Abstract
In recent years, a diverse array of unexpected neurobiological functions have been uncovered for the major cell cycle-regulated ubiquitin ligase, the anaphase-promoting complex (APC). Functions of the APC in the nervous system range from orchestrating neuronal morphogenesis and synapse development to the regulation of neuronal differentiation, survival, and metabolism. The APC acts together with the coactivating proteins Cdh1 and Cdc20 in neural cells to target specific substrates for ubiquitination and consequent degradation by the proteasome. As we continue to unravel APC functions and mechanisms in neurobiology, these studies should advance our understanding of the molecular mechanisms of neuronal connectivity, with important implications for the study of brain development and disease.
1. INTRODUCTION
The ubiquitin-proteasome system (UPS) plays a critical role in the regulation of fundamental aspects of neuronal development from the morphogenesis of axons and dendrites to the formation and refinement of synapses [1–3]. Ubiquitination is mediated by a series of biochemical steps catalyzed by an E1 ubiquitin-activating enzyme, E2 ubiquitin-conjugating enzymes, and E3 ubiquitin ligases, the latter providing substrate specificity. Among E3 ubiquitin ligases in the nervous system, the anaphase-promoting complex (APC) has emerged as a key regulator of diverse developmental processes in neurons.
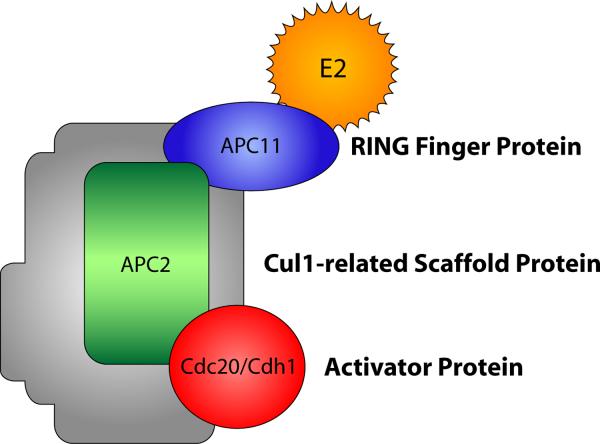
Interest in the neurobiological functions of the APC was stimulated by the initial observation that Cdh1-APC controls axon growth and patterning [4]. Subsequent studies have revealed additional functions for Cdh1-APC as well as the related ubiquitin ligase Cdc20-APC in neuronal development [3, 5–6]. The APC, independently discovered by two groups [7–8], is a large, 1.5-MDa protein complex that consists of at least 12 subunits, including the RING finger protein APC11 and the Cul1-related scaffold protein APC2, which together form the catalytic E3 ubiquitin ligase core (Fig. 1) [9–10]. Interestingly, in contrast to original hypotheses that E3 ubiquitin ligase activity of the APC occurs within the inner channel of the complex, ubiquitination appears to occur on the outside of the complex [11]. The critical APC coactivators, Cdh1 and Cdc20, stimulate the ubiquitin ligase activity of the APC and confer substrate specificity via recognition of a destruction- or D-box motif (RxxLxxxxN/D/E) in substrate proteins [9–10, 12]. Cdh1-APC can also target substrates containing KEN box (KENxxxN), A-box, or CRY box motifs [13–15]. Remarkably, despite advances in our understanding of how APC substrates are targeted and processed, the precise mechanistic basis for APC-induced ubiquitination of substrates remains poorly understood.
Figure 1.
Composition of the APC. This schematic shows the subunits of the APC with parallels to the subunit structure of the related SCF ubiquitin ligase family [76–77]. Cdh1 and Cdc20 are coactivator proteins that determine substrate specificity of the APC. The shaded area represents other subunits of the APC, including APC1, Cdc27, APC4, APC5, Cdc16, APC7, Cdc23, DOC1, Cdc26, and SWM/APC13 [10].
The functions and regulation of the APC during the cell cycle in proliferating cells have been reviewed elsewhere [9, 14, 16–19]. In this article, we will focus our attention on studies of Cdh1-APC and Cdc20-APC and their functions in the nervous system.
2. THE APC ORCHESTRATES AXON AND DENDRITE MORPHOGENESIS
Nearly a decade after its identification, unique functions for the APC in neuronal morphogenesis were uncovered [3, 5–6]. Prior to these studies, there was evidence that Cdh1 and core APC subunits were expressed in the mammalian brain [20], and later it was revealed that Cdc20 is also expressed in postmitotic neurons during development [21].
2.1. Cdh1-APC ubiquitin signaling in the nucleus controls axon growth
During the past few years, a substantial collection of studies has established a critical role for Cdh1-APC ubiquitin signaling in the control of axon growth in the mammalian brain [4–6, 22–26]. Using granule neurons of the rodent cerebellar cortex as a model system for studies of axon morphogenesis, Konishi et al. discovered that Cdh1 RNAi specifically triggers the growth of axons but not dendrites (Fig. 2) [4]. The effect of Cdh1 RNAi on axon growth is reversed by coexpression of an RNAi-resistant Cdh1 rescue construct, confirming that Cdh1 RNAi-induced axon growth is due to specific knockdown of Cdh1 rather than off-targets [4, 26]. Consistent with these findings, expression of the APC inhibitor Emi1 or a dominant-interfering form of the core APC subunit APC11 increases axon growth in granule neurons [4]. Together, these findings suggest that the ubiquitin ligase activity of Cdh1-APC inhibits axon growth. Knockdown analyses in postnatal rap pups in vivo revealed an important function for Cdh1-APC in the control of parallel fiber axon patterning in the cerebellar cortex. Cdh1 knockdown leads to parallel fiber axon defasciculation and inappropriate growth in the external granule layer (EGL) [4]. Thus, Cdh1 controls the growth and patterning of axons in the cerebellar cortex.
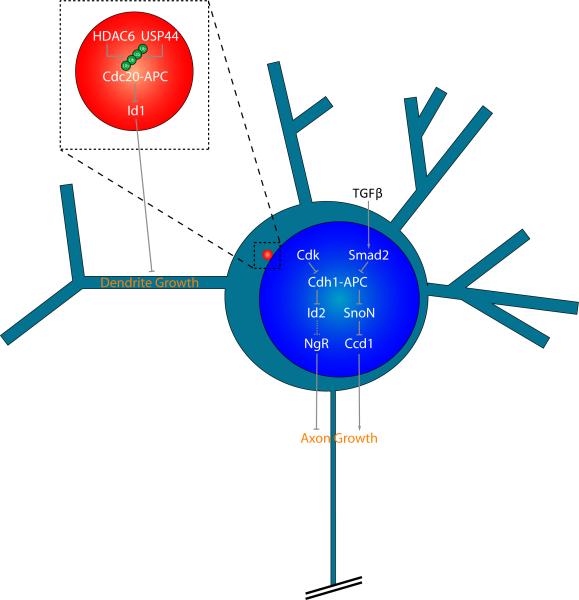
Figure 2.
Cdh1-APC and Cdc20-APC control axon and dendrite morphogenesis from distinct subcellular locales. Neuronal Cdh1-APC functions in the nucleus to regulate axon growth and patterning. Cdh1-APC targets the transcriptional regulators SnoN and Id2 for degradation, thereby restricting axon growth. SnoN and Id2 control transcription of target genes, including Ccd1 and Nogo receptor (NgR) respectively, which are localized at the axon growth cone. Upstream of Cdh1-APC, the major regulators TGFβ/SMAD2 and Cdk converge to modulate the ubiquitin ligase activity of nuclear Cdh1-APC. Activation of TGFβ stimulates the interaction of Smad2 with Cdh1 and induces Cdh1-APC-dependent degradation of SnoN. In contrast, Cdk inhibits Cdh1-APC by promoting Cdh1 phosphorylation at nine conserved sites, leading to accumulation of inactive Cdh1 in the cytoplasm. Unlike the nuclear Cdh1-APC pathway, Cdc20-APC operates at the centrosome to drive dendrite growth and elaboration. Cdc20-APC promotes the ubiquitination and degradation of the centrosomally localized protein Id1 in neurons, which inhibits dendrite growth. Polyubiquitination of Cdc20 increases the ubiquitin ligase activity of Cdc20-APC. HDAC6 stabilizes Cdc20 polyubquitination and promotes dendrite growth, while USP44 deubiqutinates Cdc20 and restrains dendrite growth, suggesting that polyubquitination of Cdc20 per se controls neuronal Cdc20-APC activity.
Additional studies have examined the signaling pathways regulating neuronal Cdh1-APC activity as well as clarified the mechanisms by which Cdh1-APC controls axon growth [22–26]. A first step in elucidating the molecular basis of Cdh1-APC function in neurons was characterizing its subcellular site of action. Although Cdh1-APC is present in both the cytoplasm and nucleus in neurons, Cdh1-APC localization in the nucleus is required for the control of axon morphogenesis [26]. Phosphorylation-dependent changes in Cdh1 subcellular localization have important consequences on Cdh1-APC function in the regulation of axon growth [22]. In particular, phosphorylation of Cdh1 at nine conserved Cdk sites promotes the accumulation of an inactive form of Cdh1 in the cytoplasm, which does not associate with the core APC subunit Cdc27 [22]. Accordingly, the inactive phosphorylated form of Cdh1 is unable to inhibit axon growth. Yet, many questions concerning the significance of Cdh1's subcellular localization in neurons remain unanswered. It remains to be determined whether Cdh1-APC at specific locales within the cytoplasm, such as at specific organelles or at the axon growth cone, might serve specific functions in axon morphogenesis or other aspects of neuronal development. In addition, the site of Cdh1-APC action in the control of axon patterning remains unknown.
An understanding of the extrinsic cues that control neuronal Cdh1-APC activity has been jump-started by studies of TGFβ-Smad signaling (Fig. 2). TGFβ promotes translocation of Smad2 or Smad3 to the nucleus, where Smad2/3 forms a complex with Cdh1 and thereby stimulates the ubiquitin ligase activity of Cdh1-APC [27–28]. Accordingly, inhibition of TGFβ/Smad2 signaling promotes axon growth, closely mimicking the effect of Cdh1 knockdown [25]. Inhibition of TGBβ signaling in neurons by the small molecule SB431542 induces the ability of axons to grow longer, even on myelin. These results suggest the exciting possibility that inhibition of TGFβ signaling or Cdh1-APC may provide a means to promote axon regeneration in the injured nervous system.
The fact that Cdh1-APC operates in the nucleus in neurons has also been essential for uncovering downstream mechanisms by which Cdh1-APC limits axon growth [26]. Because transcription factors represent critical nodes in the regulation of axon morphogenesis [6, 29–33], transcription factors are good candidates as effectors of Cdh1-APC in the control of axon growth. Stegmuller et al. identified SnoN as a key substrate of neuronal Cdh1-APC (Fig. 2) [26]. SnoN is expressed during a spatiotemporal window that coincides with axon formation and growth [34]. SnoN knockdown in granule and cortical neurons profoundly reduces axon length [26]. Additional time lapse analyses of SnoN-depleted neurons revealed that SnoN actively promotes axon elongation and growth. Knockdown studies in postnatal rat pups in vivo have uncovered an essential role for SnoN in axon development. Compared to control animals, SnoN knockdown animals have a dramatic reduction in the percentage of internal granule layer (IGL) neurons associated with parallel fiber axons in the molecular layer, demonstrating a requirement for SnoN in the growth and stabilization of granule neuron axons in vivo. Complementary biochemical analyses revealed that Cdh1 forms a complex with SnoN and stimulates the ubiquitination and degradation of SnoN in neurons. Correspondingly, SnoN knockdown suppresses Cdh1 RNAi-dependent axon growth [26], suggesting that Cdh1-APC and SnoN act together in a nuclear ubiquitin signaling pathway to orchestrate axon growth in the mammalian brain.
SnoN has been traditionally considered a corepressor of transcription [35–36]. However, in neurons, gene expression changes upon SnoN knockdown suggest an important transcriptional activating role for SnoN [23]. SnoN interacts with the transcriptional coactivator p300, and p300 is required for SnoN-dependent axon growth [23]. These results support the concept that SnoN is a versatile regulator of gene expression with activating or repressive functions that likely depend on the specific target gene or cellular context [36–38]. Ikeuchi et al. identified Ccd1 as a target gene of SnoN in neurons [23]. The levels of Ccd1 mRNA and protein are reduced upon SnoN knockdown, and SnoN is required for transcriptional activation of the Ccd1 promoter. Knockdown of Ccd1 reduces axon growth in primary granule neurons and in the cerebellar cortex in postnatal rat pups in vivo, indicating that Ccd1 is required for axon patterning in the mammalian brain. Ccd1 localizes to the actin cytoskeleton of axon terminals where it binds to mixed lineage kinase 3 (MLK3), an activator of JNK. JNK has been implicated in axon growth [39], raising the possibility that Ccd1 activation of JNK via MLK3 mediates the Ccd1 axon phenotype. Consistent with this hypothesis, Ccd1 expression stimulates JNK phosphorylation and Ccd1 knockdown reduces endogenous JNK phosphorylation. Importantly, Ccd1 knockdown suppresses the ability of SnoN to promote axon growth [23], suggesting that Ccd1 links nuclear Cdh1-APC/SnoN ubiquitin signaling and local regulation of the axon growth cone.
The transcriptional regulatory protein inhibitor of DNA binding 2 (Id2) has also been identified as an important target of neuronal Cdh1-APC in the control of axon growth (Fig. 2) [24]. Mass spectrometry analyses revealed that Id2 associates with APC1, APC5, and APC8. Similar to SnoN, Id2 has a conserved D-box, which is required for Cdh1-APC binding, ubiquitination, and degradation. Consistent with its role as a Cdh1-APC substrate, Cdh1 knockdown increases Id2 protein levels in neurons. An Id2 mutant protein in which the D-box was mutated (DBM) promotes axon growth in granule neurons [24], phenocopying the effect of Cdh1 knockdown. Id proteins inhibit the function of basic helix-loop-helix (bHLH) transcription factors [40]. Accordingly, the Id2-regulated bHLH transcription factor E47 suppresses axon growth and overrides the effect of Cdh1 knockdown or Id2-DBM expression on axon growth [24]. Thus far, SnoN and Id2 represent two critical substrates of neuronal Cdh1-APC in the regulation of axon growth. Whether additional Cdh1-APC substrates contribute to the control of axon growth remains unknown. In addition, substrates that operate downstream of Cdh1-APC in the regulation of axon patterning per se in the cerebellar cortex in vivo remain to be identified.
2.2. Cdc20-APC signaling at the centrosome drives dendrite growth and elaboration
The unanticipated role of Cdh1-APC in axon morphogenesis in neurons raised the intriguing possibility that the related APC coactivator Cdc20 may also regulate neuronal morphogenesis. Cdc20 is expressed in postmitotic neurons during development and Cdc20 levels increase with maturation [21, 41]. Kim et al. discovered that knockdown of Cdc20 impairs dendrite growth and elaboration in primary granule neurons and in the cerebellar cortex in postnatal rat pups in vivo, with little or no effect on axon growth, suggesting that Cdc20-APC and Cdh1-APC regulate distinct aspects of neuronal morphogenesis.
Cdc20-APC operates at a distinct subcellular locale from Cdh1-APC to control dendrite growth and elaboration (Fig. 2) [21]. Immunocytochemical and biochemical analyses revealed that Cdc20 is enriched in the cytoplasm and in particular at the centrosome in neurons. Structure-function analyses suggest Cdc20 contains a small fifteen amino acid centrosomal localization sequence (CLS), which is critical for Cdc20 localization at the centrosome. The CLS is required for Cdc20-APC-dependent dendrite growth and arborization. In addition, forcibly localizing Cdc20 to the centrosome, but not the nucleus, endows Cdc20 with the ability to drive dendrite growth and elaboration. Together, these results suggest that Cdc20-APC promotes dendrite development through ubiquitin signaling at the centrosome in neurons.
Investigation of the mechanisms regulating centrosomal Cdc20-APC in neurons led to consideration of the protein histone deacetylase HDAC6 [21]. In epithelial cells, HDAC6 operates at the basal body to regulate ciliary [42]. In neurons, Kim et al. found that HDAC6 binds to Cdc20 and stimulates Cdc20-APC-dependent dendrite growth and elaboration [21]. HDAC6 does not appear to influence the acetylation status of Cdc20 [21]. Instead, through its ubiquitin-binding ZnF UBP domain, HDAC6 promotes Cdc20 polyubiquitination and thereby induces the ubiquitin ligase activity of Cdc20-APC [21]. Polyubiquitination of Cdc20 increases Cdc20-APC activity [43–44]. Knockdown of the Cdc20 deubiquitinase USP44 in neurons increases Cdc20 polyubiquitination and correspondingly augments dendrite growth and arborization, suggesting that the dynamic regulation of Cdc20 polyubiquitination is critical for controlling dendrite morphogenesis (Fig. 2).
Because the ubiquitin ligase activity of Cdc20-APC is critical for its function, Kim et al. sought to identify a physiological centrosomal substrate of Cdc20-APC that inhibits dendrite elaboration. Using a candidate based approach, the authors identified Id1 as a novel substrate of neuronal Cdc20-APC (Fig. 2) [21]. Cdc20-APC binds to Id1 and stimulates its ubiquitination and degradation. Accordingly, Id1 knockdown stimulates dendrite growth and operates downstream of Cdc20 in the control of dendrite development [21]. Although Id1 is localized at the centrosome in neurons, Id1 also has established functions in the nucleus where it inhibits bHLH-dependent transcription [40]. Thus, it will be important to explore whether Id1 controls dendrite morphogenesis by regulating transcription or by acting locally at the centrosome.
3. ROLE OF THE APC IN SYNAPTIC DEVELOPMENT
Beyond its important roles in neuronal morphogenesis, the APC has also been linked to developmental processes intimately associated with neuronal connectivity. Recent studies suggest that the APC may be involved in distinct aspects of synapse development and remodeling, although a more thorough mechanistic understanding of the APC's function in these processes is still needed.
3.1. Role of Cdh1-APC in synaptic differentiation and transmission
An essential function for Cdh1-APC in synapse development was first identified in Drosophila and Caenorhabditis elegans (Fig. 3A) [45–46]. In Drosophila, Core APC components are expressed at the neuromuscular junction (NMJ) in both motor neurons and their effector muscle cells [46]. van Roessel et al. found that APC2 loss-of-function mutations in motor neurons lead to an increase in presynaptic bouton number at the NMJ in flies [46]. Motor neurons in APC2 mutant flies have increased levels of the protein Liprinα, and APC2 loss-of-function mutations in Liprinα mutants fail to augment presynaptic bouton number [46], suggesting that Liprinα operates downstream of APC2 in the regulation of synapses. Because Cdh1, APC2, and other core APC subunits are expressed at presynaptic sites at the NMJ, the authors propose that Cdh1-APC restrains presynaptic bouton number in a cell-autonomous manner [46]. In the future, it will be interesting to determine whether the related ubiquitin ligase Cdc20-APC has roles in the regulation of synaptic differentiation and transmission at the Drosophila NMJ.
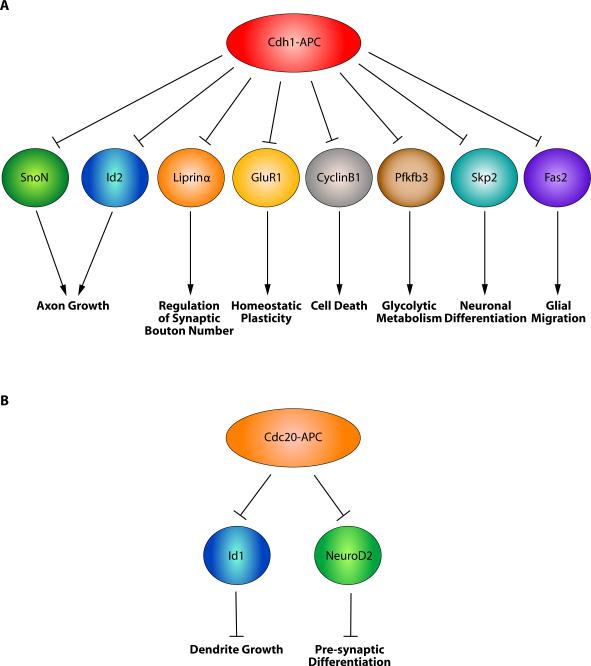
Figure 3.
Functions of the APC in synaptic development. (A) Cdh1-APC restrains presynaptic bouton number by regulating Liprinα in invertebrate model systems. Postsynaptically, Cdh1-APC controls glutamate receptor abundance in flies, worms, and mammals. A precise mechanism for APC-mediated down-regulation of glutamate receptors remains to be elucidated. (B) Cdc20-APC controls pre-synaptic differentiation in the mammalian brain. In early stages of axon growth and development, the transcription factor NeuroD2 promotes the expression of the target gene Cplx2 to suppress presynaptic differentiation. With neuronal maturation, Cdc20 expression is increased and Cdc20-APC promotes the degradation of NeuroD2, thereby reducing Cplx2 expression and stimulating the generation of functional presynaptic sites.
Additional studies have also identified a role for the APC in postsynaptic function in invertebrate and mammalian neurons. In C. elegans ventral nerve cord neurons, temperature-sensitive mutations in core APC subunits increase the abundance of the AMPA receptor subunit GLR-1 (Fig. 3A) [45]. Expression of APC subunits from the glr-1 promoter restores the width of GLR-1 synaptic puncta in APC mutant worms [45], suggesting that Cdh1-APC controls AMPA receptor levels in a cell-autonomous manner. Mutations in clathrin-mediated endocytosis block the effects of APC mutations on GLR-1 abundance, indicating that Cdh1-APC may be regulating some aspect of GLR-1 recycling in this system. Since GLR-1 lacks an obvious APC recognition motif, and GLR-1 ubiquitination is unchanged in APC mutant worms compared to wildtype worms, it is probable that Cdh1-APC indirectly regulates the abundance of GLR-1. In mammalian neurons, EphA4-mediated degradation of GluR1 has been linked to Cdh1-APC [47]. Elevated synaptic activity stimulates EphA4, which induces the polyubiquitination and degradation of GluR1 in cortical neurons. EphA4 interacts with the core APC subunit AP2 and Cdh1, and presumably activates Cdh1-APC-dependent degradation of GluR1. Consistent with this model, knockdown of either EphA4 or Cdh1 prevents the reduction in mEPSC amplitude in neurons that is associated with chronic elevated activity [47], suggesting that the EphA4-regulated Cdh1-APC signaling may regulate homeostatic plasticity. In the future, it will be interesting to determine whether Cdh1-APC signaling pathways that regulate synapse development are evolutionarily conserved across model systems, from nematodes and flies to mammals.
To probe the role of Cdh1-APC in synaptic development, other studies have taken advantage of mouse genetics to characterize Cdh1-APC function in synaptic plasticity as well as learning and memory. Using Cdh1 heterozygous mice, Li et al. found that there are no gross morphological changes in the hippocampus or other brain regions in these animals [48]. However, Cdh1 heterozygous mice have deficits in late-phase long-term potentiation (L-LTP) induced by multiple spaced trains of high-frequency stimulation. These results suggest that Cdh1-APC may play a critical role in the induction of L-LTP, presumably by inducing the ubiquitination and degradation of yet to be identified proteins that negatively regulate L-LTP. In other studies, Kuczera and colleagues found that conditional knockout of the core Cul1-related subunit APC2 in excitatory neurons of the adult forebrain impairs spatial memory in Morris water maze tests and abrogates the ability to extinguish contextual fear conditioning, consistent with a defect in hippocampal function [49]. While these results suggest that the APC may contribute to hippocampus-dependent memory and learning, the role of Cdh1-APC in synapse function and plasticity of the brain remains to be fully elucidated. In particular, the underlying molecular mechanisms of potential roles of Cdh1-APC in adaptive responses, including learning and memory, remain unknown.
3.2. Cdc20-APC ubiquitin signaling controls presynaptic differentiation
Recent studies have implicated the ubiquitin ligase Cdc20-APC in the regulation of synapse development in the mammalian brain. Yang et al. developed assays in cerebellar granule neurons that allow the study of cell-intrinsic mechanisms regulating presynaptic differentiation [50–51]. Using this system, the authors discovered that knockdown of Cdc20 or core APC components profoundly reduces the number of presynaptic sites, based on both the density of synaptic vesicle and active zone proteins (Fig. 3B). Furthermore, Cdc20 RNAi reduces the number of synapsin/PSD-95 co-clusters as well as the sites of FM4–64 uptake, suggesting that Cdc20-APC drives the differentiation of functional presynaptic sites at synapse in granule neurons. Importantly, Cdc20 knockdown in rat pups in vivo in the cerebellar cortex also impairs the differentiation of presynaptic sites.
The authors next identified the brain-enriched bHLH transcription factor NeuroD2 as a critical physiological substrate of Cdc20-APC in the regulation of presynaptic differentiation (Fig. 3B) [51]. NeuroD2 contains a D-box motif, which is required for its association with Cdc20. In addition, knockdown of endogenous Cdc20 increases NeuroD2 levels in primary granule neurons, suggesting that Cdc20-APC promotes the degradation of NeuroD2. Consistent with these data, NeuroD2 is polyubiquitinated, and the proteosomal inhibitor MG132 increases NeuroD2 levels in neurons. Correspondingly, depletion of NeuroD2 increases the density of presynaptic boutons in primary granule neurons and in the cerebellar cortex in postnatal rat pups in vivo. In epistasis analyses, NeuroD2 knockdown suppresses the effect of Cdc20 knockdown on presynaptic differentiation, suggesting that Cdc20-APC controls presynaptic differentiation in a NeuroD2-dependent manner. Collectively, these findings suggest that Cdc20-APC triggers the degradation of NeuroD2 to stimulate presynaptic differentiation.
Among the possible NeuroD2 targets, Complexin II (Cplx2) was considered as a potential physiologically relevant target in the control of presynaptic differentiation because of Cplx2's role in the control of synaptic vesicle membrane fusion in mammalian neurons and presynaptic number in Drosophila [52–53]. The authors found that expression of Cplx2 inhibits the increase in presynaptic density induced by NeuroD2 knockdown [51]. In addition, the number of functional presynaptic sites in primary granule neurons and in the cerebellar cortex in postnatal rat pups in vivo is increased upon Cplx2 knockdown. Importantly, Cplx2 knockdown also reverses the reduction in presynaptic bouton number upon Cdc20 knockdown, suggesting that Cplx2 operates downstream of Cdc20-APC signaling (Fig. 3B). Together, these results establish a novel Cdc20-APC signaling pathway that drives presynaptic differentiation in the mammalian nervous system. In future studies, it will be important to identify additional Cdc20-APC substrates as well as additional NeuroD2 targets that control presynaptic differentiation. In addition, how Cdc20-APC function in presynaptic development is regulated remains to be elucidated.
4. APC FUNCTION IN NEURONAL SURVIVAL
Several studies have reported links between the cell cycle and neuronal viability, with the overarching idea that expression and activation of cell cycle proteins associated with re-entry into the cell cycle triggers cell death in postmitotic neurons [54–56]. Since Cdh1-APC is critical for G1 maintenance [9–10], inhibition of Cdh1-APC activity might be predicted to initiate cell cycle re-entry and consequent cell death in postmitotic neurons.
Using a knockdown approach, Almeida et al. reported that Cdh1 depletion in mature primary cortical neurons triggers apoptotic cell death [57]. To further characterize the underlying mechanism, the authors examined Cyclin B1, a major substrate of Cdh1-APC in cycling cells [10]. Knockdown of Cyclin B1 suppresses Cdh1 RNAi-induced cell death, suggesting that Cdh1 prevents neuronal cell death by keeping Cyclin B1 levels low [57]. Interestingly, Cdh1 knockdown neurons appear to incorporate bromo-deoxy-uridine, a marker of active DNA synthesis and S-phase. Future studies should explore the interplay between Cdh1-APC activity, neuronal activity, and apoptosis. Notably, Cdk1, a negative regulator of Cdh1-APC activity, has been linked to activity deprivation-induced cell death [58]. Cdk1 induces neuronal apoptosis through the phosphorylation and activation of the transcription factor FOXO1 and the pro-apoptotic protein Bad [58–61]. These findings raise the intriguing possibility that in addition to FOXO1 and Bad, Cdk1 may phosphorylate Cdh1 and thereby inhibit its function, leading to neuronal apoptosis.
In primary cortical neurons, Cdh1-APC may also protect neurons from amyloid-induced apoptosis [57]. Interestingly, Cyclin B1 and Cdk1 levels are elevated in neurons from Alzheimer's disease patients [62–63]. Expression of Cdh1 inhibits β-amyloid-induced apoptosis, raising the possibility that accumulation of β-amyloid might be associated with down-regulation of Cdh1-APC activity in pathological conditions such as Alzheimer's disease [57]. Because several other neurodegenerative diseases have been associated with increased levels of Cyclin B1 [64–65], it is tempting to speculate that altered activity of Cdh1-APC may be involved in the etiology of these disorders.
To explore the role of Cdh1-APC in the regulation of stress-induced neuronal cell death, Maestre et al. used stimulation of NMDA-Rs to induce excitotoxicity in cortical neurons [66]. The authors found that glutamate may induce Cyclin B1 accumulation in the nucleus of apoptotic cells. Knockdown of Cyclin B1 appears to suppress the effect of glutamate- or NMDA-induced cell death, suggesting that excitotoxicity in postmitotic neurons depends on the nuclear accumulation of Cyclin B1. The authors also reported that inhibition of Cdh1 by RNAi or upon overexpression of Emi1 increases glutamate- or NMDA-induced apoptosis [66]. Cyclin B1 knockdown rescues the increase in apoptosis, suggesting that Cdh1 may reduce NMDAR-mediated excitotoxicity by preventing the accumulation of Cyclin B1.
5. ROLE OF THE APC IN NEURONAL DIFFERENTIATION
Because cell cycle regulators play a critical role in cellular differentiation, a role for the APC in differentiation might be anticipated. Exposure of neural stem cells (NSC) to the neurogenic substance retinoic acid induces the expression of Cdh1, leading to down-regulation of Id2 levels [67], raising the possibility that Cdh1-APC promotes cell cycle arrest and NSC differentiation into neurons. Retinoic acid induces differentiation and cell cycle exit of neuroblastoma cells [68], and concomitantly reduces markers of proliferation such as PCNA and pRb, while promoting nuclear accumulation of the Cdk inhibitor p27. Previous studies have shown that retinoic acid stabilizes p27 by inducing the degradation of the ubiquitin ligase SCF activator, Skp2, which ubiquitinates p27 [69–71]. Consistent with these observations, retinoic acid stimulates the degradation of Skp2 in parallel with the increase in p27 levels [68]. Importantly, Skp2 degradation is mediated by Cdh1-APC and occurs in a D-box-dependent manner in these cells [68]. In addition, retinoic acid treatment destabilizes Rae1, a nucleocytoplasmic inhibitor of Cdh1-APC, leading to stimulation of Cdh1-APC ubiquitin ligase activity, the degradation of Skp2, and the accumulation of p27, which culminates in cell cycle arrest and differentiation [68]. Analogous studies in PC12 cells suggest that differentiation induced by nerve growth factor stimulates the ubiquitin ligase activity of Cdh1-APC and the degradation of Skp2, leading to neurite outgrowth [72]. In the future, it will be interesting to determine whether identified targets of neuronal Cdh1-APC such as Id2 and SnoN influence the cellular transition from precursors to neurons as well as explore the roles of Rae1 and Skp2 in axon growth and patterning in postmitotic neurons.
6. THE APC MAINTAINS NEURONS IN A LOW GLYCOLYTIC STATE
Neurons have a low glycolytic rate due to low basal levels of 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase-3 (Pfkfb3). Pfkfb3 promotes the formation of fructose-2, 6-bisphosphate which activates 6-phosphofructo-1-kinase, a key regulator of glycolysis. Herrero-Mendez et al. found that Cdh1-APC promotes the KEN-box-dependent degradation of Pfkfb3 to maintain cortical neurons in a low glycolytic state [73]. Correspondingly, inhibition of Cdh1 or expression of Pfkfb3 in neurons induces activation of glycolysis at the expense of glucose oxidation through the pentose phosphate pathway. This switch in metabolism blocks the regeneration of reduced glutathione, triggering oxidative stress and apoptosis in these neurons. These results suggest that the APC is a critical regulator of neuronal metabolism. By down-regulating glycolysis in neurons, Cdh1-APC promotes the metabolism of glucose via the pentose phosphate pathway to maintain a balanced oxidative state in the cell. It will be interesting to explore whether Cdh1-APC regulates other aspects of cellular metabolism, and whether cellular metabolism in turn regulates neuronal Cdh1-APC activity.
7. APC CONTROL OF GLIAL MIGRATION
Glial migration is thought to be coordinated with exit from the cell cycle [74]. Given the importance of APC in cell cycle exit, recent studies have explored a function for the APC in migration. Using a mutagenic screen for glial migration defects in a Drosophila model system, Silies et al. identified Cdh1-APC as an important regulator of migration in glia [74]. In addition to loss-of-function mutations, null alleles of Cdh1 disrupt glial migration. The observed defects are reversed by expression of exogenous Cdh1, suggesting that Cdh1 specifically regulates glial migration. Although previous studies have implicated Cdh1-APC in glial proliferation [75], migration defects cannot be explained by changes in glial proliferation because Cdh1/Cyclin A double mutants have normal glial number but still fail to migrate normally [74]. Strikingly, expression of Cdh1 in postmitotic neurons, but not glia, rescues the migration deficit in Cdh1 mutants, suggesting that Cdh1 regulates glial migration in a non-cell autonomous manner. In addition, Cdh1-APC-mediated migration appears to depend on the ubiquitin ligase activity of Cdh1-APC. APC mutants phenocopy the glial migration defects seen in Cdh1 mutants.
A screen to identify secondary mutations that suppress migration defects in Cdh1 mutants led to the identification of the axonal protein Fasciculin2 (Fas2) as an effector of Cdh1-APC in the control of glial migration [74]. Loss-of-function mutations in Fas2 suppress the Cdh1 mutation-induced phenotype, suggesting that Cdh1-APC controls glial migration by regulating the expression of axonal Fas2. Neurons and glia express distinct Fas2 isoforms that mediate a homophilic adhesive interaction between glia and axons. Fas2 is expressed in a graded manner and Fas2 expression levels are inversely correlated with regions of glial migration. Additional mutational analyses revealed that Fas2 expression is regulated in an endocytosis-dependent manner, consistent with a model whereby Cdh1-APC promotes down-regulation of Fas2 from the cell surface by stimulating ubiquitination and subsequent endocytosis.
8. CONCLUSION
With the increasing interest and continuing progress in our understanding of the APC in neurobiology, several important overarching themes have emerged. The diverse functions of the APC are mediated by reiterative use of the ubiquitin ligase machinery at distinct temporal phases of neuronal development. The recruitment of the APC coactivators, Cdh1 and Cdc20, in different subcellular locales allows for an additional layer of control. Although diverse functions of Cdh1-APC have been uncovered (Fig. 4A), functions of the related ubiquitin ligase Cdc20-APC are just beginning to be identified (Fig. 4B). With each of these functions of the APC in neurobiology, there are several important questions that remain to be answered. It will be critical to determine the mechanisms that regulate these pathways, with particular interest and attention paid to the extrinsic cues that might ultimately be linked to the cell-intrinsic functions of the APC. Likewise, it will be important to understand how the APC and its targets regulate cytoskeletal proteins to drive changes in neuronal morphogenesis and connectivity. Finally, a major line of future research should explore the functions of the APC in the mammalian brain using mouse genetics. This approach will also allow exploration of the role of deregulation of APC function in the pathogenesis of poorly understood neurological and psychiatric disorders. Given the functions of the APC in neuronal connectivity, it is worthwhile to consider strategies that may harness manipulation of APC activity to yield therapeutic benefits for disorders of the nervous system, from developmental disorders such as mental retardation and autism spectrum disorders to neurodegenerative diseases.
Figure 4.
Neuronal substrates and functions of the APC. Although several functions of Cdh1-APC have been uncovered (A), functions of the related ubiquitin ligase Cdc20-APC are just beginning to be elucidated (B).
ACKNOWLEDGMENTS
We thank members of the Bonni laboratory for critical reading of the manuscript. This work was supported by an NIH grant to A.B. (NS051255).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Mabb AM, Ehlers MD. Ubiquitination in postsynaptic function and plasticity. Annu Rev Cell Dev Biol. 2010;26:179–210. doi: 10.1146/annurev-cellbio-100109-104129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Murphey RK, Godenschwege TA. New roles for ubiquitin in the assembly and function of neuronal circuits. Neuron. 2002;36:5–8. doi: 10.1016/s0896-6273(02)00943-1. [DOI] [PubMed] [Google Scholar]
- [3].Yang Y, Kim AH, Bonni A. The dynamic ubiquitin ligase duo: Cdh1-APC and Cdc20-APC regulate neuronal morphogenesis and connectivity. Curr Opin Neurobiol. 2010;20:92–9. doi: 10.1016/j.conb.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Konishi Y, Stegmuller J, Matsuda T, Bonni S, Bonni A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science. 2004;303:1026–30. doi: 10.1126/science.1093712. [DOI] [PubMed] [Google Scholar]
- [5].Kim AH, Bonni A. Thinking within the D box: initial identification of Cdh1-APC substrates in the nervous system. Mol Cell Neurosci. 2007;34:281–7. doi: 10.1016/j.mcn.2006.11.019. [DOI] [PubMed] [Google Scholar]
- [6].Stegmuller J, Bonni A. Moving past proliferation: new roles for Cdh1-APC in postmitotic neurons. Trends Neurosci. 2005;28:596–601. doi: 10.1016/j.tins.2005.09.003. [DOI] [PubMed] [Google Scholar]
- [7].King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, Kirschner MW. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–88. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- [8].Irniger S, Piatti S, Michaelis C, Nasmyth K. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell. 1995;81:269–78. doi: 10.1016/0092-8674(95)90337-2. [DOI] [PubMed] [Google Scholar]
- [9].Harper JW, Burton JL, Solomon MJ. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 2002;16:2179–206. doi: 10.1101/gad.1013102. [DOI] [PubMed] [Google Scholar]
- [10].Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–56. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- [11].Dube P, Herzog F, Gieffers C, Sander B, Riedel D, Muller SA, et al. Localization of the coactivator Cdh1 and the cullin subunit Apc2 in a cryo-electron microscopy model of vertebrate APC/C. Mol Cell. 2005;20:867–79. doi: 10.1016/j.molcel.2005.11.008. [DOI] [PubMed] [Google Scholar]
- [12].Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–8. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- [13].Littlepage LE, Ruderman JV. Identification of a new APC/C recognition domain, the A box, which is required for the Cdh1-dependent destruction of the kinase Aurora-A during mitotic exit. Genes Dev. 2002;16:2274–85. doi: 10.1101/gad.1007302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–65. [PMC free article] [PubMed] [Google Scholar]
- [15].Reis A, Levasseur M, Chang HY, Elliott DJ, Jones KT. The CRY box: a second APCcdh1-dependent degron in mammalian cdc20. EMBO Rep. 2006;7:1040–5. doi: 10.1038/sj.embor.7400772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Morgan DO. Regulation of the APC and the exit from mitosis. Nat Cell Biol. 1999;1:E47–53. doi: 10.1038/10039. [DOI] [PubMed] [Google Scholar]
- [17].Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–93. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- [18].Pesin JA, Orr-Weaver TL. Regulation of APC/C activators in mitosis and meiosis. Annu Rev Cell Dev Biol. 2008;24:475–99. doi: 10.1146/annurev.cellbio.041408.115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yu H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- [20].Gieffers C, Peters BH, Kramer ER, Dotti CG, Peters JM. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc Natl Acad Sci U S A. 1999;96:11317–22. doi: 10.1073/pnas.96.20.11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim AH, Puram SV, Bilimoria PM, Ikeuchi Y, Keough S, Wong M, et al. A centrosomal Cdc20-APC pathway controls dendrite morphogenesis in postmitotic neurons. Cell. 2009;136:322–36. doi: 10.1016/j.cell.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Huynh MA, Stegmuller J, Litterman N, Bonni A. Regulation of Cdh1-APC function in axon growth by Cdh1 phosphorylation. J Neurosci. 2009;29:4322–7. doi: 10.1523/JNEUROSCI.5329-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ikeuchi Y, Stegmuller J, Netherton S, Huynh MA, Masu M, Frank D, et al. A SnoN-Ccd1 pathway promotes axonal morphogenesis in the mammalian brain. J Neurosci. 2009;29:4312–21. doi: 10.1523/JNEUROSCI.0126-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lasorella A, Stegmuller J, Guardavaccaro D, Liu G, Carro MS, Rothschild G, et al. Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature. 2006;442:471–4. doi: 10.1038/nature04895. [DOI] [PubMed] [Google Scholar]
- [25].Stegmuller J, Huynh MA, Yuan Z, Konishi Y, Bonni A. TGFbeta-Smad2 signaling regulates the Cdh1-APC/SnoN pathway of axonal morphogenesis. J Neurosci. 2008;28:1961–9. doi: 10.1523/JNEUROSCI.3061-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stegmuller J, Konishi Y, Huynh MA, Yuan Z, Dibacco S, Bonni A. Cell-intrinsic regulation of axonal morphogenesis by the Cdh1-APC target SnoN. Neuron. 2006;50:389–400. doi: 10.1016/j.neuron.2006.03.034. [DOI] [PubMed] [Google Scholar]
- [27].Stroschein SL, Bonni S, Wrana JL, Luo K. Smad3 recruits the anaphase-promoting complex for ubiquitination and degradation of SnoN. Genes Dev. 2001;15:2822–36. doi: 10.1101/gad.912901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wan Y, Liu X, Kirschner MW. The anaphase-promoting complex mediates TGF-beta signaling by targeting SnoN for destruction. Mol Cell. 2001;8:1027–39. doi: 10.1016/s1097-2765(01)00382-3. [DOI] [PubMed] [Google Scholar]
- [29].Goldberg JL. Intrinsic neuronal regulation of axon and dendrite growth. Curr Opin Neurobiol. 2004;14:551–7. doi: 10.1016/j.conb.2004.08.012. [DOI] [PubMed] [Google Scholar]
- [30].Flanagan JG. Neural map specification by gradients. Curr Opin Neurobiol. 2006;16:59–66. doi: 10.1016/j.conb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- [31].Luo L, Flanagan JG. Development of continuous and discrete neural maps. Neuron. 2007;56:284–300. doi: 10.1016/j.neuron.2007.10.014. [DOI] [PubMed] [Google Scholar]
- [32].Butler SJ, Tear G. Getting axons onto the right path: the role of transcription factors in axon guidance. Development. 2007;134:439–48. doi: 10.1242/dev.02762. [DOI] [PubMed] [Google Scholar]
- [33].Polleux F, Ince-Dunn G, Ghosh A. Transcriptional regulation of vertebrate axon guidance and synapse formation. Nat Rev Neurosci. 2007;8:331–40. doi: 10.1038/nrn2118. [DOI] [PubMed] [Google Scholar]
- [34].Altman J, Bayer S. Development of the Cerebellar System: In Relation to Its Evolution, Structure, and Functions. CRC Press; New York: 1997. [Google Scholar]
- [35].Luo K. Ski and SnoN: negative regulators of TGF-beta signaling. Curr Opin Genet Dev. 2004;14:65–70. doi: 10.1016/j.gde.2003.11.003. [DOI] [PubMed] [Google Scholar]
- [36].Pot I, Bonni S. SnoN in TGF-beta signaling and cancer biology. Curr Mol Med. 2008;8:319–28. doi: 10.2174/156652408784533797. [DOI] [PubMed] [Google Scholar]
- [37].Sarker KP, Kataoka H, Chan A, Netherton SJ, Pot I, Huynh MA, et al. ING2 as a novel mediator of transforming growth factor-beta-dependent responses in epithelial cells. J Biol Chem. 2008;283:13269–79. doi: 10.1074/jbc.M708834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sarker KP, Wilson SM, Bonni S. SnoN is a cell type-specific mediator of transforming growth factor-beta responses. J Biol Chem. 2005;280:13037–46. doi: 10.1074/jbc.M409367200. [DOI] [PubMed] [Google Scholar]
- [39].Oliva AA, Jr., Atkins CM, Copenagle L, Banker GA. Activated c-Jun N-terminal kinase is required for axon formation. J Neurosci. 2006;26:9462–70. doi: 10.1523/JNEUROSCI.2625-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- [41].Puram SV, Kim AH, Bonni A. An old dog learns new tricks: a novel function for Cdc20-APC in dendrite morphogenesis in neurons. Cell Cycle. 2010;9:482–5. doi: 10.4161/cc.9.3.10558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–63. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rape M, Reddy SK, Kirschner MW. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell. 2006;124:89–103. doi: 10.1016/j.cell.2005.10.032. [DOI] [PubMed] [Google Scholar]
- [44].Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–5. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- [45].Juo P, Kaplan JM. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr Biol. 2004;14:2057–62. doi: 10.1016/j.cub.2004.11.010. [DOI] [PubMed] [Google Scholar]
- [46].van Roessel P, Elliott DA, Robinson IM, Prokop A, Brand AH. Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell. 2004;119:707–18. doi: 10.1016/j.cell.2004.11.028. [DOI] [PubMed] [Google Scholar]
- [47].Fu AK, Hung KW, Fu WY, Shen C, Chen Y, Xia J, et al. APC(Cdh1) mediates EphA4-dependent downregulation of AMPA receptors in homeostatic plasticity. Nat Neurosci. 2011;14:181–9. doi: 10.1038/nn.2715. [DOI] [PubMed] [Google Scholar]
- [48].Li M, Shin YH, Hou L, Huang X, Wei Z, Klann E, et al. The adaptor protein of the anaphae promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nat Cell Biol. 2008;10:1083–9. doi: 10.1038/ncb1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kuczera T, Stilling RM, Hsia HE, Bahari-Javan S, Irniger S, Nasmyth K, et al. The anaphase promoting complex is required for memory function in mice. Learn Mem. 2011;18:49–57. doi: 10.1101/lm.1998411. [DOI] [PubMed] [Google Scholar]
- [50].Yang Y, Bonni A. Releasing the brake on presynaptic development: Cdc20-APC triggers neuroD2 degradation to drive presynaptic differentiation. Cell Cycle. 2010;9 doi: 10.4161/cc.9.12.11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yang Y, Kim AH, Yamada T, Wu B, Bilimoria PM, Ikeuchi Y, et al. A Cdc20-APC ubiquitin signaling pathway regulates presynaptic differentiation. Science. 2009;326:575–8. doi: 10.1126/science.1177087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat Neurosci. 2007;10:1235–7. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- [53].Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N, et al. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- [54].Becker EB, Bonni A. Cell cycle regulation of neuronal apoptosis in development and disease. Prog Neurobiol. 2004;72:1–25. doi: 10.1016/j.pneurobio.2003.12.005. [DOI] [PubMed] [Google Scholar]
- [55].Greene LA, Biswas SC, Liu DX. Cell cycle molecules and vertebrate neuron death: E2F at the hub. Cell Death Differ. 2004;11:49–60. doi: 10.1038/sj.cdd.4401341. [DOI] [PubMed] [Google Scholar]
- [56].Liu DX, Greene LA. Neuronal apoptosis at the G1/S cell cycle checkpoint. Cell Tissue Res. 2001;305:217–28. doi: 10.1007/s004410100396. [DOI] [PubMed] [Google Scholar]
- [57].Almeida A, Bolanos JP, Moreno S. Cdh1/Hct1-APC is essential for the survival of postmitotic neurons. J Neurosci. 2005;25:8115–21. doi: 10.1523/JNEUROSCI.1143-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Konishi Y, Lehtinen M, Donovan N, Bonni A. Cdc2 phosphorylation of BAD links the cell cycle to the cell death machinery. Mol Cell. 2002;9:1005–16. doi: 10.1016/s1097-2765(02)00524-5. [DOI] [PubMed] [Google Scholar]
- [59].Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- [60].Konishi Y, Bonni A. The E2F-Cdc2 cell-cycle pathway specifically mediates activity deprivation-induced apoptosis of postmitotic neurons. J Neurosci. 2003;23:1649–58. doi: 10.1523/JNEUROSCI.23-05-01649.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yuan Z, Becker EB, Merlo P, Yamada T, DiBacco S, Konishi Y, et al. Activation of FOXO1 by Cdk1 in cycling cells and postmitotic neurons. Science. 2008;319:1665–8. doi: 10.1126/science.1152337. [DOI] [PubMed] [Google Scholar]
- [62].Busser J, Geldmacher DS, Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer's disease brain. J Neurosci. 1998;18:2801–7. doi: 10.1523/JNEUROSCI.18-08-02801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Vincent I, Jicha G, Rosado M, Dickson DW. Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's disease brain. J Neurosci. 1997;17:3588–98. doi: 10.1523/JNEUROSCI.17-10-03588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Husseman JW, Nochlin D, Vincent I. Mitotic activation: a convergent mechanism for a cohort of neurodegenerative diseases. Neurobiol Aging. 2000;21:815–28. doi: 10.1016/s0197-4580(00)00221-9. [DOI] [PubMed] [Google Scholar]
- [65].Smith MZ, Nagy Z, Esiri MM. Cell cycle-related protein expression in vascular dementia and Alzheimer's disease. Neurosci Lett. 1999;271:45–8. doi: 10.1016/s0304-3940(99)00509-1. [DOI] [PubMed] [Google Scholar]
- [66].Maestre C, Delgado-Esteban M, Gomez-Sanchez JC, Bolanos JP, Almeida A. Cdk5 phosphorylates Cdh1 and modulates cyclin B1 stability in excitotoxicity. EMBO J. 2008;27:2736–45. doi: 10.1038/emboj.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yao W, Qian W, Zhu C, Gui L, Qiu J, Zhang C. Cdh1-APC is involved in the differentiation of neural stem cells into neurons. Neuroreport. 2010;21:39–44. doi: 10.1097/WNR.0b013e32833312fe. [DOI] [PubMed] [Google Scholar]
- [68].Cuende J, Moreno S, Bolanos JP, Almeida A. Retinoic acid downregulates Rae1 leading to APC(Cdh1) activation and neuroblastoma SH-SY5Y differentiation. Oncogene. 2008;27:3339–44. doi: 10.1038/sj.onc.1210987. [DOI] [PubMed] [Google Scholar]
- [69].Dow R, Hendley J, Pirkmaier A, Musgrove EA, Germain D. Retinoic acid-mediated growth arrest requires ubiquitylation and degradation of the F-box protein Skp2. J Biol Chem. 2001;276:45945–51. doi: 10.1074/jbc.M103593200. [DOI] [PubMed] [Google Scholar]
- [70].Nakamura M, Matsuo T, Stauffer J, Neckers L, Thiele CJ. Retinoic acid decreases targeting of p27 for degradation via an N-myc-dependent decrease in p27 phosphorylation and an N-myc-independent decrease in Skp2. Cell Death Differ. 2003;10:230–9. doi: 10.1038/sj.cdd.4401125. [DOI] [PubMed] [Google Scholar]
- [71].Zancai P, Dal Col J, Piccinin S, Guidoboni M, Cariati R, Rizzo S, et al. Retinoic acid stabilizes p27Kip1 in EBV-immortalized lymphoblastoid B cell lines through enhanced proteasome-dependent degradation of the p45Skp2 and Cks1 proteins. Oncogene. 2005;24:2483–94. doi: 10.1038/sj.onc.1208458. [DOI] [PubMed] [Google Scholar]
- [72].Harmey D, Smith A, Simanski S, Moussa CZ, Ayad NG. The anaphase promoting complex induces substrate degradation during neuronal differentiation. J Biol Chem. 2009;284:4317–23. doi: 10.1074/jbc.M804944200. [DOI] [PubMed] [Google Scholar]
- [73].Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolanos JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–52. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- [74].Silies M, Klambt C. APC/C(Fzr/Cdh1)-dependent regulation of cell adhesion controls glial migration in the Drosophila PNS. Nat Neurosci. 2010;13:1357–64. doi: 10.1038/nn.2656. [DOI] [PubMed] [Google Scholar]
- [75].Kaplow ME, Korayem AH, Venkatesh TR. Regulation of glia number in Drosophila by Rap/Fzr, an activator of the anaphase-promoting complex, and Loco, an RGS protein. Genetics. 2008;178:2003–16. doi: 10.1534/genetics.107.086397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–51. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- [77].Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–81. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]