Abstract
Experimental evidence indicates that donor specific antibodies targeting MHC class I and class II molecules can elicit the key features of transplant vasculopathy by acting on the graft vasculature in three ways: directly activating proliferative, pro-survival, and migratory signaling in the target endothelial and smooth muscle cells; increasing expression of mitogenic factors in vascular endothelial cells, creating a potential proliferative autocrine loop; and promoting recruitment of inflammatory cells, which produce mitogenic factors and elicit chronic inflammation, proliferation, and fibrosis. Here we review the experimental literature showing the complement and Fc-independent effects of MHC class I and II antibodies on graft vascular cells which may directly contribute to the proliferative aspect of transplant vasculopathy.
Introduction
Advances in immunosuppression and patient management have greatly lowered the incidence of acute rejection in solid organ transplantation. However, long-term survival of solid organ allografts has not improved at the same rate due to chronic rejection. Based on Organ Procurement and Transplantation Network data as of August 2010, five year survival of primary cardiac and renal transplants in the United States is 70%, and by ten years post transplant, 50% of the grafts have failed. Chronic rejection is also a major limitation in lung and liver transplantation. Chronically rejected vascularized allografts develop a progressive and insidious vascular disease known as transplant or allograft vasculopathy (TV or AV). Histologically, vessels exhibit perivascular fibrosis, smooth muscle cell (SMC) proliferation and concentric neointimal thickening resulting in occlusion of the lumen. These vascular lesions are often accompanied by subendothelial lymphocytes and macrophages (1, 2).
TV manifests as bronchiolitis obliterans syndrome (BOS) in lung, cardiac allograft vasculopathy (CAV) in heart, and renal transplant arteriosclerosis in kidney transplantation. There are distinct features of disease in each organ undergoing chronic vascular rejection. In the heart, TV particularly affects the epicardial and intramyocardial arteries. The vascular lesions increase in area of the necrotic core, calcification, plaque area and burden with progression exhibit migration of SMC into the intima and neointimal thickening which reults in vessel occlusion (3). Renal arteriosclerosis results in inflammatory cell infiltration and a fibrous thickening of the vascular intima due to myofibroblast proliferation (4). This accompanies other facets of nephropathy, such as a duplication of the glomerular basement membrane and persistent capillaritis (5, 6). Chronic rejection in the lung is described as a “fibroproliferative disorder” with increased lymphocyte infiltration and disruption of the epithelium. Granulation tissue invades the small airway lumen and fibrous scarring blights the bronchioles (7–9).
Proliferation is a central feature of TV lesions. Expression of proliferating cell nuclear antigen (PCNA) is elevated in grafts with TV (10, 11). Further, increased expression of mitogenic factors, such as PDGF, TGFalpha (12), and TGFbeta (13), is observed. Chronically rejected allografts also have an increase in vascular endothelial growth factor (VEGF), an essential soluble factor which regulates angiogenesis and inflammation and is highly proliferative for vascular cells (14).
Clinical and Experimental Evidence Linking Donor Specific Antibodies to Transplant Vasculopathy
In addition to nonimmune factors, the development of TV is elicited by the alloimmune response to mismatched antigens expressed in the graft—in particular, the classical major histocompatibility molecules (MHC; also called human leukocyte antigen, HLA, in humans) on the endothelial cells (EC) lining the blood vessels of the allograft. Several in vivo animal studies have provided experimental evidence that T cell mediated alloimmunity is necessary and sufficient to cause transplant vasculopathy (15–20). In addition, donor specific antibodies (DSA) are an important clinical risk factor for development of TV (21, 22). Due to the advent of C4d staining that identifies antibody-mediated rejection, there has been a renewed interest in understanding the role of alloantibodies in TV. Indeed, animal models have shown that passive transfer of alloantibodies can mediate development of TV. When RAG-1 knockout or SCID recipient mice, immunodeficient mice which lack B and T cells, were transplanted with an MHC class I and II molecule mismatched organ and reconstituted with donor specific alloserum or MHC class I antibodies, the allografts had increased macrophage infiltration, exhibited the classic signs of antibody mediated rejection (AMR) and developed arteriosclerotic lesions and fibrosis (10, 16, 23–28). While DSA may not be necessary to cause TV, animal models of transplantation have established that MHC class I and/or II antibodies are sufficient to elicit vasculopathy in the absence of cellular immunity.
Clinical studies in cardiac, renal and lung transplantation have found significant correlations between the presence of DSA and decreased graft function, increased mortality and the incidence of chronic vascular rejection (14, 22, 29–34). The mechanisms by which antibodies may contribute to transplant arteriosclerosis are not yet fully clear. Antibody functions are mainly effected by the Fc region of the antibody, which can engage receptors on innate immune cells to provoke NK cell-mediated lysis or enhance recruitment into the tissues. Some classes of antibody can fix and activate complement through the Fc region, generating split products which are chemotactic for immune cells and which cause endothelial cell lysis, apoptosis or activation. The significance of complement during AMR has been thoroughly reviewed elsewhere (35, 36).
While Fc and complement interactions are important contributors to inflammation, particularly during acute rejection, clinical observations have shown that CAV can occur in the absence of complement split product deposition (37). This is in line with other experimental demonstrations that complement fixation is not necessary for MHC class I antibodies to activate EC in vitro or elicit vasculopathy in animal models (10, 25, 28, 38–40). These data suggest that complement fixation may not be a requisite for disease in chronic AMR (39). Therefore the complement-independent actions of antibodies on the graft will be the focus of this review.
MHC Class I Molecule-Mediated Signaling in Vascular Cells
The importance of antibodies targeting HLA molecules has been demonstrated in vivo when HLA class I antibodies but not anti-MICA antibodies stimulated SMC proliferation and consistently induced neointimal thickening in a murine recipient of a human arterial graft (10). These results agree with findings in a rat cardiac allograft model where only endothelial-reactive allosera directed against MHC molecules contributed to vasculopathy and rejection (41, 42). Accordingly, anti-endothelial cell antibodies from autoimmune sera do not induce endothelial proliferation in vitro (43). This evidence suggests that MHC antibodies function in unique manner in which the ability to induce TV may depend on the signaling capacity of the antibody target, similar to agonistic antibodies like anti-angiotensin type 1 receptor antibodies.
HLA Antibodies Directly Induce Proliferative and Survival Signaling
Antibodies which crosslink HLA class I or II molecules induce signaling in human lymphocytes, as well as EC and SMCs. The effects of alloantibodies on EC in vitro are dependent on antibody recognition and crosslinking of the target rather than interactions with target cell Fc receptors (28, 44). Although it is still unclear how the classical HLA molecule transmits its biological signal, it is well-established that crosslinking of HLA class I or II molecules with antibodies in B cells, T cells or antigen presenting cells results either in programmed cell death or activation and proliferation (45). While the mechanisms influencing the differential outcomes are incompletely understood, the pathways leading to death or proliferation in immune cells have common second messengers, including intracellular calcium, PLC, PKC and Src family kinases (46–49). By activating similar signaling pathways in the donor vasculature and inducing proliferation, HLA class I antibodies may directly promote neointimal thickening.
HLA Class I Antibodies Induce Proliferation In Vitro
While animal models using passive transfer of MHC class I antibodies or alloserum have shown repeatedly that antibodies are sufficient to elicit transplant vasculopathy, the mechanism is incompletely understood. Antibodies which crosslink HLA class I molecules consistently cause in vitro proliferation in vascular smooth muscle (10, 50) and EC (51–56). Our group was the first to report that HLA class I antibodies promoted proliferation of SMCs (50), which Galvani et al. recently confirmed using a SMC line (10). We also demonstrated that HLA class I antibodies induce proliferation in human aortic EC rendered quiescent by serum starvation (51, 52, 57), and these findings are supported by similar reports from other groups using endothelium from a variety of vascular beds (55, 56) and various assays to measure proliferation (55, 58). HLA class I antibodies also cause proliferation in airway epithelial cells, suggesting a process by which alloantibody may contribute to BOS (59, 60).
HLA Class I Antibodies Induce Phosphorylation of Signaling Proteins
Longstanding investigations by our group have sought to dissect the signaling pathways triggered by crosslinking of HLA class I molecules in endothelial and SMCs (61, 62). HLA class I molecule ligation induces signal transduction pathways which dictate mitogenesis and survival in the endothelial cell within minutes of antibody treatment, diagramed in Figure 1. One of the earliest events is the GTP loading and membrane localization of RhoA (54, 63). RhoA activation is likely key to HLA class I molecule-induced proliferation, as inhibition of Rho abrogates select downstream signals (63), simvastatin reduces HLA class I molecule-mediated proliferation of EC in vitro (54), and Rho kinase inhibitors prevent rejection and transplant arteriosclerosis in animals (64, 65).
Figure 1.
Crosslinking of HLA class I molecules by antibodies induces cell signaling pathways which promote cell growth. FAK activates paxillin, an important regulator of the cytoskeleton. FAK activation also permits complex formation with Src. PI3K-dependent activation of Akt through PDK1 is central to cell growth. Akt inhibits GSK3beta, an antagonizer of cyclins such as cyclin E, which is vital to cell cycle progression. Akt also stimulates cyclin-dependent kinase 2, which causes the tumor suppressor Rb to release the transcription factor E2F, facilitating production of genes which promote G1/S transition.
Akt targets mTOR. mTOR complex 1 is responsible for S6K phosphorylation, which in turn activates S6 ribosomal protein. 4EBP-1, an inhibitor of the translation factor eIF-4E, is phosphorylated in an mTOR dependent manner after HLA class I molecule crosslinking. Phosphorylation ultimately triggers release of eIF-4E and allows recruitment of 40S ribosomal subunits to mRNA. Cumulatively, these events result in increased protein synthesis and are central to cell proliferation.
Finally, crosslinking of HLA class I molecules results in rapid translocation of FGFR to the cell surface and increased sensitivity to growth factor. The ERK MAP kinase is phosphorylated in an mTORC2 and FGF dependent manner, and actively promotes proliferation.
After HLA class I molecule crosslinking, the tyrosine kinase Src is phosphorylated at an autophosphorylation site in the catalytic domain (57, 58). Src is a crucial signal transducer which regulates such cell functions as cytoskeletal changes, migration, mitogenesis, cell cycle progression, and cell survival. Indeed, Src inhibition during HLA class I signaling prohibits activation of most downstream events (57). We reported that focal adhesion kinase (FAK) phosphorylation permits complex formation with Src family kinases (57, 58, 63), allowing for maximal kinase activity. These phosphorylation events are dependent on Rho/Rho kinase and Src activity in HLA class I molecule signaling (57, 63).
FAK then facilitates phosphorylation of paxillin (58), an adaptor protein involved in focal adhesion formation which can recruit a variety of other signaling molecules to specific compartments in the cell. One major functional consequence of FAK and paxillin activation is cytoskeletal rearrangement and formation or stabilization of focal adhesions. We have observed the reorganization of the actin cytoskeleton after HLA class I molecule ligation, with dramatic stress fiber formation (58, 63). Cytoskeletal tension and remodeling in adherent cells is required for proliferation, as many cytoskeletal regulators including Rho and FAK additionally control cell growth (66). Indeed, inhibition of FAK by siRNA during HLA class I molecule signaling reduces proliferative capacity (44, 58). The cytoskeleton is important in regulation of endothelial permeability as well as signal transduction, since Akt/PI3K complex formation is abrogated when the cytoskeleton is disrupted (44).
HLA class I molecule ligation activates phosphatidylinositol 3 kinase (PI3K), via FAK-dependent phosphorylation of the p85 regulatory domain (44, 58, 67, 68). PI3K phosphorylates the membrane bound phospholipid phosphatidylinositol 4,5-bisphosphate (simply known as PIP2) to yield PIP3. PIP3 can recruit 3-phosphoinositide dependent protein kinase (PDK1) and Akt, to the membrane (68), where phosphorylation of Akt by PDK1 permits a second modification by mammalian target of rapamycin (mTOR), resulting in full activation. The Akt pathway is an important regulator of cell growth, survival and migration. Akt contributes to cell proliferation by setting in motion the mTOR pathway and by regulating cell cycle progression.
Akt promotes mTOR activation through inhibition of mTOR antagonists. mTOR is present in two complexes in the cell. mTOR complex 1 (mTORC1) regulates ribosomal biogenesis and protein synthesis, and comprises mTOR, raptor and GbetaL. mTOR complex 2 (mTORC2) includes mTOR, Sin1, GbetaL and rictor, and participates in cytoskeletal regulation and feedback to Akt (69). We found that after HLA class I molecule ligation, mTOR is phosphorylated and forms signaling complexes (69).
Proliferation requires de novo protein synthesis, and the factors which regulate or execute translation of mRNA are targeted by the mTOR complex. Our group reported that 4EBP-1 (or PHAS-1), an inhibitor of the translation factor eIF-4E, is phosphorylated by mTOR after HLA class I molecule crosslinking (69). This modification of 4EBP1 is considered a translation initiation signal, as it triggers release of eIF-4E translation factor. p70 S6 kinase and ribosomal protein are also substrates for mTORC1 during HLA class I molecule signaling (69–71). Activation of ribosomal proteins complements the release of the translation factor eIF-4E to enhance protein synthesis. We found that none of the above events occur after HLA class I molecule ligation when mTOR signaling is impaired, emphasizing the central role of the mTOR complex in HLA class I molecule-mediated proliferative signaling. Further, both small interfering RNA knockdown (69) and pharmacological inhibition (55) of mTOR prevent cell proliferation in response to HLA class I antibodies.
We have also demonstrated the relevance of these pathways in vivo. Histological examination of biopsies from cardiac transplant patients showed that phosphorylated S6 ribosomal protein was a more specific indicator of AMR than C4d staining, and correlated with circulating levels of HLA class II antibodies (71). Our group also used an animal model to confirm that activation of these pathways, including S6RP, S6K, ERK, mTOR, and Akt, was significantly increased during chronic AMR (69).
HLA Class I Antibodies Increase Cell Sensitivity to Growth Factor
As described, cells treated with HLA class I antibodies proliferate in the absence of exogenous growth signals. In addition to immediate activation of growth-promoting signaling, HLA class I antibody-stimulated vascular cells also acquire proliferative capacity by augmenting their sensitivity to growth factors. Our group reported that treatment of EC with HLA class I antibodies led to rapid translocation of the fibroblast growth factor receptor (FGFR) at the cell surface and redistribution within the cell (50, 57).
The increase in surface FGFR results in amplified sensitivity to bFGF. The upregulation of FGFR is expected to be central to the resulting proliferative response of the cells, since addition of bFGF to the culture medium significantly potentiates cell growth in response to HLA class I antibodies and because neutralizing antibody to soluble bFGF abrogates HLA class I-induced cell proliferation (50–52). FGFR signaling converges on ERK, a MAP kinase. Indeed, our group found that ERK is phosphorylated at two key sites after HLA class I molecule ligation (72), in an mTORC2 dependent manner, which allows dimerization and nuclear translocation. Since activation of ERK appears to be tied to FGFR signaling, while activation of Akt is not dependent on FGFR, we hypothesize that FGFR signaling must act in parallel with other HLA class I molecule-mediated signaling events (44).
HLA Class I Antibodies Promote Cell Cycle Progression
HLA class I molecule signaling also regulates cell cycle progression. One inhibitor of cell survival and proliferation, glycogen synthase kinase 3 beta (GSK3beta) is targeted by Akt to promote growth. Akt is known to inactivate GSK3beta (54), thereby relieving inhibition of cyclins, translation initiator eIF2B and other cell cycle regulators. It has also been reported that the activity of another cell cycle inhibitor is altered after HLA class I molecule crosslinking. Nath et al. showed that Rb was inactivated as a result of the action of cyclin dependent kinase 2 (Cdk2). Further, the inactivation of Rb was likely mediated by FGFR signaling, since inhibition of bFGF abolished the observed effect (73).
Cell Survival Pathways
While HLA class I and II antibodies can cause apoptosis in lymphocytes, stimulation of EC with a low dose of HLA class I antibodies or alloserum promotes cell survival and resistance to death (44, 67, 74). Akt lies at the heart of endothelial survival in the presence of HLA class I antibodies, as illustrated in Figure 2. We reported that after HLA class I molecule crosslinking, the pro-apoptotic protein Bad is phosphorylated at two Akt-responsive sites, which results in its sequestration from the mitochondria by the 14-3-3 proteins (44). Our group and others also showed that the expression of anti-apoptotic proteins Bcl-2 and Bcl-xL are increased downstream of the PI3K/Akt/mTOR signaling axis (44, 68, 69). These Bcl family members suppress programmed cell death by regulating mitochondrial membrane permeability to prevent activation of caspases. Biopsies from patients with circulating DSA and mouse cardiac allografts undergoing AMR also had increased Bcl-2 expression, confirming these findings in vivo (28, 44, 74).
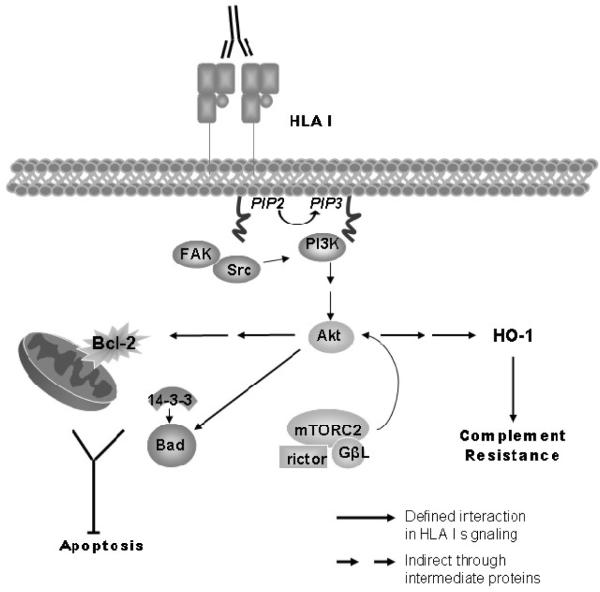
Figure 2.
Crosslinking of HLA class I molecules with antibodies activates Akt, which promotes cell survival. PI3K catalyzes phosphorylation of PIP2 to yield PIP3 which can interact with a discrete region known as the pleckstrin homology domain. Akt and PDK1 localize to the membrane in response to PIP3, where PDK1 phosphorylates Akt at Thr308. Akt promotes mTORC1 activation, while mTORC2 phosphorylates Akt at Ser473. Akt increases Bcl-2 and Bcl-xL levels in the cell and phosphorylates Bad, a pro-apoptotic mediator, which allows 14-3-3 proteins to bind. This association with 14-3-3 prevents Bad antagonism of Bcl-2 and Bcl-xL proteins and promotes cell survival. Finally, cells treated with HLA class I antibody upregulate the antioxidant HO-1 in a PI3K dependent manner, which contributes to the cells' resistance to complement mediated lysis.
Other genes which protect from cell death during stress are upregulated after HLA class I molecule ligation. For example, expression of heme oxygenase 1 (HO-1), a factor which is cytoprotective against oxidative stress and other forms of cellular injury, is increased in endothelium after HLA class I molecule ligation and confers resistance to complement-mediated lysis (67, 75). Evidence also points to a protective effect of intragraft HO-1 against rejection and TV (76–78).
Cell survival and proliferative pathways are concurrently active and share key mediators, such as PI3K/Akt/mTOR pathway. Akt is the central signaling hub whose pleiotropic effects are accomplished by a broad array of targets. Akt activation imparts resistance to death signals (67) by modulating the balance between pro- and anti-apoptotic factors. The Akt signal also causes proliferation by regulating the activity of cyclins, thereby driving cell cycle progression, and diverges into the two mTOR complexes to influence protein synthesis. It is therefore difficult to dissect the signaling which increases cell viability from that which promotes active division because, while the outcomes are distinct, they are also strongly linked.
HLA Class I Molecule Signal Transduction
How classical HLA molecules signal is still a matter of investigation. While they do not have intrinsic kinase activity, it is postulated that HLA molecules associate with other proteins at the membrane which are capable transducing signals, an arrangement common to many receptors. For example, MHC class I molecules have been coimmunoprecipitated with growth factor receptors (IGFR and EGFR) (79, 80), whereas HLA class II molecules have been shown to interact with a tetraspanin network which links them to integrins such as integrin alpha4beta1 and alpha6beta1 (81). Indeed the signaling cascade induced by crosslinking of the HLA class I molecule is markedly similar to integrin and growth factor receptor signaling.
In order to explain the ability of HLA class I molecules to transduce signals in EC, our lab investigated the function of integrins in HLA class I molecule-mediated signaling. A molecular association between HLA class I molecules and the integrin subunit beta4 was found, which was increased following antibody ligation of HLA class I molecules. Deletion of the cytoplasmic domain of the HLA class I molecule, which is required for the association with integrin beta4, suppressed HLA class I molecule signaling. HLA class I molecules required integrin beta4 expression in order to cause phosphorylation of Src, Akt and ERK after crosslinking. Furthermore, knockdown of integrin beta4 abolished proliferation in response to HLA class I antibodies, demonstrating a dependency of HLA class I molecules on integrin beta4 for induction of cell growth. Importantly, we also uncovered a previously unknown role for the HLA class I molecule in integrin beta4 signaling, revealing for the first time that HLA class I molecule expression is required for integrin beta4-mediated functions such as migration and ERK phosphorylation (82). These studies give fresh and valuable insight into the molecular mechanisms by which HLA class I molecules stimulate cellular proliferation.
MHC Antibodies Activate Transcription and Synthesis of Mitogenic Mediators
MHC class I antibodies have also been observed to induce transcription factor activity and production of cytokines in EC. Cytokine production could initiate autocrine signaling that reiterates the endogenous proliferative signaling, and promotes recruitment of immune cells.
The Role of NF-kappaB
NF-kappaB is one of the most well-recognized transcription factors in inflammation. It controls cell growth and apoptosis, as well as production of inflammatory mediators in a variety of cells, and plays an important role in endothelial cell activation (83). Treatment of EC with HLA class I antibodies increases mRNA and protein expression of NF-kappaB dependent gene targets, such as cytokines and anti-apoptotic factors, suggesting a role in AMR. Traditional models of NF-kappaB signaling involve degradation of the inhibitory IkappaB proteins, which sequester the NF-kappaB transcription factor in the cytoplasm. Serine phosphorylation of IkappaBalpha causes release of NF-kappaB, ubiquitination and degradation of IkappaB, resulting in translocation of NFkappaB into the nucleus.
HLA class I antibodies activate NF-kappaB in immune cells (84). Ligation of HLA class I by antibody increased the DNA binding ability of NF-kappaB and tyrosine phosphorylation of IkappaBalpha in both human umbilical vein EC (HUVEC) and cardiac microvascular EC (53). However, the investigators were not able to consistently show degradation of IkappaBalpha with different HLA class I antibodies. Another group likewise failed to show a reduction in protein levels of IkappaBalpha which would correspond to its degradation (54). It may be that HLA class I ligation activates a less familiar NF-kappaB pathway, such as the tyrosine phosphorylation and nondegradory dissociation described in VEGF signaling (85).
In addition to directing inflammatory responses, NF-kappaB regulates the decision between death and survival in a variety of cell types (86). Experimental evidence suggests that suppression of NF-kappaB in the allograft by anti-inflammatory proteins may reduce neointimal thickening and extend graft survival during chronic rejection (77, 87). Mechanistic studies of NF-kappaB are needed to understand its connection to the processes which promote vasculopathy during AMR. Further, it is almost certain that other transcription factors are active during HLA class I signaling and are waiting to be discovered.
Induction of Proliferative Factors
While complement split products can induce expression of inflammatory factors in EC (88, 89), MHC class I antibodies can elicit this response in the absence of complement. Rahimi et al. showed that high doses of both complement fixing and noncomplement fixing MHC class I antibodies can induce expression of KC in a murine endothelial cell line (SVEC-10) (38, 39). This finding is supported by the reported induction of interleukin-8 (IL-8, the human homolog of KC) in arterial EC after treatment with HLA class I antibodies (56). IL-8 is well-characterized as a chemokine which attracts neutrophils and other leukocytes to promote their recruitment to sites of inflammation. In addition to its function as a chemokine, IL-8 has an autocrine proliferative effect on EC (90).
Tissue factor (TF) plays a role as a procoagulant and has also been implicated in neointimal hyperplasia, fibrosis, EC and SMC proliferation (91, 92). In a rat cardiac transplant model of chronic rejection, TF was increased in the coronary intima (93), and inhibition of TF expression reduced neointimal thickening (94). In addition, patients exhibiting vasculopathy had nearly eight-fold higher expression of TF in endocardial biopsy than those without vasculopathy (95), which was determined to be predictive of TV (96). Naji et al. treated HUVEC with allele-specific HLA-A antibodies in vitro and showed that TF was increased (97), demonstrating that HLA class I antibodies can directly induce this important mediator.
VEGF is a key regulator of vascular function. In addition to its importance during vascular development, VEGF also induces proliferation and survival signaling in EC. Several reports have shown that VEGF is upregulated in human cardiac transplant biopsies with evidence of TV, and localizes to EC (14, 98). Further, VEGF expression combined with macrophage infiltration increases the risk for development of TV by 2.5-fold (14, 98). A rat model of chronic rejection revealed the importance of VEGF signaling in chronic transplant rejection. It was found that overexpression of VEGF in the graft exacerbated intimal thickening while antagonism decreased vessel occlusion and mononuclear cell infiltration (99, 100). HUVEC treated with HLA class I antibodies in vitro increase production of VEGF mRNA. In addition, HLA class I-mediated cell migration was dependent on VEGFR2, implicating an autocrine signaling pathway in which secreted VEGF alters the endothelial cell phenotype (55). Therefore, VEGF plays a prominent role in antibody-mediated vasculopathy and may be the direct result of HLA antibody effects.
In addition to acting on EC to induce factors which promote vascular cell growth and migration, HLA class I antibodies may target other cells to elicit vascular disease. Rat SMCs produce PDGF, FGF-2 and IGF-1 upon exposure to donor specific antibodies (101). Additionally, airway epithelial cells treated with HLA class I antibodies elaborated growth factors which promote fibrosis, such as PDGF and bFGF, and stimulated proliferation of fibroblasts (102). This study highlights the effects of HLA class I antibodies during BOS in lung allografts. The authors postulated that expression of these growth factors promotes survival and proliferation of the SMCs in an autocrine fashion.
The mediators produced by EC upon MHC class I stimulation are summarized in Table 1. Importantly, while many studies reported that MHC class I antibodies cause both cytokine secretion and proliferation of EC, none have conclusively demonstrated the direct contribution of cytokine production to MHC class I molecule-induced proliferation. Further work should be correlative and focus on directly identifying the contribution of each cytokine to in vitro proliferation and the development of vasculopathy in vivo. The induction of the above factors by initial MHC class I molecule signaling may reinforce the proliferative signaling observed following ligation, by creating autocrine feedback from a highly proliferative cytokine milieu.
Table 1.
Summary of cytokines and growth factors induced by MHC I antibodies in endothelial cells, their functions and role in allograft rejection.
| Cytokine | Effect on Vascular Cells | Inflammatory Effect | Observations in Clinical Rejection | In vivo work showing its role in TV | Primary In Vitro Reference |
|---|---|---|---|---|---|
| IL-8 | Causes autocrine proliferation of EC (90) | Promotes neutrophil and monocyte recruitment and activation (117) | Strong expression in high grade rejection heart biopsies (118) | None reported | MHC I Ab induce KC (38, 39) and IL-8 (56) production from ECs |
| Tissue factor | Induces mitogenic factors (91) Regulates EC proliferation and apoptosis (92) |
Initiator of coagulant cascade Leukocyte diapedesis Promotes fibrin deposition | Px with TV had higher expression in endomyocardial biopsy (95) Early biopsy staining predictive of TV (96) |
Upregulated in rat coronary intima and adventitia, of EC and mononuclear cell origin (93) Inhibition of TF expression reduces intimal thickening (94) |
Increased TF mRNA and protein in HUVEC after HLA I Ab treatment (97) |
| IL-6 | Induces production of VEGF (119) and PDGF Promotes SMC and EC migration (120) and proliferation (121) |
Regulates EC adhesion molecule expression and permeability (122) | Increased in plasma of patients with TV (123) Strong correlation between intragraft mRNA and histological rejection (124) |
None reported | Human EC produce IL-6 after HLA I Ab treatment (56) |
| VEGF | Causes EC proliferation and migration SMC chemoattractant (125–127) |
Regulates vascular permeability (128) Induces EC adhesion molecules (129–131) Monocyte chemokine (132) |
Upregulated in biopsies from patients with TV (14, 98) | VEGFR antagonism reduced TV and decreased mononuclear cell recruitment (100) Overexpression of VEGF increased intimal thickening and macrophage infiltration (99) |
Treatment of HUVEC with HLA I Ab increased VEGF mRNA (55) |
| MCP-1 (CCL2) | None reported (133) | Monocyte chemokine (117) | Increased in plasma of patients with TV (123) | Increased expression in graft when recipient was treated with anti-donor Ab (38) Increased intragraft MCP-1 preceded intimal thickening (134) |
Murine EC line produces MCP-1 after treatment with MHC I Ab (38, 56) |
| RANTES (CCL5) | None reported | Chemotactic for T cells and monocytes | Increased in graft vessels in patients with graft atherosclerosis (135) and rejection (136) | Expression correlated with mononuclear infiltration and intimal thickening (134, 136) | Murine EC line produces RANTES after treatment with MHC I Ab (38) |
MHC Antibodies Promote Recruitment of Leukocytes
Macrophages in Vasculopathy
Mononuclear cell infiltration is a key feature of AMR, and macrophages are found in high frequency in the thickened intima of chronically rejected grafts from multiple species. Macrophages have been extensively studied in atherosclerosis, where they play an active pathogenic role. For example, macrophages can promote alteration of extracellular matrix production by SMC (103) and can directly cause endothelial cell proliferation by releasing a variety of growth factors and cytokines (104).
Mononuclear cells are not only a hallmark of chronic rejection, but they also are influential in the pathophysiology of rejection. Depletion of macrophages or transplant into a macrophage deficient allograft recipient significantly reduces intimal thickening (17, 105, 106). Further, antagonism of MCP-1, a chemokine signal important to monocyte recruitment from the blood into the tissue, increases graft survival and decreases transplant vasculopathy in an animal model of lung transplantation (107). Macrophage-derived factors in the graft neointima can contribute to local inflammation by causing tissue damage, fibrosis and SMC proliferation (108, 109). While the potential for macrophages to cause progression of arterial lesions is clear, evidence for a causative effect of macrophage function in TV is mostly indirect.
HLA Class I Antibodies Cause Leukocyte Recruitment by Endothelial Cells
Leukocyte recruitment from the blood by EC is a complex cascade with discrete stages directed by an array of adhesion molecules and chemokines. The first step, known as “rolling,” is a low affinity interaction between the leukocyte and endothelial cell mediated by selectins. Subsequently, high affinity integrin receptors on the leukocyte convert rolling to firm adhesion and arrest. Finally, remodeling of the endothelial barrier and polarization of leukocyte receptors permits extravasation into the subendothelial space.
There is mounting proof that antibodies can trigger endothelial cell recruitment of immune cells to the graft. Passive transfer of MHC class I antibody into an immunodeficient recipient of a mismatched allograft elicits strong macrophage and perivascular cellular infiltration (23, 28, 38, 110). When EC are coated with antibody, leukocyte receptors for antibody Fc regions (FcR) can enhance leukocyte recruitment by initiating inside-out signaling which supports integrin-mediated firm adhesion and arrest (111). While Fc functions can be a significant factor in leukocyte-endothelial interactions during rejection, as demonstrated by Lee et al. (38), other work suggests that the Fc is not necessarily required for antibodies to elicit leukocyte recruitment. Treatment of the immunodeficient allograft recipient with an F(ab′)2 fragment of the MHC class I antibody does not preclude recruitment of leukocytes into the graft (28, 38). This observation is strongly indicative of functions mediated by the antibody independent of Fc interactions or complement, in which alloantibody-induced signaling in EC increase endothelial adhesivity.
HLA class I antibodies may cause leukocyte recruitment through induction of adhesion molecules and chemokines in the endothelium. While previous work by our group ruled out the upregulation of the endothelial adhesion molecules as ICAM-1, VCAM-1 and E-selectin (112), ligation of HLA class I molecules causes release of endothelial vesicles known as Weibel-Palade bodies and rapid presentation of P-selectin, which facilitated leukocytic cell adherence (113). P-selectin participates in neutrophil and macrophage recruitment during inflammation, and study of chronic rejection in rat cardiac allografts uncovered a correlation between intimal thickening and increased P-selectin expression in the intima (114, 115).
Cytokine production by MHC class I-activated EC could initiate an autocrine inflammatory loop to promote leukocyte recruitment. MHC class I antibodies cause cultured EC to produce a variety of inflammatory cytokines, including IL-6 and IL-1beta, and chemokines, such as MCP-1, VEGF and IL-8 (38, 55, 56). These factors could contribute to the mobilization of leukocytes into the graft, either by directly guiding leukocyte migration or by enhancing and extending endothelial cell activation (summarized in Table 1). For example, intragraft expression of VEGF facilitates recruitment of mononuclear cells directly and augments endothelial expression of other chemokines, thereby amplifying the rejection response (116).
Certainly macrophage functions and the leukocyte recruitment cascade represent important targets in chronic allograft vasculopathy. Antagonism of mononuclear cell recruitment or the endothelial signaling which promotes recruitment may reduce lesion burden (17).
Conclusions and Future Directions
In summary, HLA class I antibodies can activate a variety of signaling pathways in vascular endothelial and SMCs which may contribute to transplant vasculopathy. As shown in Figure 3, HLA class I antibodies cause immediate activation of proliferative and survival pathways. They also induce production of mitogenic factors which may reinforce the proliferative milieu, and inflammatory mediators which can recruit leukocytes to the graft. We propose that these effects compound during chronic AMR to provoke proliferation of the graft vasculature, culminating in TV.
Figure 3.
Three-step model of the Fc independent effects of HLA class I antibodies on vascular endothelium. HLA class I molecule ligation immediately activates cell signaling pathways which cause cell cycle progression, promote cell survival and protein synthesis, and ultimately induce cellular proliferation. HLA class I molecule crosslinking also activates transcription, and endothelial cells begin producing inflammatory and proliferative factors such as IL-8, VEGF and MCP-1 after a lag period. Finally, HLA class I molecule signaling mobilizes endothelial vesicles known as Weibel-Palade bodies (WPb), resulting in presentation of the adhesion molecule P-selectin on the cell surface and increasing the binding of leukocytes. Immune cells such as T cells and macrophages can release mediators in the neointima which cause endothelial and smooth muscle cell proliferation and vascular remodeling.
While extensive work has yielded insight into the effects of DSA on the graft vasculature, much more investigation is needed to fully understand how HLA antibodies contribute to transplant arteriosclerosis. In particular, the intracellular signaling pathways should be further explored in order to ascertain the potential therapeutic benefits of modulating this system and to identify other possible biomarkers of AMR which may be early indicators of vasculopathy. Additionally, the specific contribution of cytokines and other mitogenic factors should be clarified with correlative studies, as cytokine milieu and microenvironments are known to influence disease outcomes. Finally, it will be important to more specifically investigate the role of macrophages in vasculopathy, as has been done in atherosclerosis, to fully reveal their contribution to the pathogenesis of chronic rejection.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases Grant RO1 AI 042819 and NIH U01AI077821 and the National Heart Lung and Blood Institute Grant RO1 HL 090995 to E. F. R, and the Ruth L. Kirschstein National Research Service Award T32HL69766 to N. M. V.
List of Abbreviations
- HLA
human leukocyte antigen
- MHC
major histocompatibility complex
- TV
Transplant vasculopathy
- BOS
bronchiolitis obliterans syndrome
- CAV
cardiac allograft vasculopathy
- VEGF
vascular endothelial growth factor
- PCNA
proliferating cell nuclear antigen
- RAG
recombinase activating gene
- DSA
donor specific antibodies
- AMR
antibody mediated rejection
- FAK
focal adhesion kinase
- PI3K
phospho inositol 3 kinase
- PDK1
3 phosphoinositide dependent protein kinase
- mTOR
mammalian target of rapamycin
- FGFR
fibroblast growth factor receptor
- EC
endothelial cell
- SMC
smooth muscle cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1.Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy 2010. J Heart Lung Transplant. 2010;29:717–27. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell RN, Libby P. Vascular Remodeling in Transplant Vasculopathy. Circ Res. 2007;100:967–78. doi: 10.1161/01.RES.0000261982.76892.09. [DOI] [PubMed] [Google Scholar]
- 3.de la Torre Hernandez JM, Vazquez de Prada JA, Burgos V, et al. Virtual histology intravascular ultrasound assessment of cardiac allograft vasculopathy from 1 to 20 years after heart transplantation. J Heart Lung Transplant. 2009;28:156–62. doi: 10.1016/j.healun.2008.11.915. [DOI] [PubMed] [Google Scholar]
- 4.Colvin RB. Humoral Immunity in Kidney Transplantation. Contrib Nephrol. 2009;162:75–86. [PubMed] [Google Scholar]
- 5.Cosio FG, Gloor JM, Sethi S, Stegall MD. Transplant glomerulopathy. Am J Transplant. 2008;8:492–96. doi: 10.1111/j.1600-6143.2007.02104.x. [DOI] [PubMed] [Google Scholar]
- 6.Gloor J, Cosio F, Lager DJ, Stegall MD. The spectrum of antibody-mediated renal allograft injury: implications for treatment. Am J Transplant. 2008;8:1367–73. doi: 10.1111/j.1600-6143.2008.02262.x. [DOI] [PubMed] [Google Scholar]
- 7.Shilling RA, Wilkes DS. Immunobiology of chronic lung allograft dysfunction: new insights from the bench and beyond. Am J Transplant. 2009;9:1714–18. doi: 10.1111/j.1600-6143.2009.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166:440–44. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- 9.McDyer JF. Human and murine obliterative bronchiolitis in transplant. Proc Am Thorac Soc. 2007;4:37–43. doi: 10.1513/pats.200605-107JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galvani S, Augé N, Calise D, et al. HLA Class I antibodies provoke graft arteriosclerosis in human arteries transplanted into SCID/Beige mice. Am J Transplant. 2009;9:2607–14. doi: 10.1111/j.1600-6143.2009.02804.x. [DOI] [PubMed] [Google Scholar]
- 11.Orth SR, Odoni G, Karkoszka H, et al. Combination treatment with an ETA-receptor blocker and an ACE inhibitor is not superior to the respective monotherapies in attenuating chronic transplant vasculopathy in different aorta allotransplantation rat models. Nephrol Dial Transplant. 2003;18:62–69. doi: 10.1093/ndt/18.1.62. [DOI] [PubMed] [Google Scholar]
- 12.Hosenpud JD, Morris TE, Shipley GD, et al. Cardiac allograft vasculopathy : preferential regulation of endothelial cell-derived mesenchymal growth factors in response to a donor-specific cell-mediated allogeneic response. Transplantation. 1996;6:939–48. doi: 10.1097/00007890-199603270-00017. [DOI] [PubMed] [Google Scholar]
- 13.Aziz T, Hasleton P, Hann AW, et al. Transforming growth factor beta in relation to cardiac allograft vasculopathy after heart transplantation. J Thorac Cardiovasc Surg. 2000;119:700–08. doi: 10.1016/s0022-5223(00)70004-3. [DOI] [PubMed] [Google Scholar]
- 14.Bayliss J, Bailey M, Leet A, et al. Late onset antibody-mediated rejection and endothelial localization of Vascular Endothelial Growth Factor are associated with development of cardiac allograft vasculopathy. Transplantation. 2008;86:991–97. doi: 10.1097/TP.0b013e318186d734. [DOI] [PubMed] [Google Scholar]
- 15.Gareau A, Hirsch GM, Lee TDG, et al. Contribution of B cells and antibody to cardiac allograft vasculopathy. Transplantation. 2009;88:470–77. doi: 10.1097/TP.0b013e3181b076cc. [DOI] [PubMed] [Google Scholar]
- 16.Soleimani B, Katopodis A, Wieczorek G, et al. Smooth muscle cell proliferation but not neointimal formation is dependent on alloantibody in a murine model of intimal hyperplasia. Clin Exp Immunol. 2006;146:509–17. doi: 10.1111/j.1365-2249.2006.03237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi C, Lee WS, He Q, et al. Immunologic basis of transplant-associated arteriosclerosis. Proc Nat Acad Sci USA. 1996;93:4051–56. doi: 10.1073/pnas.93.9.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaro AI, Liwski RS, Zhou J, et al. CD8+ T cells mediate aortic allograft vasculopathy by direct killing and an interferon dependent indirect pathway. Cardiovasc Res. 2005;65:283–91. doi: 10.1016/j.cardiores.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Nejat S, Zaki A, Hirsch GM, et al. CD8(+) T cells mediate aortic allograft vasculopathy under conditions of calcineurin immunosuppression: role of IFN-gamma and CTL mediators. Transpl Immunol. 2008;19:103–11. doi: 10.1016/j.trim.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Allan JS, Choo JK, Vesga L, et al. Cardiac Allograft vasculopathy is abrogated by anti-CD8 monoclonal antibody therapy. Ann Thorac Surg. 1997;64:1019–25. doi: 10.1016/s0003-4975(97)00796-0. [DOI] [PubMed] [Google Scholar]
- 21.Reed EF, Demetris AJ, Hammond E, et al. Acute Antibody-mediated Rejection of Cardiac Transplants. J Heart Lung Transplant. 2006;25:153–59. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Terasaki PI, Ozawa M. Predicting Kidney Graft Failure by MHC Antibodies: a Prospective Trial. Am J Transplant. 2004;4:438–43. doi: 10.1111/j.1600-6143.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 23.Russell PS, Chase CM, Winn H, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. II. Importance of humoral immunity. J Immunol. 1994;152:5135–41. [PubMed] [Google Scholar]
- 24.Russell PS, Chase CM, Colvin RB. Alloantibody- and T cell-mediated immunity in the pathogenesis of transplant arteriosclerosis: Lack of progression to sclerotic lesions in B cell-deficient mice. Transplantation. 1997;64:1531–36. doi: 10.1097/00007890-199712150-00005. [DOI] [PubMed] [Google Scholar]
- 25.Uehara S, Chase CM, Cornell LD, et al. Chronic cardiac transplant arteriopathy in mice: relationship of alloantibody, C4d deposition and neointimal fibrosis. Am J Transplant. 2007;7:57–65. doi: 10.1111/j.1600-6143.2006.01599.x. [DOI] [PubMed] [Google Scholar]
- 26.Gareau AJ, Nashan B, Lee TDG. The role of alloantibody in the development of cardiac allograft vasculopathy. J Heart Lung Transplant International Society for Heart and Lung Transplantation Twenty-Ninth Annual Meeting and Scientific Sessions, Palais des Congres, Paris, France. 2009;28(Suppl 1):S115. [Google Scholar]
- 27.Maruyama T, Jaramillo A, Narayanan K, et al. Induction of obliterative airway disease by anti-HLA class I antibodies. Am J Transplant. 2005;5:2126–34. doi: 10.1111/j.1600-6143.2005.00999.x. [DOI] [PubMed] [Google Scholar]
- 28.Jindra PT, Hsueh A, Hong L, et al. Anti-MHC class I antibody activation of proliferation and survival signaling in murine cardiac allografts. J Immunol. 2008;180:2214–24. doi: 10.4049/jimmunol.180.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davenport A, Younie ME, Parsons JEM, et al. Development of cytotoxic antibodies following renal allograft transplantation is associated with reduced graft survival due to chronic vascular rejection. Nephrol Dial Transplant. 1994;9:1315–19. [PubMed] [Google Scholar]
- 30.Abe M, Kawai T, Futatsuyama K, et al. Postoperative production of anti-donor antibody and chronic rejection in renal transplantation. Transplantation. 1997;63:616–1619. doi: 10.1097/00007890-199706150-00014. [DOI] [PubMed] [Google Scholar]
- 31.Piazza A, Poggi E, Borrelli L, et al. Impact of donor-specific antibodies on chronic rejection occurrence and graft loss in renal transplantation: Posttransplant analysis using flow cytometric techniques. Transplantation. 2001;71:1106–12. doi: 10.1097/00007890-200104270-00017. [DOI] [PubMed] [Google Scholar]
- 32.Lee P-C, Terasaki PI, Takemoto SK, et al. All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation. 2002;74:1192–4. doi: 10.1097/00007890-200210270-00025. [DOI] [PubMed] [Google Scholar]
- 33.Worthington JE, Martin S, Al-Husseini DM, et al. Posttransplantation production of donor HLA-specific antibodies as a predictor of renal transplant outcome. Transplantation. 2003;75:1034–40. doi: 10.1097/01.TP.0000055833.65192.3B. [DOI] [PubMed] [Google Scholar]
- 34.Cardarelli F, Pascual M, Tolkoff-Rubin N, et al. Prevalence and significance of anti-HLA and donor-specific antibodies long-term after renal transplantation. Transpl Int. 2005;18:532–40. doi: 10.1111/j.1432-2277.2005.00085.x. [DOI] [PubMed] [Google Scholar]
- 35.Wehner J, Morrell CN, Reynolds T, et al. Antibody and complement in transplant vasculopathy. Circ Res. 2007;100:191–203. doi: 10.1161/01.RES.0000255032.33661.88. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin WM, III, Qian Z, Ota H, et al. Complement as a mediator of vascular inflammation and activation in allografts. J Heart Lung Transpl. 2000;19:723–30. doi: 10.1016/s1053-2498(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 37.Smith RN, Brousaides N, Grazette L, et al. C4d Deposition in Cardiac Allografts Correlates With Alloantibody. J Heart Lung Transplant. 2005;24(9):1202–10. doi: 10.1016/j.healun.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Lee C-Y, Lotfi-Emran S, Erdinc M, et al. The Involvement of FcR Mechanisms in Antibody-Mediated Rejection. Transplantation. 2007;84:1324–34. doi: 10.1097/01.tp.0000287457.54761.53. [DOI] [PubMed] [Google Scholar]
- 39.Rahimi S, Qian Z, Layton J, et al. Non-complement- and complement-activating antibodies synergize to cause rejection of cardiac allografts. Am J of Transplant. 2004;4:326–34. doi: 10.1111/j.1600-6143.2004.00334.x. [DOI] [PubMed] [Google Scholar]
- 40.Hirohashi T, Uehara S, Chase CM, et al. Complement independent antibody-mediated endarteritis and transplant arteriopathy in mice. Am J Transplant. 2010;10:510–17. doi: 10.1111/j.1600-6143.2009.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derhaag JG, Duijvestijn AM, Damoiseaux JG, et al. Effects of antibody reactivity to major histocompatibility complex (MHC) and non-MHC alloantigens on graft endothelial cells in heart allograft rejection. Transplantation. 2000;69:1899–906. doi: 10.1097/00007890-200005150-00027. [DOI] [PubMed] [Google Scholar]
- 42.Duijvestijn AM, Derhaag JG, van Breda Vriesman PJC. Complement activation by anti-endothelial cell antibodies in MHC-mismatched and MHC-matched heart allograft rejection: Anti-MHC-, but not anti non-MHC alloantibodies are effective in complement activation. Transpl Int. 2000;13:363–71. doi: 10.1007/s001470050715. [DOI] [PubMed] [Google Scholar]
- 43.Direskeneli H, Keser G, D'Cruz D, et al. Anti-endothelial cell antibodies, endothelial proliferation and von Willebrand factor antigen in Behçet's disease. Clin Rheum. 1995;14:55–61. doi: 10.1007/BF02208085. [DOI] [PubMed] [Google Scholar]
- 44.Jin Y-P, Fishbein MC, Said JW, et al. Anti-HLA class I antibody-mediated activation of the PI3K/Akt signaling pathway and induction of Bcl-2 and Bcl-xL expression in endothelial cells. Hum Immunol. 2004;65:291–302. doi: 10.1016/j.humimm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Truman J-P, Choqueux C, Charron D, et al. HLA Class II molecule signal transduction leads to either apoptosis or activation via two different pathways. Cell Immunol. 1996;172:149–57. doi: 10.1006/cimm.1996.0227. [DOI] [PubMed] [Google Scholar]
- 46.Skov S. Intracellular signal transduction mediated by ligation of MHC class 1 molecules. Tissue Antigens. 1998;51:215–18. [PubMed] [Google Scholar]
- 47.Skov S, Bregenholt S, Claesson M. MHC class I ligation of human T cells activates the ZAP70 and p56lck tyrosine kinases, leads to an alternative phenotype of the TCR/CD3 zeta- chain, and induces apoptosis. J Immunol. 1997;158:3189–96. [PubMed] [Google Scholar]
- 48.Rich T, Lawler S, Lord J, et al. HLA class II-induced translocation of PKC alpha and PKC beta II isoforms is abrogated following truncation of DR beta cytoplasmic domains. J Immunol. 1997;159:3792–98. [PubMed] [Google Scholar]
- 49.Haylett RS, Koch N, Rink L. MHC class II molecules activate NFAT and the ERK group of MAPK through distinct signaling pathways in B cells. Eur J Immunol. 2009;39:1947–55. doi: 10.1002/eji.200838992. [DOI] [PubMed] [Google Scholar]
- 50.Bian H, Harris P, Reed EF. Ligation of HLA class I molecules on smooth muscle cells with anti-HLA antibodies induces tyrosine phosphorylation, fibroblast growth factor receptor expression and cell proliferation. Int Immunol. 1998;10:1315–23. doi: 10.1093/intimm/10.9.1315. [DOI] [PubMed] [Google Scholar]
- 51.Harris P, Bian H, Reed EF. Induction of high affinity fibroblast growth factor receptor expression and proliferation in human endothelial cells by anti-HLA antibodies: a possible mechanism for transplant atherosclerosis. J Immunol. 1997;159:5697–704. [PubMed] [Google Scholar]
- 52.Bian H, Reed EF. Alloantibody-Mediated Class I signal transduction in endothelial cells and smooth muscle cells: Enhancement by IFN-{gamma} and TNF-{alpha} J Immunol. 1999;163:1010–18. [PubMed] [Google Scholar]
- 53.Smith JD, Lawson C, Yacoub MH, et al. Activation of NF-{kappa}B in human endothelial cells induced by monoclonal and allospecific HLA antibodies. Int Immunol. 2000;12:563–71. doi: 10.1093/intimm/12.4.563. [DOI] [PubMed] [Google Scholar]
- 54.Coupel S, Leboeuf F, Boulday G, et al. RhoA Activation mediates phosphatidylinositol 3-kinase-dependent proliferation of human vascular endothelial cells: an alloimmune mechanism of chronic allograft nephropathy. J Am Soc Nephrol. 2004;15:2429–39. doi: 10.1097/01.ASN.0000138237.42675.45. [DOI] [PubMed] [Google Scholar]
- 55.Bieri M, Oroszlan M, Farkas A, et al. Anti-HLA I antibodies induce VEGF production by endothelial cells, which increases proliferation and paracellular permeability. Internat J Biochem Cell Biol. 2009;41:2422–30. doi: 10.1016/j.biocel.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Reyes-Vargas E, Pavlov IY, Martins TB, et al. Binding of anti-HLA class I antibody to endothelial cells produce an inflammatory cytokine secretory pattern. J Clin Lab Anal. 2009;23:157–60. doi: 10.1002/jcla.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin Y-P, Singh RP, Du Z-Y, et al. Ligation of HLA Class I molecules on endothelial cells induces phosphorylation of src, paxillin, and focal adhesion kinase in an actin-dependent manner. J Immunol. 2002;168:5415–23. doi: 10.4049/jimmunol.168.11.5415. [DOI] [PubMed] [Google Scholar]
- 58.Jin Y-P, Korin Y, Zhang X, et al. RNA interference elucidates the role of focal adhesion kinase in HLA Class I-mediated focal adhesion complex formation and proliferation in human endothelial cells. J Immunol. 2007;178:7911–22. doi: 10.4049/jimmunol.178.12.7911. [DOI] [PubMed] [Google Scholar]
- 59.Reznik SI, Jaramillo A, Zhang L, et al. Anti-HLA antibody binding to HLA class I molecules induces proliferation of airway epithelial cells: A potential mechanism for bronchiolitis obliterans syndrome. J Thor Cardiovasc Sur. 2000;119:39–45. doi: 10.1016/s0022-5223(00)70215-7. [DOI] [PubMed] [Google Scholar]
- 60.Jaramillo A, Smith CR, Maruyama T, et al. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Hum Immunol. 2003;64:521–29. doi: 10.1016/s0198-8859(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 61.Li F, Atz ME, Reed EF. Human leukocyte antigen antibodies in chronic transplant vasculopathy--mechanisms and pathways. Curr Opin Immunol. 2009;21:557–62. doi: 10.1016/j.coi.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Atz ME, Reed EF. Role of anti-MHC class I antibody in facilitating transplant accommodation. Crit Rev Immunol. 2008;28:485–511. doi: 10.1615/critrevimmunol.v28.i6.20. [DOI] [PubMed] [Google Scholar]
- 63.Lepin EJ, Jin Y-P, Barwe SP, et al. HLA class I signal transduction is dependent on Rho GTPase and ROK. Biochem Biophys Res Com. 2004;323:213–17. doi: 10.1016/j.bbrc.2004.08.082. [DOI] [PubMed] [Google Scholar]
- 64.Ohki S, Iizuka K, Ishikawa S, et al. A highly selective inhibitor of Rho-associated coiled-coil forming protein kinase, Y-27632, prolongs cardiac allograft survival of the BALB/c-to-C3H/He mouse model. J Heart Lung Transplant. 2001;20:956–63. doi: 10.1016/s1053-2498(01)00292-3. [DOI] [PubMed] [Google Scholar]
- 65.Hattori T, Shimokawa H, Higashi M, et al. Long-term treatment with a specific Rho-kinase inhibitor suppresses cardiac allograft vasculopathy in mice. Circ Res. 2004;94:46–52. doi: 10.1161/01.RES.0000107196.21335.2B. [DOI] [PubMed] [Google Scholar]
- 66.Mammoto A, Ingber DE. Cytoskeletal control of growth and cell fate switching. Curr Opin Cell Biol. 2009;21:864–70. doi: 10.1016/j.ceb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Narayanan K, Jaramillo A, Phelan DL, et al. Pre-exposure to sub-saturating concentrations of HLA class I antibodies confers resistance to endothelial cells against antibody complement-mediated lysis by regulating Bad through the phosphatidylinositol 3-kinase/Akt pathway. Eur J Immunol. 2004;34:2303–12. doi: 10.1002/eji.200324843. [DOI] [PubMed] [Google Scholar]
- 68.Narayanan K, Jendrisak MD, Phelan DL, et al. HLA class I antibody mediated accommodation of endothelial cells via the activation of PI3K/cAMP dependent PKA pathway. Transplant Immunol. 2006;15:187–97. doi: 10.1016/j.trim.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Jindra PT, Jin Y-P, Rozengurt E, et al. HLA Class I antibody-mediated endothelial cell proliferation via the mTOR pathway. J Immunol. 2008;180:2357–66. doi: 10.4049/jimmunol.180.4.2357. [DOI] [PubMed] [Google Scholar]
- 70.Jindra PT, Reed EF. MHC class I signaling via anti-HLA antibodies induce the mTOR/S6 kinase signaling pathway. Hum Immunol. 2005;66(Suppl 1):83. [Google Scholar]
- 71.Lepin EJ, Zhang Q, Zhang X, et al. Phosphorylated s6 ribosomal protein: a novel biomarker of antibody-mediated rejection in heart allografts. Am J Transplant. 2006;6:1560–71. doi: 10.1111/j.1600-6143.2006.01355.x. [DOI] [PubMed] [Google Scholar]
- 72.Jindra PT, Jin Y-P, Jacamo R, et al. MHC class I and integrin ligation induce ERK activation via an mTORC2-dependent pathway. Biochem Biophys Res Com. 2008;369:781–87. doi: 10.1016/j.bbrc.2008.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nath N, Bian H, Reed EF, et al. HLA Class I-mediated induction of cell proliferation involves cyclin E-mediated inactivation of Rb function and induction of E2F activity. J Immunol. 1999;162:5351–58. [PubMed] [Google Scholar]
- 74.Salama AD, Delikouras A, Pusey CD, et al. Transplant Accommodation in Highly Sensitized Patients: A Potential Role for Bcl-xL and Alloantibody. Am J Transplant. 2001;1:260–69. doi: 10.1034/j.1600-6143.2001.001003260.x. [DOI] [PubMed] [Google Scholar]
- 75.Iwasaki K, Miwa Y, Haneda M, et al. Significance of HLA class I antibody-induced antioxidant gene expression for endothelial cell protection against complement attack. Biochem Biophys Res Com. 2010;391:1210–15. doi: 10.1016/j.bbrc.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 76.Hancock W, Buelow R, Sayegh M, et al. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nature Med. 1998;4:1392–96. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 77.Du D, Chang S, Chen B, et al. Adenovirus-mediated Heme oxygenase transfer inhibits graft arteriosclerosis in rat aortic transplants. Transplant Proc. 2007;39:3446–48. doi: 10.1016/j.transproceed.2007.03.114. [DOI] [PubMed] [Google Scholar]
- 78.Kinderlerer AR, Pombo Gregoire I, Hamdulay SS, et al. Heme oxygenase-1 expression enhances vascular endothelial resistance to complement-mediated injury through induction of decay-accelerating factor: a role for increased bilirubin and ferritin. Blood. 2009;113:1598–607. doi: 10.1182/blood-2008-04-152934. [DOI] [PubMed] [Google Scholar]
- 79.Fehlmann M, Peyron JF, Samson M, et al. Molecular association between major histocompatibility complex class I antigens and insulin receptors in mouse liver membranes. Proc Nat Acad Sci. 1985;82:8634–37. doi: 10.1073/pnas.82.24.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramalingam TS, Chakrabarti A, Edidin M. Interaction of Class I Human Leukocyte Antigen (HLA-I) molecules with insulin receptors and its effect on the insulin-signaling cascade. Mol Biol Cell. 1997;8:2463–74. doi: 10.1091/mbc.8.12.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rubinstein E, Naour FL, Lagaudrière-Gesbert C, et al. CD9, CD63, CD81, and CD82 are components of a surface tetraspan network connected to HLA-DR and VLA integrins. Eur J Immunol. 1996;26:2657–65. doi: 10.1002/eji.1830261117. [DOI] [PubMed] [Google Scholar]
- 82.Zhang X, Reed EF. MHC Class I Molecules Partner with Integrin beta4 to Stimulate Endothelial Cell Proliferation and Migration. Science Signaling. doi: 10.1126/scisignal.2001158. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Madge LA, Pober JS. TNF Signaling in Vascular Endothelial Cells. Exper Mol Path. 2001;70:317–25. doi: 10.1006/exmp.2001.2368. [DOI] [PubMed] [Google Scholar]
- 84.Turco MC, Romano MF, Lamberti A, et al. Induction of nuclear factor kB/Rel nuclear activity in human peripheral blood T lymphocytes by anti-HLA class I monoclonal antibodies. Tissue Antigens. 1997;50:1–7. doi: 10.1111/j.1399-0039.1997.tb02826.x. [DOI] [PubMed] [Google Scholar]
- 85.Grosjean J, Kiriakidis S, Reilly K, et al. Vascular endothelial growth factor signalling in endothelial cell survival: A role for NF[kappa]B. Biochem Biophys Res Com. 2006;340:984–94. doi: 10.1016/j.bbrc.2005.12.095. [DOI] [PubMed] [Google Scholar]
- 86.Luo J-L, Kamata H, Karin M. IKK/NF-kB signaling: balancing life and death: a new approach to cancer therapy. J Clin Invest. 2005;115:2625–32. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Daniel S, Patel VI, Shrikhande GV, et al. The universal NF-kappaB inhibitor A20 protects from transplant vasculopathy by differentially affecting apoptosis in endothelial and smooth muscle cells. Transplant Proc. 2006;38:3225–27. doi: 10.1016/j.transproceed.2006.10.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saadi S, Holzknecht RA, Patte CP, et al. Complement-mediated regulation of tissue factor activity in endothelium. J Exp Med. 1995;182:1807–14. doi: 10.1084/jem.182.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Selvan RS, Kapadia HB, Platt JL. Complement-induced expression of chemokine genes in endothelium: Regulation by IL-1-dependent and -independent mechanisms. J Immunol. 1998;161:4388–95. [PubMed] [Google Scholar]
- 90.Li A, Varney ML, Valasek J, et al. Autocrine role of Interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis. 2005;8:63–71. doi: 10.1007/s10456-005-5208-4. [DOI] [PubMed] [Google Scholar]
- 91.Versteeg HH, Peppelenbosch MP, Spek CA. Tissue factor signal transduction in angiogenesis. Carcinogenesis. 2003;24:1009–13. doi: 10.1093/carcin/bgg039. [DOI] [PubMed] [Google Scholar]
- 92.Pradier A, Ettelaie C. The influence of exogenous tissue factor on the regulators of proliferation and apoptosis in endothelial cells. J Vasc Res. 2008;45:19–32. doi: 10.1159/000109074. [DOI] [PubMed] [Google Scholar]
- 93.Holschermann H, Bohle RM, Zeller H, et al. In situ detection of tissue factor within the coronary intima in rat cardiac allograft vasculopathy. Am J Pathol. 1999;154:211–20. doi: 10.1016/S0002-9440(10)65267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holschermann H, Bohle RM, Schmidt H, et al. Hirudin reduces tissue factor expression and attenuates graft arteriosclerosis in rat cardiac allografts. Circulation. 2000;102:357–63. doi: 10.1161/01.cir.102.3.357. [DOI] [PubMed] [Google Scholar]
- 95.Yamani MH, Masri CS, Ratliff NB, et al. The role of vitronectin receptor ({alpha}v{beta}3) and tissue factor in the pathogenesis of transplant coronary vasculopathy. J Am Coll Cardiol. 2002;39:804–10. doi: 10.1016/s0735-1097(01)01823-x. [DOI] [PubMed] [Google Scholar]
- 96.Yen MH, Pilkington G, Starling RC, et al. Increased tissue factor expression predicts development of cardiac allograft vasculopathy. Circulation. 2002;106:1379–83. doi: 10.1161/01.cir.0000028588.73765.b4. [DOI] [PubMed] [Google Scholar]
- 97.Naji A, Deschaseaux F, Racadot E, et al. Induction of tissue factor expression on human umbilical vein endothelial cells by cell-specific HLA Class I Antibody: Preliminary data. Transplant Proc. 2005;37:2892–93. doi: 10.1016/j.transproceed.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 98.Reinders MEJ, Fang JC, Wong W, et al. Expression patterns of vascular endothelial growth factor in human cardiac allografts: association with rejection. Transplantation. 2003;76:224–30. doi: 10.1097/01.TP.0000071363.55007.D0. [DOI] [PubMed] [Google Scholar]
- 99.Lemstrom KB, Krebs R, Nykanen AI, et al. Vascular Endothelial Growth Factor enhances cardiac allograft arteriosclerosis. Circulation. 2002;105:2524–30. doi: 10.1161/01.cir.0000016821.76177.d2. [DOI] [PubMed] [Google Scholar]
- 100.Raisky O, Nykanen AI, Krebs R, et al. VEGFR-1 and -2 regulate inflammation, myocardial angiogenesis, and arteriosclerosis in chronically rejecting cardiac allografts. Arterioscler Thromb Vasc Biol. 2007;27:819–25. doi: 10.1161/01.ATV.0000260001.55955.6c. [DOI] [PubMed] [Google Scholar]
- 101.Thaunat O, Louedec L, Dai J, et al. Direct and indirect effects of alloantibodies link neointimal and medial remodeling in graft arteriosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2359–65. doi: 10.1161/01.ATV.0000241980.09816.ac. [DOI] [PubMed] [Google Scholar]
- 102.Jaramillo A, Zhang L, Mohanakumar T. Binding of anti-HLA class I antibodies to airway epithelial cells induces activation and growth factor production and indirectly upregulates lung fibroblast proliferation. J Heart Lung Transplant. 2001;20:166. doi: 10.1016/s1053-2498(00)00304-1. [DOI] [PubMed] [Google Scholar]
- 103.Edwards I, Wagner W, Owens R. Macrophage secretory products selectively stimulate dermatan sulfate proteoglycan production in cultured arterial smooth muscle cells. Am J Pathol. 1990;136:609–21. [PMC free article] [PubMed] [Google Scholar]
- 104.Schubert SY, Benarroch A, Ostvang J, et al. Regulation of endothelial cell proliferation by primary monocytes. Arterioscler Thromb Vasc Biol. 2008;28:97–104. doi: 10.1161/ATVBAHA.107.157537. [DOI] [PubMed] [Google Scholar]
- 105.Azuma H, Nadeau KC, Ishibashi M, et al. Prevention of functional, structural, and molecular changes of chronic rejection of rat renal allografts by a specific macrophage inhibitor. Transplantation. 1995;60:1577–82. doi: 10.1097/00007890-199560120-00034. [DOI] [PubMed] [Google Scholar]
- 106.Kitchens WH, Chase CM, Uehara S, et al. Macrophage depletion suppresses cardiac allograft vasculopathy in mice. Am J Transplant. 2007;7:2675–82. doi: 10.1111/j.1600-6143.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- 107.Belperio JA, Keane MP, Burdick MD, et al. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108:547–56. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Russell ME. Macrophages in chronic rejection and graft vasculopathy: A diverse and dynamic cell with myriad roles. Transplantation Reviews. 1999;13:157–68. [Google Scholar]
- 109.Wyburn KR, Jose MD, Wu H, et al. The role of macrophages in allograft rejection. Transplantation. 2005;80:1641–47. doi: 10.1097/01.tp.0000173903.26886.20. editorial. [DOI] [PubMed] [Google Scholar]
- 110.Morrell CN, Murata K, Swaim AM, et al. In vivo platelet-endothelial cell interactions in response to Major Histocompatibility Complex alloantibody. Circ Res. 2008;102:777–85. doi: 10.1161/CIRCRESAHA.107.170332. [DOI] [PubMed] [Google Scholar]
- 111.Ortiz-Stern A, Rosales C. Cross-talk between Fc receptors and integrins. Immunol Lett. 2003;90:137–43. doi: 10.1016/j.imlet.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 112.Bian H, Reed EF. Anti-HLA class I antibodies transduce signals in endothelial cells resulting in FGF receptor translocation, down-regulation of ICAM-1 and cell proliferation. Transplant Proc. 2001;33:311. doi: 10.1016/s0041-1345(00)02022-4. [DOI] [PubMed] [Google Scholar]
- 113.Yamakuchi M, Kirkiles-Smith NC, Ferlito M, et al. Antibody to human leukocyte antigen triggers endothelial exocytosis. Proc Nat Acad Sci. 2007;104:1301–06. doi: 10.1073/pnas.0602035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koskinen PK, Lemstrom KB. Adhesion molecule P-Selectin and Vascular Cell Adhesion Molecule-1 in enhanced heart allograft arteriosclerosis in the rat. Circulation. 1997;95:191–96. doi: 10.1161/01.cir.95.1.191. [DOI] [PubMed] [Google Scholar]
- 115.Phillips JW, Barringhaus KG, Sanders JM, et al. Single injection of P-Selectin or P-Selectin Glycoprotein Ligand-1 monoclonal antibody blocks neointima formation after arterial injury in Apolipoprotein E-Deficient Mice. Circulation. 2003;107:2244–49. doi: 10.1161/01.CIR.0000065604.56839.18. [DOI] [PubMed] [Google Scholar]
- 116.Reinders MEJ, Masayuki Sho, Izawa A, et al. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J Clin Invest. 2003;112:1655–65. doi: 10.1172/JCI17712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gerszten RE, Garcia-Zepeda EA, Lim Y-C, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–23. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 118.Van Hoffen E, Van Wichen D, Stuji I, et al. In situ expression of cytokines in human heart allografts. Am J Pathol. 1996;149:1991–2003. [PMC free article] [PubMed] [Google Scholar]
- 119.Cohen T, Nahari D, Cerem LW, et al. Interleukin 6 induces the expression of Vascular Endothelial Growth Factor. J Biol Chem. 1996;271:736–41. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 120.Wang Z, Newman WH. Smooth muscle cell migration stimulated by Interleukin 6 is associated with cytoskeletal reorganization. J Surg Res. 2003;111:261–66. doi: 10.1016/s0022-4804(03)00087-8. [DOI] [PubMed] [Google Scholar]
- 121.Holzinger C, Weissinger E, Zuckermann A, et al. Effects of interleukin-1, -2, -4, -6, interferon-gamma and granulocyte/macrophage colony stimulating factor on human vascular endothelial cells. Immunol Lett. 1993;35:109–17. doi: 10.1016/0165-2478(93)90078-g. [DOI] [PubMed] [Google Scholar]
- 122.Maruo N, Morita I, Shirao M, et al. IL-6 increases endothelial permeability in vitro. Endocrinol. 1992;131:710–14. doi: 10.1210/endo.131.2.1639018. [DOI] [PubMed] [Google Scholar]
- 123.Gullestad L, Simonsen S, Ueland T, et al. Possible role of proinflammatory cytokines in heart allograft coronary artery disease. Am J Cardiol. 1999;84:99–1003. doi: 10.1016/s0002-9149(99)00487-7. [DOI] [PubMed] [Google Scholar]
- 124.Zhao XM, Frist WH, Yeoh TK, et al. Expression of cytokine genes in human cardiac allografts: correlation of IL-6 and transforming growth factor-beta (TGF-beta) with histological rejection. Clin Exp Immunol. 1993;93:448–51. doi: 10.1111/j.1365-2249.1993.tb08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clauss M, Gerlach M, Gerlach H, et al. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990;172:1535–45. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grosskreutz CL, Anand-Apte B, Dupláa C, et al. Vascular Endothelial Growth Factor-induced migration of vascular smooth muscle cells in vitro. Microvasc Res. 1999;58:128–36. doi: 10.1006/mvre.1999.2171. [DOI] [PubMed] [Google Scholar]
- 127.Bernatchez PN, Soker S, Sirois MG. Vascular Endothelial Growth Factor Effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis Is Flk-1-dependent. J Biol Chem. 1999;274:31047–54. doi: 10.1074/jbc.274.43.31047. [DOI] [PubMed] [Google Scholar]
- 128.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the [beta]-arrestin-dependent endocytosis of VE-cadherin. Nature. 2006;8:1223–34. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 129.Kim I, Moon S-O, Hoon Kim S, et al. Vascular Endothelial Growth Factor expression of Intercellular Adhesion Molecule 1 (ICAM-1), Vascular Cell Adhesion Molecule 1 (VCAM-1), and E-selectin through Nuclear Factor-kB activation in endothelial cells. J Biol Chem. 2001;276:7614–20. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 130.Zittermann SI, Issekutz AC. Endothelial growth factors VEGF and bFGF differentially enhance monocyte and neutrophil recruitment to inflammation. J Leukoc Biol. 2006;80:247–57. doi: 10.1189/jlb.1205718. [DOI] [PubMed] [Google Scholar]
- 131.Matsushita K, Yamakuchi M, Morrell CN, et al. Vascular endothelial growth factor regulation of Weibel-Palade-body exocytosis. Blood. 2005;105:207–14. doi: 10.1182/blood-2004-04-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barleon B, Sozzani S, Zhou D, et al. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–43. [PubMed] [Google Scholar]
- 133.Weber KSC, Nelson PJ, Grone H-J, et al. Expression of CCR2 by endothelial cells : Implications for MCP-1 mediated wound injury repair and in vivo inflammatory activation of endothelium. Arterioscler Thromb Vasc Biol. 1999;19:2085–93. doi: 10.1161/01.atv.19.9.2085. [DOI] [PubMed] [Google Scholar]
- 134.Yun JJ, Fischbein MP, Laks H, et al. Early and late chemokine production correlates with cellular recruitment in cardiac allograft vasculopathy. Transplantation. 2000;69:2515–24. doi: 10.1097/00007890-200006270-00009. [DOI] [PubMed] [Google Scholar]
- 135.Pattison JM, Nelson PJ, Huie P, et al. RANTES chemokine expression in transplant-associated accelerated atherosclerosis. J Heart Lung Transplant. 1996;15:1194–9. [PubMed] [Google Scholar]
- 136.Yun JJ, Fischbein MP, Laks H, et al. RANTES production during development of cardiac allograft vasculopathy. Transplantation. 2001;70:1649–56. doi: 10.1097/00007890-200106150-00026. [DOI] [PubMed] [Google Scholar]