Abstract
Two synthetic DNA sequences, carrying no other known E. coli promoter element than the consensus hexamers (CH) TTGACA (CH-35) and TATAAT CH(-10), spaced by 17 bp, were inserted in pBR329, in a position enabling transcription of the complete Cmr gene. The region upstream of the Cmr transcription start was carefully cleared of w.t. promoter elements (full deletion of the wild type (w.t.) Cmr promoter upstream +2 and large portion of an upstream coding sequence). Both synthetic promoters, which differ only by the sequences of the spacers (non consensus, constrained in AT or GC) support in vivo high level Cmr gene expression. The GC rich spacer is associated with transcription start at the usual +1 position, but with the AT rich spacer, transcription starts at several places, mainly in CH(-10). Rearranged promoter sequences derived from the synthetic ones upon transformation with partly ligated plasmids, yield new insights on the role of the standard CH pair, the size of the spacer and the sequence downstream of CH(-10).
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama T., Takanami M., Ohtsuka E., Taniyama Y., Marumoto R., Sato H., Ikehara M. Essential structure of E. coli promoter: effect of spacer length between the two consensus sequences on promoter function. Nucleic Acids Res. 1983 Sep 10;11(17):5855–5864. doi: 10.1093/nar/11.17.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auble D. T., Allen T. L., deHaseth P. L. Promoter recognition by Escherichia coli RNA polymerase. Effects of substitutions in the spacer DNA separating the -10 and -35 regions. J Biol Chem. 1986 Aug 25;261(24):11202–11206. [PubMed] [Google Scholar]
- Bossi L., Smith D. M. Conformational change in the DNA associated with an unusual promoter mutation in a tRNA operon of Salmonella. Cell. 1984 Dec;39(3 Pt 2):643–652. doi: 10.1016/0092-8674(84)90471-9. [DOI] [PubMed] [Google Scholar]
- Brosius J., Erfle M., Storella J. Spacing of the -10 and -35 regions in the tac promoter. Effect on its in vivo activity. J Biol Chem. 1985 Mar 25;260(6):3539–3541. [PubMed] [Google Scholar]
- Conley E. C., Saunders V. A., Saunders J. R. Deletion and rearrangement of plasmid DNA during transformation of Escherichia coli with linear plasmid molecules. Nucleic Acids Res. 1986 Nov 25;14(22):8905–8917. doi: 10.1093/nar/14.22.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
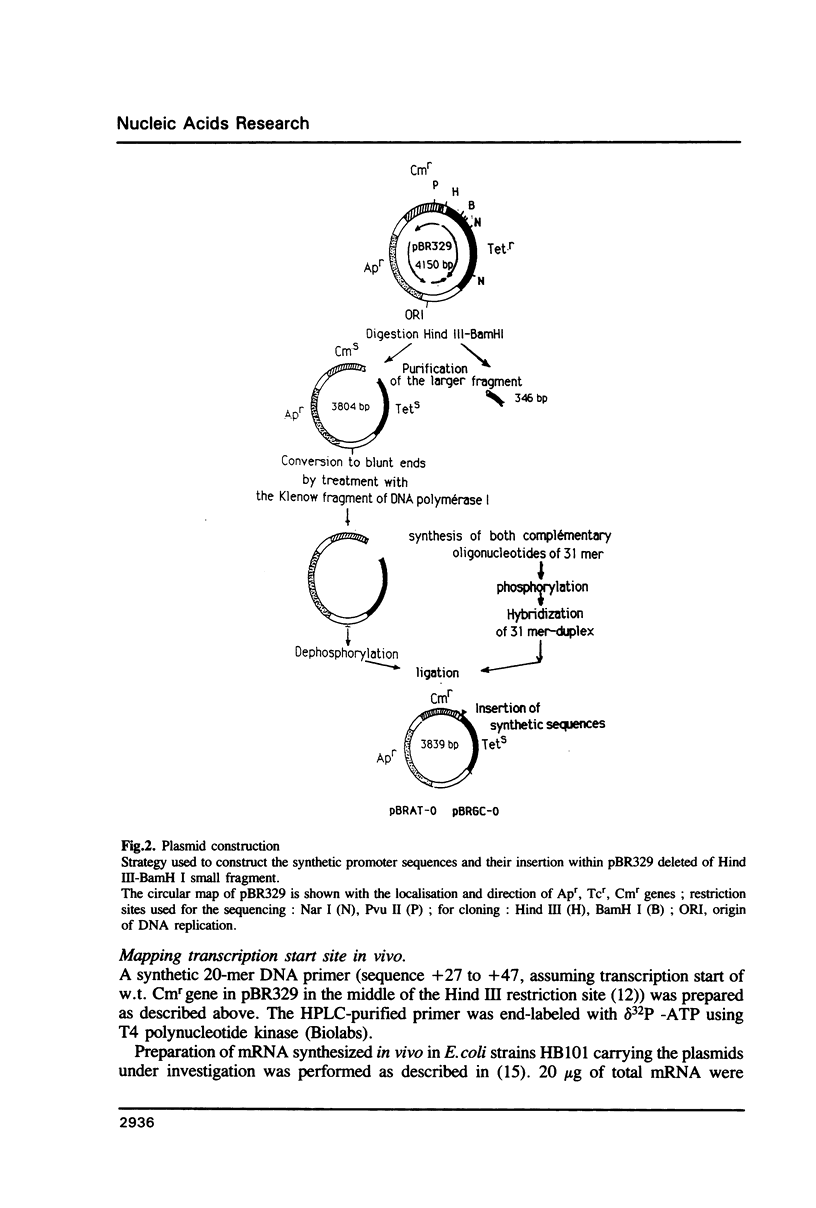
- Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. VI. Plasmid pBR329, a new derivative of pBR328 lacking the 482-base-pair inverted duplication. Gene. 1982 Jan;17(1):79–89. doi: 10.1016/0378-1119(82)90103-2. [DOI] [PubMed] [Google Scholar]
- Deuschle U., Kammerer W., Gentz R., Bujard H. Promoters of Escherichia coli: a hierarchy of in vivo strength indicates alternate structures. EMBO J. 1986 Nov;5(11):2987–2994. doi: 10.1002/j.1460-2075.1986.tb04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Valentin G., Ehrlich R. Dynamic and structural characterisation of multiple steps during complex formation between E. coli RNA polymerase and the tetR promoter from pSC101. Nucleic Acids Res. 1987 Jan 26;15(2):575–594. doi: 10.1093/nar/15.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Valentin G., Ehrlich R. Far upstream sequences of the bla promoter from TN3 are involved in complexation with E. coli RNA-polymerase. Nucleic Acids Res. 1988 Mar 25;16(5):2031–2044. doi: 10.1093/nar/16.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Loeb L. A. DNA sequences of random origin as probes of Escherichia coli promoter architecture. J Biol Chem. 1988 Oct 15;263(29):14724–14731. [PubMed] [Google Scholar]
- Jacquet M. A., Ehrlich R. In vivo and in vitro effect of mutations in tetA promoter from pSC101: insertion of poly(dA.dT) stretch in the spacer region does not inactivate the promoter. Biochimie. 1985 Sep;67(9):987–997. doi: 10.1016/s0300-9084(85)80293-5. [DOI] [PubMed] [Google Scholar]
- Keilty S., Rosenberg M. Constitutive function of a positively regulated promoter reveals new sequences essential for activity. J Biol Chem. 1987 May 5;262(13):6389–6395. [PubMed] [Google Scholar]
- Knaus R., Bujard H. PL of coliphage lambda: an alternative solution for an efficient promoter. EMBO J. 1988 Sep;7(9):2919–2923. doi: 10.1002/j.1460-2075.1988.tb03150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzer M., Bujard H. Promoters largely determine the efficiency of repressor action. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8973–8977. doi: 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcaud H., Gabarro-Arpa J., Ehrlich R., Reiss C. An algorithm for studying cooperative transitions in DNA. Nucleic Acids Res. 1986 Jan 10;14(1):551–558. doi: 10.1093/nar/14.1.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Sugimoto K., Sugisaki H., Takanami M. DNA regions essential for the function of a bacteriophage fd promoter. Nucleic Acids Res. 1977 Jul;4(7):2213–2222. doi: 10.1093/nar/4.7.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnambalam S., Webster C., Bingham A., Busby S. Transcription initiation at the Escherichia coli galactose operon promoters in the absence of the normal -35 region sequences. J Biol Chem. 1986 Dec 5;261(34):16043–16048. [PubMed] [Google Scholar]
- Rossi J. J., Soberon X., Marumoto Y., McMahon J., Itakura K. Biological expression of an Escherichia coli consensus sequence promoter and some mutant derivatives. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3203–3207. doi: 10.1073/pnas.80.11.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano J. E., Gralla J. D. Spacer mutations in the lac ps promoter. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1069–1072. doi: 10.1073/pnas.79.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L., Ehrenfeucht A. Use of the 'Perceptron' algorithm to distinguish translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2997–3011. doi: 10.1093/nar/10.9.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. M., Reznikoff W. S. Deletion analysis of RNA polymerase interaction sites in the Escherichia coli lactose operon regulatory region. J Mol Biol. 1986 Apr 20;188(4):545–553. doi: 10.1016/s0022-2836(86)80004-3. [DOI] [PubMed] [Google Scholar]




