Abstract
Background & Aims
Our previous studies showed that CD133, EpCAM and aldehyde dehydrogenase (ALDH) are useful markers to identify cancer stem cells (CSCs) in hepatocellular carcinoma (HCC) tissues. The present study aims to evaluate chemosensitivity and invasion capability of HCC based on CSC marker profiles, and to explore underlying molecular mechanisms.
Methods
Hepatoma cell lines were separated into subpopulations according to CD133, EpCAM and ALDH expression profiles. Epithelial mesenchymal transition (EMT) and hedgehog (Hh) signaling were examined to identify their links with chemoresistance and aggressive invasion.
Results
Well-differentiated cell lines were positive for CD133+/ALDHhigh and CD133+/EpCAM+ at 1.5–15% and 2.3–8.3%; whereas, poorly-differentiated cells were almost all negative for these markers. FACS-enriched CD133+/ALDHhigh and CD133+/EpCAM+ Hep3B and Huh-7 cells formed more spheroids in vitro. CD133−/ALDHlow HLE cells were more resistant to cisplatin, doxorubicin or sorafenib than their positive counterparts. CD133−/EpCAM− Huh-7 cells or CD133−/ALDH− HLE cells exhibited a higher invasion rate than their positive counterparts. HLE and HLF cells acquired EMT in double negative subpopulations. Hh activity in Huh-7 CD133−/EpCAM− cells was higher than in their positive counterparts, and the inhibition of Hh activity by cyclopamine resulted in reduced cell proliferation.
Conclusions
Well-differentiated CD133+/ALDHhigh or CD133+/EpCAM+ cells appear to be a CSC/initiating subpopulation; whereas, in poorly-differentiated hepatoma cells, EMT and enhanced hedgehog signaling activity may be responsible for their chemoresistance and invasion. These findings underscore the significance of EMT and enhanced Hh signaling in liver cancer stem or initiating cells.
Keywords: Hepatocellular carcinoma, Cancer stem cells, Chemoresistance, Epithelial mesenchymal transition, Hedgehog signaling
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third most deadly and the fifth most common malignancy worldwide, with an estimation of 600,000 new cases per year [1]. Surgical removal and liver transplantation remain the most effective therapy for HCC. Although various adjuvant approaches to treat non-resectable HCC are available, the efficacy and three year survival rate (30–40%) are not promising [2].
Cancer stem cells (CSCs) are a newly identified subpopulation that possesses stem cell properties, and may differentiate into heterogeneous progenies of malignant cells [3]. CSCs are thought to be the cells that are least sensitive to chemotherapy or radiotherapy, and develop resistance to pharmacologic, biologic or radiotherapy [4]. These cells are probably the source for tumor metastasis and relapse. A single CD133+ cell from a colon cancer was able to form a tumor in the renal capsule of immuno-deficient mice [5]. High aldehyde dehydrogenase (ALDH) activity is a common feature of stem cells, and bone marrow-derived progenitor cells with high ALDH activity displayed great engrafting potential in mouse liver [6]. EpCAM-positive cells have been implicated as initiating/stem cells in pancreatic cancer [7]. In a recent study, we have identified a cluster of CD133+/ALDHhigh cells in human HCC tissue, especially in invaded vessels, and it was demonstrated that combined staining of CD133, ALDH, EpCAM, as well as CD44 and CD90 could identify CSCs in HCC [8].
In order to further investigate the relationship between phenotypic characteristics of CSCs and tumorigenicity, chemosensitivity or invasion capability, we separated subpopulations of hepatoma cells according to their CSC marker expression profile. It was found that CD133+/ALDHhigh and CD133+/EpCAM+ cells were more tumorigenic, whereas, CD133−/ALDHlow cells were more chemoresistant and metastatic as compared to their positive counterparts. We further investigated the markers of epithelial mesenchymal transition (EMT) and hedgehog (Hh) signaling activity, and found that the occurrence of EMT and enhanced Hh signaling activity appear to be responsible for the chemoresistance, aggressive invasion and proliferation seen in CD133−/ALDHlow hepatoma cell lines. Our study underscores the significance of EMT and the Hh signaling pathway in liver cancer stem or initiating cells.
METHODS AND MATERIALS
Cell culture
HepG2, Hep3B, Huh-7 and SKHep1 hepatoma cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). HLE and HLF cells were kindly provided by Health Science Research Resources Bank, Tokyo, Japan. HepG2 and Hep3B cells were cultured as reported previously [9].
Fluorescence-activated cell sorting (FACS)
CD133+/ALDHhigh and CD133−/ALDHlow subpopulations in HepG2, Hep3B, Huh-7, SKHep-1, HLE and HLF cell lines were enriched by FACS using allophycocyanin (APC)-conjugated monoclonal antibodies against human CD133 (Miltenyi Biotec Inc, Auburn, CA, USA) and an Aldefluor kit (STEMCELL Technologies, Vancouver, BC, Canada), which labels ALDH-positive cells in green fluorescence as we have described [6]. CD133+/EpCAM+ and CD133−/EpCAM− subpopulations in Hep3B, Huh-7, HLE and HLF cell lines were enriched using APC-conjugated monoclonal antibodies against human CD133 and FITC-conjugated monoclonal antibodies against human EpCAM (Dako North America, Inc. Carpinteria, CA, USA). The staining protocol followed a report [6] with modification. Briefly, detached cells were blocked with 20% rabbit serum, and incubated with monoclonal antibodies (CD133/EpCAM). Then, cells were suspended in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) until sorting. For CD133/ALDH staining, after being labeled with APC-conjugated antibodies against CD133, cells were further incubated with Aldefluor substrate. All labeled cells were sorted in a high speed Cytopeia Influx Cell Sorter (BD Biosciences, San Jose, CA, USA). After sorting, hepatoma subpopulations were cultured in regular medium for the assays described below (see the supplemental information for methods not included in this part).
Formation of spheroid cores and immunofluorescent staining
FACS-enriched subpopulations of 2×103 cells were seeded in 24-well low attachment culture plates, and cultured for 3 weeks. Then spheroid cores were counted, fixed in 4% PBS-buffered paraformaldehyde, and incubated with the primary antibodies against CD133, α-fetal protein (AFP), and proliferating cell nuclear antigen (PCNA) in combinations for double staining as described by us [8]. Stained spheroids were visualized under a Zeiss LSM510 laser scanning confocal microscope (Oberkochen, Germany).
Western blotting analysis
Total protein and the subcellular fractions of the membrane, cytosolic, and nuclear soluble proteins were extracted from FACS-isolated cell subpopulations using corresponding Extraction kits (Pierce Biotech, Rockford, IL, USA). Protein content was measured with a BCA protein assay (Pierce Biotech, Rockford IL, USA). Western blotting assay was performed as reported previously [10] and the membranes were blotted separately with primary antibodies: monoclonal anti-E-cadherin, anti-vimentin and anti-Zeb1 (Santa Cruz Biotechnologies, Santa Cruz, CA, USA), polyclonal rabbit anti-GLI2 (Abcam), monoclonal anti-snail (Sigma), monoclonal anti-Patched/PTCH1 (Abcam). Monoclonal anti-β-actin (Sigma), anti-integrin β1 (Santa Cruz) and anti-TATA box binding protein (Abcam) were used as loading controls for cytosolic, membrane and nuclear fractions.
Hh signaling activity by a GLI-lux reporter system
In order to determine Hh signaling activity in FACS-enriched hepatoma subpopulations, we used a GLI-lux reporter system, in which the firefly luciferase gene is driven by the GLI promoter [11]. FACS-purified Huh-7 CD133+/EpCAM+ or CD133−/EpCAM− subpopulations were transfected with the GLI-Lux reporter gene plasmid using FuGENE 6 (Roche Applied Science, Indianapolis, IN, USA). The medium was replaced with medium containing cyclopamine (CPM) (Sigma), an Hh signaling pathway inhibitor, 12 hrs after transfection. Transfected cells were lysed 48h after transfection using reporter lysis buffer. Luciferase activity was determined as we have previously reported [12].
Xenograft formation in immunodeficient mice
All animal experiments were performed in compliance with the NIH Guidelines for experimental animals, and the animal protocol was approved by the UC Davis Institutional Animal Care and Use Committee (IACUC). Immediately following FACS enrichment, 2000 cells from the CD133+/EpCAM+ and CD133−/EpCAM− Hep3B and Huh-7 subpopulations were implanted in the left renal capsule of immunodeficient NOD-SCID-IL2Rγ−/− mice with an aseptic surgical procedure under an anesthetic cocktail consisting of xylazine (5–10 mg/kg) and ketamine (50–100 mg/kg, i.p.). The implanted mice were observed for up to 4 months, and xenograft formation was recorded.
Statistical analysis
All the experiments were performed three times with a minimum of triplicates. The data were expressed as means ± standard error of the mean (SEM), and analyzed by student t test for difference between two groups or variance analysis and further Newman-Keuls tests for multiple comparisons among groups. The occurrence of xenograft tumors in NOD-SCID mice in different groups was analyzed by the Chi-square test. p<0.05 was considered as statistically significant.
RESULTS
FACS enrichment of hepatoma subpopulations
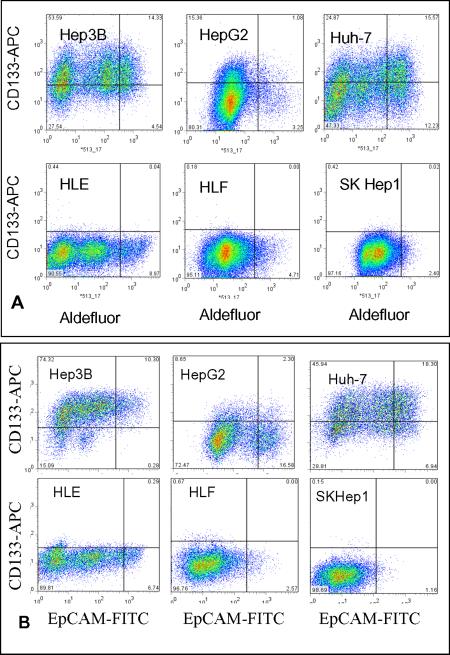
We employed well-characterized hepatoma cell lines, HepG2, Hep3B, Huh-7, HLE, HLF and SKHep1, in which HepG2, Hep3B and Huh-7 hepatoma cells are well-differentiated with a high level hepatic-specific gene expression. In contrast, the remaining three were poorly-differentiated with low levels of hepatic gene expression. We first separated CD133+/ALDHhigh and CD133−/ALDHlow subpopulations by FACS enrichment. It is clear in Fig. 1A that well-differentiated hepatoma cell lines displayed a relatively higher CD133 expression than poorly-differentiated lines. As a result, the CD133+/ALDHhigh subpopulation in Hep3B, HepG2 and Huh-7 cells was 13.4, 1.1 and 15.6%, respectively; whereas poorly differentiated HLE, HLF and SKHep1 cells were almost all negative for these two markers. EpCAM has been suggested to be a CSC marker in epithelial-derived malignancies. We further separated CD133+/EpCAM+ or CD133−/EpCAM− subpopulations in these cell lines. As shown in Fig. 1B, CD133+/EpCAM+ subpopulations in Hep3B, HepG2 and Huh-7 were 10.3, 2.3 and 18.3% positive; in contrast, HLE, HLF and SKHep1 cells were almost all negative for these two markers. We were not able to obtain a CD133+/EpCAM+ subpopulation from these poorly-differentiated cell lines. Instead, we obtained CD133−/EpCAM+ and CD133−/EpCAM− subpopulations for HLE and HLF. Thus, the separation of hepatoma cells according to their CSC marker profile provided subpopulations unique for the further determination of tumorigenicity, chemoresistance and invasive capability, as well as signaling pathways affecting these critical aspects of oncogenesis and progression.
Figure 1. Flow cytometric analysis of hepatoma cells.
A. CD133+/ALDHhigh cells in Hepatoma cell lines. B. CD133+/EpCAM+ cells in hepatoma cell lines. CD133+/ALDHhigh and CD133−/ALDHlow or CD133+/EpCAM+ and CD133−/EpCAM− cells were separated by FACS after staining with APC-conjugated anti-CD133 antibody and an Adelfluor kit (A) or with APC-conjugated anti-CD133 and FITC-conjugated anti-EpCAM antibodies (B).
Tumorigenicity of FACS-enriched subpopulations
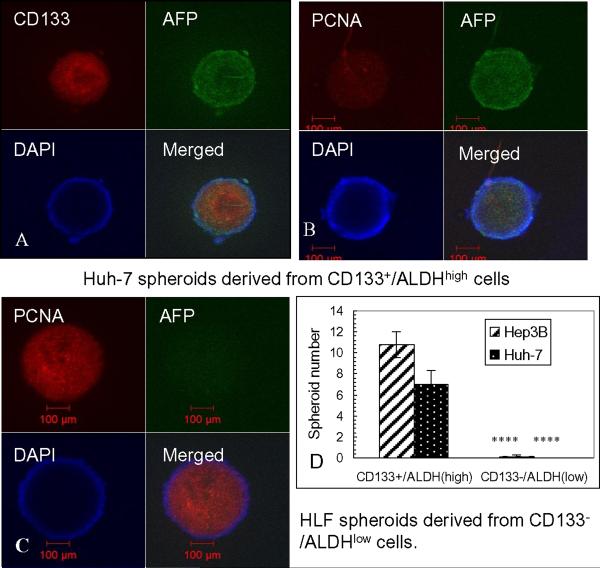
As shown in Fig. 2D, CD133+/ALDHhigh Huh-7 and Hep3B cells formed a higher number of spheroids than their double negative counterparts (p<0.001). Immunohistochemical staining showed that Huh-7 spheroids were CD133 and AFP-positive, as well as PCNA-positive, a marker of cell proliferation (Fig. 2A, B). HLF spheroids were CD133 (not shown) and AFP-negative, but PCNA positive (Fig. 2C). They were able to form large spheroids. In separate experiments, after FACS enrichment, 2000 cells of Huh-7 and Hep3B subpopulations were injected under the renal capsule of immunodeficient NOD-SCID IL-2Rγ−/− mice for xenograft formation. After 3–4 months, there was a trend towards more xenografts having formed in NOD-SCID mice injected with CD133+/EpCAM+ Huh-7 and Hep3B cells than in the double negative subpopulation-injected mice (p>0.05) (Table 1). Flow cytometric analysis of cells isolated from a xenograft tumor derived from CD133+/EpCAM+ Huh-7 cells showed that 90.4% of cells were still CD133+/EpCAM+, indicating that these cells maintained their phenotypes in vivo. Thus, it appeared that in well-differentiated hepatoma cell lines, CD133 expression is associated with higher levels of spheroid formation; however, in poorly-differentiated cell lines, CD133 expression is not essential for spheroid formation.
Figure 2. Spheroid formation of hepatoma subpopulations after FACS enrichment.
A, B and C: Spheroid cores were formed after seeding on low-attachable plates for 3 weeks, and co-stained with antibodies against AFP and CD133 or PCNA. D. Summary of spheroid formation in Hep3B and Huh-7 subpopulations 3 weeks after FACS enrichment.
Table 1.
Xenograft formation from FACS-enriched hepatoma subpopulations in NOD-SCID mice
| Hepatoma subpopulations | NOD-SCID mice injected | No. of mice formed tumors | P value |
|---|---|---|---|
| Huh-7 CD133+/EpCAM+ | 5 | 2 | |
| Huh-7 CD133−/EpCAM− | 5 | 0 | p>0.05 |
| Hep3B CD133+/EpCAM+ | 5 | 4 | |
| Hep3B CD133−/EpCAM− | 5 | 1 | p>0.05 |
Enhanced chemoresistance in HLE CD133−/ALDHlow subpopulation
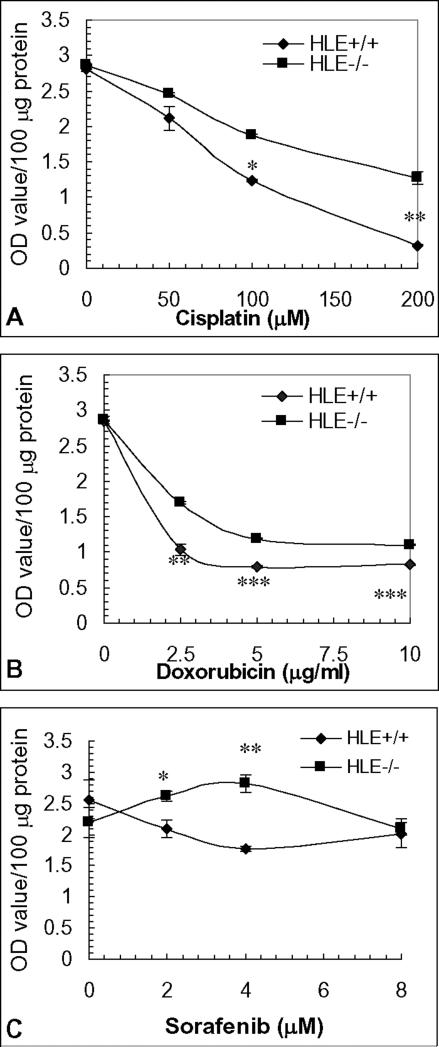
Viable cell numbers were determined spectrophotometrically using a WST-1 reagent after exposure to cisplatin and doxorubicin, both of which are included in transarterial chemoembolization (TACE) as one of adjuvant therapies, or sorafenib, a newly proven multikinase inhibitor targeting vascular endothelial growth factor (VEGF)-mediated angiogenesis. As shown in Fig. 3, more CD133−/ALDHlow HLE cells survived in a 24 hour-exposure to cisplatin, doxorubicin or sorafenib in comparison to the CD133+/ALDHhigh subpopulation. As the major subpopulation of poorly-differentiated hepatoma cell lines, the chemoresistance of CD133−/ALDHlow HLE cells may be comparable to insensitivity to chemotherapy of poorly-differentiated HCC.
Figure 3. Chemosensitivity of FACS-enriched HLE subpopulations.
Chemosensitivity of HLE subpopulations to cisplatin (A), doxorubicin (B) and sorafenib (C) was assayed with WST-1 reagent after treatment for 24 hours and expressed as optical density value per 100 μg protein content. *, **, *** p<0.05, 0.01 and 0.005 compared to HLE CD133+/ALDHhigh subpopulation.
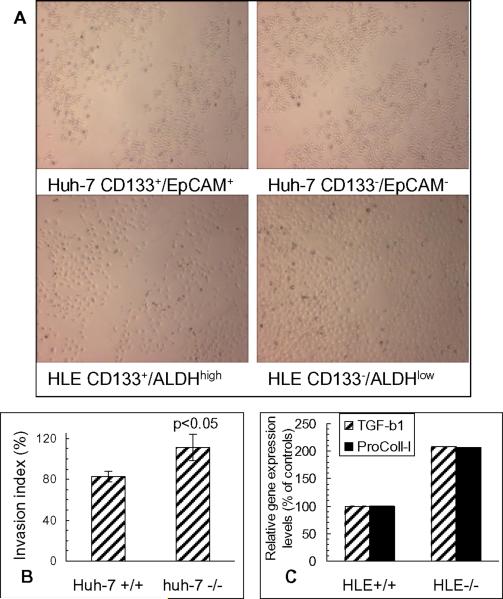
Enhanced invasive capability and wound healing in CD133-negative subpopulations
FACS-enriched Huh-7 and HLE subpopulations were subjected to in vitro Matrigel invasion and wound healing assays. As shown in Fig. 4A, both CD133−/EpCAM− Huh-7 cells and CD133−/ALDHlow HLE cells repaired the wounds quicker than their counterparts. Moreover, the in vitro Matrigel invasion assay demonstrated that more Huh-7 CD133−/EpCAM− cells crossed the separation membrane in the transwells than CD133+/EpCAM+ cells (p<0.05) (Fig. 4B). These results indicate that CD133− cells are not only chemoresistant, but also are more invasive in comparison with CD133+ cells, especially in poorly-differentiated HLE subpopulations.
Figure 4. Invasion capability and gene expression of TGF-β1 and procollagen type I in hepatoma subpopulations.
A. Wound healing after scraping in Huh-7 and HLE subpopulations. B. Matrigel invasion assay of Huh-7 subpopulations. C. Gene expression of TGF-β1 and procollagen type I by quantitative RT-PCR.
Acquisition of EMT in CD133− and poorly-differentiated subpopulations
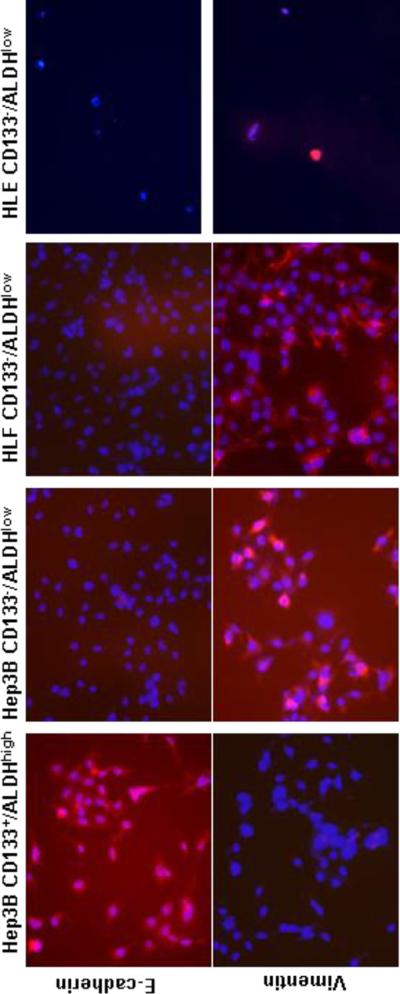
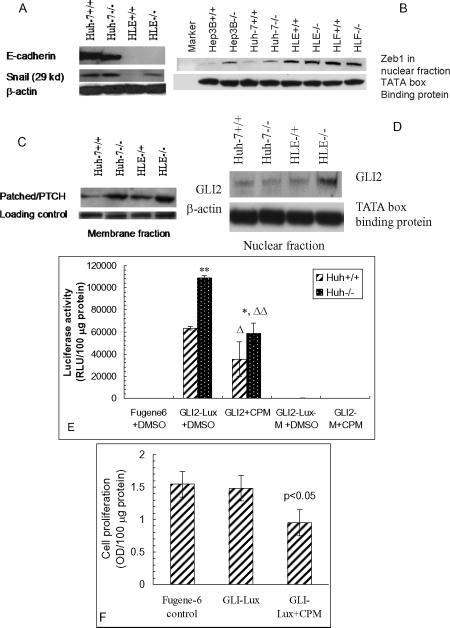
Having established that the CD133−/ALDHlow HLE subpopulation is chemoresistant, and possesses an enhanced invasive capability, we explored the underlying molecular mechanisms. We first stained subpopulations with E-cadherin, a cell surface adhering molecule, and vimentin, a cytoskeleton protein, and found that Hep3B CD133+/ALDHhigh cells were E-cadherin-positive, and vimentin-negative. Whereas, Hep3B CD133−/ALDHlow cells exhibited the opposite E-cadherin and vimentin expression profile, similar to CD133−/ALDHlow HLE and HLF cells (Fig. 5). They lost E-cadherin expression, but vimentin staining was obviously positive. This staining profile verified the acquisition of EMT in the Hep3B CD133−/ALDHlow subpopulation and in HLE and HLF cells, and was consistent with more TGF-β1 and procollagen type I (α1) expression in CD133−/ALDHlow HLE subpopulation (Fig. 4C). Snail is a transcription factor, which modulates the acquisition of EMT in many epithelial cells. Higher snail protein levels were found in CD133−/ALDHlow HLE and Huh-7 subpopulations than their double-positive counterparts as determined by Western blotting (Fig. 6A). The HLE double-negative subpopulation was almost completely negative for E-cadherin. CD133−/ALDHlow Huh-7 and Hep3B subpopulations appeared to express more Zeb1, another transcription factor modulating EMT occurrence in a nuclear fraction than their double-positive counterparts. Zeb1 nuclear expression was clearly positive in both poorly-differentiated HLE and HLF subpopulations when TATA box binding protein was used as a loading control of the nuclear fraction in Western blot analysis (Fig. 6B). In summary, both immunohistochemical staining and Western blot analysis of E-cadherin and two transcription factors, snail and Zeb1, demonstrated that in well-differentiated hepatoma cell lines, CD133−/ALDHlow subpopulations underwent EMT acquisition, whereas, poorly-differentiated hepatoma cell lines had already undergone EMT, especially in the CD133−/ALDHlow subpopulations.
Figure 5. Acquisition of EMT in CD133−/ALDHlowHep3B, HLE and HLF cells.
FACS-enriched CD133+/ALDHhigh or CD133−/ALDHlow subpopulations were stained with either Cy5-conjugated monoclonal antibodies against E-cadherin or vimentin in red. Cy5-stained images were overlaid with DAPI nuclear counter-staining in blue. Hep3B and HLF (20×), HLE (10×).
Figure 6. Western blot analysis of EMT markers and Hh signaling activity in Huh-7 and HLE subpopulations.
A. Western blot image of E-cadherin and snail in Huh-7 and HLE subpopulations. β-actin was used as a loading control. B. Zeb1 levels in various hepatoma subpopulations. TATA box binding protein was used as a nuclear protein loading control. C. PTCH1 levels in the membrane fraction of Huh-7 subpopulations were determined by Western blot analysis with integrin β1 as a loading control. D. GLI2 levels in nuclear fractions of Huh-7 and HLE subpopulations. E. Hh signaling activity after transfection with GLI-lux plasmid and mutated plasmid (GLI-Lux-M) in the presence or absence of cyclopamine (5 μM CPM). Luciferase activity was determined one day after transfection of the plasmids. ** p <0.01 compared to Huh-7 CD133+/EpCAM+ subpopulation. Δ, ΔΔ p <0.05 and 0.01 compared to GLI2-Lux+DMSO. DMSO was used to dissolve CPM, and included in transfection controls. F. Inhibition of cell proliferation by cyclopamine in Huh-7 CD133+/EpCAM+ subpopulation. Cell proliferation was determined after transfection with the GLI-Lux plasmid using the WST-1 reagent and expressed by optical density per 100 μg protein. Fugene-6 was included as a transfection reagent control.
Enhanced Hh signaling activity in CD133-negative hepatoma populations
Hh signaling activation has been shown to lead to EMT. After defining the association of EMT acquisition with chemoresistance and enhanced invasion capability in poorly-differentiated HLE subpopulations, we investigated whether the Hh signaling activation contributes to EMT and down-stream effects in hepatoma cells. We first determined that more Patched/PTCH1, the receptor for Hh ligand, was expressed in Huh-7 CD133−/EpCAM− than in CD133+/EpCAM+ cells, and more in CD133−/EpCAM− than CD133−/EpCAM+ HLE cells in the membrane fraction by Western blot analysis (Fig. 6C). Then, we found that GLI2, a transcription factor in the Hh signaling pathway, was increased in the nuclear fraction of HLE CD133−/EpCAM− cells compared to CD133−/EpCAM+ cells (Fig. 6D). After that, we transfected Huh-7 subpopulations with a GLI-lux reporter system, and determined luciferase activity one day after transfection. It was found that luciferase activity was much higher in Huh-7 CD133−/EpCAM− cells than their double-positive counterparts, while cells transfected with mutated GLI-Lux did not show any luciferase activity (Fig. 6E). Moreover, when transfected cells were treated with an Hh signaling inhibitor, cyclopamine (CPM), both cell proliferation as determined by SWT-1 reagent and luciferase activity were markedly inhibited by the treatment (Fig. 6E, F). These findings demonstrated that the Hh signaling activity in either CD133−/EpCAM− Huh-7 or HLE cells was higher than CD133+/EpCAM+ or CD133−/EpCAM+ cells, and that enhanced Hh signaling activity may be responsible for the acquisition of EMT, as well as cell proliferation, chemoresistance and aggressive invasion capability in these cells.
DISCUSSION
The concept of CSCs is a new paradigm of cancer biology explaining many existing basic and clinical challenges. However controversies exist in terms of which cell markers are specific for CSCs, whether CSCs are prominent in cancer tissues, and even whether CSCs are the origin for cancer development. These controversies make the identification of CSCs in HCC more complicated. It is generally accepted that CSCs are probably the progenitor cells that undergo unknown genetic mutations, lose potential for tissue repair, but retain stem cell characteristics, such as self renewal and plasticity to differentiate into different cell types during various stages of oncogenicity and treatment [3, 13]. With this regard, it is our goal to search for CSCs in HCC tissues and to define which subpopulations in heterogeneous malignant cells represent the characteristics of CSCs in HCC.
In our previous studies, we were able to co-localize CD133+ and ALDH+ cells in HCC tissue, especially in the areas of connective tissues and in vessels with HCC invasion [8]. These cells were also positive for CD44 and CD90. Thus, we believe that these markers are useful in identifying CSCs in HCC tissue. However, these markers are not specific for CSCs; they are also positive for stem or progenitor cells in chronic liver injury with fibrosis/cirrhosis [8, 14]. In the present study, we separated each of six hepatoma cell lines into subpopulations according to their CSC marker expression profile, and defined their phenotypic characteristics, such as tumorigenicity, chemoresistance and invasive capability. More CD133+/ALDHhigh or CD133+/EpCAM+ cells were separated from well-differentiated Hep3B, HepG2 and Huh-7 lines than from poorly-differentiated HLE, HLF and SKHep1 lines. In fact, HLE, HLF and SKHep1 cells had very low levels of CD133 positivity (Fig. 1A&B). We also found that CD133+/ALDHhigh Hep3B and Huh-7 cells formed more spheroids than their CD133−/ALDHlow counterparts, whereas, CD133−/ALDHlow HLE, HLF and SHHep1 subpopulations formed large spheroids (Fig. 2). Implantation of 2000 freshly FACS-enriched CD133+/EpCAM+ Hep3B and Huh-7 cells under the renal capsules of immunodeficient NOD-SCID IL2Rγ−/− mice for 3–4 months resulted in 4 out 5 Hep3B and 2 out of 5 Huh-7 CD133+/EpCAM+-injected mice forming xenografts, whereas, one xenograft was formed in mice after implantation of CD133−/EpCAM− Hep3B and no xenograft was seen in mice receiving implantation of CD133−/EpCAM− Huh-7 cells. These data are consistent with recent reports [15, 16] that CD133+/ALDHhigh or CD133+/EpCAM+ subpopulations were more tumorigenic both in vitro and in vivo. These hepatoma cells are characterized as well-differentiated with high levels of hepatic gene expression levels, and most are AFP-positive. Thus, concerning tumorigenicity, this subpopulation has been considered as CSCs or initiating cells (TICs) with high Wnt/β-catenin signaling activity [16].
We further evaluated the chemoresistance of FACS-enriched subpopulations, and found that CD133−/ALDHlow HLE cells were less sensitive to cisplatin, doxorubicin and sorafenib than their CD133+/ALDHhigh subpopulation during 24 hrs of the treatments. The in vitro Matrigel invasion assay showed that Huh-7 CD133−/EpCAM− cells were more invasive than their positive counterparts, and CD133−/ALDHlow HLE cells were able to repair scraping wounds faster than their double positive counterparts. Thus, these findings indicate that CD133−/ALDHlow or CD133−/EpCAM− cells were more chemoresistant and invasive, which is in accordance with clinical observations that poorly-differentiated HCC is more refractory to chemotherapy and has a poor prognosis [17] and that the development of insensitivity to epithelial growth factor receptor inhibitors (erlotinib, gefitinib and cetuximab) was a result of EMT acquisition [18]. Therefore, we hypothesized that the insensitivity to chemotherapy and more invasive capability in CD133−/ALDHlow or CD133−/EpCAM− cells could be attributed to the EMT acquisition, and further investigated the occurrence of EMT and its regulations through Hh signaling pathways.
We performed immunocytochemical staining, and found that well-differentiated CD133+/ALDHhigh cells are E-cadherin-positive and vimentin-negative, whereas their CD133−/ALDHlow counterparts had an opposite E-cadherin and vimentin expression profile. In contrast, poorly-differentiated CD133−/ALDHlow cells were E-cadherin-negative and vimentin-positive (Fig. 5). These findings were supported by more TGF-β1 and procollagen type I (α1) expression in the HLE CD133−/ALDHlow subpopulation. The fact that no E-cadherin was found in CD133−/ALDHlow HLE and HLF subpopulations by Western blot analysis, more GLI2 in the nuclear fraction of cell lysates, more Zeb1 expression in CD133−/EpCAM− Hep3B and Huh-7 cells than their double positive counterparts, and that Zeb1 was positive for both CD133−/EpCAM+ or CD133−/EpCAM− HLE and HLF subpopulations also confirms the findings (Fig. 6). These findings support the concept that there was EMT acquisition in poorly-differentiated hepatoma cell lines, especially in CD133−/ALDHlow or CD133−/EpCAM− subpopulations, and that the EMT acquisition may be responsible for their chemoresistance and aggressive invasion. The loss of epithelial characteristics and the acquisition of the mesenchymal phenotype are the typical phenotypic changes in EMT, which may enhance mobility and the invasive predisposition of cancer cells. The increased migratory activity is mediated by repression of cell to cell adhesion proteins, such as E-cadherin, and the induction of invasion-associated matrix metalloproteinase (MMP) production [19]. Currently, it is known that EMT can be induced by TGF-β1 via activation of a transcription factor, snail, by forming a complex with smad3 and smad4 [20]. Zeb1 is a zinc finger E-box transcription factor that negatively modulates the E-cadherin expression in epithelial cells through inhibition of the miRNA200 family. Zeb1 appears to be a critical factor for the initiation and dissemination of pancreatic and colorectal cancer cells [21].
The Hh signaling pathway regulates body patterning and organ development during embryo development. In adults, the Hh pathway is mainly quiescent with the exception of roles in tissue maintenance and repair, and its inappropriate reactivation has been linked to several human cancers, such as breast cancer [22]. The activation of the Hh signaling pathway occurs when Hh binds to its receptor (PTCH1), and when GLI2/3A is translocated into the nucleus to activate target genes, such as snail, bcl2, and cyclin D, etc. leading to proliferation, EMT and cell survival [23]. The activation of the Hh signaling pathway has been found in cholangiocytes and hepatocytes in models of primary biliary cirrhosis (PBC) and non-alcoholic steatohepatitis (NASH) in which the acquisition of EMT in hepatocytes and cholangiocytes contributes to the development of hepatic fibrosis [24]. To our knowledge, no previous study has explored Hh signaling involvement in poorly-differentiated hepatoma cells, and its link with EMT in CD133−/ALDHlow or CD133−/EpCAM− hepatoma cells. Hence, we hypothesized that the activation of Hh signaling pathway may be responsible for the acquisition of EMT and chemoresistance seen in poorly-differentiated hepatoma cells. To test this hypothesis, we first determined Hh signaling activation by the identification of PTCH1 in the membrane fraction and GLI2 translocation in nuclear extracts in the HLE CD133−/ALDHlow subpopulation. Then, we transfected a GLI-lux reporter system in FACS-enriched hepatoma subpopulations, and found that CD133−/EpCAM− Huh-7 cells displayed much higher luciferase activity than CD133+/EpCAM+. Mutated GLI-Lux plasmid transfection did not cause any elevation in luciferase activity in either subpopulation. Moreover, a specific Hh inhibitor, cyclopamine (CPM) not only significantly blocked Hh signaling activity, but also inhibited cell proliferation of CD133+/EpCAM+ Huh-7 cells. These data for the first time demonstrated that Hh signaling is critical for tumorigenesis of CSC subpopulations. Blocking Hh signaling with a small molecule, such as HhAntag (Cur-691), which has been used to eliminate medulloblastoma in mice [25], would be a new approach in molecular target of therapy in eradicating chemoresistant subpopulations of HCC; in vivo experiments are needed to further prove this therapeutic strategy. In summary, these findings establish a new basis for reclassification of HCC specimens based on the acquisition of EMT and Hh signaling activity levels, and aid in the development of individual treatment strategies based on HCC molecular classification [26].
In conclusion, in well-differentiated hepatoma cells, CD133+/ALDHhigh or CD133+/EpCAM+ cells appear to be a CSC/initiating subpopulation; whereas, in poorly-differentiated hepatoma cell lines, EMT and enhanced hedgehog signaling activity may be responsible for their chemoresistance and invasion. Our data suggest that novel molecular therapy targeting transcription factors of EMT and Hh signaling pathways could be promising in eradicating chemoresistant poorly-differentiated subpopulations in HCC.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to Dr. Hiroshi Sasaki in RIKEN Center for Developmental Biology, Kobe, Hyogo, Japan for providing a GLI-Lux reporter system.
Financial support: The study was supported by the NIH grant (R01 DK075415) and the California Institute of Regenerative Medicine (CIRM, RC1-00359) to MAZ; as well as UC Davis Stem Cell Program Start-up Funding for JN, and Technology Transfer fund from UC Davis Medical Center to JW.
Abbreviations used in this manuscript:
- ALDH
aldehyde dehydrogenase
- APC
allophycocyanin
- CPM
cyclopamine
- CSCs
cancer stem cells
- DAPI
4',6-diamidino-2-phenylindole
- EMT
epithelial mesenchymal transition
- FACS
fluorescence-activated cell sorting
- FITC
fluorescein isothiocyanate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HCC
hepatocellular carcinoma
- Hh
hedgehog
- PCNA
proliferating cell nuclear antigen
- TGF-β1
transforming growth factor-β1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- [2].Tanaka S, Arii S. Molecularly targeted therapy for hepatocellular carcinoma. Cancer Sci. 2009;100:1–8. doi: 10.1111/j.1349-7006.2008.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- [4].Park CY, Tseng D, Weissman IL. Cancer stem cell-directed therapies: recent data from the laboratory and clinic. Mol Ther. 2009;17:219–230. doi: 10.1038/mt.2008.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- [6].Zhou P, Hohm S, Olusanya Y, Hess DA, Nolta J. Human progenitor cells with high aldehyde dehydrogenase activity efficiently engraft into damaged liver in a novel model. Hepatology. 2009;49:1992–2000. doi: 10.1002/hep.22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Salnikov AV, Groth A, Apel A, Kallifatidis G, Beckermann BM, Khamidjanov A, et al. Targeting of cancer stem cell marker EpCAM by bispecific antibody EpCAMxCD3 inhibits pancreatic carcinoma. J Cell Mol Med. 2009;13:4023–4033. doi: 10.1111/j.1582-4934.2009.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lingala S, Cui YY, Chen X, Ruebner BH, Qian XF, Zern MA, Wu J. Immunohistochemical staining of cancer stem cell markers in hepatocellular carcinoma. Exp Mol Pathol. 2010;89:27–35. doi: 10.1016/j.yexmp.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wu J, Liu L, Yen RD, Catana A, Nantz MH, Zern MA. Liposome-mediated extracellular superoxide dismutase gene delivery protects against acute liver injury in mice. Hepatology. 2004;40:195–204. doi: 10.1002/hep.20288. [DOI] [PubMed] [Google Scholar]
- [10].Chen XL, Murad M, Cui YY, Yao LJ, Venugopal SK, Dawson K, Wu J. Transplantation. 2010. miRNA signatures in remaining liver of 50% partial hepatectomy and small size liver graft in rat recipients. in press. [DOI] [PubMed] [Google Scholar]
- [11].Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–1322. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- [12].Liu L, Zern MA, Lizarzaburu ME, Nantz MH, Wu J. Poly(cationic lipid)-mediated in vivo gene delivery to mouse liver. Gene Ther. 2003;10:180–187. doi: 10.1038/sj.gt.3301861. [DOI] [PubMed] [Google Scholar]
- [13].Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oliva J, French BA, Qing X, French SW. The identification of stem cells in human liver diseases and hepatocellular carcinoma. Exp Mol Pathol. 2010;88:331–340. doi: 10.1016/j.yexmp.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, Guan XY. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- [16].Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Perez-Saborido B, de los Galanes SJ, Meneu-Diaz JC, Romero CJ, Elola-Olaso AM, Suarez YF, et al. Tumor recurrence after liver transplantation for hepatocellular carcinoma: recurrence pathway and prognostic factors. Transplant Proc. 2007;39:2304–2307. doi: 10.1016/j.transproceed.2007.06.059. [DOI] [PubMed] [Google Scholar]
- [18].Fuchs BC, Fujii T, Dorfman JD, Goodwin JM, Zhu AX, Lanuti M, Tanabe KK. Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res. 2008;68:2391–2399. doi: 10.1158/0008-5472.CAN-07-2460. [DOI] [PubMed] [Google Scholar]
- [19].Zhao XL, Sun T, Che N, Sun D, Zhao N, Dong XY, et al. Promotion of hepatocellular carcinoma metastasis through matrix metalloproteinase activation by epithelial-mesenchymal transition regulator twist1. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01052.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, et al. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11:943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- [22].Kasper M, Jaks V, Fiaschi M, Toftgard R. Hedgehog signalling in breast cancer. Carcinogenesis. 2009;30:903–911. doi: 10.1093/carcin/bgp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Scales SJ, de Sauvage FJ. Mechanisms of hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- [24].Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- [26].Hoshida Y, Toffanin S, Lachenmayer A, Villanueva A, Minguez B, Llovet JM. Molecular classification and novel targets in hepatocellular carcinoma: recent advancements. Sem Liver Dis. 2010;30:35–51. doi: 10.1055/s-0030-1247131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.