5). The symbols for statistical significance are defined as described in the legend of Fig. 5
Discussion
In this study, we used organ-culture system of differentiated arterial vessels as more physiological model than cell culture to evaluate the cellular and molecular mechanism, by which chronic treatment with growth-stimulant FBS impairs contractility, morphological alteration and DNA synthesis (Lindqvist et al. 1999; Murata et al. 2005). This study presents three important findings. First, the ROCK inhibitor effectively maintains the structural and functional integrity of small arteries during chronic FBS-supplemented organ-culture. The drug protects not only endothelial cells, but also smooth muscle cells. Secondly, the FBS-induced gradual contraction of mesenteric arteries over several days is mediated mainly through the over-expression of RhoA rather than ROCK1 or ROCK2. Thirdly, removal of Y-27632 after chronic treatment leads to accelerated contraction with associated changes in MYPT1 phosphorylation, suggesting a rebound from the over-expression of RhoA that was not averted by the inhibitor.
Endothelial cells respond to various physical and chemical stimuli mainly by producing NO that regulates vascular tone and permeability (Félétou and Vanhoutte 2006). Up-regulated RhoA/ROCK signaling suppresses eNOS expression and inhibits eNOS activity, leading to impaired NO production. Thus, ROCK inhibitors have been proposed to serve as a therapeutic agent for several cardiovascular diseases (Noma et al. 2006; Shimokawa and Takeshita 2005; Loirand et al. 2006). Ozaki, Karaki and their colleagues (Ozaki and Karaki 2002; Murata et al. 2005) demonstrated that in organ-cultured arterial rings chronic treatment with dexamethasone prevented FBS-induced endothelial detachment, reduction in eNOS expression, and decreases in endothelium-dependent relaxation. Glucocorticoids, however, even at high concentrations, fail to inhibit FBS-induced arterial contraction, thickening of vessel walls, disorientation of smooth muscle cells, and reduction of high K+-induced contraction. These observations suggest that dexamethasone has a protective effect against endothelial but not smooth muscle dysfunction in organ-cultured artery. In contrast, the present study shows that chronic treatment with Y-27632 protects almost all structural and functional integrities of both endothelial and smooth muscle cells. H-1152, another ROCK inhibitor, and simvastatin also significantly produced an inhibitory effect on FBS-induced contraction, although the inhibitory magnitude of the statin was smaller than those of ROCK inhibitors. After culture in the presence of FBS for 7 days, contracted arterial rings relaxed after 1-day treatment with Y-27632, suggesting that the ROCK inhibitor not only prevents, but also restores FBS-induced vascular contraction. These results clearly suggest that ROCK inhibitors counteract the up-regulated RhoA/ROCK signaling pathway in endothelial cells to restore NO production and in smooth muscle cells to reactivate MLCP. Both effects together may efficiently relax contracted arterial rings in the FBS-supplemented organ culture for 7 days. Y-27632 has more preventive effects than the drugs that affect only endothelial cells. Therefore, patients with vascular dysfunction due to up-regulated RhoA/ROCK signaling may have a better response to agents effecting both endothelial and smooth muscle cell types compared to those for either cell type.
Chronic FBS treatment markedly enhanced RhoA expression in rabbit mesenteric arteries. Phosphorylation of MYPT1 at ROCK-specific Thr853 was significantly increased with FBS treatment and decreased with ROCK inhibitor Y-27632. Assuming that Thr853 phosphorylation level at D0 in fresh mesenteric artery at rest is similar to that (0.29 ± 0.09 mol of Pi/mol of protein (Dimopoulos et al. 2007)) reported in fresh femoral artery of the same animal, the value at D7 in the absence and presence of Y-27632 corresponds to 0.41 and 0.18 mol of Pi/mol, respectively. This suggests that ROCK activity is significantly up-regulated and down-regulated in FBS organ-culture conditions without and with Y-27632, respectively. Even though MYPT1 was significantly phosphorylated, the contraction induced by the FBS treatment was slow and weak, suggesting that the essential Ca2+/calmodulin/ MLCK/MLC-phosphorylation signaling pathway is not activated in FBS organ-culture conditions without contractile agonists. It is also possible that a partial shift from contractile smooth muscle myosin II to slow and weak nonmuscle myosin II phenotype occurs during remodeling in organ-culture (Arner et al. 2003; Owens et al. 2004). In addition, even though RhoA and ROCK are up-regulated and MYPT1 is phosphorylated in FBS organ-culture conditions, the extent of agonist-induced contraction is significantly lower after the FBS treatment (Fig. 3A). This is at least partly due to about 50% reduction in expression of MLC and CPI-17 (Fig. 2B), both of which play an essential role in the development of physiological vascular smooth muscle contraction (see Dimopoulos et al. 2007). The FBS-free organ-culture in DMEM for 7 days used as a control culture condition still induced a small but significant vasoconstriction (Fig. 1B), a significant reduction in expression of CPI-17 (Fig. 2B) in spite of no up-regulation of RhoA and ROCK, and also a diminution in agonist—but not high K+-induced contraction (Fig. 3A), suggesting that the control organ-culture condition used was still apart from that in vivo. These might be due to a lack of intra-luminal pressure with pulsatile components (Lehoux et al. 2005) and/or endogenous vasoactive components.
In rabbit mesenteric artery, interestingly, chronic treatment with Y-27632 further increased FBS-induced RhoA over-expression, also elevated expression MYPT1 and ROCK1 but not ROCK2, and prevented the reduction of MLC expression, suggesting the existence of a compensatory mechanism for the Y-27632-induced inhibition of RhoA/ROCK signaling. ROCK2 expression was not affected by either the presence or the absence of Y-27632 in rabbit artery, suggesting that the vascular abnormality induced by chronic FBS treatment under ex vivo conditions is primarily mediated through the RhoA/ROCK1, rather than RhoA/ROCK2, signaling pathway in this animal (Noma et al. 2008). In mesenteric arteries from wild-type, Rock1+/− and Rock2+/− mice, RhoA was similarly over-expressed during FBS-containing organ-culture for 6 days while ROCK1 and ROCK2 expression was significantly suppressed in Rock1+/− and Rock2+/− arteries, respectively, compared to that of wild-type. The time course of FBS-induced contraction, however, was similar among arteries from different animal species, including ROCK1 and ROCK2 heterozygous mice, suggesting that marked over-expression of RhoA, but not ROCK, is primarily responsible for the chronic FBS-induced contraction. Another possibility is that expression of either ROCK isoform to 50% of the wild-type levels is sufficient to play a pathophysiological role in FBS-induced contraction when RhoA is up-regulated. Y-27632 and H-1152 also potently inhibit PRK2 (PKN2) (Bain et al. 2007), which is also downstream of RhoA (Mukai 2003). The possibility that PRK2 is involved in the FBS-induced vascular abnormality remains to be investigated.
After 7 days of organ-culture in the presence of FBS, an additional 1-day treatment with Y-27632, even in the continuous presence of FBS, reduced the increased MYPT1 Thr853 phosphorylation and relaxed contracted arterial rings. This result agrees with the hypothesis that ROCK inhibitors can both prevent and rescue vascular dysfunction through production of NO in endothelial cells and restoration of MLCP activity in smooth muscle cells (Noma et al. 2006; Shimokawa and Takeshita 2005; Loirand et al. 2006). However, inhibition of ROCK with Y-27632 did not restore the artery to its original condition with regard to protein expression level. In rabbit mesenteric artery, chronic treatment with Y-27632 and FBS increased expression levels of RhoA, ROCK1 and MYPT1 to higher levels than those of FBS alone, and prevented the FBS-induced reduction of MLC expression. As a result, the sudden withdrawal of Y-27632 from the culture medium induced a contraction of the artery within a day and increased MYPT1 phosphorylation by 3.3-fold compared to that in the presence of Y-27632, demonstrating a rebound of ROCK activity after withdrawal of the inhibitor. In mouse mesenteric artery, however, treatment with Y-27632 and FBS did not significantly increase RhoA and ROCK1/2 expression compared to that in the presence of FBS alone, suggesting little rebound of ROCK activity in mouse artery. The difference in effect of Y-27632 on protein expression between rabbit and mouse mesenteric arteries is not clear but may be related to species difference. Although no rebound of blood pressure was observed after withdrawal of a novel ROCK inhibitor in spontaneous hypertensive rat models (Löhn et al. 2009), such a rebound effect of drugs should be carefully investigated in humans.
In conclusion, the findings of the present study provide structural and functional evidence that inactivation of RhoA/ROCK signaling prevents FBS-supplemented organ-culture-induced arterial damage against both endothelial and smooth muscle dysfunctions, and thus effectively maintains the relaxed state, agonist-induced contractility and endothelium-dependent relaxation. Although the inhibition of RhoA/ROCK function could offer a therapeutic means for RhoA/ROCK-mediated vascular diseases, withdrawal of the drug should be applied with caution.
Acknowledgments
We thank Drs. James Sherley and Albert Wang of the BBRI for their comments on the manuscript. This work was supported by National Institute of Health grant R01 HL070881 to TK and HL052233, HL080187, and DK085006 to JKL.
Abbreviations
- ACh
Acetylcholine
- eNOS
Endothelial nitric oxide synthase
- ET-1
Endothelin-1
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- MLC
Myosin light chain
- MLCK
MLC kinase
- MLCP
MLC phosphatase
- MYPT1
Myosin phosphatase targeting subunit 1
- PDBu
Phorbol 12,13-dibutyrate
- PE
Phenylephrine
- ROCK
Rho-associated kinase (Rho-kinase)
- SNP
Sodium nitroprusside
Contributor Information
Yang Hoon Huh, Boston Biomedical Research Institute, 64 Grove St, Watertown, MA 02472, USA.
Qian Zhou, Brigham & Women’s Hospital, 65 Landsdowne Street, Cambridge, MA 02139, USA.
James K. Liao, Brigham & Women’s Hospital, 65 Landsdowne Street, Cambridge, MA 02139, USA
Toshio Kitazawa, Email: Kitazawa@bbri.org, Boston Biomedical Research Institute, 64 Grove St, Watertown, MA 02472, USA.
References
- Arner A, Lofgren M, Morano I. Smooth, slow and smart muscle motors. J Muscle Res Cell Motil. 2003;24:165–173. doi: 10.1023/a:1026001513928. [DOI] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- De Mey JGR, Uitendaal MP, Boonen HCM, Vrijdag MJJF, Daemen MJAP, Struyker-Boudier HAJ. Acute and long-term effects of tissue culture on contractile reactivity in renal arteries of the rat. Circ Res. 1989;65:1125–1135. doi: 10.1161/01.res.65.4.1125. [DOI] [PubMed] [Google Scholar]
- Dimopoulos G, Semba S, Kitazawa K, Eto M, Kitazawa T. Ca2+-dependant rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ Res. 2007;100:121–129. doi: 10.1161/01.RES.0000253902.90489.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelial dysfunction: a multi-faceted disorder. Am J Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res. 2007;100:607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol. 2003;546:879–889. doi: 10.1113/jphysiol.2002.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T, Semba S, Huh YH, Kitazawa K, Eto M. Nitric oxide-induced biphasic mechanism of vascular relaxation via dephosphorylation of CPI-17 and MYPT1. J Physiol. 2009;587:3587–3603. doi: 10.1113/jphysiol.2009.172189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehoux S, Esposito B, Merval R, Tedgui A. Differential regulation of vascular focal adhesion kinase by steady stretch and pulsatility. Circulation. 2005;111:643–649. doi: 10.1161/01.CIR.0000154548.16191.2F. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Nordström I, Malmqvist U, Nordenfelt P, Hellstrand P. Long-term effects of Ca2+ on structure and contractility of vascular smooth muscle. Am J Physiol. 1999;277:C64–C73. doi: 10.1152/ajpcell.1999.277.1.C64. [DOI] [PubMed] [Google Scholar]
- Löhn M, Plettenburg O, Ivashchenko Y, Kannt A, Hofmeister A, Kadereit D, Schaefer M, Linz W, Kohlmann M, Herbert JM, Janiac P, O’Connor SE, Ruetten H. Pharmacological characterization of SAR407899, a novel rho-kinase inhibitor. Hypertension. 2009;54:676–683. doi: 10.1161/HYPERTENSIONAHA.109.134353. [DOI] [PubMed] [Google Scholar]
- Loirand G, Guérin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- Merrilees MJ, Scott L. Organ culture of rat carotid artery: maintenance of morphological characteristics and of pattern of matrix synthesis. In Vitro. 1982;18:900–910. doi: 10.1007/BF02796346. [DOI] [PubMed] [Google Scholar]
- Mukai H. The structure and function of PKN, a protein kinase having a catalytic domain homologous to that of PKC. J Biochem. 2003;133:17–27. doi: 10.1093/jb/mvg019. [DOI] [PubMed] [Google Scholar]
- Murata T, Suzuki N, Yamawaki H, Sato K, Hori M, Karaki H, Ozaki H. Dexamethasone prevents impairment of endothelium-dependent relaxation in arteries cultured with fetal bovine serum. Eur J Pharmacol. 2005;515:134–141. doi: 10.1016/j.ejphar.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol. 2006;290:C661–C668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu P-Y, Wang H, Ahl D, Sawada N, Okamoto R, Okamoto R, Hiroi Y, Shimizu K, Luscinskas FW, Sun J, Liao JK. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest. 2008;118:1632–1644. doi: 10.1172/JCI29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Karaki H. Organ culture as a useful method for studying the biology of blood vessels and other smooth muscle tissues. Jpn J Pharmacol. 2002;89:93–100. doi: 10.1254/jjp.89.93. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Suzuki M, Hidaka H. The novel and specific Rho-kinase inhibitor (S)-(+)-2-methyl-1-[(4-methyl-5-isoquino-line)sulfonyl]-homopiperazine as a probing molecule for Rho-kinase-involved pathway. Pharmacol Ther. 2002;93:225–232. doi: 10.1016/s0163-7258(02)00191-2. [DOI] [PubMed] [Google Scholar]
- Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997;99:2814–2816. doi: 10.1172/JCI119472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa H, Rashid M. Development of Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol Sci. 2007;28:296–302. doi: 10.1016/j.tips.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25:1767–1775. doi: 10.1161/01.ATV.0000176193.83629.c8. [DOI] [PubMed] [Google Scholar]
- Thorne GD, Paul RJ. Effects of organ culture on arterial gene expression and hypoxic relaxation: role of the ryanodine receptor. Am J Physiol. 2003;284:C999–C1005. doi: 10.1152/ajpcell.00158.2002. [DOI] [PubMed] [Google Scholar]
- Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23:5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Velasco G, Armstrong C, Morrice N, Frame S, Cohen P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr850 induces its dissociation from myosin. FEBS Lett. 2002;527:101–104. doi: 10.1016/s0014-5793(02)03175-7. [DOI] [PubMed] [Google Scholar]
- Woodsome TP, Polzin A, Kitazawa K, Eto M, Kitazawa T. Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J Cell Sci. 2006;119:1769–1780. doi: 10.1242/jcs.02805. [DOI] [PubMed] [Google Scholar]